Abstract
Intraperitoneal injection of CCK reduces food intake and triggers a behavioral pattern similar to natural satiation. Reduction of food intake by CCK is mediated by vagal afferents that innervate the stomach and small intestine. These afferents synapse in the hindbrain nucleus of the solitary tract (NTS) where gastrointestinal satiation signals are processed. Previously, we demonstrated that intraperitoneal (IP) administration of either competitive or noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonists attenuates reduction of food intake by CCK. However, because vagal afferents themselves express NMDA receptors at both central and peripheral endings, our results did not speak to the question of whether NMDA receptors in the brain play an essential role in reduction of feeding by CCK. We hypothesized that activation of NMDA receptors in the NTS is necessary for reduction of food intake by CCK. To test this hypothesis, we measured food intake following IP CCK, subsequent to NMDA receptor antagonist injections into the fourth ventricle, directly into the NTS or subcutaneously. We found that either fourth-ventricle or NTS injection of the noncompetitive NMDA receptor antagonist MK-801 was sufficient to inhibit CCK-induced reduction of feeding, while the same antagonist doses injected subcutaneously did not. Similarly fourth ventricle injection of d-3-(2-carboxypiperazin-4-yl)-1-propenyl-1-phosphoric acid (d-CPPene), a competitive NMDA receptor antagonist, also blocked reduction of food intake following IP CCK. Finally, d-CPPene injected into the fourth ventricle attenuated CCK-induced expression of nuclear c-Fos immunoreactivity in the dorsal vagal complex. We conclude that activation of NMDA receptors in the hindbrain is necessary for the reduction of food intake by CCK. Hindbrain NMDA receptors could comprise a critical avenue for control and modulation of satiation signals to influence food intake and energy balance.
Keywords: vagus, satiation, gut-brain peptides
satiation is the process by which food entering the gastrointestinal tract gradually reduces food intake, eventually resulting in termination of a meal. Previous reports from our group and others indicate that systemic administration of antagonists of N-methyl-d-aspartate-type glutamate receptors (NMDAr antagonists) delays satiation and increases meal size (see, for example, Refs. 8, 9, and 25). Additionally, we demonstrated that injecting NMDAr antagonists directly into the nucleus of the solitary tract (NTS), where vagal afferents from the gastrointestinal tract synapse, increases meal size (19, 23, 46) and that lesions of the NTS abolish this effect (47). These observations suggest that NMDA receptors, perhaps in the NTS, participate in vagally mediated control of food intake.
The hypothesis that NMDA receptors participate in the process of satiation is supported by our prior reports that peripherally administered NMDA receptor antagonists attenuate reduction of food intake induced by CCK (11, 19), a gut peptide known to reduce food intake by activating abdominal vagal afferents (43, 44). However, functional evidence suggests that NMDA receptors on peripheral vagal afferent fibers modulate gastrointestinal sensory information (42). Consequently, it is not clear whether systemically administered NMDA receptor antagonists attenuate reduction of food intake by interfering with CCK-induced vagal activation in the periphery, or by altering vagal communication in the hindbrain. In the experiments reported here, we tested the hypothesis that antagonism of NMDA receptors in the hindbrain, near the site of vagal afferent termination, would be sufficient to prevent reduction of food intake and neuronal activation following intraperitoneal (IP) injection of CCK.
We found that competitive and noncompetitive NMDA receptor antagonists injected into the fourth ventricle or directly into the NTS, at doses that had no effect when injected subcutaneously or intraperitoneally, blocked reduction of food intake by IP CCK. We also found that fourth-ventricle administration of a competitive NMDA receptor antagonist attenuated CCK-induced increase in c-Fos expression in the hindbrain. Taken together, these results indicate that antagonism of NMDA receptors in the hindbrain is sufficient to attenuate reduction of food intake by CCK, and are consistent with the hypothesis that hindbrain NMDA receptors participate in control of food intake by modulating vagally mediated satiation signals in the hindbrain.
METHODS
Subjects.
Male adult Sprague-Dawley rats (Simonsen Laboratories, Gilroy, CA) were individually housed in hanging wire mesh cages in a vivarium under conditions of controlled illumination (12:12-h light-dark cycle; lights out 1800), humidity (70%), and temperature (22°C). Rats weighed 280–350 g at the beginning of experiments. Rats were handled daily and habituated to laboratory conditions before surgery or testing began, and they had ad libitum access to pelleted rodent diet (Teklad, Kent, WA) and water except during experiments and overnight fasts, as described below. All animal housing and experiments reported here were conducted in compliance with the National Institutes of Health's Guide for Care and Use of Laboratory Animals under a protocol approved by the Washington State University Institutional Animal Care and Use Committee.
Drugs.
MK-801 (Sigma, St. Louis, MO), d-3-(2-carboxypiperazin-4-yl)-1-propenyl-1-phosphoric acid (d-CPPene, Tocris, Ellisville, MO), and sulfated cholecystokinin octapeptide (CCK-8, Peptides International, Louisville, KY) were dissolved in sterile 0.9% NaCl. Peripheral injections of MK-801 and d-CPPene were made subcutaneously in a volume of 0.4 ml. CCK-8 was injected intraperitoneally in a volume of 1.0 ml/kg BW. Central injections of MK-801 and d-CPPene into the fourth ventricle (4V; 3 μl) or into the NTS (100 nl) were made via a 26-gauge guide cannula using a 33-gauge injector attached to a 5-μl Hamilton syringe. Sterile 0.9% NaCl injections served as the vehicle controls in all experiments.
Surgical procedures.
Rats were anesthetized with 50 mg/kg ketamine, (Pfizer, New York, NY), 25 mg/kg xylazine (Vedco, St. Joseph, MO), and 2 mg/kg acepromazine (Boehringer Ingelheim Vetmedica, St. Joseph, MO), placed in a stereotaxic instrument, with head leveled between lambda and bregma, and implanted with 26-gauge stainless-steel guide cannulas (McMaster-Carr, Santa Fe Springs, CA) aimed for the rostral 4V (−10.5 mm caudal from bregma, −6.05 mm ventral from surface of the dura, and 0.0 mm from midline) or left medial NTS (0.1 mm caudal to the occipital crest 0.8 mm lateral to the midline, 7.8 mm ventral to the surface of the skull). Cannulas were secured to the skull using stainless-steel screws and methacrylate (Orthojet, Patterson Dental Supply, Spokane, WA). Immediately following surgery, the analgesic flunixin meglumine (2.5 mg/kg; MWI Veterinary Supply, Meridian, ID) and the antibiotic procaine penicillin G (300,000 units/kg; Norbrook, Lenexa, KS) were administered subcutaneously. Rats were allowed 2 wk postsurgical recovery, after which they all exceeded their presurgical body weights.
Effects of fourth-ventricular vs. peripheral administration of MK-801 on CCK-mediated reduction of chow intake.
Rats (n = 15) were adapted to eat a meal of pelleted rodent diet over 30 min, following an overnight 15-h fast. Briefly, food, but not water, was removed 1 h prior to lights out, and a weighed amount was returned between 0900 and 1000 the following day. Food intake and spillage were measured over the ensuing 30 min, after which the rats were allowed ad libitum access to both food and water for at least the next 48 h, before another overnight fast was imposed. On experimental days, each rat received a 3-μl injection of either 0.9% NaCl or 6 μg MK-801 in 3 μl 0.9% NaCl via its 4V cannula. This dose of MK-801 was chosen because our previously published (46) and unpublished work indicate that doses of this order reliably increase food intake without producing locomotor side effects. Five minutes after the 4V injection, animals received an IP injection of either CCK-8 (2 μg/kg in 0.9% NaCl) or 0.9% NaCl. Following the IP injection, the rats were given immediate access to a preweighed amount of pelleted rodent diet, and intake less spillage was recorded for 30 min, as described above. After completion of testing with 4V injections, 8 of the same 15 rats were tested with subcutaneous MK-801, according to the same procedure described above, except that instead of receiving MK-801 or NaCl via the 4V cannulas, the rats were injected with 0.4 ml 0.9% NaCl or 6 μg MK-801 in 0.4 ml 0.9% NaCl subcutaneously, and 30-min food intake was measured, as described above. Each rat received the following testing injection combinations in the following order in both the 4V injection and subcutaneous injection tests: 4V NaCl/IP NaCl, 4V NaCl/IP CCK, 4V MK-801/IP CCK, 4V MK-801/IP NaCl, and 4V NaCl/IP NaCl. For data analysis and presentation, intakes following the first and last NaCl/NaCl tests were averaged.
Effects of administration of MK-801 into the NTS on CCK-mediated reduction of chow intake.
A separate group of rats (n = 14) implanted unilaterally with 26-gauge cannulas aimed for the NTS was used to assess the effect of injection of MK-801 directly into the NTS on reduction of food intake by IP CCK-8. Using an experimental protocol similar to that employed for 4V injections, we administered 500 ng of MK-801 in either 100 nl 0.9% NaCl for NTS injection or in 0.4 ml 0.9% NaCl for subcutaneous injections. As before, injections of an equivalent volume of 0.9% NaCl comprised the control procedure. Once again, testing for response to subcutaneous injections was conducted after completion of testing for effects of intra-NTS injections. As in the 4V MK-801 experiment above, each animal received the following testing injection combinations in the following order in both the NTS injection and subcutaneous injection tests: SC NaCl/IP NaCl, SC NaCl/IP CCK, SC MK-801/IP CCK, SC MK-801/IP NaCl, and SC NaCl/IP NaCl. For data analysis and presentation, intakes following the first and last NaCl/NaCl tests were averaged.
Effect of fourth ventricular vs. peripheral administration of d-CPPene on CCK-mediated reduction of chow intake.
To examine the effect of hindbrain administration of a competitive NMDA receptor antagonist on reduction of food intake by CCK, d-CPPene (40 ng in 3 μl) was injected into the 4V (n = 11) following an overnight (16–20 h) fast, as described above. We chose the 40-ng dose of d-CPPene based on our prior dose-response studies with centrally administered competitive NMDA antagonists, including d-CPPene (18). Treatment combinations were as follows: 4V NaCl/IP NaCl, 4V NaCl/IP CCK, 4V d-CPPene/IP CCK, 4V NaCl/IP d-CPPene, and 4V NaCl/IP NaCl. d-CPPene was always administered 1 min before IP CCK-8 (2 μg/kg). Immediately after CCK-8 injection, a preweighed amount of pelleted rodent diet was presented, and intake less waste was recorded at 30 min post-CCK-8 injection. Intakes for the first and last NaCl/NaCl tests were averaged for the purposes of data presentation and analysis.
Using rats from the same group tested for effects of 4V d-CPPene, we also examined the ability of IP d-CPPene to prevent CCK-induced reduction of food intake. Our prior published work indicated that an IP dose of 2 mg/kg, but not 0.5 or 1 mg/kg, of d-CPPene increases meal size in rats (19). Furthermore, we found that the 2 mg/kg dose was sufficient to attenuate reduction of food intake by IP CCK (19). Therefore, in the current experiment, we used a protocol identical to that used for testing the effects of 4V and subcutaneous NMDA antagonists on CCK-induced reduction of food intake, to examine the efficacy of d-CPPene (1 mg/kg; n = 5) and d-CPPene (2 mg/kg; n = 6) for the ability to attenuate reduction of food intake by CCK-8 (2 μg/kg ip). In so doing, we had the opportunity to replicate our prior results, using the 2 mg/kg ip dose, while using the 1 mg ip dose as a very conservative control for potential peripheral effects of our 4V-injected d-CPPene. The order of testing was the same as described in all previous experiments, except d-CPPene or 0.9% NaCl was injected intraperitoneally in a volume of 1 ml/kg. Thirty-minute food intake was measured as described above.
Effects of 4V administration of d-CPPene on CCK-induced expression of hindbrain c-Fos-immunoreactivity.
To determine whether antagonism of hindbrain NMDA receptors would attenuate CCK-induced activation of neurons in the dorsal vagal complex, as indicated by increased expression of hindbrain c-Fos immunoreactivity, a group of naïve rats (n = 14) was divided in four subgroups of 3–5 rats each. After an overnight (15 h) fast, each subgroup received one of the following treatments: 4V NaCl/IP NaCl (n = 3), 4V NaCl/IP CCK (n = 3), 4V d-CPPene/IP CCK (n = 5), and 4V d-CPPene/IP NaCl (n = 3). Fourth-ventricle injections of d-CPPene (40 ng) or 0.9% NaCl were in volumes of 3 μl and were followed immediately by an IP injection of either CCK-8 (10 μg/kg) or 0.9% NaCl. We chose to use a CCK-8 dose of 10 μg/kg, rather than the 2 μg/kg dose that we used for our behavioral studies, to induce a maximal amount of c-Fos and reduce variability in expression between animals. Rats were not given food after injections. Rather, 90 min after IP injection, animals were rapidly and deeply anesthetized with isoflurane, exsanguinated, and perfused intracardially with 0.1 M phosphate buffer NaCl followed by 4% paraformaldehyde (#19202; Electron Microscopy, Hatfield, PA) in 0.1 M phosphate buffer (pH 7.4). Immediately after perfusion, brains were collected, blocked, post-fixed in the same fixative for 2 h, and cryoprotected in 0.1 M phosphate buffer containing 25% sucrose overnight at 4°C. Thirty-micrometer coronal sections through the hindbrain were cut in a cryostat for immunohistochemical detection, and quantification of c-Fos-immunoreactive neuronal nuclei, as previously described (15, 35). Briefly, sections were incubated for 24 h at room temperature in primary antiserum raised in rabbit against a peptide representing amino acids 4–17 of human c-Fos (Ab-5, 1:10,000; EMD Biosciences Gibstown, NJ). Then, sections were washed and incubated overnight in biotintylated donkey anti-rabbit serum (#711-065-152, 1:500; Jackson ImmunoResearch Laboratories, West Grove, PA). The sections were washed again and incubated for 3 h in avidin conjugated to ExtrAvidin (#e2886, 1:1,500; Sigma). The avidin-biotin-antibody complex was revealed histochemically using a nickel-intensified diaminobenzidine reaction (Sigma). After being mounted on slides, cleared, and cover-slipped, hindbrain sections were examined microscopically and counts of c-Fos-immunopositive nuclei were made. The counts for each section were done manually using four sections per rat; one section at each of four different brain levels, corresponding to 14.1 mm, 13.8 mm, 13.6 mm, and 13.3 mm caudal to bregma, according to the stereotaxic atlas of Paxinos and Watson (30). c-Fos-positive nuclei in the commissural NTS (cNTS), medial NTS (mNTS), area postrema (AP), and the dorsal motor nucleus of the vagus (DMV) were counted bilaterally in each section for each rat. The numbers of c-Fos-immunoreactive nuclei are presented as averages for each brain area across all of the rostrocaudal levels at which the particular structure appeared.
Statistics.
Data from all experiments was subjected to statistical analysis using appropriate one- or two-way repeated-measures ANOVA, followed by the Holme-Sidak test. In behavioral experiments, the repeated factor was treatment condition, whereas in our immunohistochemical experiment, the repeated factor was brain area analyzed. The confidence limit for statistical significance was set at P < 0.05. However, wherever actual confidence limits were substantially less than 0.05, those P values are provided.
RESULTS
Prevention of CCK-8-induced reduction of food intake by 4V or NTS injection of MK-801.
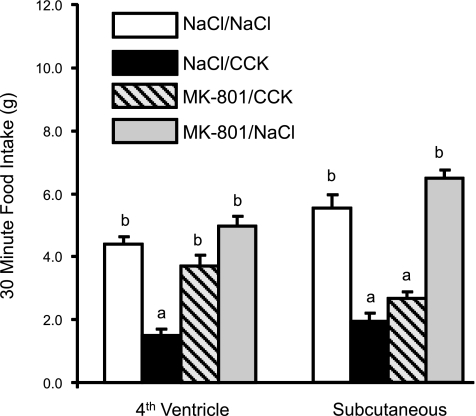
Figure 1 illustrates the effect of 4V or subcutaneous injection of MK-801 (6 μg/rat) on reduction of 30-min food intake following IP injection of CCK-8 (2 μg/kg). There were significant differences between treatment conditions in the 4V injection experiment [F(3,14) = 44.301, P < 0.001]. Thirty-minute food intake following 4V NaCl and CCK-8 (2 μg/kg ip) was reduced compared with intake following 4V NaCl and IP NaCl (P < 0.01). Intake after 4V MK-801/IP CCK-8 did not differ from intake after 4V NaCl/IP NaCl, but it was significantly greater than intake after 4V NaCl/IP CCK (P < 0.001). There also were significant differences between treatment conditions following subcutaneous injections of MK-801 or NaCl in combination with IP CCK or NaCl [F (3,7) = 79.5, P < 0.001]. Specifically, compared with SC NaCl/IP NaCl, 30-min food intake was significantly reduced following SC NaCl/IP CCK (P < 0.001). However, subcutaneous injection of MK-801 (6 μg) did not attenuate reduction of food intake by IP CCK. Intake after SC MK-801/IP CCK was significantly reduced compared with intake after SC NaCl/IP NaCl (P < 0.001) and was not significantly different from intake after SC NaCl/IP CCK.
Fig. 1.
CCK-8-induced reduction of 30-min food intake following fourth-ventricle or subcutaneous injection of the noncompetitive N-methyl-d-aspartate (NMDA) channel blocker, MK-801. Rats were deprived of food, but not water, overnight, and then injected with either 0.9% NaCl or MK-801 (6 μg/rat) either via the fourth ventricle or subcutaneously. Five minutes later, they received IP injections of either 0.9% NaCl or CCK-8 (2 μg/kg) and then were immediately presented with weighed pelleted rodent diet in their home cages. Fourth ventricle, but not subcutaneous, MK-801 injection prevented reduction of food intake by CCK-8. a,bDissimilar letters above bars indicate significantly different intakes (P < 0.001).
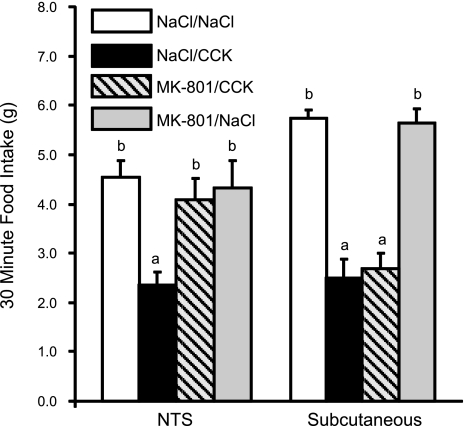
Figure 2 illustrates the effects of injecting of MK-801 (500 ng/rat) directly into the NTS or subcutaneously on reduction of 30-min food intake following IP injection of CCK-8 (2 μg/kg). Histological examination revealed that 9 of 14 rats with cannulas aimed for the dorsal vagal complex had cannula tips located in the medial NTS or in the dorsal aspect of the DMV. The remaining 5 cannula placements were located in the cerebellum and were not included in statistical analysis for effects of NTS injections on reduction of food intake by IP CCK-8. Administration of MK-801 (500 ng) directly into the nucleus of the solitary tract prior to IP CCK-8 prevented CCK-induced reduction of food intake [F (3,8) = 5.199, P = 0.007] (Fig. 2). Food intake following NTS MK-801/IP CCK-8 injection did not differ significantly from the NTS NaCl/IP NaCl condition, but it was significantly greater than following NTS NaCl/IP CCK (P < 0.01). When we injected the same 500-ng dose of MK-801 subcutaneously prior to CCK administration, reduction of food intake by IP CCK was not attenuated. Results from 4V and NTS injections of MK-801 indicate that noncompetitive blockade of hindbrain NMDA channels prevents CCK-induced reduction of food intake at doses that are not effective when injected subcutaneously.
Fig. 2.
CCK-8-induced reduction of 30-min food intake following nucleus of the solitary tract (NTS) or subcutaneous injection of the noncompetitive NMDA channel blocker, MK-801. Injection of MK-801 (50 ng) into the NTS, but not subcutaneously, prevented reduction of food intake by CCK-8. a,bDissimilar letters above bars indicate significantly different intakes (P < 0.01).
Prevention of CCK-8-induced reduction of food intake by 4V injection of d-CPPene.
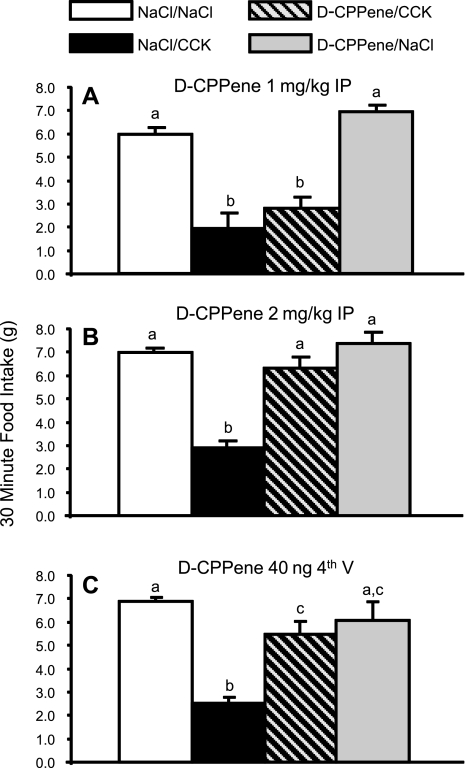
Unlike MK-801, a noncompetitive blocker of the NMDA receptor ion channel, d-CPPene competes with glutamate for its binding site on the NR2A and NR2B channel subunits. Figure 3C illustrates the effects of 4V d-CPPene (40 ng/rat) on reduction of 30-min food intake following IP injection of CCK-8 (2 μg/kg). There were significant differences in food intake between treatment conditions when d-CPPene or NaCl was administered via the 4V followed by either intraperitoneally administered CCK-8 or NaCl [F (3,9) = 41.851, P < 0.001]. Thirty-minute food intake following 4V NaCl/IP CCK was significantly reduced compared with 4V NaCl/IP NaCl. Fourth-ventricle injection of d-CPPene (40 ng) prevented reduction of food intake by IP CCK. Specifically, intake following 4V d-CPPene/IP CCK did not differ from intake after 4V NaCl/IP NaCl. In experiments combining IP injection of d-CPPene at a dose of 1 mg/kg with IP CCK-8 (2 μg/kg), CCK-8 significantly reduced 30-min food intake [F (3,4) = 29.214, P < 0.001]. However, IP d-CPPene 1 mg/kg did not significantly attenuate reduction of food intake by IP CCK-8 (Fig. 3A). IP CCK-8 (2 μg/kg) reduced food intake [F(3,5)=23.139, P < 0.001, in another experiment in which the IP d-CPPene dose was increased to 2 mg/kg]. However, reduction of 30-min food intake by IP CCK was prevented by 2 mg/kg d-CPPene. Intake after IP d-CPPene/IP CCK-8 was significantly greater than after IP NaCl/IP CCK-8 (P < 0.001) and did not differ from IP NaCl/IP NaCl. Thus, 4V administration of a competitive NMDA receptor antagonist prevented reduction of food intake by IP CCK-8 at a dose two orders of magnitude lower than a dose that was effective when injected intraperitoneally.
Fig. 3.
Prevention of CCK-8-induced reduction of food intake by fourth ventricle or IP injection of the competitive NMDA receptor antagonist d-CPPene. Intraperitoneal injection of 1 mg/kg d-3-(2-carboxypiperazin-4-yl)-1-propenyl-1-phosphoric acid (d-CPPene) had no effect on reduction of food intake by IP CCK-8 (A), while increasing the IP dose of d-CPPene to 2 mg/kg prevented CCK-8-induced reduction of food intake (B). Fourth-ventricle injection of 40 ng d-CPPene, a dose two orders of magnitude below the effective IP dose, prevented reduction of food intake by IP CCK-8. a,b,cDissimilar letters above bars indicate significantly different intakes (P < 0.001).
Attenuation of CCK-8-induced increase in hindbrain c-Fos immunoreactivity by 4V injection of d-CPPene.
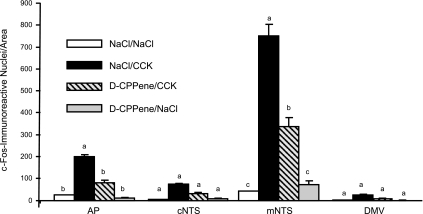
In an experiment to determine whether 4V d-CPPene (40 ng) could attenuate increased expression of hindbrain c-Fos immunoreactivity following IP CCK-8 (10 μg/kg), there were significant effects of treatment on hindbrain c-Fos immunoreactivity [F(3,10)= 80.430, P < 0.01]. Moreover, there was significant interaction between treatment and brain area [F(3,9)=53.090, P < 0.001]. Compared with IP NaCl, IP CCK-8 increased c-Fos immunoreactivity in the mNTS and AP (P < 0.01), but not in the cNTS or DMV. Following 4V d-CPPene, c-Fos immunoreactivity in both the AP and NTS was significantly reduced compared with 4V NaCl/IP CCK-8 (P < 0.01), but it remained significantly elevated compared with 4V NaCl/IP NaCl (Figs. 4 and 5).
Fig. 4.
Effect of fourth-ventricle injection of NMDA receptor antagonist, d-CPPene, on number of c-Fos-immunoreactive nuclei in the hindbrain dorsal vagal complex 90 min after IP injection of CCK-8. c-Fos-immunoreactive nuclei were counted in coronal sections from 3 to 5 rats per treatment condition, at four rostrocaudal levels of the hindbrain, as established using the stereotaxic atlas of Paxinos and Watson (30). Counts were made for the following areas of interest at each rostrocaudal level where the given area occurs: area postrema (AP), the commissural NTS (cNTS), the medial NTS (mNTS), and the dorsal motor nucleus of the vagus (DMV). The counts for areas of interest at all levels were summed for each animal, and averages and standard errors were calculated for each area under each treatment condition. a,b,cDissimilar letters above bars indicate significantly different numbers of c-Fos-immunoreactive nuclei (P < 0.01).
Fig. 5.
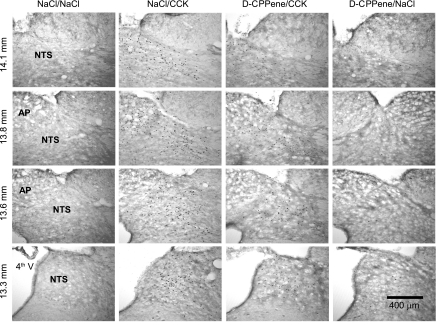
Representative images of dorsal hindbrain sections stained to reveal c-Fos-immunoreactivity following fourth-ventricle d-CPPene and IP CCK. Column labels indicate treatment conditions, and row labels indicate the distance caudal to bregma for sections in that row. 4th V, fourth ventricle; NTS, nucleus of the solitary tract, including the medial and commissural subnuclei; AP, area postrema.
DISCUSSION
Previously published findings from our group reveal that systemic administration of NMDA antagonists attenuates reduction of food intake by IP CCK (11, 19) and reduces CCK-induced c-Fos expression in the mNTS (19). These observations indicate that NMDA-type glutamate receptors participate in vagally mediated reduction of food intake. However, they do not provide any information regarding the localization of NMDA receptors that participate in CCK-induced reduction of food intake. This issue is not trivial because NMDA receptors are expressed by vagal afferent neurons in the nodose ganglia (12, 13), as well as by central vagal afferent terminals and postsynaptic neurons in the NTS (1). Moreover, electrophysiological experiments suggest that peripheral NMDA receptors may modulate gastrointestinal vagal afferent activity (42). Therefore, it is plausible that NMDA receptor antagonists could alter the behavioral responses to CCK through actions at peripheral vagal afferent sites. Our results reported here show that reduction of 30-min food intake by IP CCK is prevented by hindbrain administration of NMDA receptor antagonists. Moreover, we demonstrate that the same antagonist doses that prevent CCK-induced reduction of food intake when injected into the hindbrain fail to attenuate CCK's effect when injected subcutaneously or intraperitoneally. Therefore, our results indicate that central NMDA receptors play an important, and perhaps essential, role in control of food intake by CCK.
Our current results strongly support the hypothesis that hindbrain NMDA receptors are necessary participants in CCK-induced activation of mNTS neurons and reduction of food intake. Nevertheless, our results do not rule out additional participation from peripherally located NMDA receptors. Circumstantial evidence continues to suggest a possible contribution to CCK-induced reduction of feeding by peripheral NMDA receptors. As mentioned above, virtually all vagal afferent neurons, including those that innervate the upper GI tract, express NMDA receptor subunit immunoreactivity (12, 13). Furthermore, electrical activity recorded from the distal ends of cut vagal afferents is modulated by NMDA receptors (42), suggesting that receptors in peripheral vagal afferent membranes may participate in relaying sensory information to the brain. Immunoreactivity for glutamate and vesicular glutamate transporter (VGLUT) has been reported in neurons of enteric plexus of the guinea pig and rat small intestines (45, 51). Likewise, immunoreactivity to glutamate has been reported in the intrinsic and extrinsic innervations of the human digestive tract (15), and there is compelling evidence that at least some gastrointestinal vagal afferent terminals are immunoreactive for VGLUTs (32). Finally, calcium-dependent glutamate release has been demonstrated in the enteric nervous system (35). In summary, vagal afferent processes innervating the gastrointestinal tract express NMDA receptor subunits, and there appear to be multiple sources of glutamate that could act on peripheral vagal afferent processes. Therefore, our results do not obviate the possibility that peripheral, as well as central, NMDA receptors could participate in control of food intake by vagal signals from the GI tract, such as CCK.
At present, the cellular location of hindbrain NMDA receptors that are necessary for CCK-induced reduction of food intake is uncertain. NMDA receptor subunit immunoreactivity and mRNA have been observed in hindbrain neurons (5, 7, 21, 31, 49, 50), including those in the NTS. Therefore, it is plausible that NMDA antagonists attenuate reduction of food intake by CCK through an action on intrinsic NTS neurons. On the other hand, vagal afferents themselves express NMDA receptors (12, 13, 42), and ultrastructural studies have revealed immunoreactivity for the NR1 subunit on vagal afferent terminals in the NTS (1). Hence, it is possible that NMDA receptor antagonists administered into the hindbrain attenuate CCK-induced reduction of food intake by acting presynaptically on central vagal afferent processes. In support of this hypothesis, we have previously reported that administration of NMDA receptor antagonists into the NTS increases food intake (18, 23, 46). Most interesting, however, is the fact that after degeneration of central vagal afferent terminals, NTS injection of the antagonist does not increase food intake (17). These results are consistent with the working hypothesis that at least some hindbrain NMDA receptors participating in reduction of food intake by vagal signals, including CCK, are located presynaptically on vagal afferent processes in the NTS.
The cellular mechanisms by which antagonism of hindbrain NMDA receptors could attenuate reduction of CCK-induced reduction of food intake remain speculative. However, one possibility is that antagonism of presynaptic or extrasynaptic NMDA receptors reduces transmitter release from vagal afferent terminals in the NTS. This sort of modulation of neurotransmitter release by activation of presynaptic or extrasynaptic NMDA receptors is well documented in the central nervous system, including in the visual cortex (41), entorhinal cortex (6), and glutamatergic somatosensory afferent terminals in the spinal dorsal horn (3, 22, 39). Furthermore, at least one synapse has been described in which NMDA channels provide obligatory calcium influx to trigger glutamate release (10). In this instance, a glutamatergic axoaxonal input, with axonal postsynaptic NMDA receptors, is required in order for an invading action potential to trigger glutamate release from the innervated terminal. Conceivably, descending projections from brain areas, such as the paraventricular hypothalamic nucleus (52) or the amygdala (40), might provide glutamatergic axoaxonal synapses on preterminal vagal afferent endings, which could modulate transmitter release from vagal afferent terminals. Alternatively, extrasynaptic vagal afferent NMDA receptors could be activated by glial glutamate release (16, 38), thereby enhancing their excitability and increasing transmitter release from vagal afferent terminals when invading action potentials depolarize them.
One might think that positive modulation of glutamate release from vagal afferent terminals by NMDA receptors located on the afferents themselves is a recipe for runaway positive feedback. However, it should not be assumed that vagal afferent NMDA receptors are auto-receptors for vagally released glutamate. If NMDA receptors that modulate transmitter release from primary vagal afferents were located on portions of the preterminal axon, apart from the site where glutamate is supplied to NTS postsynaptic neurons, then uncontrolled positive feedback would be obviated. Unfortunately, ultrastructural and electrophysiological examinations of NMDA receptor distributions and function on vagal afferents have not reached the necessary level of resolution to evaluate this hypothesis.
Vagal afferents that are activated by CCK synapse on neurons in the NTS of the dorsal vagal complex (34, 37) and increased c-Fos immunoreactivity following CCK injection are interpreted to reflect the increase in vagal afferent activation that is evoked by CCK (29). We found that 4V administration of a competitive NMDA receptor antagonist, d-CPPene, attenuated the increase in nuclear c-Fos immunoreactivity in the dorsal vagal complex following CCK injection, supporting the interpretation that NMDA antagonists attenuate reduction of feeding by CCK by reducing vagal afferent activation of postsynaptic neurons in the NTS. It is worth noting, however, that while d-CPPene reduced CCK-induced NTS c-Fos expression, it did not return it to levels expressed by vehicle-treated control rats or by rats that received d-CPPene, but not CCK. There are several potential explanations for the fact that attenuation of CCK-induced c-Fos expression is significant, but incomplete. First, most electrophysiological experiments indicate that vagal afferent activation of fast excitatory conductances in NTS neurons is mediated primarily by AMPA/kainate-type glutamate receptors (2). However, NMDA receptors may play a modulatory role for some, but perhaps not all, vagal afferent inputs. For example, NMDA receptor currents in some neurons are responsible for extending the time period during which postsynaptic neurons are depolarized following primary activation of AMPA/kainate-type glutamate receptors (2). If this mechanism pertains to CCK-induced activation of vagal afferents, then antagonism of hindbrain NMDA receptors might reduce, but not abolish, increased excitation of NTS neurons excited by CCK. An alternative possibility is that NMDA receptors are necessary for excitation of some, but not all, CCK-responsive vagal circuits. In this case, one would predict that NMDA receptors, while critical for CCK-induced c-Fos expression in neurons that participate in control of food intake, might not be essential for activation of neurons that mediate other responses to CCK. In support of this hypothesis, it is pertinent to note that reduction of food intake and inhibition of gastric emptying by CCK both are mediated by vagal afferent neurons (33, 34, 36, 43, 44). However, as we previously reported, NMDA receptor antagonism attenuates only CCK-induced reduction of food intake, but not CCK-induced inhibition of gastric emptying (11). A third possibility is that d-CPPene doses that were able to block the behavioral consequences of exogenous CCK administration did not eliminate release of afferent transmitter entirely, but rather reduced release of a peptide cotransmitter necessary for the behavioral effects of CCK. A fourth alternative explanation for incomplete reduction of CCK-induced c-Fos immunoreactivity relates to the fact that d-CPPene competes with glutamate for binding to the NR2(A/B) subunit of the NMDA receptor. Because we administered the antagonist as a bolus prior to injection of CCK, it is possible that effective competitive concentrations of d-CPPene were present at the receptors for only a portion of the time during which CCK was stimulating glutamate release and, therefore, only reduced NMDA receptor activation during part of the period of vagal activation. A test of this last hypothesis theoretically is possible using a noncompetitive, open-channel, antagonist, like MK-801. However, we have observed that MK-801, by itself, induces very high levels of c-Fos expression throughout the brain, including in the DVC. Therefore, we have not found it useful to substitute this antagonist in experiments where c-Fos expression is the dependent variable.
Perspectives and Significance
The results of our experiments indicate that injections of NMDA receptor antagonists into the NTS, where vagal afferents terminate, reverse reduction of food intake by systemically administered CCK. Consistent with reduction of CCK-evoked vagally mediated NTS excitation, 4V NMDA receptor antagonist injection also reduce CCK-induced c-Fos immunoreactivity in the NTS. These results indicate that NMDA receptors in the dorsal vagal complex of the hindbrain play an essential role in vagally mediated control of food intake by CCK. The cellular mechanisms that account for such dramatic attenuation of CCK's effects are not yet clear. However, NMDA receptors located on vagal afferents and/or on NTS neurons, could provide a means for glutamatergic inputs from other brain regions to adjust the strength of gastrointestinal satiation signals at the point where they enter the brain. Moreover, it is possible that activation of hindbrain NMDA receptors may trigger plastic changes in vagal afferent/NTS synapses, thereby altering vagal satiation signals. NMDA receptor channels are calcium channels (14). Hence, their activation not only affects membrane charge transfer, but also provides increased intracellular calcium that initiates signaling cascades, protein phosphorylations, and transcriptional changes, leading to alterations in neuronal function that may occur over seconds, minutes, and hours (20, 26, 48). Consequently, NMDA receptor activation can translate brief neuronal volleys into sustained changes in neuronal and network activity that influence behaviors. The classical example of this sort of NMDA receptor-mediated plasticity is, of course, long-term potentiation (4, 27, 28), which now is recognized in many CNS structures beyond the hippocampus (24), perhaps including the NTS. Consequently, hindbrain NMDA receptors on or near vagal afferent endings comprise an exciting avenue of investigation into the neural substrates of satiation and could provide an opportunity for therapeutic intervention in satiation mechanisms.
GRANTS
This work was supported by National Institute of Digestive and Kidney Diseases Grant DK-52849 and the National Institute of Neurological Diseases and Stroke Grant NS-20561.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Aicher SA, Sharma S, Pickel VM. N-methyl-d-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience 91: 119–132, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius—common denominators. Chem Senses 21: 387–395, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci 24: 2774–2781, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature 363: 347–350, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Benke D, Wenzel A, Scheuer L, Fritschey JM, Mohler H. Immunobiochemical characterization of the NMDA-receptor subunit NR1 in the developing and adult rat brain. J Recept Signal Transduct Res 15: 393–411, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Berretta N, Jones RS. Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience 75: 339–344, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Broussard DL, Wiedner EB, Li X, Altschuler SM. NMDAR1 mRNA expression in the brainstem circuit controlling esophageal peristalsis. Brain Res Mol Brain Res 27: 329–332, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav 56: 145–149, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Burns GA, Ritter RC. Visceral afferent participation in delayed satiation following NMDA receptor blockade. Physiol Behav 65: 361–366, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Cochilla AJ, Alford S. NMDA receptor-mediated control of presynaptic calcium and neurotransmitter release. J Neurosci 19: 193–205, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Covasa M, Ritter RC, Burns GA. NMDA receptor blockade attenuates CCK-induced reduction of real feeding but not sham feeding. Am J Physiol Regul Integr Comp Physiol 286: R826–R831, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Czaja K, Ritter RC, Burns GA. N-methyl-d-aspartate receptor subunit phenotypes of vagal afferent neurons in nodose ganglia of the rat. J Comp Neurol 496: 877–885, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Czaja K, Ritter RC, Burns GA. Vagal afferent neurons projecting to the stomach and small intestine exhibit multiple N-methyl-d-aspartate receptor subunit phenotypes. Brain Res 1119: 86–93, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999 [PubMed] [Google Scholar]
- 15. Ewald P, Neuhuber WL, Raab M. Vesicular glutamate transporter 1 immunoreactivity in extrinsic and intrinsic innervation of the rat esophagus. Histochem Cell Biol 125: 377–395, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Fellin T, Pascual O, Haydon PG. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology 21: 208–215, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol 289: R1504–R1511, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. Blockade of hindbrain NMDA receptors containing NR2 subunits increases sucrose intake. Am J Physiol Regul Integr Comp Physiol 296: R921–R928, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. NMDA NR2 receptors participate in CCK-induced reduction of food intake and hindbrain neuronal activation. Brain Res 1266: 37–44, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Haddad JJ. N-methyl-d-aspartate (NMDA) and the regulation of mitogen-activated protein kinase (MAPK) signaling pathways: a revolving neurochemical axis for therapeutic intervention? Prog Neurobiol 77: 252–282, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Huang J, Wang H, Pickel VM. Rostrocaudal variation in targeting of N-methyl-d-aspartate and mu- opioid receptors in the rat medial nucleus of the solitary tract. J Comp Neurol 421: 400–411, 2000 [PubMed] [Google Scholar]
- 22. Huettner JE, Kerchner GA, Zhuo M. Glutamate and the presynaptic control of spinal sensory transmission. Neuroscientist 8: 89–92, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Hung CY, Covasa M, Ritter RC, Burns GA. Hindbrain administration of NMDA receptor antagonist AP-5 increases food intake in the rat. Am J Physiol Regul Integr Comp Physiol 290: R642–R651, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Ikeda H, Kiritoshi T, Murase K. Synaptic plasticity in the spinal dorsal horn. Neurosci Res 64: 133–136, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Jahng JW, Houpt TA. MK801 increases feeding and decreases drinking in nondeprived, freely feeding rats. Pharmacol Biochem Behav 68: 181–186, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58: 951–961, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacDonald JF, Jackson MF, Beazely MA. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim Biophys Acta 1768: 941–951, 2007 [DOI] [PubMed] [Google Scholar]
- 28. MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol 18: 71–84, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res 770: 277–288, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. San Diego: Academic, 1997 [Google Scholar]
- 31. Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci 14: 667–696, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raab M, Neuhuber WL. Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function? Cell Tiss Res 312: 141–148, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Raybould HE. Capsaicin-sensitive vagal afferents and CCK in inhibition of gastric motor function induced by intestinal nutrients. Peptides 12: 1279–1283, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Raybould HE, Gayton RJ, Dockray GJ. Mechanisms of action of peripherally administered cholecystokinin octapeptide on brain stem neurons in the rat. J Neurosci 8: 3018–3024, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reis HJ, Massensini AR, Prado MA, Gomez RS, Gomez MV, Romano-Silva MA. Calcium channels coupled to depolarization-evoked glutamate release in the myenteric plexus of guinea-pig ileum. Neuroscience 101: 237–242, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 248: R501–R504, 1985 [DOI] [PubMed] [Google Scholar]
- 37. Ritter RC, Ritter S, Ewart WR, Wingate DL. Capsaicin attenuates hindbrain neuron responses to circulating cholecystokinin. Am J Physiol Regul Integr Comp Physiol 257: R1162–R1168, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez Diaz M, Alonso TJ, Perdomo Diaz J, Gonzalez Hernandez T, Castro Fuentes R, Sabate M, Garcia Dopico J. Glial regulation of nonsynaptic extracellular glutamate in the substantia nigra. Glia 49: 134–142, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Rustioni A. Modulation of sensory input to the spinal cord by presynaptic ionotropic glutamate receptors. Arch Ital Biol 143: 103–112, 2005 [PubMed] [Google Scholar]
- 40. Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32: 450–456, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39: 641–654, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Slattery JA, Page AJ, Dorian CL, Brierley SM, Blackshaw LA. Potentiation of mouse vagal afferent mechanosensitivity by ionotropic and metabotropic glutamate receptors. J Physiol 577: 295–306, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol 249: R638–R641, 1985 [DOI] [PubMed] [Google Scholar]
- 44. South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 9: 601–612, 1988 [DOI] [PubMed] [Google Scholar]
- 45. Tong Q, Ma J, Kirchgessner AL. Vesicular glutamate transporter 2 in the brain-gut axis. Neuroreport 12: 3929–3934, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Treece BR, Covasa M, Ritter RC, Burns GA. Delay in meal termination follows blockade of N-methyl-d-aspartate receptors in the dorsal hindbrain. Brain Res 810: 34–40, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Treece BR, Ritter RC, Burns GA. Lesions of the dorsal vagal complex abolish increases in meal size induced by NMDA receptor blockade. Brain Res 872: 37–43, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem 100: 1–11, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Wang YH, Bosy TZ, Yasuda RP, Grayson R, Vicini S, Pizzorusso T, Wolfe BB. Characterization of NMDA receptor subunit-specific antibodies: distribution of NR2A and NR2B receptor subunits in rat brain and ontogenic profile in the cerebellum. J Neurochem 65: 176–183, 1995 [DOI] [PubMed] [Google Scholar]
- 50. Wenzel A, Villa M, Mohler H, Benke D. Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem 66: 1240–1248, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Wiley JW, Lu YX, Owyang C. Evidence for a glutamatergic neural pathway in the myenteric plexus. Am J Physiol Gastrointest Liver Physiol 261: G693–G700, 1991 [DOI] [PubMed] [Google Scholar]
- 52. Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG, Jr, Berthoud HR. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am J Physiol Regul Integr Comp Physiol 298: R720–R728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]