Abstract
Although it is well established that the renal endothelin (ET-1) system plays an important role in regulating sodium excretion and blood pressure through activation of renal medullary ETB receptors, the role of this system in Dahl salt-sensitive (DS) hypertension is unclear. The purpose of this study was to determine whether the DS rat has abnormalities in the renal medullary endothelin system when maintained on a high sodium intake. The data indicate that Dahl salt-resistant rats (DR) on a high-salt diet had a six-fold higher urinary endothelin excretion than in the DR rats with low Na+ intake (17.8 ± 4 pg/day vs. 112 ± 44 pg/day). In sharp contrast, urinary endothelin levels increased only twofold in DS rats in response to a high Na+ intake (13 ± 2 pg/day vs. 29.8 ± 5.5 pg/day). Medullary endothelin concentration in DS rats on a high-Na+ diet was also significantly lower than DR rats on a high-Na+ diet (31 ± 2.8 pg/mg vs. 70.9 ± 5 pg/mg). Furthermore, DS rats had a significant reduction in medullary ETB receptor expression compared with DR rats while on a high-Na+ diet. Finally, chronic infusion of ET-1 directly into the renal medulla blunted Dahl salt-sensitive hypertension. These data indicate that a decrease in medullary production of ET-1 in the DS rat could play an important role in the development of salt-sensitive hypertension observed in the DS rat.
Keywords: kidney, blood pressure, hypertension
endothelin-1 (et-1) was first characterized in 1988 as a potent vasoconstrictor released by endothelial cells (26). Two receptor subtypes have been since identified, ETA and ETB (24). Both of these receptors are located in the kidney with the highest concentration of ETB receptors existing within the medulla (11). Although the role of ETA receptors has been well characterized in the pathophysiology of experimental hypertension, the physiological importance of ETB receptors in modulating sodium excretion and blood pressure regulation in salt-sensitive hypertension is unclear. ETB activation inhibits sodium transport (15, 18, 19), and growing evidence suggests a critical role for the renal medullary endothelin system in the integrated response to a high-salt diet (6, 20–21). For example, Pollock and others (4)have shown that renal ET-1 production is enhanced in response to 1 wk of a high-sodium diet, and intramedullary blockade of ETB receptors for 7 days leads to salt-sensitive hypertension. In addition, specific knockout of the ETB receptor gene and the ET-1 gene in the medullary collecting duct produces salt-sensitive hypertension (1, 6).
Although there is growing evidence from a number of laboratories that the renal endothelin system via ETB receptor activation plays an important role in modulating renal pressure natriuresis and blood pressure regulation, very little is known about potential abnormalities in renal endothelin system in models of salt-sensitive hypertension. Our laboratory and others have reported that there is a rightward shift in the pressure natriuresis relationship in Dahl salt-sensitive rats (DS) (12). One potential mechanism for the abnormal pressure natriuresis relationship is a defect in the renal medullary endothelin system, resulting from either an abnormality in renal endothelin production or an altered receptor distribution and signaling. Although several previous studies have reported alterations in renal cortical endothelin production in models of salt-sensitive spontaneous hypertension, the endothelin system was assessed only after long-standing hypertension and renal injury, a condition that is known to stimulate endothelin release (7, 9). Thus, very little is known about potential abnormalities in renal endothelin system in response to increases in sodium intake in the early phases of hypertension in salt-sensitive hypertension models. Therefore, the purpose of this study was to determine whether the DS rat has abnormalities in the components of the renal medullary endothelin system when maintained on a high-sodium intake for 7 days, prior to the development of hypertension. Another goal of our study was to determine whether chronic intramedullary infusion of ET-1 blunts the hypertensive response to high Na+ intake in DS rats maintained on a high-sodium intake for 3 wk.
MATERIALS AND METHODS
This study was performed using male age-matched (8–10 wk old), DS, and Dahl salt-resistant (DR) rats purchased from Harlan. Animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals and were approved by the Institutional Animal Care and Use Committee at University of Mississippi Medical Center.
Experimental protocol 1: effect of 7 days of a high-salt diet on ET-1 production and receptor expression in DR and DS rats.
Animals were randomly placed into the following groups: DR rats on low-salt diet (LS; 0.3% NaCl), DR rats on high-salt diet (HS; 8% NaCl), DS rats on LS, and DS on HS (n = 6). The animals were allowed water ad libitum. After 6 days on their respective diets, the animals were placed in metabolic cages for 24-h urine collection. Urine was collected, and on day 7, rats were instrumented with carotid catheters for arterial pressure measurement. On day 8, pressure was measured over a 20-min period using DataQ systems analysis program, and the average over this time period was taken. Immediately following, tissues were collected and snap frozen in liquid nitrogen and stored at −80°C until molecular assays were performed.
Experimental protocol 2: effect of chronic infusion of ET-1 on blood pressure in Dahl rats.
Male DR and DS (300 g) rats were randomly distributed into the following groups: 1) DR vehicle (n = 8), 2) DR ET-1 (n = 8), 3) DS vehicle (n = 6), and 4) DS ET-1 (n = 5). The rats were maintained on a low Na+ diet (0.3% NaCl) prior to experiment. Each rat was uninephrectomized, and a chronic indwelling catheter was placed in the medullary interstitium of the other kidney and secured to the renal capsule. Next, the rats were instrumented with a telemetry probe (Data Sciences International) for 24-h blood pressure measurements. The rats were allowed to heal until blood pressure was stabilized. Next, the rats were placed on a HS diet and tethered; then infusion of vehicle (saline) or ET-1 (2 ng·kg−1·min−1 at 0.600 μl/min) was carried out for 15 days.
Extraction of urinary ET-1.
Equal volume of 20% acetic acid was added to the sample and centrifuged at 3,000 rpm for 10 min at 4°C. A 200-mg C18 Sep-Pak was equilibrated with one column reservoir volume (CV) methanol followed by one CV water and one CV 10% methanol. Supernatant was applied to the Sep-Pak column and washed with one CV 10% acetic acid followed by two separate washes with ethyl acetate. Washes were discarded. Sample was eluted in 3 ml methanol/0.05 M ammonium bicarbonate and collected in polyethylene tubes. The sample was evaporated to dryness using a centrifugal concentrator under a vacuum and reconstituted with assay buffer provided by the manufacturer (R&D Systems). Endothelin concentrations were measured using Quantiglo ET-1 ELISA (manufactured by R&D Systems).
Extraction of tissue ET-1.
Tissue was snap frozen in liquid nitrogen and stored at −80°C. Frozen samples were pulverized in liquid nitrogen and homogenized for 60 s in 10 volumes of 1 M acetic acid containing 10 μg/ml of pepstatin. Samples were heated for 10 min at 100°C, placed on ice, and centrifuged at 3,500 rpm for 45 min at 4°C. Supernatant was stored at −80°C and was used for ET-1 quantification. Samples were standardized to total protein using BCA protein kit (Pierce Biotechnology).
Measurement of urinary and tissue ET-1.
Endothelin concentrations were measured using Quantiglo ET-1 ELISA (R&D Systems) using directions supplied by the company.
Western blot analysis of endothelin receptors.
Protein was extracted from renal medullary tissue by crunching frozen tissue with a mortar and pestle, then agitation in RIPA buffer containing 10 μl/ml of a protease inhibitor cocktail, PMSF, and sodium orthovanadate (Santa Cruz Biotechnology). Concentration of total protein was acquired using a BCA protein kit (Pierce Biotechnology). Protein lysates (50 μg total protein) were mixed with an equal volume of SDS-Laemmli sample buffer (Bio-Rad). Samples were denatured at 95°C for 5 min and chilled on ice for 2 min. They were then loaded into a Criterion gel (Bio-Rad) containing a 4–20% gradient SDS/PAGE gel in running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS) and ran for 1.5 h at 120 V. The gels were then equilibrated in transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol) for 5 min. Next, the protein was transferred onto a 0.2 μm nitrocellulose membrane in transfer buffer at room temperature for 2 h at 40 V. Membranes were blocked for 1 h with blocking buffer (Odyssey). Primary antibody (Rabbit anti-ETB, Abcam) was diluted in blocking buffer (1:500) and incubated overnight at 4°C. The membrane was then washed three times for 5 min. Membranes were then incubated for 45 min at room temperature with gentle agitation in secondary antibody (Donkey anti-rabbit conjugated to IRDye 700DX, 1:5,000) diluted in blocking buffer. Blots were then washed three times in TBS for 5 min per wash. Blots were scanned on an Odyssey infrared imaging system (LiCor) for detection of the individual fluorophores. The blots were then stripped using NewBlot Western stripping buffer (Licor), washed, and rehybridized using an ETA receptor antibody (Rabbit polyclonal to ETA receptor, 1:500; Abcam) using the same secondary antibody, as previously described. After attaining blots for both receptor subtypes, the blots were rehybridized for β-actin in the same manner, as previously described. All ETB and ETA bands were normalized to β-actin control bands. Antibodies (Mouse anti-β-actin, 1:5,000 primary and donkey anti-mouse conjugated to IRDye 800DX, 1:5,000) for β-actin detection were obtained from Abcam and Rockland Immunochemical, respectively. Quantification of the Western blot images was carried out with the National Institutes of Health's ImageJ software package.
Statistical analysis.
All data are expressed as means ± SE. In experiment 1, comparisons for multigroup analysis were performed by two-way ANOVA and by using Tukey's post hoc test for comparison between groups. The criterion for significant differences between groups of study was P < 0.05. For experiment 2, a two-way repeated-measures ANOVA was performed, and furthermore, each day was compared with the baseline measurements at day 4 by Student's t-test.
RESULTS
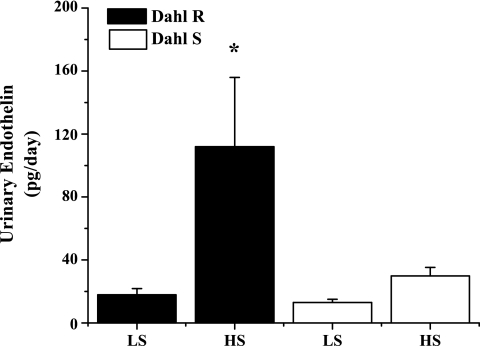
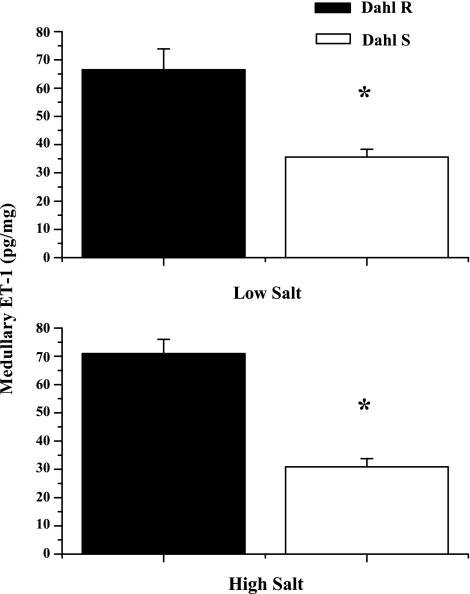
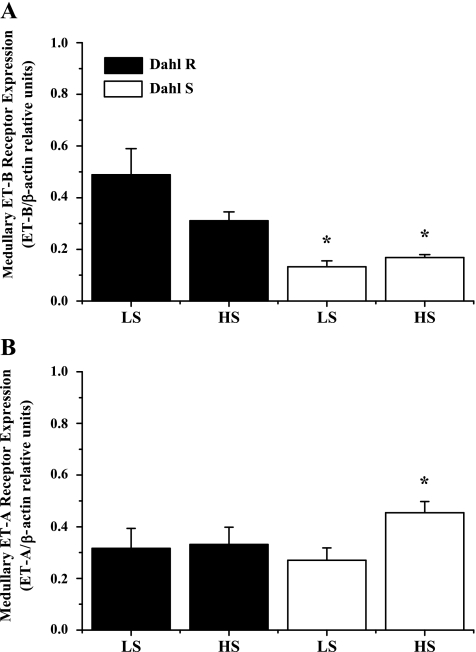
To determine whether medullary ET-1 plays a role in the development of DS hypertension, we determined urinary ET-1, which is indicative of renal production, in DS and DR rats on low- and high-salt diets for 1 wk, prior to an increase in pressure in the DS. As can be seen in Fig. 1, DR rats placed on a HS diet had a five-fold increase in ET-1 production (17.8 ± 4 pg/day on NS diet vs. 112 ± 44 pg/day on HS, P < .05); however, this increase was significantly blunted in the DS (29.8 ± 5.5 pg/ml HS and 13 ± 2 pg/day NS). Also, as indicated in Fig. 2, there is a significant reduction in medullary ET-1 in DS rats compared with DR (70.9 ± 5 DR vs. 30.9 ± 2.8 DS). Finally, our data indicate in Fig. 3 that DS rats have significantly lower medullary ETB receptor expression compared with DR (0.13 ± 0.02 DS on LS and 0.16 ± 0.01 DS on HS ETB/β-actin relative units vs. 0.48 ± 0.22 DR on HS and 0.31 ± 0.08 DR on LS ETB/β-actin relative units, n = 6), while also having a significant elevation in ETA receptor expression in response to a high-salt diet (0.31 ± 0.03 DS on LS vs. 0.49 ± 0.10 DS on HS ETB/β-actin relative units; n = 6). The data indicate that a failure of the DS rat to produce ET-1 and a reduction in ETB receptor expression in the renal medulla may be important initiating factors in the development of the hypertension observed in this model.
Fig. 1.
Urinary ET-1 excretion is blunted in Dahl salt-sensitive (Dahl S) rats in response to high Na+ intake. When Dahl salt-resistant (Dahl R; n = 6) rats are placed on a high-salt (HS) diet, urinary endothelin-1 (ET-1) increases six-fold; however, the increase in urinary ET-1 excretion was significantly attenuated in Dahl S. *Significant difference, P < 0.05 vs. all other groups.
Fig. 2.
Renal medullary tissue levels of ET-1 were significantly reduced in Dahl S rats, compared with Dahl R rats, maintained on either low-salt (LS; top) or high-salt (HS; bottom) diet for 7 days. *Significant difference, P < 0.05 vs. all other groups.
Fig. 3.
A: Dahl S rats have significantly lower ETB receptor expression than Dahl R rats. There is a tendency for receptor expression to decrease in Dahl R rats in response to a HS diet; however, there is no change in response to changes in dietary salt intake in Dahl S rats. B: in response to a HS diet for 1 wk, there is no significant difference in renal medullary ETA receptor expression in Dahl R rats. However, Dahl S rats have a 70% increase in ETA receptor expression after 1 wk on a HS diet. *Significant difference, P < 0.05 vs. Dahl S on a LS diet.
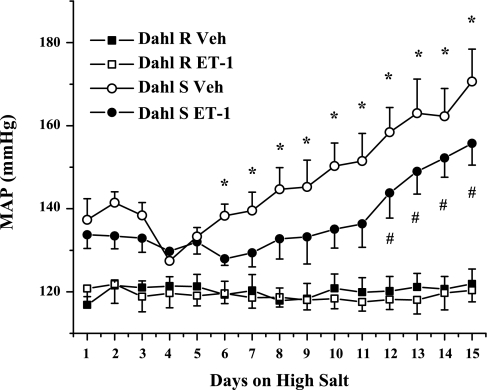
Finally, we wanted to determine whether chronic intramuscular infusion of ET-1 would attenuate DS hypertension. As seen in Fig. 4, there is a clear trend for a reduction in blood pressure (P = 0.11) in response to infusion of ET-1 directly into the renal medulla of DS rats. Furthermore, we found that treatment with ET-1 delayed the onset of hypertension in response to a high-salt intake observed in this model. There was no effect of treatment in DR rats.
Fig. 4.
In response to chronic infusion of ET-1 directly into the renal medulla, the hypertensive response to a HS diet is delayed in Dahl S rats, while there is no effect on Dahl R. *Significant difference, P < 0.05 vs. day 4 vehicle infused; #significant difference, P < 0.05 vs. day 4 ET-1 infused.
DISCUSSION
A role for renal medullary derived ET-1 in the control of renal excretory function has accumulated over recent years (1, 8, 21). Multiple studies indicate the importance of this system becomes greater as Na+ intake is increased (21). However, the role of the renal medullary ET-1 system in different forms of genetic and experimental salt-sensitive hypertension has yet to be fully elucidated. ET-1 can either elicit a prohypertensive, antinatriuretic effect by activating ETA receptors in the kidneys (mainly due its cortical actions in the kidney, where most of the ETA receptors are located) or an antihypertensive, natriuretic effect via ETB receptor activation (mainly in the renal medulla where most of the ETB receptors are located). Thus, the ability of ET-1 to influence blood pressure regulation and renal function is highly dependent on where ET-1 is produced and which ET receptor type is activated.
The main goal of our study was to determine whether abnormalities in renal medullary ET production (and possibly ETB receptors levels) play a role in the early phases of hypertension in response to salt in the DS rats. To determine whether DS rats have altered renal production of ET-1 in the early phases of DS hypertension, we examined the effect of 7 days of a high-salt diet on urinary ET-1 excretion. It is important to note that only moderate elevations in blood pressure develop after 1 wk on a high-salt diet in this model (12); therefore, observations in this study were made prior to elevations in blood pressure. There were two major findings in this study. First, in response to a high-salt diet, DR rats had a significant six-fold increase in urinary ET-1 excretion, whereas DS rats only had a twofold elevation. Since, urinary ET-1 is indicative of renal production (3), we can conclude that the DS rat has a markedly blunted renal production of ET-1 compared with the DR. Secondly, DS rats had significantly lower tissue levels of ET-1 in the renal medulla than the DR maintained on a HS intake. This finding is in contrast to a previous study by Barton et al. (2) that reported that DS rats have higher levels of ET-1 within the renal medulla in response to a HS diet (2). There may be multiple reasons for the differences in findings, including differences in the length of sodium intake (1 wk vs. 8 wk), differences in blood pressure, and differences in methodologies used to measure tissue endothelin levels (2). Collectively, our data indicate that DS rats have a significant reduction in renal medullary ET-1 production in response to a HS diet in the early phases of DS hypertension.
In addition to a reduction in renal medullary ET-1 production, the DS rat has alterations in renal medullary ET-1 receptor expression during the early phases of DS hypertension. Our data indicate that DS rats have significant reductions in medullary ETB receptors compared with DR rats. Furthermore, in response to a HS diet, medullary ETA receptors are upregulated in DS rats. The significance of this depends on which cell type this increase occurs. There is evidence that the ETA receptor may have natriuretic properties and can play an adaptive role in the absence of ETB receptor function (17). However, if ETA receptors were upregulated on vascular cells, this would contribute further to the hypertension in this model, especially with a lack of ETB receptors to offset the constrictor properties of ETA receptors.
Since we showed that DS rats have significant reductions in renal ET-1 production in response to a high-salt intake, the next step was to determine the chronic consequences of these effects. Since we proposed that reductions in renal medullary ET-1 contributes to DS hypertension, we hypothesized that chronic infusion of ET-1 directly to the renal medulla would blunt the salt-sensitive hypertension in this model by restoring the defect in intramedullary levels of ET-1. Indeed, intramedullary infusion of ET-1 led to a reduction in mean arterial pressure (MAP) in DS rats, while having no effect in DR rats. While ET-1 did not completely abolish the hypertension long term, intramedullary infusion of ET-1 delayed the onset of hypertension in response to a HS diet in DS rats because there was no significant change in arterial pressure until day 11, when MAP began to rise equally in both groups. It is hypothesized that this effect is due to the activation of natriuretic ETB receptors, thus leading to a reduction of blood pressure in the initial phase of Dahl salt-sensitive hypertension. Our data indicate that renal medullary infusion of ET-1 did not completely blunt the increase in pressure in response to a HS diet in DS rats. There are several potential explanations for this response. For instance, there are many other factors that have been implicated in the pathogenesis of DS hypertension, such as decreased synthesis of nitric oxide (10) and 20-hydroxyeicosatetraenoic acid (23). Thus, intramedullary infusion ET-1 alone would only correct one of the defects responsible for the hypertension and, therefore, would not be expected to totally prevent the hypertension in DS rats.
While we observed significant increases in urinary ET-1 excretion and renal medullary ET-1 concentration in the DR rats in response to increases in Na+ intake, the cellular and/or molecular mechanism underlying the defective ET-1 response to Na+ intake in DS rats remains unknown. ET-1 is thought to be released in response to increased medullary tonicity (25) or tubular flow (16); however, other factors may be involved. Whether medullary tonicity or tubular flow-induced production of ET-1 is altered in DS rats is unclear. Thus, research into the cellular and/or molecular mechanism underlying the defective ET-1 response to Na+ intake in DS rats warrants further investigation.
Although our findings suggest that a decrease in medullary production of ET-1 in the DS rat could play an important role in the early phases in the development of salt-sensitive hypertension observed in the DS rat, several lines of evidence suggest that ET-1 via ETA receptor activation may contribute to late and established phases of salt-sensitive hypertension. DS rats placed on a HS diet are characterized by attenuated pressure natriuresis, development of hypertension, extensive glomerulosclerosis, renal arteriolar, and tubular injury, as well as progressive renal injury (2, 12). ET-1 levels are increased in the renal cortex of DS rats compared with DR rats, and a positive correlation between ET-1 generation in the renal cortex and the extent of glomerulosclerosis has been reported in DS hypertensive rats (2) . Also supporting a role of ET-1 in DS hypertension is the finding that acute infusion of a nonselective ETA-ETB receptor antagonist directly into the renal interstitium improved renal hemodynamic and excretory function in DS rats but not in DR rats (13). Moreover, chronic blockade of ETA receptors attenuated the hypertension and proteinuria and ameliorated the glomerular and tubular damage associated with HS intake in DS rats (2, 12). An important unanswered question is whether the beneficial effect of the ET blockade in reducing renal injury is mediated through lower blood pressure or through direct renal mechanisms.
In conclusion, we report that the DS rat has several abnormalities in the renal medullary endothelin system when maintained on a HS intake during the early phases of DS hypertension. We found that the DS rat has an attenuated renal medullary ET synthesis response to a HS diet during the early phases of DS hypertension. In contrast to the DR rat, which had a six-fold higher urinary endothelin excretion in response to a high Na+ diet, urinary endothelin levels increased only twofold in DS rats. We also report that medullary endothelin concentration in DS rats on a high-salt diet was also significantly lower than DR rats on a high-salt intake. Finally, DS rats had a significant reduction in medullary ETB receptor expression compared with DR rats on a high-salt intake. In summary, our data indicate that a decrease in medullary production of ET-1 in the DS rat could play an important role in the early phases of salt-sensitive hypertension observed in the DS rat.
Perspectives and Significance
Although salt-sensitive hypertension accounts for a significant portion of human essential hypertension, the mechanisms underlying the pathogenesis of this form of hypertension remains to be fully elucidated. The current study suggests that in an experimental model of salt-sensitive hypertension, the DS rat, defects in the renal endothelin system may contribute to the pathogenesis of salt-sensitive hypertension. Moreover, our data suggest that the renal medullary ETB receptors may be a potential target for treatment of hypertension. Thus far, clinical studies utilizing ET-1 receptor blockers have shown little effect on blood pressure in hypertensive patients (22); however, the early ET-1 receptor blockers used were either combined ETA/ETB blockers or were ETA blockers, which lacked in vivo specificity (4). Because of the natriuretic and antihypertensive properties of ETB receptors, blockade of ETB receptors in these studies may have counteracted any beneficial effects of ETA receptor blockade. More recently, sitaxsentan, a very specific ETA blocker, was shown to reduce blood pressure and proteinuria in patients with chronic kidney disease (5). Therefore, determining the importance of ET-1 in human essential hypertension deserves further investigation.
GRANTS
This work was supported by National Institutes of Health Grant HL51971 to J. P. Granger and American Heart Association Grant 09PRE2250470 to J. S. Speed.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Also, the authors would like to thank the Mississippi Functional Genomics Network.
REFERENCES
- 1. Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest 114: 504–511, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton M, Vos I, Shaw S, Boer P, D'Uscio LV, Grone HJ, Rabelink TJ, Lattmann T, Moreau P, Luscher TF. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and glomerulosclerosis. J Am Soc Nephrol 11: 835–845, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Benigni A, Perico N, Gaspari F, Zoja C, Bellizzi L, Gabanelli M, Remuzzi G. Increased renal endothelin production in rats with reduced renal mass. Am J Physiol Renal Fluid Electrolyte Physiol 260: F331–F339, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 52: 452–459, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Dhaun N, MacIntyre IM, Kerr D, Melville V, Johnston NR, Haughie S, Goddard J, Webb DJ. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension 57: 772–779, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol 291: F1274–F1280, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Goligorsky MS, Iijima K, Morgan M, Yanagisawa M, Masaki T, Lin L, Nasjletti A, Kaskel F, Frazer M, Badr KF. Role of endothelin in the development of Dahl hypertension. J Cardiovasc Pharmacol 17 Suppl 7: S484–S491, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem 284: 1454–1460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikeda T, Ohta H, Okada M, Kawai N, Nakao R, Siegl PK, Kobayashi T, Maeda S, Miyauchi T, Nishikibe M. Pathophysiological roles of endothelin-1 in Dahl salt-sensitive hypertension. Hypertension 34: 514–519, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Ikeda Y, Saito K, Kim JI, Yokoyama M. Nitric oxide synthase isoform activities in kidney of Dahl salt-sensitive rats. Hypertension 26: 1030–1034, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Jones CR, Hiley CR, Pelton JT, Miller RC. Autoradiographic localisation of endothelin binding sites in kidney. Eur J Pharmacol 163: 379–382, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Kassab S, Miller MT, Novak J, Reckelhoff J, Clower B, Granger JP. Endothelin-A receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 31: 397–402, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Kassab S, Novak J, Miller T, Kirchner K, Granger J. Role of endothelin in mediating the attenuated renal hemodynamics in Dahl salt-sensitive hypertension. Hypertension 30: 682–686, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Kohan DE. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETB receptor on Na+-K+ ATPase activity by extracellular Ca2+ entry and Ca2+ release from the endoplasmic reticulum in renal proximal tubule cells. Hypertens Res 32: 846–852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyon-Roberts B, Strait KA, van Peursem E, Kittikulsuth W, Pollock JS, Pollock DM, Kohan DE. Flow regulation of collecting duct endothelin-1 production. Am J Physiol Renal Physiol 300: F650–F656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14–3-3/Nedd4–2. J Am Soc Nephrol 21: 833–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ET(B) receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Pollock DM. Renal endothelin in hypertension. Curr Opin Nephrol Hypertens 9: 157–164, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Rich S, McLaughlin VV. Endothelin receptor blockers in cardiovascular disease. Circulation 108: 2184–2190, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens 10: 63S–67S, 1997 [PubMed] [Google Scholar]
- 24. Rubanyi GM, Polokoff MA. Endothelins: molecular biology: biochemistry, pharmacology, physiology, pathophysiology. Pharmacol Rev 46: 325–415, 1994 [PubMed] [Google Scholar]
- 25. Stricklett PK, Strait KA, Kohan DE. Novel mechanism for regulation of endothelin synthesis: role of extracellular pH. Cell Physiol Biochem 21: 117–122, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988 [DOI] [PubMed] [Google Scholar]