Abstract
Acute heat stress activates visceral sympathetic nerve discharge (SND) in young rats, and the functional integrity of the rostral ventrolateral medulla (RVLM) is required for sustaining visceral sympathoexcitation during peak increases in internal body temperature (Tc). However, RVLM mechanisms mediating SND activation to hyperthermia remain unknown. In the present study, we investigated the role of RVLM ionotropic excitatory amino acid receptors in mediating visceral SND activation to heat stress in anesthetized, young rats. The effects of bilateral RVLM kynurenic acid (Kyn; 2.7 and 5.4 nmol), saline, or muscimol (400–800 pmol) microinjections on renal SND and splenic SND responses to heat stress were determined at peak hyperthermia (Tc 41.5°C), during progressive hyperthermia (Tc 40°C), and at the initiation of heating (Tc increased from 38 to 38.5°C). RVLM Kyn microinjections did not reduce renal and splenic SND recorded during progressive or peak hyperthermia and did not attenuate SND activation at the initiation of heating. In fact, renal and splenic SND tended to be or were significantly increased following RVLM Kyn microinjections at the initiation of heating and during hyperthermia (40 and 41.5°C). RVLM muscimol microinjections at 39, 40, and 41.5°C resulted in immediate reductions in SND. These data indicate that RVLM ionotropic glutamate receptors are required for mediating visceral sympathoexcitation to acute heating and suggest that acute heating activates an RVLM ionotropic excitatory amino acid receptor dependent inhibitory input, which reduces the level of visceral SND to heating.
Keywords: rostral ventrolateral medulla, sympathetic nerve discharge, kynurenic acid, acute heat stress
the rostral ventrolateral medulla (RVLM) is a brain stem region that plays a critical role in regulation of sympathetic nerve discharge (SND) (3, 10, 11, 15, 17, 22, 38, 54–56). RVLM neurons receive both inhibitory and excitatory synaptic inputs, mediated to a large extent by γ-amino butyric acid (GABA) and excitatory amino acid (EAA) receptors, respectively (3, 11, 15, 17). RVLM muscimol (GABAA receptor agonist) microinjections decrease SND and arterial blood pressure (7, 14, 16, 18, 50, 51), whereas RVLM microinjections of the EAA transmitter, l-glutamate, increase SND and arterial blood pressure (18, 48). Blockade of RVLM GABA receptors increases arterial blood pressure and SND (17, 36, 41), supporting the existence of a tonic inhibitory tone that appears to arise primarily from GABAergic inputs to the RVLM from the caudal ventrolateral medulla (CVLM) (3, 10, 11, 15, 17, 22, 38, 54–56). In contrast, bilateral RVLM microinjections of kynurenic acid (Kyn), an ionotropic EAA receptor antagonist, exert little effect on resting levels of mean arterial pressure (MAP) in the rat (22, 32, 57, 59).
Several investigations have studied the functional balance of excitatory and inhibitory synaptic inputs to RVLM presympathetic neurons under basal conditions (17, 22). Ito and Sved (22) reported that, after injection of muscimol into the CVLM, blockade of RVLM ionotropic EAA receptors (Kyn microinjection) reduced MAP to levels similar to those produced by blockade of autonomic ganglionic transmission. To explain these results, Ito and Sved (22) proposed a model suggesting that, under basal conditions, a tonically active, Kyn-sensitive input to the RVLM simultaneously excites sympathoexcitatory neurons and activates a counterbalancing inhibition of sympathoexcitatory neurons, an effect that likely includes a projection from the RVLM to the CVLM and subsequent excitation of an inhibitory projection from the CVLM to the RVLM. Thus, under basal conditions, blockade of RVLM ionotropic EAA receptors may remove both tonic excitatory and inhibitory influences on RVLM sympathoexcitatory neurons, resulting in little or no change in MAP (22). These observations provided consideration for the existence of a novel RVLM glutamate-activated inhibitory pathway. Horiuchi et al. (17) reported that bilateral inactivation of CVLM neurons significantly increased MAP and renal SND; however, subsequent RVLM Kyn microinjections did not substantially affect renal SND and did not reduce MAP to levels consistent with ganglionic blockade. These investigators concluded that the renal sympathoexcitation observed under conditions when inputs from the CVLM to the RVLM are blocked, at the same time that EAA drive to the RVLM has been removed, is likely dependent either on non-EAA inputs from a source other than the CVLM, or on the autoactivity of RVLM neurons (17). These studies provided seminal observations demonstrating the complexity of RVLM mechanisms regulating basal levels of MAP and SND.
Recent studies have investigated RVLM mechanisms contributing to sympathetic activation in several pathophysiological states and in response to various physiological stressors (2, 6, 8, 20, 21, 32, 39, 42, 59). Bilateral RVLM Kyn microinjections reduce MAP in hypertensive, but not normotensive, rats (8, 20, 21) and reduce MAP and renal SND in congestive heart failure, but not sham heart failure, rats (59). RVLM Kyn microinjections reduce MAP in water-deprived, but not water-replete, rats (6), block splanchnic sympathoexcitatory responses to peripheral chemoreceptor stimulation (42), attenuate increases in MAP evoked by electrical stimulation of sciatic nerve afferents (32), reduce lumbar SND activation to hyperinsulinemia (2), and attenuate the initial increases in MAP and renal SND to air-jet stress (39). On the other hand, RVLM Kyn microinjections do not affect plateau cardiovascular and SND responses to air-jet stress (39) and do not alter SND activation to stimulation of central chemoreceptors (42). Collectively, these results demonstrate that RVLM ionotropic EAA receptors play a key role in mediating sympathoexcitation in various pathophysiological conditions and in response to specific physiological interventions; however, their involvement is not ubiquitous and may depend on the type of experimental intervention, or even specific components (e.g., onset, plateau) within a specific intervention.
Hyperthermia is an environmental stressor that markedly alters efferent SND. Elevations in internal body temperature (Tc) decrease SND directed to the caudal ventral artery in anesthetized rats (23), increase muscle SND in conscious humans (9, 44) and splanchnic SND in conscious rats (34), and increase the level of activity directed to visceral organs in young anesthetized rats (25–30). In a recent study, Hosking et al. (18) reported that bilateral inhibition of RVLM synaptic activation by muscimol microinjections after increasing Tc to 41.5°C, as well as interruption of RVLM synaptic activation and axonal transmission by bilateral microinjections of lidocaine at 41.5°C, produced significant reductions in hyperthermia-induced SND activation. These data suggest that the functional integrity of RVLM neural circuits is required for sustaining a substantial amount of the visceral sympathoexcitation during peak increases in Tc (18); however, RVLM mechanisms mediating sympathetic activation to hyperthermia have not been investigated.
In the present study, we determined the effects of bilateral RVLM Kyn microinjections on visceral SND (renal and splenic) responses during three phases of heat stress: peak hyperthermia (Tc increased from 38 to 41.5°C), during progressive hyperthermia (Tc increased from 38 to 40°C), and at the initiation of acute heating (pretreatment before increasing Tc from 38 to 38.5°C). We speculated that RVLM Kyn microinjections would influence SND responses to acute heating in at least one of three ways. First, visceral sympathoexcitation to acute heating may be dependent on activation of RVLM ionotropic EAA receptors; therefore, RVLM Kyn microinjections would be expected to markedly reduce or attenuate visceral SND responses to acute heating. Second, RVLM Kyn microinjections may exert minimal effects on visceral SND responses to acute heating, suggesting that heating-induced excitation of visceral SND is not dependent on activation of RVLM ionotropic glutamate receptors. Third, visceral SND activation to acute heating may be enhanced after RVLM Kyn microinjections, suggesting an inhibitory role for glutamatergic activation of RVLM ionotropic EAA receptors during heating.
METHODS
The experimental procedures and protocols used in this investigation were performed in accordance with the American Physiological Society's guiding principles for research involving animals and approved by the Institutional Animal Care and Use Committee at Kansas State University.
General Procedures
Experiments were completed using male Sprague-Dawley rats (300–350 g). Anesthesia was induced by isoflurane (3%) and maintained during surgical procedures using isoflurane (1.5–2.5%), α-chloralose (80 mg/kg ip), and urethane (800 mg/kg ip). A catheter was placed in the femoral vein for the intravenous administration of maintenance doses of α-chloralose (35–45 mg·kg−1·h−1). Maintenance doses of urethane (200 mg/kg every 4 h) were administered intraperitoneally. The trachea was cannulated with a polyethylene-240 catheter, and rats were paralyzed with gallamine triethiodide (5–10 mg/kg iv, initial dose; 10–15 mg·kg−1·h−1, maintenance dose) and artificially ventilated (24). Femoral arterial pressure was monitored using a pressure transducer connected to a blood pressure analyzer. Heart rate (HR) was derived from the pulsatile arterial pressure output of the blood pressure analyzer. Tc was measured with a thermistor probe inserted ∼5–6 cm into the colon and was kept at 38.0°C during surgical interventions by a temperature-controlled table.
Sympathetic Nerve Recordings
Activity was recorded biphasically with a platinum bipolar electrode after capacity-coupled preamplification (band pass 30–3,000 Hz) from the central end of cut or distally crushed renal and splenic sympathetic nerves. The left renal and splenic nerves were isolated retroperitoneally, and nerve-electrode preparations were covered with silicone gel to prevent exposure to room air. The sympathetic nerve potentials were full-wave rectified and integrated (time constant 10 ms), which produced a smooth tracing of the synchronized discharges. Total power in renal SND and splenic SND was quantified as volts × seconds (V·s) (18), and SND recordings were corrected for background noise after administration of the ganglionic blocker, chlorisondamine (5 mg/kg iv) (18).
The adequacy of anesthesia during the initial surgical procedures was indicated by the absence of a withdrawal reflex in response to mechanical stimulation of the tail or hindlimb. The adequacy of anesthesia after the establishment of SND recordings and following initiation of neuromuscular blockade was indicated by an inability of mechanical stimulation of the hindlimb or tail to increase SND or MAP.
RVLM Microinjections
Rats were placed in a stereotaxic apparatus, the incisor bar was set at −11 mm (50), and the brain stem was exposed. As described by Ito and Sved (22), target stereotaxic coordinates for the RVLM were 1.6–2.0 mm rostral to calamus scriptorius, 1.7–2.1 mm lateral from midline, and 2.6–3.2 mm below the dorsal surface of the brain. RVLM microinjections were completed using multibarrel glass micropipettes (3 barrels, outside tip diameter 40–70 μm) filled with appropriate drugs, with the pipette angled 20° rostrally (50). Microinjection volumes were determined with the aid of an operating microscope by measuring the displacement of the fluid meniscus in the micropipette barrel with respect to a horizontal grid. The RVLM was functionally identified by completing glutamate (10 mM, 40 nl) (41) microinjections across a range of coordinates to locate the RVLM site producing the largest increase in SND and MAP.
Experimental Protocols
Following completion of surgical interventions, rats were allowed to stabilize for 60 min before initiation of experimental protocols. End-tidal CO2 was measured using a micro-capnometer and was maintained near 4.0% during experimental interventions by adjusting the frequency of ventilation. Tc was increased at an approximate rate of 0.08–0.09°C/min using a heat lamp during heating experiments. Four experimental protocols involving RVLM microinjections were completed, and microinjectate volume was 100 nl for each microinjection. With the exception of a few experiments [e.g., RVLM N-methyl-d-aspartic acid (NMDA) microinjections at different levels of Tc], experiments associated with each of the individual protocols (I–IV) were completed in separate rats, and microinjection experiments within each protocol were completed in separate rats. SND (renal and splenic), MAP, and HR were recorded continuously during each experiment. At the conclusion of each experiment, rats were euthanized via an overdose of methohexital sodium (Brevital, 150 mg/kg iv).
Protocol I.
To determine the role of RVLM ionotropic EAAs in sustaining visceral sympathoexcitation at peak hyperthermia, bilateral RVLM Kyn microinjections (2.7 nmol, n = 5; 5.4 nmol, n = 6) were administered after Tc had been increased to 41.5°C. Volume control experiments involved determining SND responses to bilateral RVLM saline microinjections (n = 4) at a Tc of 41.5°C. To determine the role of RVLM synaptic activation in sustaining visceral sympathoexcitation at peak hyperthermia, bilateral RVLM muscimol microinjections (400–800 pmol, n = 5) were completed at a Tc of 41.5°C.
Protocol II.
Experiments completed in this protocol were designed to determine the role of RVLM ionotropic EAAs in mediating SND responses during progressive hyperthermia. Bilateral RVLM microinjections of Kyn (2.7 nmol, n = 7; 5.4 nmol, n = 4) or saline (n = 5) were administered during acute heating at a Tc of 40°C. To determine the role of RVLM synaptic activation in mediating visceral sympathoexcitation during progressive hyperthermia, bilateral RVLM muscimol microinjections (400–800 pmol, n = 3) were completed at a Tc of 40°C.
Protocol III.
Experiments completed in this protocol were designed to determine the role of RVLM ionotropic EAAs in mediating SND activation at the onset of acute heating. At a baseline Tc of 38°C, rats were pretreated with bilateral RVLM microinjections of either Kyn (2.7 nmol, n = 15; 5.4 nmol, n = 5) or saline (n = 9). Five minutes after completion of RVLM microinjections, acute heating was initiated, and Tc was increased from 38 to 38.5°C over the course of 10 min. Temperature control experiments were completed by determining SND responses to bilateral microinjections of either Kyn (2.7 nmol, n = 3; 5.4 nmol, n = 6) or saline (n = 7), with Tc maintained at 38°C for 15 min. Bilateral RVLM muscimol microinjections (400–800 pmol, n = 3) were completed at a Tc of 39°C to determine the contribution of RVLM synaptic activation to renal and splenic SND soon after the onset of acute heating.
Protocol IV.
The effectiveness of RVLM Kyn microinjections to block ionotropic EAA receptors was confirmed by completing two separate experimental interventions. 1) With Tc maintained at 38.5°C (n = 2), 40°C (n = 1), and 41.5°C (n = 4), SND and MAP responses to bilateral RVLM microinjections of NMDA (20–40 pmol, n = 7) were determined before and after (5–15 min) RVLM Kyn microinjections (2.7 nmol, n = 4; 5.4 nmol, n = 3). 2) The somatosympathetic reflex, which is known to be mediated via activation of RVLM ionotropic EAA receptors (31, 58), was tested before and after bilateral RVLM Kyn microinjections. The sciatic nerve was exposed, immersed in mineral oil, and sectioned. The central end of the nerve was placed on bipolar stimulating electrodes, and the somatosympathetic reflex was elicited by recording SND responses to electrical stimulation (square-wave pulse, 200–300 μA, 1-ms duration) of the sciatic nerve (31, 58). Sciatic nerve stimulation trials were completed before and 5–15 min after (5–10 min after stimulation, n = 3; 10–15 min after stimulation, n = 3) RVLM microinjections of 2.7 nmol Kyn (MAP, n = 3; renal SND, n = 3; splenic SND, n = 2) and 5.4 nmol Kyn (MAP, n = 3; renal SND, n = 3; splenic SND, n = 2). In two experiments, MAP and renal SND responses to sciatic nerve stimulation were determined before and 20 min after RVLM Kyn microinjections (2.7 nmol, n = 1; 5.4 nmol, n = 1). Control experiments involved the completion (15–20 min apart) of successive trials of sciatic nerve stimulation without intervening RVLM Kyn microinjections (n = 3).
Data and Statistical Analysis
Values are means ± SE. Renal and splenic SND are expressed as percent change from baseline values (control), using the formula [(experimental SND − control SND)/control SND] × 100. Statistical analyses included Student t-tests and analysis of variance techniques with a repeated-measures design, followed by Bonferroni post hoc tests. The overall level of statistical significance was P < 0.05.
RESULTS
Identification of the RVLM
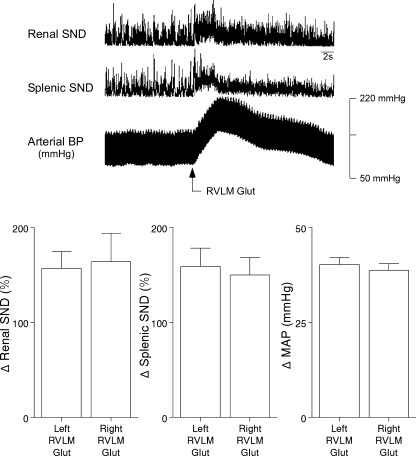
The RVLM was functionally identified by demonstrating that bilateral microinjections of l-glutamate (10 mM, 40 nl) at target coordinates elicited increases in SND and arterial blood pressure. Figure 1 (top) shows abrupt increases in renal SND, splenic SND, and arterial blood pressure produced by microinjection of l-glutamate in the left RVLM from a representative experiment. Average increases in renal SND, splenic SND, and MAP to l-glutamate microinjections in right and left RVLM sites are summarized in Fig. 1 (bottom).
Fig. 1.
Top: traces of renal sympathetic nerve discharge (SND), splenic SND, and arterial blood pressure (BP) from a representative experiment recorded before and after unilateral microinjection of l-glutamate into the rostral ventrolateral medulla (RVLM Glut). Horizontal calibration is 2 s. Bottom: average increases (Δ) in renal SND (left), splenic SND (middle), and mean arterial pressure (MAP; right) to Glut microinjections in left and right RVLM sites. Values are means ± SE.
Protocol I: Effects of RVLM Kyn Microinjections on SND Regulation at Peak Hyperthermia
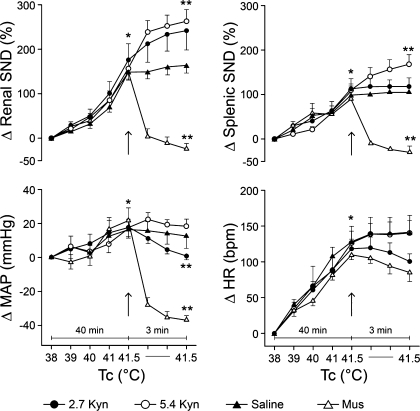
Summarized renal SND (top left), splenic SND (top right), MAP (bottom left), and HR (bottom right) data are shown in Fig. 2. Tc was increased from 38 to 41.5°C in 40 min and maintained at this level for 3 min after RVLM Kyn (2.7 and 5.4 nmol), saline, and muscimol (400–800 pmol) microinjections (designated by arrows). Each measured variable was significantly increased from preheating control levels after Tc was elevated to 41.5°C. At peak hyperthermia (41.5°C), renal SND and splenic SND were abruptly reduced following RVLM muscimol microinjections, remained unchanged in response to RVLM saline microinjections, and either remained unchanged or were significantly increased in response to RVLM Kyn microinjections. At 41.5°C, MAP was reduced following RVLM muscimol microinjections, remained unchanged in response to RVLM saline microinjections, and either remained unchanged (Kyn, 5.4 nmol) or was significantly reduced (Kyn, 2.7 nmol) after RVLM Kyn microinjections. HR was not significantly changed after RVLM Kyn, muscimol, or saline microinjections at 41.5°C.
Fig. 2.
Changes in renal SND (top left), splenic SND (top right), MAP (bottom left), and heart rate [HR; beats per min (bpm); bottom right] during acute heating that increased internal body temperature (Tc) from 38 to 41.5°C over 40 min, and for 3 min following RVLM microinjections of kynurenic acid (Kyn; 2.7 nmol, n = 5; 5.4 nmol, n = 6), muscimol (Mus; 400–800 pmol, n = 5), or saline (n = 4) (designated by arrows) (Tc maintained at 41.5°C for the 3-min postinjection period). Values are means ± SE. *Kyn (2.7 and 5.4 nmol), Mus, and saline significantly different from control (38°C) at peak hyperthermia (41.5°C). **Significantly different from peak-heating levels after completion of RVLM microinjections. Baseline MAP values: 2.7 Kyn, 115 ± 5 mmHg; 5.4 Kyn, 106 ± 3 mmHg; Mus, 92 ± 3 mmHg; saline, 93 ± 3 mmHg. Baseline HR values: 2.7 Kyn, 411 ± 20 beats/min; 5.4 Kyn, 375 ± 11 beats/min; Mus, 395 ± 13 beats/min; saline, 414 ± 8 beats/min.
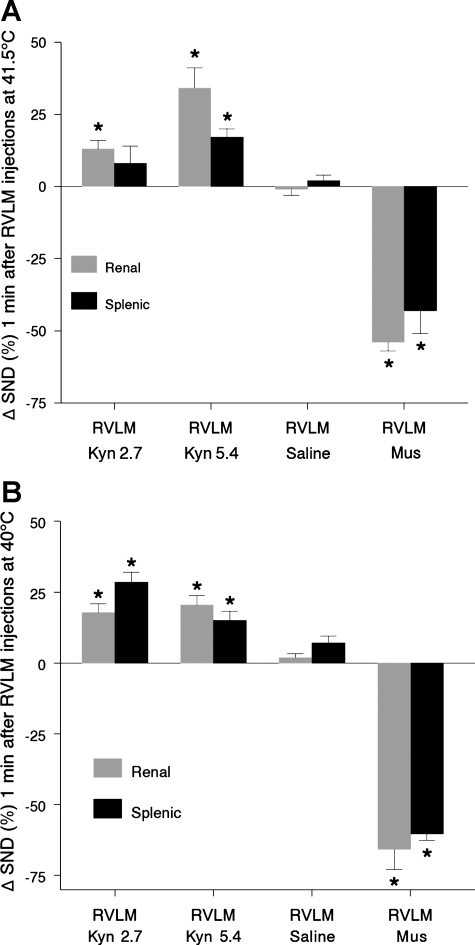
The effect of RVLM Kyn, saline, and muscimol microinjections on SND at peak hyperthermia was further analyzed by expressing SND values recorded 1 min after RVLM microinjections at 41.5°C as a percent change from levels recorded at 41.5°C immediately before microinjections. Results of this analysis are summarized in Fig. 3A. Renal SND (shaded bars) was significantly increased 1 min after RVLM Kyn microinjections at 2.7 and 5.4 nmol, whereas splenic SND (solid bars) was significantly increased after RVLM Kyn microinjections at 5.4 nmol. Renal and splenic SND remained unchanged after RVLM saline microinjections and were significantly reduced 1 min after RVLM muscimol microinjections.
Fig. 3.
A: changes in renal SND and splenic SND from levels recorded at 41.5°C 1 min after completion of bilateral RVLM Kyn (2.7 nmol, n = 5; 5.4 nmol, n = 6), saline (n = 4), and Mus microinjections (400–800 pmol, n = 5) (Tc maintained at 41.5°C 1 min after completion of RVLM microinjections). Values are means ± SE. *Significantly different from peak-heating levels at 1 min postmicroinjection. B: changes in renal SND and splenic SND from levels recorded at 40°C 1 min after completion of bilateral RVLM Kyn (2.7 nmol, n = 7; 5.4 nmol, n = 4), saline (n = 5), and Mus (400–800 pmol, n = 3) microinjections (Tc maintained at 40°C 1 min after completion of RVLM microinjections). *Significantly different from values recorded at 40°C at 1 min postmicroinjection.
Protocol II: Effects of RVLM Kyn Microinjections on SND Regulation During Progressive Hyperthermia
The effect of RVLM Kyn, saline, and muscimol microinjections on SND during progressive hyperthermia was analyzed by expressing SND values recorded 1 min after RVLM microinjections at 40°C as a percent change from levels recorded at 40°C immediately before microinjections (Fig. 3B). Renal SND (shaded bars) and splenic SND (solid bars) were significantly increased 1 min after RVLM Kyn microinjections (2.7 and 5.4 nmol), remained unchanged after RVLM saline microinjections, and were significantly reduced after RVLM muscimol microinjections (400–800 pmol) at 40°C. MAP and HR were not significantly changed 1 min after RVLM Kyn (2.7 and 5.4 nmol) or saline microinjections at 40°C (data not shown), whereas RVLM muscimol microinjections at 40°C reduced MAP (22 ± 4 mmHg).
Protocol III: Effects of RVLM kyn Microinjections on SND Regulation at the Initiation of Acute Heating
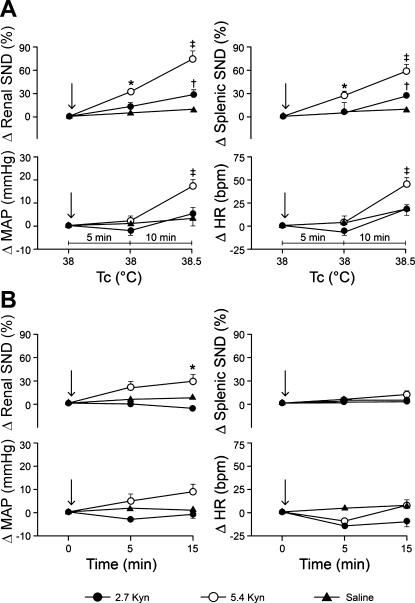
Figure 4A shows summarized changes in renal and splenic SND (top) and MAP and HR (bottom) at a Tc of 38°C 5 min after rats received bilateral RVLM microinjections of Kyn (2.7 or 5.4 nmol) or saline (designated by arrows) (microinjections completed at 38°C) and during the initiation of acute heating that increased Tc from 38 to 38.5°C over a period of 10 min. With Tc maintained at 38°C, renal and splenic SND were significantly increased 5 min after RVLM Kyn microinjections at 5.4 nmol, but remained unchanged after RVLM Kyn microinjections at 2.7 nmol and after RVLM saline microinjections (Fig. 4A, top). With the onset of acute heating (Tc increased from 38 to 38.5°C), renal and splenic SND were significantly higher in RVLM Kyn-treated (2.7 and 5.4 nmol) compared with RVLM saline-treated rats, and in rats treated with RVLM Kyn at 5.4 nmol compared with 2.7 nmol (Fig. 4A, top). MAP and HR remained unchanged 5 min after RVLM Kyn (2.7 and 5.4 nmol) and saline microinjections with Tc maintained at 38°C (Fig. 4A, bottom). With the onset of acute heating, MAP and HR were significantly higher in rats treated with RVLM Kyn at 5.4 nmol compared with 2.7 nmol, and in rats treated with RVLM Kyn at 5.4 nmol compared with RVLM saline-treated rats (Fig. 4A, bottom). RVLM muscimol microinjections (400–800 pmol, n = 3) completed at 39°C in separate experiments significantly reduced renal (−64 ± 8%, P < 0.05) and splenic SND (−66 ± 7%, P < 0.05).
Fig. 4.
A: changes in renal SND and splenic SND (top) and MAP and HR (bottom) at a Tc of 38°C 5 min after rats were treated with bilateral RVLM microinjections of Kyn (2.7 nmol, n = 15; 5.4 nmol, n = 5) or saline (n = 9) (designated by arrows; microinjections completed at 38°C), and during the initiation of acute heating that increased Tc from 38 to 38.5°C (10 min). Values are means ± SE. *Renal SND and splenic SND significantly different from preinjection control levels 5 min after RVLM Kyn microinjections at 5.4 nmol. †RVLM Kyn (2.7 nmol) significantly different from RVLM saline at 38.5°C for renal SND and splenic SND. ‡RVLM Kyn (5.4 nmol) significantly different from RVLM Kyn (2.7 nmol) and RVLM saline at 38.5°C for renal SND, splenic SND, MAP, and HR. B: changes in renal SND and splenic SND (top) and MAP and HR (bottom) in rats treated at a Tc of 38°C with bilateral RVLM microinjections of either Kyn (2.7 nmol, n = 3; 5.4 nmol, n = 6) or saline (n = 7) (designated by arrows), and maintained at this Tc for 15 min after RVLM microinjections. Values are means ± SE. *Renal SND significantly different from preinjection control levels after RVLM Kyn microinjections at 5.4 nmol. Baseline MAP values: 2.7 Kyn + heat, 110 ± 3 mmHg; 5.4 Kyn + heat, 109 ± 4 mmHg; saline + heat, 106 ± 3 mmHg; 2.7 Kyn time control, 119 ± 2 mmHg; 5.4 Kyn time control, 120 ± 9 mmHg; saline time control, 114 ± 3 mmHg. Baseline HR values: 2.7 Kyn + heat, 415 ± 7 beats/min; 5.4 Kyn + heat, 410 ± 17 beats/min; saline + heat, 394 ± 9 beats/min; 2.7 Kyn time control, 428 ± 5 beats/min; 5.4 Kyn time control, 422 ± 15 beats/min; saline time control, 391 ± 10 beats/min.
Figure 4B shows summarized renal and splenic SND (top) and MAP and HR (bottom) responses in rats treated at a Tc of 38°C with bilateral RVLM microinjections of Kyn (2.7 or 5.4 nmol) or saline (designated by arrows), and maintained at this Tc for 15 min after RVLM microinjections. Renal SND remained unchanged from control after RVLM Kyn microinjections at 2.7 nmol and after RVLM saline microinjections, but was significantly increased from control levels 15 min after RVLM Kyn microinjections at 5.4 nmol (Fig. 4B, top). Splenic SND, MAP, and HR remained unchanged from control after RVLM Kyn (2.7 and 5.4 nmol) and saline microinjections (Fig. 4B, top and bottom).
Protocol IV: Effects of RVLM kyn Microinjections on SND Responses to RVLM NMDA Microinjections and to Sciatic Nerve Stimulation
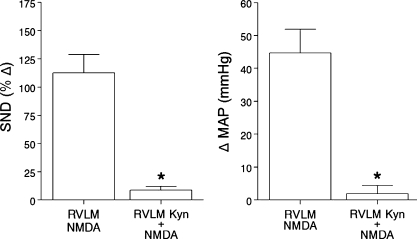
Consecutive bilateral RVLM microinjections of NMDA (20–40 pmol) were completed before and after (5–15 min) RVLM Kyn microinjections in experiments with Tc maintained at 38.5°C (n = 2), 40°C (n = 1), and 41.5°C (n = 4). The initial RVLM NMDA microinjections markedly increased SND (renal and splenic combined) and MAP (Fig. 5, RVLM NMDA). SND and MAP responses were significantly reduced when the second RVLM NMDA microinjections were completed after the RVLM was treated with Kyn (2.7 nmol, n = 4; 5.4 nmol, n = 3) (Fig. 5, RVLM Kyn + NMDA).
Fig. 5.
Average increases in SND (renal and splenic data combined for presentation; left) and MAP (right) to RVLM N-methyl-d-aspartic acid (NMDA; 20–40 pmol) microinjections alone (RVLM NMDA, data presented on the left side of each panel, n = 7) and to RVLM NMDA microinjections (20–40 pmol) after pretreatment of the RVLM with Kyn (2.7 nmol, n = 4; 5.4 nmol, n = 3; RVLM Kyn + NMDA, data presented on the right side of each panel). Values are means ± SE. *RVLM Kyn + NMDA significantly different from RVLM NMDA.
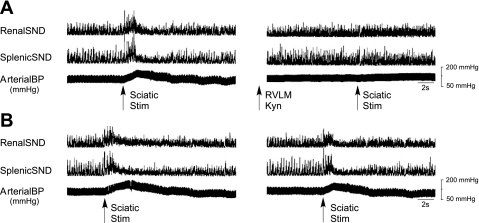
Figure 6A shows traces of renal SND, splenic SND, and arterial blood pressure responses to successive trials of sciatic nerve stimulation in a representative experiment. The initial sciatic nerve stimulation trial (left) induced marked increases in SND and arterial blood pressure, responses that were not induced by the second sciatic nerve stimulation trial (right), which was completed after bilateral RVLM Kyn (5.4 nmol) microinjections. Sciatic nerve stimulation trials were completed before and 5–15 min after RVLM microinjections of 2.7 and 5.4 nmol Kyn (Table 1). As expected, the initial sciatic nerve stimulation trial induced substantial increases in MAP, renal SND, and splenic SND (Table 1), whereas, after RVLM Kyn injections at 2.7 and 5.4 nmol, the second sciatic nerve stimulation trial induced modest increases or decreases from control levels in MAP and SND (Table 1). In contrast, stimulation-induced increases in MAP (initial stimulation, +23 ± 3 mmHg; second stimulation, +24 ± 2 mmHg) and SND (initial stimulation, +68 ± 17%; second stimulation, +59 ± 14%, renal and splenic SND combined for presentation) did not substantially differ when successive sciatic nerve stimulation trials were completed (15–20 min apart) without intervening RVLM Kyn microinjections (n = 3) (Fig. 6B shows a representative example).
Fig. 6.
Traces of renal SND, splenic SND, and arterial BP recorded before, during, and after successive sciatic nerve stimulation trials from two representative experiments. A: bilateral RVLM Kyn microinjections (5.4 nmol) were completed between successive sciatic nerve stimulation trials. B: successive sciatic nerve stimulation trials were completed without intervening RVLM Kyn microinjections. Horizontal calibration is 2 s.
Table 1.
Changes in MAP, renal SND, and splenic SND to sciatic nerve stimulation before and after RVLM Kyn (2.7 and 5.4 nmol) microinjections
| n | Stimulation Before RVLM Kyn | Stimulation After RVLM Kyn | |
|---|---|---|---|
| 2.7 nmol RVLM Kyn | |||
| ΔMAP, mmHg | 3 | +26 ± 2 | +5 ± 2 |
| ΔRenal SND, % | 3 | +76 ± 10 | −8 ± 10 |
| ΔSplenic SND, % | 2 | +88 ± 29 | +7 ± 2 |
| 5.4 nmol RVLM Kyn | |||
| ΔMAP, mmHg | 3 | +21 ± 1 | +6 ± 5 |
| ΔRenal SND, % | 3 | +45 ± 10 | −27 ± 5 |
| ΔSplenic SND, % | 2 | +56 ± 18 | +14 ± 26 |
Values are means ± SE;
n, no. of rats. RVLM, rostral ventrolateral medulla; Kyn, kynurenic acid; Δ, change; MAP, mean arterial pressure; SND, sympathetic nerve discharge.
In two experiments, MAP and renal SND responses to sciatic nerve stimulation were determined before and 20 min after RVLM Kyn microinjections (2.7 nmol, n = 1; 5.4 nmol, n = 1). The initial stimulation trial increased MAP (stimulation before 2.7 nmol Kyn, +16 mmHg; stimulation before 5.4 nmol Kyn, +21 mmHg) and renal SND (stimulation before 2.7 nmol Kyn, +64%; stimulation before 5.4 nmol Kyn, +81%) from control levels. In contrast, the second stimulation trial completed 20 min after bilateral RVLM Kyn microinjections produced either no change or modest reductions from control levels in MAP (stimulation after 2.7 nmol Kyn, −2 mmHg; stimulation after 5.4 nmol Kyn, −4 mmHg) and renal SND (stimulation after 2.7 nmol Kyn, −11%; stimulation after 5.4 nmol Kyn, −21%).
DISCUSSION
The present study revealed two novel findings regarding RVLM regulation of visceral sympathoexcitation to acute heating. First, RVLM Kyn microinjections did not reduce levels of renal and splenic SND recorded during progressive (Tc = 40°C) or peak (Tc = 41.5°C) hyperthermia and did not attenuate SND activation at the onset of heating. The resistance of heating-induced visceral sympathoexcitation to RVLM Kyn indicates that RVLM ionotropic glutamate receptors are not required for mediating sympathetic activation to acute heating, a significant finding based on the substantial increases in visceral SND produced by progressive hyperthermia. It is important to note that bilateral RVLM muscimol microinjections at the initiation, progressive activation, and peak phases of acute heating produced significant reductions in visceral SND, demonstrating that the integrity of RVLM neural circuits is required for mediating visceral sympathoexcitatory responses to acute heat stress. Second, after increasing Tc to 40 and 41.5°C, RVLM Kyn microinjections tended to increase, or significantly increased, levels of renal and splenic SND, and the SND responsiveness at the initiation of heating was significantly enhanced after pretreatment of the RVLM with Kyn. The increase in visceral SND following RVLM Kyn microinjections at the onset of heating and during hyperthermia suggests that acute heating activates an ionotropic EAA receptor-dependent inhibitory input to RVLM neural circuits, which reduces the level of visceral SND to heating.
Based on the established role of RVLM ionotropic EAA receptors in cardiovascular and SND regulation in pathophysiological states characterized by sustained activation of sympathetic nerve outflow (8, 20, 21, 59) and in SND and arterial blood pressure responses to numerous acute physical stressors (2, 6, 32, 39, 42), activation of RVLM ionotropic glutamate receptors has often been considered obligatory for mediating stimulus-induced cardiovascular and SND responses. However, the current results demonstrate that SND activation at the onset, progressive activation, and peak phases of acute heating is resistant to antagonism of RVLM ionotropic glutamate receptors. This finding suggests a high degree of intrinsic complexity in the regulation of RVLM neural circuits and supports several existing lines of evidence demonstrating functional specificity in the RVLM regulation of SND. For example, Kiely and Gordon (32) reported that RVLM Kyn microinjections eliminated MAP responses to sciatic nerve stimulation, but not to electrical stimulation of hypothalamic sites, indicating that cardiovascular responses to selected stimuli can be mediated via mechanisms other than activation of RVLM ionotropic EAA receptors. Similarly, RVLM Kyn microinjections block splanchnic sympathoexcitatory responses to stimulation of peripheral, but not central, chemoreceptors (42), demonstrating stimulus specificity in the involvement of RVLM ionotropic glutamate receptors in mediating SND activation. Moreover, Mayorov and Head (39) reported that RVLM Kyn injections attenuate the initial increases in renal SND and arterial blood pressure to emotional stress, but exert little effect on the sustained responses to this stressor, indicating that SND responses to specific stimuli can be mediated via multiple receptor systems and/or neural pathways, including, at least in part, via activation of RVLM ionotropic glutamate receptors.
The results of several investigations have reported that blockade of RVLM ionotropic glutamate receptors in the rat exerts little effect on resting levels of MAP (22, 32, 57, 59), leading to the suggestion that RVLM presympathetic neurons do not receive substantial tonic excitatory input from ionotropic EAA receptors. However, Ito and Sved (22) proposed that, under basal conditions, blockade of RVLM inotropic EAA receptors removes both tonic excitatory and inhibitory influences on RVLM sympathoexcitatory neurons, resulting in little or no change in sympathetic nerve outflow or MAP, and supporting the idea that central regulation of basal SND may include a novel RVLM glutamate-activated inhibitory pathway. The results of the present study extend these findings by demonstrating for the first time that visceral SND activation to acute heating is enhanced after RVLM Kyn microinjections, providing support for an inhibitory role for glutamatergic activation of RVLM ionotropic EAA receptors during heating. The presence of a counterbalancing inhibitory input to RVLM neural circuits during heat stress suggests a high degree of regulatory finesse in SND regulation during this environmental stressor. The present results also suggest that RVLM ionotropic glutamate receptors provide a degree of inhibitory tone to the basal regulation of SND as renal and splenic SND were significantly increased from control levels following RVLM Kyn microinjections at 5.4 nmol. It has been previously reported that, in anesthetized rats, arterial blood pressure and lumbar SND are significantly increased following RVLM Kyn microinjections (43), and arterial blood pressure is increased after blockade of RVLM NMDA receptors (31). It should be noted that several dose-associated response differences were observed in the present study. For example, basal SND was increased following RVLM Kyn microinjections at 5.4 nmol, but not 2.7 nmol, MAP was reduced at peak hyperthermia after RVLM Kyn microinjections at 2.7 nmol, but not 5.4 nmol, and, following RVLM Kyn microinjections of 2.7 nmol, both renal and splenic SND were significantly increased during progressive, but not peak, hyperthermia. Because of differences in the level of SND activation (basal vs. acute heating), levels of Tc (initiation, progressive hyperthermia, peak hyperthermia), and experimental variables affected (MAP, renal SND, splenic SND), it is difficult to draw definitive conclusions from the present data regarding dose-related Kyn effects on RVLM SND regulation.
The resistance of heating-induced visceral sympathoexcitation to RVLM Kyn indicates that SND activation to acute heating is not dependent on RVLM ionotropic glutamate receptors, suggesting a role for alternative mechanisms. The excitatory effect of heating on sympathetic neural circuits could result from an intrinsic thermal sensitivity of RVLM presympathetic neurons, although this would require a substantial degree of local neuronal selectivity as acute heating does not increase the level of activity in all sympathetic nerves, and, in fact, increased Tc decreases SND to the caudal ventral artery in the anesthetized rat (23). Alternatively or in addition, activation of RVLM neurons could be dependent on synaptic inputs from cells that do not operate via ionotropic glutamate receptors. Moreover, it is well established that the endogenous RVLM GABAergic system is tonically inhibitory to SND (3, 10, 11, 15); therefore, it is plausible that withdrawal of GABAergic tone to RVLM presympathetic neurons, i.e., disinhibition of sympathetic neural circuits, could contribute to heating-induced sympathetic activation.
Although the primary rationale for completing the present study was based on the established findings that progressive hyperthermia produces marked activation of visceral SND (25–30) and that maintenance of sympathetic activation at peak hyperthermia is dependent on the integrity of RVLM neural circuits (18), acute heating was chosen as the physiological stimulus in the present study for several additional reasons. First, heating-induced sympathoexcitatory responses play a critical role in mediating cardiovascular responses to increased Tc. For example, blockade of autonomic ganglionic transmission during hyperthermia reduces arterial pressure to values less than those produced by ganglionic blockade at normothermia (26), suggesting that sympathetic activation is important for counteracting vasodilatory influences during acute heating. Second, heat illness/stroke are medical emergencies that are characterized by sympathetic nervous system dysregulation and cardiovascular alterations (19, 33, 35, 37). Heat-related pathophysiological complications contribute to high levels of mortality worldwide (1, 4, 5, 12, 13, 47, 52), and there is a high likelihood that heat stoke is often misdiagnosed or underreported, especially in less developed regions of the world, thereby substantially underestimating the morbidity and mortality effects of heat waves (12). Importantly, the frequency and intensity of heat waves throughout the world have increased, and climate models predict an even higher incidence of severe heat waves (40, 45–47, 49). Third, excess mortality from hyperthermia and cardiovascular disease occurs in aged humans during heat waves (1, 13), and SND responses to hyperthermia are attenuated in aged compared with young F344 rats (28) and in young heart failure compared with young sham heart failure rats (29), demonstrating that individually both advanced age and heart failure affect sympathetic nervous system responsivity to acute heating. However, mechanisms mediating attenuated SND responses to heating in aged rats and in young rats with heart failure remain virtually unknown, due primarily to the fact that little information is known regarding central neurotransmitter/receptor mechanisms mediating SND responses to acute heat stress in young, healthy rats.
The present study was completed using an anesthetized preparation because, at least in our hands, it is difficult to complete brain stem microinjections while simultaneously recording the discharges in multiple sympathetic nerves in a conscious preparation. Importantly, SND responses to acute heat stress are qualitatively similar in awake and conscious preparations, as heat stress increases SND in conscious humans (9, 44), conscious rats (34), and young, anesthetized rats (25–30), whereas SND remains unchanged during acute heating in conscious (53) and anesthetized (28) aged F344 rats. Consistent with our laboratory's previous findings (18), we are confident that the RVLM was successfully targeted in the present study, as demonstrated by the observations that bilateral microinjections of l-glutamate at target coordinates elicited marked increases in SND and arterial blood pressure, and that RVLM muscimol microinjections during heating produced significant reductions in visceral SND and arterial blood pressure. However, as with any microinjection study, it is difficult to precisely assess the diffusion area of the microinjectate; therefore, we cannot exclude the possibility that adjoining medullary areas may play a role in mediating the observed SND responses. Although RVLM Kyn microinjections did not reduce or attenuate visceral SND responses to acute heating, three lines of evidence indicate that the Kyn microinjections were efficacious. First, RVLM Kyn blocked the visceral sympathoexcitation elicited via stimulation of sciatic nerve afferents. Second, RVLM Kyn effectively antagonized sympathoexcitatory and pressor responses produced by microinjection of NMDA into the RVLM, consistent with the results of a previous study by Kiely and Gordon (32). Third, because it was speculated that the visceral sympathoexcitation to acute heating may be dependent on activation of RVLM ionotropic EAA receptors, we considered the possibility that the excitation of RVLM neural circuits during acute heating may be so profound that Kyn microinjections at 2.7 nmol do not sufficiently block RVLM ionotropic glutamate receptors. If this was the case, then RVLM Kyn microinjections at a dose of 5.4 nmol would be expected to produce a more efficacious blockade of ionotropic glutamate receptors, leading to Kyn-induced reductions in SND and MAP during heating. However, the present results demonstrate that RVLM Kyn microinjections at 5.4 nmol did not reduce SND or MAP at the onset of acute heating or during progressive and peak hyperthermia.
Perspectives and Significance
The sympathetic nervous system plays a critical role in mediating physiological changes to acute physical stress. Hyperthermia is an excellent intervention for studying regulation of central sympathetic nerve outflow, because visceral SND is markedly activated during increased Tc, brain stem neural circuits are critically involved in mediating SND responses to acute heating, heat stroke is associated with sympathetic nervous system dysregulation and changes in arterial blood pressure, subjects with compensated cardiovascular systems and the aged are particularly vulnerable to mortality in heat waves, and SND responses to acute heating are substantially altered in aged subjects (humans and rats). However, a dearth of information exists regarding central mechanisms mediating SND responses to acute heating in young or aged subjects. The present results indicate that heating-induced visceral sympathoexcitation in young rats occurs in the presence of pharmacological antagonism of RVLM inotropic glutamate receptors, and that sympathetic activation to heating tends to be or is enhanced after RVLM Kyn microinjections. These data provide insight into the dynamic nature of RVLM sympathetic neural circuits involved in mediating sympathetic activation to acute physical stress and support the notion that regulation of RVLM sympathetic neural circuits involves a high level of intrinsic complexity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-091342 and HL-092392.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Argaud L, Ferry T, Le QH, Marfisi A, Ciorba D, Achache P, Ducluzeau R, Robert D. Short- and long-term outcomes of heatstroke following the 2003 heat wave in Lyon, France. Arch Intern Med 167: 2177–2183, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barman SM. Brainstem control of cardiovascular function. In: Brainstem Mechanisms of Behavior, edited by Klemm WR, Vertes RP. New York: Wiley, 1990, p. 353–381 [Google Scholar]
- 4. Bouchama A, Dehbi M, Mohamed G, Matthies F, Shoukri M, Menne B. Prognostic factors in heat wave related deaths: a meta-analysis. Arch Intern Med 167: 2170–2176, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Bouchama A, Knochel JP. Heat stroke. N Engl J Med 346: 1978–1988, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Brooks VL, Freeman KL, Clow KA. Excitatory amino acids in rostral ventrolateral medulla support blood pressure during water deprivation in rats. Am J Physiol Heart Circ Physiol 286: H1642–H1648, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126: 229–240, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Carvalho TH, Bergamaschi CT, Lopes OU, Campos RR. Role of endogenous angiotensin II on glutamatergic actions in the rostral ventrolateral medulla in Goldblatt hypertensive rats. Hypertension 42: 707–712, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 277: H2348–H2352, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177: 209–218, 2003 [DOI] [PubMed] [Google Scholar]
- 12. deSouza AL. Global warming and heatstroke. Indian J Med Res 128: 574–576, 2008 [PubMed] [Google Scholar]
- 13. Dematte JE, O'Mara K, Buescher J, Whitney CG, Forsythe S, McNamee T, Adiga RB, Ndukwu IM. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann Intern Med 129: 173–181, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol 280: H2891–H2901, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Horiuchi J, Dampney RA. Dependence of sympathetic vasomotor tone on bilateral inputs from the rostral ventrolateral medulla in the rabbit: role of baroreceptor reflexes. Neurosci Lett 248: 113–116, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Horiuchi J, Killinger S, Dampney RA. Contribution to sympathetic vasomotor tone of tonic glutamatergic inputs to neurons in the RVLM. Am J Physiol Regul Integr Comp Physiol 287: R1335–R1343, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hosking KG, Fels RJ, Kenney MJ. Inhibition of RVLM synaptic activation at peak hyperthermia reduces visceral sympathetic nerve discharge. Auton Neurosci 150: 104–110, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang YP, Lin MT, Chen JS, Wu PY. Naltrexone protects against hypotension, hyperthermia, and beta-endorphin overproduction during heatstroke in the rat. J Pharm Sci 97: 519–524, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension 35: 413–417, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension 37: 687–691, 2001 [PubMed] [Google Scholar]
- 22. Ito S, Sved AF. Tonic glutamate-mediated control of rostral ventrolateral medulla and sympathetic vasomotor tone. Am J Physiol Regul Integr Comp Physiol 273: R487–R494, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Johnson CD, Gilbey MP. Sympathetic activity recorded from the rat caudal ventral artery in vivo. J Physiol 476: 437–442, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenney MJ. Frequency characteristics of sympathetic nerve discharge in anesthetized rats. Am J Physiol Regul Integr Comp Physiol 267: R830–R840, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Kenney MJ, Barney CC, Hirai T, Gisolfi CV. Sympathetic nerve responses to hyperthermia in the anesthetized rat. J Appl Physiol 78: 881–889, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Kenney MJ, Claassen DE, Bishop MR, Fels RJ. Regulation of the sympathetic nerve discharge bursting pattern during heat stress. Am J Physiol Regul Integr Comp Physiol 275: R1992–R2001, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Kenney MJ, Fels RJ. Forebrain and brain stem neural circuits contribute to altered sympathetic responses to heating in senescent rats. J Appl Physiol 95: 1986–1993, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kenney MJ, Fels RJ. Sympathetic nerve regulation to heating is altered in senescent rats. Am J Physiol Regul Integr Comp Physiol 283: R513–R520, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Kenney MJ, Musch TI, Weiss ML. Renal sympathetic nerve regulation to heating is altered in rats with heart failure. Am J Physiol Heart Circ Physiol 280: H2868–H2875, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Kenney MJ, Pickar JG, Weiss ML, Saindon CS, Fels RJ. Effects of midbrain and spinal cord transections on sympathetic nerve responses to heating. Am J Physiol Regul Integr Comp Physiol 278: R1329–R1338, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Kiely JM, Gordon FJ. Non-NMDA receptors in the rostral ventrolateral medulla mediate somatosympathetic pressor responses. J Auton Nerv Syst 43: 231–239, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses. Am J Physiol Heart Circ Physiol 267: H1549–H1556, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Kregel KC, Gisolfi CV. Circulatory responses to heat after celiac ganglionectomy or adrenal demedullation. J Appl Physiol 66: 1359–1363, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Kregel KC, Stauss H, Unger T. Modulation of autonomic nervous system adjustments to heat stress by central ANG II receptor antagonism. Am J Physiol Regul Integr Comp Physiol 266: R1985–R1991, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Kregel KC, Wall PT, Gisolfi CV. Peripheral vascular responses to hyperthermia in the rat. J Appl Physiol 64: 2582–2588, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Kvochina L, Hasser EM, Heesch CM. Pregnancy increases baroreflex-independent GABAergic inhibition of the RVLM in rats. Am J Physiol Regul Integr Comp Physiol 293: R2295–R2305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li PL, Chao YM, Chan SH, Chan JY. Potentiation of baroreceptor reflex response by heat shock protein 70 in nucleus tractus solitarii confers cardiovascular protection during heatstroke. Circulation 103: 2114–2119, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Loewy A. Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Loewy A, Spyer K. New York: Oxford University Press, 1990, p. 28–43 [Google Scholar]
- 39. Mayorov DN, Head GA. Ionotropic glutamate receptors in the rostral ventrolateral medulla mediate sympathetic responses to acute stress in conscious rabbits. Auton Neurosci 98: 20–23, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305: 994–997, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Moffitt JA, Heesch CM, Hasser EM. Increased GABAA inhibition of the RVLM after hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol 283: R604–R614, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Moreira TS, Takakura AC, Colombari E, Guyenet PG. Central chemoreceptors and sympathetic vasomotor outflow. J Physiol 577: 369–386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol 102: 803–813, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst 63: 61–67, 1997 [DOI] [PubMed] [Google Scholar]
- 45. O'Neill MS, Ebi KL. Temperature extremes and health: impacts of climate variability and change in the United States. J Occup Environ Med 51: 13–25, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Parry ML, Canziani JP, et al. IPCC 2007: Summary of policymakers. In: Climate Change 2007: Impacts, Adaptation and Vulnerability. New York: Cambridge University Press, 2007 [Google Scholar]
- 47. Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature 438: 310–317, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Sakima A, Yamazato M, Sesoko S, Muratani H, Fukiyama K. Cardiovascular and sympathetic effects of l-glutamate and glycine injected into the rostral ventrolateral medulla of conscious rats. Hypertens Res 23: 633–641, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Schar C, Jendritzky G. Climate change: hot news from summer 2003. Nature 432: 559–560, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol 289: R1746–R1755, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 529: 221–236, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shibolet S, Lancaster MC, Danon Y. Heat stroke: a review. Aviat Space Environ Med 47: 280–301, 1976 [PubMed] [Google Scholar]
- 53. Stauss HM, Morgan DA, Anderson KE, Massett MP, Kregel KC. Modulation of baroreflex sensitivity and spectral power of blood pressure by heat stress and aging. Am J Physiol Heart Circ Physiol 272: H776–H784, 1997 [DOI] [PubMed] [Google Scholar]
- 54. Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989 [DOI] [PubMed] [Google Scholar]
- 55. Sun MK. Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol 47: 157–233, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Sun MK. Pharmacology of reticulospinal vasomotor neurons in cardiovascular regulation. Pharmacol Rev 48: 465–494, 1996 [PubMed] [Google Scholar]
- 57. Sun MK, Guyenet PG. Hypothalamic glutamatergic input to medullary sympathoexcitatory neurons in rats. Am J Physiol Regul Integr Comp Physiol 251: R798–R810, 1986 [DOI] [PubMed] [Google Scholar]
- 58. Tagawa T, Horiuchi J, Potts PD, Dampney RA. Sympathoinhibition after angiotensin receptor blockade in the rostral ventrolateral medulla is independent of glutamate and gamma-aminobutyric acid receptors. J Auton Nerv Syst 77: 21–30, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Wang WZ, Gao L, Wang HJ, Zucker IH, Wang W. Tonic glutamatergic input in the rostral ventrolateral medulla is increased in rats with chronic heart failure. Hypertension 53: 370–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]