Abstract
We have recently shown that a multi-mineral extract from the marine red algae, Lithothamnion calcareum, suppresses colon polyp formation and inflammation in mice. In the present study, we used intact human colon tissue in organ culture to compare responses initiated by Ca2+ supplementation versus the multi-mineral extract. Normal human colon tissue was treated for 2 d in culture with various concentrations of calcium or the mineral-rich extract. The tissue was then prepared for histology/immunohistochemistry, and the culture supernatants were assayed for levels of type I procollagen and type I collagen. At higher Ca2+ concentrations or with the mineral-rich extract, proliferation of epithelial cells at the base and walls of the mucosal crypts was suppressed, as visualized by reduced Ki67 staining. E-cadherin, a marker of differentiation, was more strongly expressed at the upper third of the crypt and at the luminal surface. Treatment with Ca2+ or with the multi-mineral extract influenced collagen turnover, with decreased procollagen and increased type I collagen. These data suggest that calcium or mineral-rich extract has the capacity to (1) promote differentiation in human colon tissue in organ culture and (2) modulate stromal function as assessed by increased levels of type I collagen. Taken together, these data suggest that human colon tissue in organ culture (supporting in vivo finding in mice) will provide a valuable model for the preclinical assessment of agents that regulate growth and differentiation in the colonic mucosa.
Keywords: Organ culture, Colon, Differentiation, Calcium, Multi-mineral
Introduction
Colon cancer is the third most common cause of cancer mortality in the world (American Cancer Society 2009). Colon cancer develops over years or decades (Burgart 2002). Molecular changes in colonic epithelial cells give rise to areas of hyperplasia called aberrant crypt foci (ACF; Takayama et al. 2001). ACF can be visualized histologically (review by Gupta et al. 2007) and are believed to be the precursor lesions for most adenomatous polyps. Adenomatous polyps are neoplastic growths in which glandular structures are still evident, but the glands are enlarged and disorganized. They may exist for years as premalignant lesions and are the major target picked up in routine colonoscopy procedures. Some polyps contain malignant cells, in which case the lesion may be referred to as carcinoma in situ. All of these precursor lesions can be distinguished from invasive colon cancer by their confinement to the mucosal space, i.e., the malignancy has not penetrated the submucosa.
Cancer chemoprevention can be defined as the use of chemical moieties to prevent the conversion of histologically normal cells into early premalignant lesions and to prevent the progression of premalignant lesions into invasive cancer. Empirical evidence exists for effective chemoprevention in the colon with Ca2+ (alone or in conjunction with vitamin D). This is based on epidemiological findings and the results from Ca2+ supplementation trials in humans, as well as interventional studies in experimental animals. Mechanistic studies have defined pathways (via Ca2+-induced differentiation) that contribute to chemoprevention. While Ca2+ supplementation reduces polyp formation in the colon, efficacy is far from complete, and additional chemopreventive agents are needed (Beaty et al. 1993; Bostick et al. 1993; Kampman et al. 1994, 2000; Baron et al. 1999; Holt et al. 2001; Lamprecht and Lopkin 2003; Grau et al. 2007).
A major obstacle in identifying and testing potentially useful agents is the lack of effective models in which to assess chemopreventive activity. This is especially true of agents that are not approved for human use. One possible solution involves the use of human colon tissue in organ culture. Previous studies (Autrup 1980; Senior et al. 1982; Lipkin 1985; Moorghen et al. 1996; Kesisoglou et al. 2006), including one from our own laboratory (Dame et al. 2010), have demonstrated that human colon tissue can be maintained for a period of time under conditions in which histological and biochemical features are preserved. In our previous study, we showed that markers of growth and differentiation, including Ki67 (proliferation marker), E-cadherin, and the extracellular calcium-sensing receptor (differentiation markers), were detectable and positionally expressed in human colon tissue after 2 d in culture. The present study continues our earlier effort. Here, we demonstrate that supplementation with Ca2+ above the normal level of 1.5 mM (two to five times with calcium chloride) or supplementation with a multi-mineral extract from the red marine algae Lithothamnion calcareum (containing comparable levels of Ca2+, in addition to 72 trace metals) decreases Ki67 expression and leads to the increased expression of E-cadherin. These findings suggest that human colon tissue in organ culture can provide a useful model with which to conduct preclinical chemoprevention studies.
Materials and Methods
Colon tissue
Organ culture of human colon tissue was conducted as in our previous work (Dame 2010). Human colon tissue specimens (split thickness) were obtained from surgical resections performed at the University of Michigan Hospitals. The tissue was from beyond the margin of disease and was grossly and histologically normal. It was provided by the Tissue Procurement Core Laboratory with all identifiers removed. As a result, this study has been reviewed by IRBMED at the University of Michigan and determined not to involve human subjects. A total of six specimens were cultured, from four females and two males, between the ages of 28 and 80+yr.
Culture medium
As described earlier (Dame 2010), the culture medium used here is based on a serum-free modification of earlier formulations from Kesisoglou et al. (2006), Schmiedlin-Ren (1993), and Autrup et al. (1978). The basal medium consists of a mixture of 80% CMRL medium 1066 (Invitrogen, Grand Island, NY) and 20% Hams F-12 nutrient mixture (Invitrogen) with: 25 mM glucose (Sigma-Aldrich, St. Louis, MO), 2 mM GlutaMax I (Invitrogen), 0.1 μM sodium selenite (Sigma-Aldrich), 3 μM zinc sulfate (Sigma-Aldrich), 145 nM menadione sodium bisulfate-vitamin K (M2518, Sigma-Aldrich), 45 nM (+)-α-tocopherol acetate (T1157, Sigma-Aldrich), and 50 μg/ml gentamicin (Invitrogen). The medium is made serum-free with modifications from Moorghen et al. (1996): 3 μg/ml hydrocortisone (H0888, Sigma-Aldrich), 50 ng/ml glucagon (G3157, Sigma-Aldrich), 0.5 ng/ml 3,3′,5-triiodo-l-thyronine sodium salt (T5516, Sigma-Aldrich), 1 mg/ml bovine serum albumin (8806, Sigma-Aldrich), and 10 μg/ml insulin from bovine pancreas (I6634, Sigma-Aldrich). We further supplemented with 50 μg/ml bovine pituitary extract (P1476, Sigma-Aldrich).
Organ culture protocol
The tissue was washed three times and dissected in cold phosphate-buffered saline (Invitrogen). The mucosa and submucosa were gently teased away from the muscularis propria and then cut into pieces approximately 3–4 mm3 in size. The pieces were placed, luminal side up, on a 100-μm cell strainer (BD Biosciences, Bedford, MA) which was inserted into a 35-mm2 well of a six-well dish (Corning, Corning, NY). The pieces were partially submerged by adding the medium until it was approximately 1 mm above the strainer membrane (6.2 ml total volume). The dish was placed in a modular incubator chamber (Billups-Rothenberg Inc., Del Mar, CA), gassed for 20 min with 5% CO2 and 95% O2, and then incubated at 37°C. At 24 h, the chamber was re-gassed. At the end of the 2-d incubation, the medium was collected for analysis and tissue was fixed in 10% buffered formalin for histology.
Treatment protocol
The tissue was treated for 48 h with supraphysiological levels of calcium (CaCl2; Sigma-Aldrich) or with a multi-mineral extract. The concentration range of calcium approximates that which is seen in the colon for patients on high-calcium supplementation (2400 mg/d; Baron et al. 1999). The multi-mineral extract is derived from the mineral-rich fronds of the marine red algal, L. calcareum (Pallas), also known as Phymatolithon calcareum (Pallas) (Adey and McKibbin 1970). The fronds are deposited as skeletal remains on the Atlantic seabed at the coastal regions of southwest Ireland and northwest Iceland. The mineralized remains are harvested, separated from extraneous materials, sterilized, dried, and milled under ISO and HACCP certification. The extract is produced as a natural food supplement (GRAS 00028), Aquamin® (Marigot, Cork, Ireland) and is available for human consumption in Europe, Asia, Australia, and North America. The mineral extract contains 12% calcium, 1% magnesium, and measurable levels of 72 other trace minerals. The mineral composition of the algae extract is shown in Table 1.
Table 1.
Composition of the multi-mineral extract of Litho-thamnion calcareum
| Element | μg/g | Element | μg/g | Element | μg/g | Element | μg/g |
|---|---|---|---|---|---|---|---|
| Aluminum | 291 | Fluoride | 7.28 | Neodymium | 0.034 | Strontium | 1,810 |
| Antimony | 6.74 | Gadolinium | 0.109 | Nickel | 1.48 | Sulfur | 5,700 |
| Arsenic | <0.2 | Gallium | 2.48 | Niobium | 0.142 | Tantalum | 0.06 |
| Barium | 64.2 | Germanium | 0.207 | Osmium | <0.05 | Tellurium | 0.048 |
| Beryllium | 0.306 | Gold | <0.01 | Palladium | <0.01 | Terbium | 0.03 |
| Bismuth | 0.081 | Hafnium | <0.03 | Phosphorus | 310 | Thallium | 0.088 |
| Boron | 39.5 | Holmium | <0.05 | Platinum | <0.01 | Thorium | <0.02 |
| Bromine | 10.1 | Indium | 0.052 | Potassium | 5,176 | Thulium | 0.061 |
| Cadmium | 0.07 | Iodine | 32.8 | Praseodymium | 0.228 | Tin | 0.197 |
| Calcium | 351,500 | Iridium | <0.05 | Rhenium | <0.05 | Titanium | 27.8 |
| Carbon | 122,000 | Iron | 915 | Rhodium | <0.01 | Tungsten | 0.188 |
| Cerium | 2.17 | Lanthanum | 0.372 | Rubidium | 1.95 | Vanadium | 37.5 |
| Cesium | 0.096 | Lead | 0.158 | Ruthenium | 0.088 | Ytterbium | 0.096 |
| Chloride | 910 | Lithium | 2.77 | Samarium | 0.529 | Yttrium | 1.22 |
| Chromium | 0.82 | Lutetium | 0.065 | Scandium | 0.041 | Zinc | 15.8 |
| Cobalt | 0.082 | Magnesium | 25,800 | Selenium | 0.672 | Zirconium | 0.339 |
| Copper | 4.89 | Manganese | 57.5 | Silicon | 504 | ||
| Dysprosium | 0.078 | Mercury | 0.008 | Silver | 0.25 | ||
| Europium | 0.051 | Molybdenum | 0.052 | Sodium | 4,150 |
2008 Test Certificate for Aquamin®, by Advanced Laboratories (Salt Lake City, UT), for client Marigot (Ireland)
Immunohistology
Tissue from six patients was treated for 2 d in organ culture, then formalin-fixed and paraffin-embedded. Five-micrometer sections were prepared and stained with hematoxylin and eosin. In addition, tissue sections were immunostained for Ki67 and E-cadherin (antibodies from Chemicon Inc., Temecula, CA). Both of the antibodies were known to react with their cognate antigens in formalin-fixed, paraffin-embedded tissue. Tissue sections were scored semi-quantitatively for staining intensity at the base and at the sides of the crypts. The slides were blinded and then scored by two separate individuals for relative expression.
Type I procollagen and type I collagen
Culture fluids were assayed for type I procollagen by enzyme-linked immunosorbent assay (Takara Bio, Inc., Otsu, Shiga, Japan) as described previously (Varani et al. 2007). Type I procollagen contains the N- and C-terminal peptide sequences that are present at synthesis and, therefore, provides a measure of newly synthesized collagen precursor. Culture fluids were assayed for type I collagen by Western blotting. Samples were separated in SDS-PAGE without SDS under non-reducing conditions. Protein was transferred to nitro-cellulose membranes and probed with a rabbit antihuman type I collagen antibody (Abcam LTD, Cambridge, MA). After blocking with a 5% nonfat milk solution in Tris-buffered saline with 0.1% Tween (TTBS) for 1 h at room temperature, membranes were incubated overnight at 4°C with the desired antibody and diluted 1:10,000 in 5% nonfat milk/TTBS. Thereafter, the membranes were washed with TTBS and bound antibody detected using the Super-Signal Westpico chemiluminescent substrate (Pierce Biotechnology, Inc., acquired by Thermo Fisher Scientific, Inc., Rockford, IL). Images were scanned, digitized, and quantified. Before loading the gels, protein levels in each sample were determined using the DC Protein Assay kit (BioRad, Hercules, CA) and equal amounts of protein were loaded onto each lane.
Results
Histological features of human colon tissue in organ culture treated with increasing amounts of calcium or multi-mineral extract
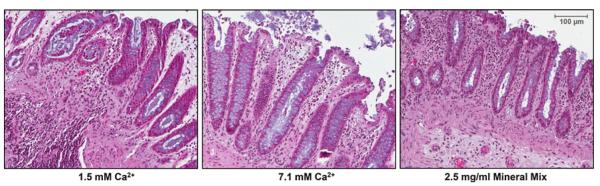
Figure 1 shows tissue treated in organ culture for 2 d. At the physiological Ca2+ level of 1.5 mM, the tissue appears healthy with normal crypt structure, including goblet cells. The tissue treated with high calcium (7.1 mM) or with multi-mineral extract (containing 7.1 mM calcium) shows the same healthy histological organization with good mucin production. The multi-mineral extract though appears to especially promote stromal integrity, as can be seen by the density of the lamina propria surrounding the crypts (Fig. 1, right panel).
Figure 1.
Histological features of human colon tissue in organ culture treated with calcium or multi-mineral extract. Formalin-fixed tissue is hematoxylin- and eosin-stained. Normal colon crypt structure, and stromal integrity, is maintained after 2 d of treatment. Original magnification, ×150.
Effects of calcium and mineral supplementation on proliferation marker Ki67
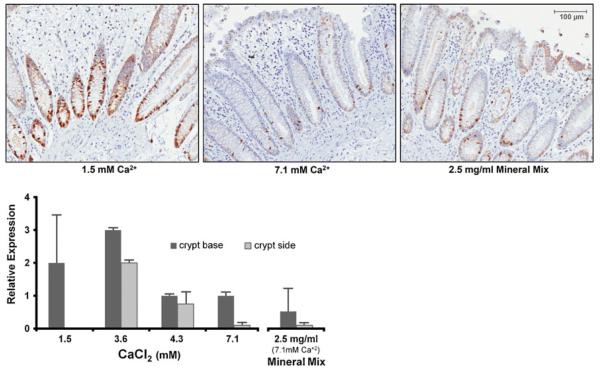
Tissue maintained in physiological calcium (1.5 mM) expressed the proliferation marker Ki67 at the base and sides of the crypts where cells are actively cycling from stem to transit amplifying cells (Fig. 2). As the calcium concentration is increased in the culture medium, Ki67 staining decreased (middle panel). The multi-mineral extract caused a comparable reduction in the cell cycling marker (right panel).
Figure 2.
Immunoperoxidase staining for Ki67 expression in human colon tissue treated with calcium or multi-mineral extract. After 2 d in culture, cell cycling in the crypt is evident by expression of Ki67. Treatments with higher concentrations of calcium or with multi-mineral extract suppress proliferation of crypt cells at sides and base. The figure represents averages ± ranges of two independent experiments. Original magnification, ×150.
Effects of calcium and mineral supplementation on a marker of differentiation
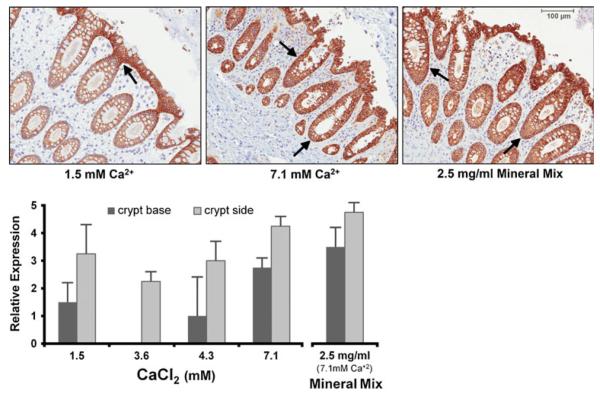
After 2 d in culture, tissue treated with calcium or the multi-mineral extract showed increased expression of membrane-associated E-cadherin (Fig. 3). At physiological Ca2+, E-cadherin was most highly expressed at the luminal surface and entrance to the crypts. As the calcium concentration was increased, E-cadherin staining was elevated at regions further down into the crypt (middle panel). The multi-mineral extract was also associated with increased differentiation throughout the crypt, as seen by the staining intensity of E-cadherin (right panel).
Figure 3.
Immunoperoxidase staining for E-cadherin expression in human colon tissue treated with calcium or multi-mineral extract. After 2 d in culture, differentiation of the epithelium is assessed by expression of E-cadherin. Treatments with higher concentrations of calcium or with multi-mineral extract induce differentiation further into the crypt—at sides and base. Arrows indicate areas of intense staining (luminal surface, crypt side, and crypt base). The figure represents averages ± ranges of four independent experiments. Original magnification, ×150.
Type I procollagen and type I collagen levels of colon tissue treated with calcium and mineral supplementation
Two-day tissue culture supernatants from control and treated tissue were assayed for procollagen by ELISA. At higher Ca2+ concentrations, and at the multi-mineral extract calcium equivalents, procollagen levels were dramatically reduced (Fig. 4). Soluble type I collagen though, as measured by Western blot analysis, increased with calcium and multi-mineral treatments (Fig. 5). As type I procollagen levels were reduced, type I collagen increased.
Figure 4.
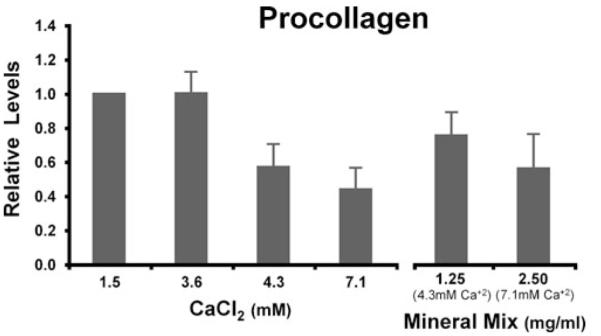
Type I procollagen levels of human colon tissue treated with calcium or multi-mineral extract. Two-day culture supernatants from treated tissue were assayed for procollagen by ELISA. Procollagen levels were dramatically reduced with high calcium or multi-mineral extract treatment. The figure represents averages ± ranges of six independent experiments.
Figure 5.
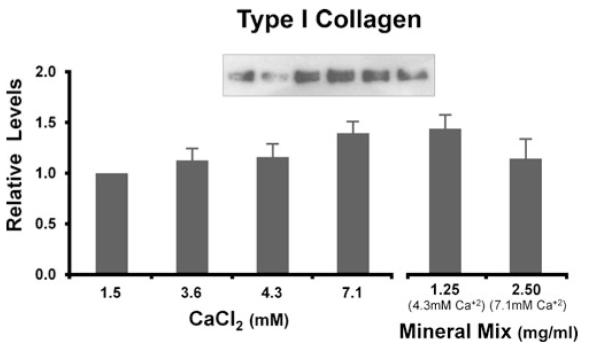
Type I collagen levels of human colon tissue treated with calcium or multi-mineral extract. Two-day culture supernatants from treated tissue were assayed for soluble type I collagen by Western blot analysis. Type I collagen increased with high calcium or multi-mineral extract treatment. The figure represents averages ± ranges of five independent experiments.
Discussion
When human colon tissue is surgically removed, histological, immunohistological, and biochemical characteristics can be readily established in the freshly obtained tissue. However, the opportunity is lost to modulate the characteristics experimentally. Thus, we are left with analytical information but cannot carry out interventional studies. In order to study (i.e., modulate) properties of interest, researchers have utilized cultured cell lines, multicellular organotypic models, xenografts, or animal models. While these models support interventional studies, none of these can fully replicate the complex events of early tumor development in the human colon. If intact human colon tissue could be maintained in organ culture under conditions that preserve the in situ phenotype, and if tissue phenotype could be modulated with the appropriate interventions, this would provide a sophisticated tissue model to mimic the in vivo situation. Previous studies by a number of groups have shown that histologic features of normal colonic mucosa can be preserved in organ culture for a period of several days (Autrup 1980; Senior et al. 1982; Lipkin 1985; Moorghen et al. 1996; Kesisoglou et al. 2006). In our recent study, we demonstrated that premalignant/malignant colon tissue, as well as histologically normal tissue, was amenable to in vitro culture (Dame 2010). Thus, we asked whether interventional studies could be carried out in organ-cultured colon tissue and would the findings in this model reflect what might be observed in vivo.
To begin to answer this question, we examined the effects of Ca2+ supplementation on markers of growth and differentiation in organ cultures of histologically normal human colon tissue over a 2-d period. Previous studies with colon epithelial cells in monolayer culture have demonstrated that Ca2+ supplementation of the medium reduces proliferation and increases differentiation (Kallay et al. 1997; Chakrabarty et al. 2003, 2005; Bhagavathula et al. 2005, 2007). Here, we show that Ki67 expression (proliferation marker) is reduced in normal colon tissue in the presence of a two to fivefold increase in Ca2+. Concomitantly, a marker of differentiation, E-cadherin, is increased under the same conditions. Altered expression of growth and differentiation markers suggest that colon epithelial cells in organ culture are responsive to Ca2+ supplementation, just as are cells in monolayer culture. Of interest is that the increased concentration in extracellular Ca2+ used in these studies (i.e., Ca2+ level increased from 1.5 mM to 3.6, 4.3, and 7.1 mM) is consistent with the level of Ca2+ supplementation found to be effective in vivo. In one widely cited study, Baron et al. (1999) demonstrated that supplementation with approximately 2,400 mg of ionized Ca2+ per d (with an assumed dietary intake of 800–1,000 mg/d) reduced polyp formation after a period of 1 yr. A 4-yr follow-up study demonstrated that the protective effects of the initial treatment were still visible (Grau et al. 2007).
In addition to assessing the effects of Ca2+ alone in this model, we also examined a multi-mineral extract. For these studies, colon tissue was treated with 1.25 and 2.5 mg/ml of a multi-mineral extract from the marine red algae, L. calcareum. The extract is derived from the skeletonized remains of the algal fronds which accumulate on the seabed off the coasts of southwest Ireland and northwest Iceland. The remains are harvested, separated from extraneous materials, sterilized, dried, and milled under ISO and HACCP certification. The extract is produced as a natural food supplement (GRAS 00028), Aquamin® (Marigot, Cork, Ireland) and is available for human consumption in Europe, Asia, Australia, and North America. The mineral extract contains 12% calcium, 1% magnesium, and measurable levels of 72 other trace minerals. In recent animal studies, our laboratory has found that it may have chemopreventive characteristics. In these studies, we found that the multi-mineral extract had unique properties that distinguish it from its calcium-containing effects alone. In a long-term diet study with mice, the multi-mineral extract was linked to a reduction in polyp formation and inflammation in the gastrointestinal tract (Aslam et al. 2010). We believe that this might be attributed to the fact that the multi-mineral extract, in addition to calcium, contains numerous trace metals, of which 13 of the 15 lanthanide metals are represented. In separate cell culture studies with colon cancer lines, we found that the lanthanide ionic metals mimic calcium by inducing differentiation and reducing cell proliferation, but at 100- to 1,000-fold less concentrations than seen with calcium (Aslam et al. 2009).
These promising results in mice and cell culture begged for further studies with intact human colon tissue. Here, in the organ culture model, we found similar changes with multi-mineral extract treatment as seen with Ca2+ supplementation—reduced cell proliferation (Ki67 staining) and increased differentiation (E-cadherin staining) of the crypt epithelium. Thus, although there was no dramatic change in histological features of the epithelium, 2-d treatments in organ culture were sufficient to observe both calcium- and multi-mineral extract-driven changes in whole tissue colonic epithelium of human.
In addition to the above markers for epithelial health, we examined changes in the stromal element. We focused on parameters of collagen turnover by measuring related protein levels in the 2-d culture supernatant. To our surprise, type I procollagen was dramatically reduced with high calcium or multi-mineral extract treatment. Type I collagen though concomitantly increased. We postulate that with the treatments, procollagen is further incorporated into intact whole collagen. Interestingly, as seen by histology, the multi-mineral extract appears to uniquely improve the integrity of the stroma surrounding the crypt structures in the lamina propria.
We conclude that (1) the organ culture model herein further elucidates in human tissue those calcium and multi-mineral extract-induced changes seen in mice and cultured cells; (2) short-term (i.e., 2 d) treatment of human colon tissue in organ culture is sufficient to see immunohistochemical changes reflective of improved differentiation; and (3) the multi-mineral extract, rich in lanthanides and other trace metals, may enhance growth control properties of calcium.
In recent years, the scientific community has begun to ask very sophisticated questions relevant to understanding colon cancer initiation, progression, prevention, and therapeutics. We need equally sophisticated tissue models in which to address these questions. Human colon tissue in organ culture is a valuable model for the study and preclinical assessment of agents that regulate growth and differentiation in the colonic mucosa.
Acknowledgments
This study was supported, in part, by grant 1R21CA140760 01 from the USPHAS. The authors would like to thank Deborah Postiff and Justin Reagan of the Tissue Procurement Core Laboratory, Comprehensive Cancer Center (Cancer Center Support Grant 5 P30 CA46592), as the source of the tissue specimens; Lisa Riggs (Histology Core) for her help with the preparation of tissue for histological examination; and Ron Craig (Histomorphometry Core) for his ScanScope service and assistance. Core laboratories are supported by the Department of Pathology at the University of Michigan.
References
- Adey WH, McKibbin DL. Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo. Bot. 1970 Mar;13(2):100–106. doi:10.1515/botm.1970.13.2.100. [Google Scholar]
- American Cancer Society Leading sites of new cancer and deaths—2009 estimate. Cancer facts & figures. 2009 http://www.cancer.org/downloads/stt/CFF2009_LeadingSites_Est_6.pdf. cited June 27, 2010; 2009.
- Aslam MN, Bhagavathula N, Paruchuri T, Hu X, Chakrabarty S, Varani J. Growth-inhibitory effects of a mineralized extract from the red marine algae, Lithothamnion calcareum, on Ca(2+)-sensitive and Ca(2+)-resistant human colon carcinoma cells. Cancer Lett. 2009;283(2):186–192. doi: 10.1016/j.canlet.2009.03.037. PMID: 19394137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam MN, Paruchuri T, Bhagavathula N, Varani J. A mineral-rich red algae extract inhibits polyp formation and inflammation in the gastrointestinal tract of mice on a high-fat diet. Integr. Cancer. Ther. 2010;9(1):93–99. doi: 10.1177/1534735409360360. PMID: 20150219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autrup H. Explant culture of human colon. Methods Cell. Biol. 1980;21B:385–401. doi: 10.1016/s0091-679x(08)60694-9. PMID: 7191040. [DOI] [PubMed] [Google Scholar]
- Autrup H, Barrett LA, Jackson FE, Jesudason ML, Stoner G, Phelps P, Trump BF, Harris CC. Explant culture of human colon. Gastroenterology. 1978;74(6):1248–1257. PMID: 648817. [PubMed] [Google Scholar]
- Baron JA, Beach M, Mandel JS, Van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, Greenberg ER, Calcium Polyp Prevention Study Group Calcium supplements for the prevention of colorectal adenomas. N. Engl. J. Med. 1999;340(2):101–107. doi: 10.1056/NEJM199901143400204. PMID: 9887161. [DOI] [PubMed] [Google Scholar]
- Beaty MM, Lee EY, Giauert HP. Influence of dietary calcium on colon epithelial proliferation and 1,2-dimethyhydrazine-induced colonic cancer in rats fed high fat diets. J. Nutr. 1993;123(1):144–152. doi: 10.1093/jn/123.1.144. PMID: 8421225. [DOI] [PubMed] [Google Scholar]
- Bhagavathula N, Hanosh AW, Nerusu KC, Appelman H, Chakrabarty S, Varani J. Regulation of E-cadherin and β-catenin by Ca2+ in colon carcinoma is dependent on calcium-sensing receptor expression and function. Int. J. Cancer. 2007;121(7):1455–1462. doi: 10.1002/ijc.22858. PMID: 17557293. [DOI] [PubMed] [Google Scholar]
- Bhagavathula N, Kelley EA, Reddy M, Nerusu KC, Leonard C, Fay K, Chakrabarty S, Varani J. Upregulation of calcium-sensing receptor and mitogen-activated protein kinase signaling in the regulation of growth and differentiation in colon carcinoma. Brit. J. Cancer. 2005;93(12):1364–1371. doi: 10.1038/sj.bjc.6602852. PMID: 16278666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am. J. Epidermiol. 1993;137(12):1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. PMID: 8333412. [DOI] [PubMed] [Google Scholar]
- Burgart LJ. Colorectal polyps and other precursor lesions. Need for an expanded view. Gastroenterol. Clin. N. Am. 2002;31(4):959–970. doi: 10.1016/s0889-8553(02)00056-0. PMID: 12489272. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of β-catenin/TCF activation. Cancer Res. 2003;63(1):67–71. PMID: 12517779. [PubMed] [Google Scholar]
- Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca2+ and 1,25-dihydroxyvitamin D3. Cancer Res. 2005;65(2):493–498. PMID: 15695391. [PubMed] [Google Scholar]
- Dame M, Bhagavathula N, Mankey C, DaSilva M, Paruchuri T, Aslam MN, Varani J. Human colon tissue in organ culture: preservation of normal and neoplastic characteristics. In Vitro Cell Dev. Biol. Anim. 2010;46(2):114–122. doi: 10.1007/s11626-009-9247-9. PMID: 19915935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Sandler RS, Wallace K, Haile RW, Church TR, Beck GJ, Summers RW, Barry EL, Cole BF, Snover DC, Rothstein R, Mandel JS. Prolonged effect of calcium supplementation on risk of colorectal adenomas in a randomized trial. J. Natl. Cancer Inst. 2007;99:129–136. doi: 10.1093/jnci/djk016. PMID: 17227996. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Pretlow TP, Schoen RE. Aberrant crypt foci: what we know and what we need to know. Clin. Gastroenterol. Hepatol. 2007;5(5):526–533. doi: 10.1016/j.cgh.2007.02.014. PMID: 17433788. [DOI] [PubMed] [Google Scholar]
- Holt PR, Wolper C, Moss SF, Yang K, Lipkin M. Comparison of calcium supplementation or low-fat dairy foods on epithelial cell proliferation and differentiation. Nutr. Cancer. 2001;41(1–2):150–155. doi: 10.1080/01635581.2001.9680626. PMID: 12094618. [DOI] [PubMed] [Google Scholar]
- Kallay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, Cross HS. Calcium-dependent c-myc proto-oncogene expression and proliferation of CACO-2 cells: a role for luminal extracellular calcium-sensing receptor. Biochem. Biophys. Res. Commun. 1997;232:80–83. doi: 10.1006/bbrc.1997.6225. PMID: 9125156. [DOI] [PubMed] [Google Scholar]
- Kampman E, Giovannucci E, Van Veer P, Rimm E, Stampfer MJ, Colditz GA, Kok FJ, Willett WC. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am. J. Epidemiol. 1994;139(1):16–29. doi: 10.1093/oxfordjournals.aje.a116931. PMID: 8296771. [DOI] [PubMed] [Google Scholar]
- Kampman E, Slattery ML, Caan B, Potter JD. Calcium, vitamin D, sunshine exposure, dairy products and colon cancer risk. Cancer Causes Control. 2000;11(5):459–466. doi: 10.1023/a:1008914108739. PMID: 10877339. [DOI] [PubMed] [Google Scholar]
- Kesisoglou F, Schmiedlin-Ren P, Fleisher D, Roessler B, Zimmermann EM. Restituting intestinal epithelial cells exhibit increased transducibility by adenoviral vectors. J. Gene Med. 2006;8(12):1379–1392. doi: 10.1002/jgm.981. PMID: 17133338. [DOI] [PubMed] [Google Scholar]
- Lamprecht SA, Lopkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat. Rev. Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. PMID: 12894248. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Growth and development of gastrointestinal cells. Annu. Rev. Physiol. 1985;47:175–197. doi: 10.1146/annurev.ph.47.030185.001135. PMID: 3888073. [DOI] [PubMed] [Google Scholar]
- Moorghen M, Chapman M, Appleton DR. An organ-culture method for human colorectal mucosa using serum-free medium. J. Pathol. 1996;180(1):102–105. doi: 10.1002/(SICI)1096-9896(199609)180:1<102::AID-PATH613>3.0.CO;2-S. PMID: 8943824. [DOI] [PubMed] [Google Scholar]
- Schmiedlin-Ren P, Benedict PE, Dobbins WO, Ghosh M, Kolars JC, Watkins PB. Cultured adult rat jejunal explants as a model for studying regulation of CYP3A. Biochem. Pharmacol. 1993;46:905–918. doi: 10.1016/0006-2952(93)90501-m. PMID: 8373442. [DOI] [PubMed] [Google Scholar]
- Senior PV, Pritchett CJ, Sunter JP, Appleton DR, Watson AJ. Crypt regeneration in adult human colonic mucosa during prolonged organ culture. J. Anat. 1982;134(3):459–469. PMID: 7107511. [PMC free article] [PubMed] [Google Scholar]
- Takayama T, Ohi M, Hayashi T, Miyanishi K, Nobuoka A, Nakajima T, Satoh T, Takimoto R, Kato J, Sakamaki S, Niitsu Y. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121(3):599–611. doi: 10.1053/gast.2001.27203. PMID: 11522744. [DOI] [PubMed] [Google Scholar]
- Varani J, Fay K, Perone P. MDI 301, a nonirritating retinoid, induces changes in human skin that underlie repair. Arch. Dermatol. Res. 2007;298(9):439–448. doi: 10.1007/s00403-006-0720-y. PMID: 17146625. [DOI] [PMC free article] [PubMed] [Google Scholar]