Abstract
The objective of the present study was to explore the ability of eosinophils to present Strongyloides stercoralis antigen in naive and immunized mice. Antigen-pulsed eosinophils were injected intraperitoneally into naive or immunized mice, and then mice were examined for antigen-specific immune responses. A single inoculation of antigen-pulsed eosinophils was sufficient to prime naive mice and to boost immunized mice for antigen-specific T helper cell type 2 (Th2) immune responses with increased interleukin (IL)– 4 and IL-5 production. Mice inoculated 3 times with live eosinophils pulsed with antigen showed significant increases in parasite antigen–specific immunoglobulin (Ig) M and IgG levels in their serum. Antigen-pulsed eosinophils deficient in major histocompatibility complex class II molecules or antigen-pulsed dead eosinophils failed to induce immune responses, thereby demonstrating the requirement for direct interaction between eosinophils and T cells. These experiments demonstrate that eosinophils function as antigen-presenting cells for the induction of the primary and the expansion of the secondary Th2 immune responses to S. stercoralis in mice.
Eosinophils are multifunctional cells that possess potent cytotoxic and proinflammatory capabilities [1, 2]. In addition, eosinophils have been shown to function as antigen-presenting cells (APCs) in experimental allergy model systems [3]. Antigen-loaded eosinophils present antigen to primed T cells and increase Th2 cytokine production [4, 5]. Eosinophils migrate into local lymph nodes and localize in the T cell–rich paracortical zones, where they stimulate the expansion of CD4+ T cells. Antigen-loaded eosinophils also promote the production of interleukin (IL)–5 when placed in culture with antigen-specific CD4+ T cells isolated from allergic mice [5].
Eosinophils are commonly associated with helminth infections, in which their role has been characterized as defending the host against nonphagocytosable parasites. Because eosinophils are in the proximity of helminth parasites at the initial stage of infection [6–9], it is possible that these cells capture antigens from the worms, migrate to T cell–rich regions, and present antigens to T cells to initiate antigen-specific T cell responses. The demonstration that eosinophils recovered from mice infected with the nematode Brugia malayi express high levels of major histocompatibility complex (MHC) class II molecules supports the hypothesis that these cells are capable of antigen presentation [10]. Moreover, granulocyte-macrophage colony-stimulating factor (GM-CSF)–activated eosinophils are also capable of acting as a specific APC to a T cell clone derived from mice infected with the cestode Mesocestoides corti [11]. Further evidence that eosinophils are capable of acting as APCs in the immune response to parasitic infections comes from in vitro studies of the nematode parasite Strongyloides stercoralis. Eosinophils pulsed with S. stercoralis antigen stimulated antigen-specific primed T cells and CD4+ T cells to increase IL-5 production. Blocking of MHC class II expression on eosinophils inhibited their ability to induce IL-5 production by CD4+ T cells in culture. Antigen-pulsed eosinophils were also able to prime naive T cells and CD4+ T cells in culture and polarize them into IL-5–producing Th2 cells similar to those induced by antigen-loaded dendritic cells. The observation that eosinophils are capable of inducing a primary response to the infection in vitro suggests that eosinophils may function as APCs for the induction of adaptive immunity in vivo [12].
Protective immunity to S. stercoralis in mice depends on various components of the immune system, including eosinophils [6], neutrophils [13], complement [14], B-1a B cells for IgM antibody production [15], and CD4+ Th2 cells for IL-4 and IL-5 production [16]. Eosinophils play a crucial role during both innate and adaptive immunity [6, 17]. IL-5−/− mice, which are incapable of augmenting blood and tissue eosinophil levels, failed to develop adaptive protective immunity to infection with S. stercoralis. However, adoptive transfer of eosinophils into IL-5−/− mice at the time of immunization with live S. stercoralis larvae reconstituted their ability to develop adaptive protective immunity against the infection [6]. It was hypothesized that the transferred eosinophils functioned as APCs, and this hypothesis was subsequently supported by data demonstrating that eosinophils possess the ability to act as APCs for S. stercoralis and can initiate the adaptive protective immune response in vitro [12].
The goal of the present study was to explore in vivo the antigen-presenting ability of eosinophils during S. stercoralis infection in mice. Purified eosinophils exposed to S. stercoralis antigens were inoculated intraperitoneally in naive mice, and then specific T cell and B cell immune responses against S. stercoralis were measured. These experiments demonstrated that eosinophils presented antigen by a MHC class II– dependent mechanism, resulting in both Th2 cytokine production and antigen-specific antibody responses to S. stercoralis.
MATERIALS AND METHODS
Experimental animals and parasites
C57BL/6J mice were purchased from Jackson Laboratory. The IL-5 transgenic mice (mouse line NJ.1638) were bred at Thomas Jefferson University [18]. IL-5 transgenic mice deficient in MHC class II molecules were generated by cross-breeding IL-5 transgenic mice (C57BL/6 background) with MHC class II– deficient mice (ABBN12 mice, C57BL/6 background; obtained from Taconic Farms). All of the mice used in the experiments were 6–8 weeks old. Primers required for genotyping the IL-5 transgenic/MHC class II– deficient mice were purchased from Sigma (Sigma Chemical Co.) and were used in accordance with the polymerase chain reaction protocol provided by Taconic. S. stercoralis larvae (L3) were obtained from charcoal cultures of fresh stool samples from a laboratory dog infected with the parasite, according to methods described elsewhere [19].
Antigen preparation
Soluble larval antigens from S. stercoralis (L3) were prepared as described elsewhere [20].
Isolation of eosinophils
Eosinophils were isolated from spleens of naive IL-5 transgenic mice or IL-5 transgenic/MHC class II–deficient mice, according to a method described elsewhere for isolating the cells from blood [21]. Spleen cells were separated on a density-gradient Percoll column by centrifugation, the eosinophil/lymphocyte layer was transferred to a tube containing 2% bovine serum albumin (BSA)–PBS, and erythrocytes were eliminated by hypotonic shock. Cells were incubated with anti–mouse CD90 microbeads (Miltenyi Biotec) to eliminate T cells and with anti–mouse CD45R microbeads (Miltenyi Biotec) to eliminate B cells, and eosinophils were collected after passage through a magnetic cell sorter (Miltenyi Biotec). Cells were placed in a Cytospin 3 apparatus (Shandon) and stained for differential counts with a Hema 3 stain set (Fisher Diagnostics), to check for purity. Isolated eosinophils used in the experiments were 100% viable.
Generation of dendritic cells and macrophages
Dendritic cells and macrophages were generated from bone marrow recovered from the femurs and tibias of C57BL/6J mice. Dendritic cells were generated as described elsewhere [22]. In brief, cells were seeded into petri dishes at 2 × 105/mL in 10 mL of RPMI 1640 medium supplemented with 10% heat-inactivated and filtered fetal bovine serum (FBS), 2 mmol/L L-glutamine (Life Technologies), 100 U/mL penicillin plus 100 μg/mL streptomycin (Life Technologies), and 50 μmol/L 2-mercaptoethanol (2-ME; Sigma), with the addition of 20 ng/mL GM-CSF (Peprotech). On day 3, 10 mL of medium containing 20 ng/mL GM-CSF was added. On day 6, 10 mL of culture supernatant was removed and replaced with 10 mL of fresh culture medium containing 20 ng/mL GM-CSF. For generation of immature dendritic cells, plates were fed on day 8 as on day 6, but only 5 ng/mL GM-CSF was added in fresh medium, and cells were harvested 18 h later (day 9).
To generate macrophages from bone marrow cells, cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Sigma) supplemented with 20% heat-inactivated and filtered fetal calf serum (FCS; HyClone), 2 mmol/L L-glutamine, 100 U/mL penicillin plus 100 μg/mL streptomycin, and 50 μmol/L 2-ME, with the addition of 30% L929 cell– conditioned medium as a source of macrophage colony-stimulating factor (M-CSF) [23]. On day 3, culture supernatants were removed and replaced with fresh medium. On day 6, cells were harvested using ice-cold PBS. Dendritic cells and macrophages were treated with S. stercoralis antigens (100 μg/mL) for the final 18-h incubation, to activate the cells.
Trafficking of carboxyfluorescein succinimide ester (CFSE)–labeled eosinophils
Purified eosinophils were labeled with CFSE (Molecular Probes), as described elsewhere [24] with minor modifications. Eosinophils were incubated with 2.0 μmol/L CFSE, labeling was terminated by adding 2.0 mL of FBS, and a total of 5 × 106 labeled cells were inoculated intraperitoneally into naive mice. Spleens were recovered 36 h later and evaluated for the presence of CFSE-labeled eosinophils, by use of a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson).
Determination of contaminating macrophages and dendritic cells in eosinophil preparations
Purified eosinophil preparations were placed in fluorescence-activated cell sorter buffer (1× PBS, 0.2% BSA fraction V, and 4 mmol/L sodium azide), and the Fc receptors on cells were blocked by use of Fc Block CD16/CD32 antibody (BD Pharmingen). The percentage of contaminating dendritic cells and macrophages in the eosinophil preparations was determined by measuring the expression of 33D1 antigen, a mouse dendritic cell–specific surface marker, and CD115, a receptor for M-CSF. Cells were stained with phycoerythrin-labeled anti-33D1 monoclonal antibody (MAb) and biotinylated anti-CD115 MAb, and streptavidin-allophycocyanin conjugate was used to detect biotinylated anti-CD115 antibody (all immunofluorescent staining reagents were purchased from Bioscience). Samples were analyzed using a FACSCalibur flow cytometer and CellQuest software.
Determination of the ability of eosinophils, dendritic cells, and macrophages to prime naive mice and to initiate immune responses
Purified eosinophils were treated with 100 μg/mL S. stercoralis antigens or 1.0 μg/mL lipopolysaccharide (LPS) for 18 h at 37°C in RPMI 1640 medium containing 2 ng/mL GM-CSF. A single dose of 5 × 105antigen-pulsed eosinophils was inoculated intraperitoneally into naive C57BL/6J mice. Untreated eosinophils were inoculated into mice that were used as controls. On day 10, mice were killed, and splenocytes were cultured in vitro to determine the ability of antigen-pulsed eosinophils to prime lymphocytes and initiate immune responses. In some experiments, naive C57BL/6J mice were inoculated with a mixture of macrophages (2 × 104; 4% of the eosinophil population) and dendritic cells (1 × 104; 2% of the eosinophil population), to measure the effect of the small number of contaminating professional APCs present in the purified eosinophil preparations.
Proliferation of T cells
Spleen cells were incubated with 2.0 μmol/L CFSE, labeling was terminated by the addition of 2.0 mL of FBS, and the cells were washed twice and resuspended in complete RPMI 1640 medium [25]. Cells were plated at a concentration of 2 × 106 cells/well in a final volume of 200 μL in 96-well ∪-bottom plates (Costar) in the presence of anti-CD3 MAb (BD Pharmingen) for 5 days and were analyzed for lymphocyte proliferation by use of a FACSCalibur flow cytometer.
Determination of the ability of eosinophils to stimulate primed T cells
C57BL/6J mice were immunized with live S. stercoralis L3, as described elsewhere [19]. The immunization protocol consisted of 2 subcutaneous injections with 5000 live L3 administered 2 weeks apart. Four weeks after the second injection, the immunized mice were inoculated intraperitoneally with a single dose of 5 × 105antigen-pulsed eosinophils. Immunized mice inoculated with untreated eosinophils were used as controls. On day 3, mice were killed, and spleens were aseptically removed, made into single-cell suspensions, and cultured in vitro in the presence of S. stercoralis antigen.
MHC class II restriction for antigen presentation by live eosinophils
To determine whether eosinophils interact directly with T cells, naive mice were inoculated with 5 × 105 MHC class II– deficient eosinophils or freeze-killed normal eosinophils.
Spleen cell stimulation and cytokine analysis
Spleen cells, cultured for 3 days at 2 × 106 cells/well in 96-well plates, were restimulated with S. stercoralis antigens in the presence of anti–IL-4Rα MAb (BD Pharmingen) in DMEM supplemented with 10% heat-inactivated and filtered FCS, 2 mmol/L L-glutamine, 100-U/mL penicillin plus 100-μg/mL streptomycin, and 50 μmol/L 2-ME. Culture supernatants were analyzed for IL-5 and IL-4 production by sandwich ELISAs, using appropriately matched MAbs (TRFK-5 and TRFK-4 for measuring IL-5 and BVD6-24G2 and BVD4-1D11 for measuring IL-4; BD Pharmingen) for capture and detection. Incubation with extravidin peroxidase (Sigma) followed by the ABTS peroxidase substrate (Kirkegaard & Perry Laboratories) resulted in color reaction, which was measured at 405 nm.
Antibody ELISA
Three doses of 1 × 106 antigen-pulsed eosinophils were inoculated intraperitoneally 1 week apart into naive C57BL/6J mice. Serum samples were collected from the mice on day 28, and production of S. stercoralis–specific antibodies was determined by ELISA according to a method that has been described elsewhere [20]. In brief, 96-well plates were coated with 50 μL of S. stercoralis antigens at 10 μg/mL in PBS. Test samples were placed in wells at serial dilutions, and biotinylated goat anti–mouse IgM (Vector Laboratories) and IgG (BD Pharmingen) antibodies were added. Extravidin peroxidase followed by the peroxidase substrate ABTS resulted in color reaction, which was measured at 405 nm.
Statistical analysis
Statistical analysis of the data was performed using multivariate general linear hypothesis multifactorial analysis of variance, with Systat software (version 11; Systat). Fisher’s least significant difference test was performed for post hoc analyses. Differences for which P ≤ .05 were considered to be significant.
RESULTS
Determination that intraperitoneally inoculated eosinophils can migrate to the spleen and stimulate T cell proliferation
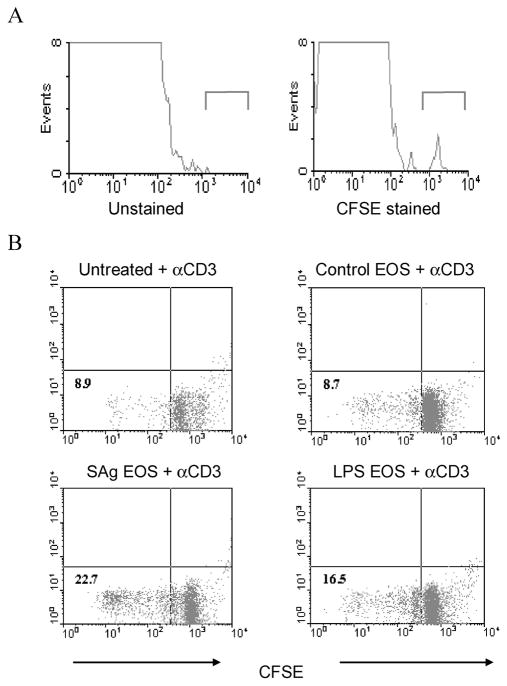
Purified eosinophils labeled with CFSE were inoculated intraperitoneally into naive mice to assess the potential of eosinophils to migrate to lymphoid organs. Spleens recovered from these mice were made into single-cell suspensions and examined for the presence of CFSE-labeled eosinophils. Results of flow cytometry indicated that eosinophils migrated from the peritoneal cavity to the spleen (figure 1A). Eosinophils exposed to either parasite antigen or LPS as a control antigen were inoculated intraperitoneally into naive mice to determine whether eosinophils could stimulate T cell proliferation in vivo. Spleen cells recovered from the inoculated mice were labeled with CFSE and then cultured in the presence of anti-CD3 MAb to examine T cell proliferation. Spleen cells from mice inoculated with eosinophils pulsed with parasite antigen or LPS had increased proliferation in the presence of anti-CD3 MAb, compared with cells from untreated mice or cells from mice that had received untreated control eosinophils (figure 1B). It was therefore concluded that antigen-pulsed eosinophils migrate to the lymphoid organs and can stimulate T cell proliferation.
Figure 1.
Migration to the spleen and stimulation of T cell proliferation by intraperitoneally injected purified eosinophils. In panel A, 5 × 106 carboxyfluorescein succinimide ester (CFSE)–labeled eosinophils were inoculated intraperitoneally into naive C57BL/6J mice. Spleens were recovered 36 h later, made into single-cell suspensions, and evaluated for the presence of CFSE-labeled eosinophils by use of a FACSCalibur flow cytometer. Representative data from 1 of 2 separate experiments are shown. In panel B, 5 × 105 control eosinophils (indicated as “EOS” in the figure), eosinophils pulsed with Strongyloides stercoralis antigen (SAg), or eosinophils pulsed with lipopolysaccharide (LPS) were intraperitoneally inoculated into naive C57BL/6J mice. On day 10, spleen cells were labeled with CFSE and cultured in the presence of anti-CD3 antibody (αCD3) to demonstrate T cell proliferation. Representative data from 1 of 3 separate experiments are shown.
Priming of naive and memory splenocytes for Th2 cytokine production by antigen-pulsed eosinophils
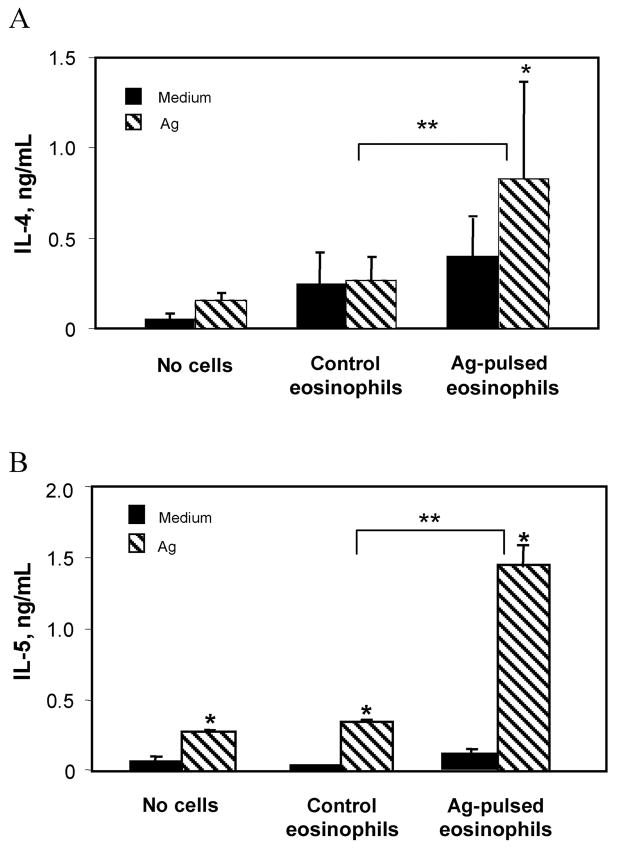
Experiments were performed to analyze cytokine production by spleen cells recovered from naive mice injected with antigen-pulsed eosinophils. Spleen cells were cultured in the presence of S. stercoralis antigens, and the production of IL-4 and IL-5 was measured. The production of IL-4 (figure 2A) and IL-5 (figure 2B) by cells isolated from mice inoculated with antigen-pulsed eosinophils was significantly increased, compared with that in control mice. The ability of eosinophils to stimulate antigen-specific memory T cells was evaluated by inoculating antigen-pulsed eosinophils into immunized mice. Antigen-pulsed eosinophils stimulated memory cells, as shown by significantly elevated IL-4 (figure 3A) and IL-5 (figure 3B) production by cells recovered from mice receiving the antigen-pulsed eosinophils.
Figure 2.
Stimulation of naive splenocytes for Th2 cytokine production by antigen (Ag)–pulsed eosinophils. Spleen cells from C57BL/6J mice inoculated with untreated or Ag-pulsed eosinophils were cultured in the presence of Strongyloides stercoralis Ag (75 μg/mL) at 37°C for 72 h, and supernatants were screened for interleukin (IL)– 4 (A) and IL-5 (B) production by ELISA. Data are mean ± SD values from 4–5 mice per group and are representative of results from 3 separate experiments. Asterisks indicate a statistically significant difference (P ≤.05) between restimulation with medium and restimulation with Ag (*) and between control and Ag-pulsed eosinophils (**).
Figure 3.
Restimulation of memory cells for increased interleukin (IL)– 4 and IL-5 production by antigen (Ag)–pulsed eosinophils. Spleen cells from immunized C57BL/6J mice inoculated with untreated or Ag-pulsed eosinophils were cultured in the presence of Strongyloides stercoralis Ag (75 μg/mL) at 37°C for 72 h, and supernatants were screened for IL-4 (A) and IL-5 (B) production by ELISA. Data are mean ± SD values from of 4 –5 mice per group and are representative of results from 3 separate experiments. Asterisks indicate a statistically significant difference (P ≤.05) between restimulation with medium and restimulation with Ag (*) and between control and Ag-pulsed eosinophils (**).
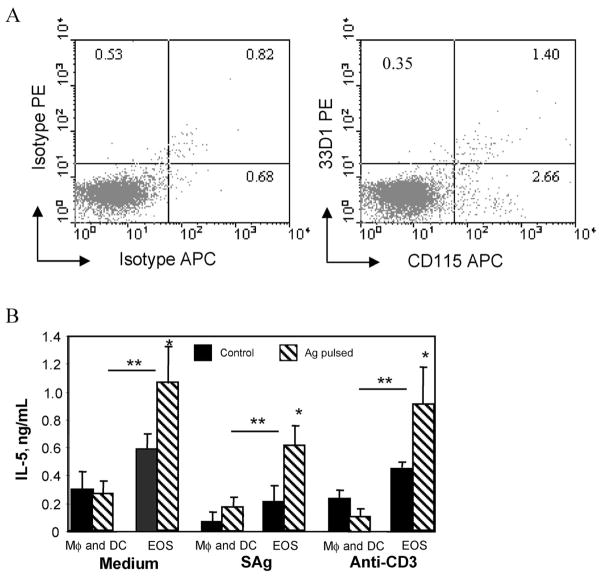
The purified eosinophils used in the experiments described above contained ~5% contaminating cells, consisting of a mixture of neutrophils and mononuclear cells, based on differential cell-staining analyses. Flow cytometry analyses demonstrated that the purified eosinophils specifically contained the professional APCs macrophages (2.6%) and dendritic cells (0.4%) (figure 4A). Experiments were performed to determine whether the immune responses induced by the purified eosinophils were actually induced by the small number of contaminating macrophages and dendritic cells. Naive mice were inoculated either with 5 × 105 purified antigen-pulsed eosinophils or with a mixture of 2 × 104 antigen-pulsed macrophages (4% of the inoculated eosinophil population) and 1 × 104 antigen-pulsed dendritic cells (2% of the inoculated eosinophil population). The mixture of antigen-pulsed dendritic cells and macrophages did not increase IL-5 production by spleen cells recovered from treated mice, compared with the response of spleen cells recovered from mice that received the pulsed eosinophils, which had significantly increased IL-5 production (figure 4B). These experiments demonstrate that the induction of IL-5 production by naive T cells was due to the presentation of antigen by eosinophils, not the small number of contaminating macrophages and dendritic cells present in the eosinophil population.
Figure 4.
Antigen (Ag) presentation to T cells is not the result of small nos. of contaminating dendritic cells and macrophages within the eosinophil preparation. In panel A, purified eosinophils were stained with labeled antibody isotype controls or phycoerythrin (PE)–labeled anti-33D1 monoclonal antibody (MAb) (anti–mouse dendritic cell–surface marker), and biotinylated anti-CD115 MAb (anti–mouse receptor for macrophage colony-stimulating factor) with streptavidin-allophycocyanin (APC) conjugate was used to detect biotinylated anti-CD115 antibody. Samples were analyzed using a FACSCalibur flow cytometer for quantifying the contaminating dendritic cells and macrophages. In panel B, naive C57BL/6J mice were inoculated with eosinophils (indicated as “EOS” in the figure) or a mixture of Strongyloides stercoralis Ag-pulsed macrophages (indicated as “Mφ” in the figure) (2 × 104; 4% of the eosinophil population), and dendritic cells (indicated as “DC” in the figure) (1 × 104; 2% of the eosinophil population) were used to measure the effects of a small no. of contaminating professional Ag-presenting cells present in the eosinophil preparations. Spleen cells from these mice were cultured in the presence of either S. stercoralis Ag (SAg) or anti-CD3 antibody, and supernatants were screened for IL-5 production by ELISA. Spleen cells were also cultured in medium alone to determine the baseline secretion of IL-5. Data are mean ± SD values from 4 mice per group and are representative of the results from 2 separate experiments. Asterisks indicate a statistically significant difference (P ≤.05) between restimulation with medium and restimulation with Ag (*) and between Ag-stimulated eosinophils and either macrophages or dendritic cells (**).
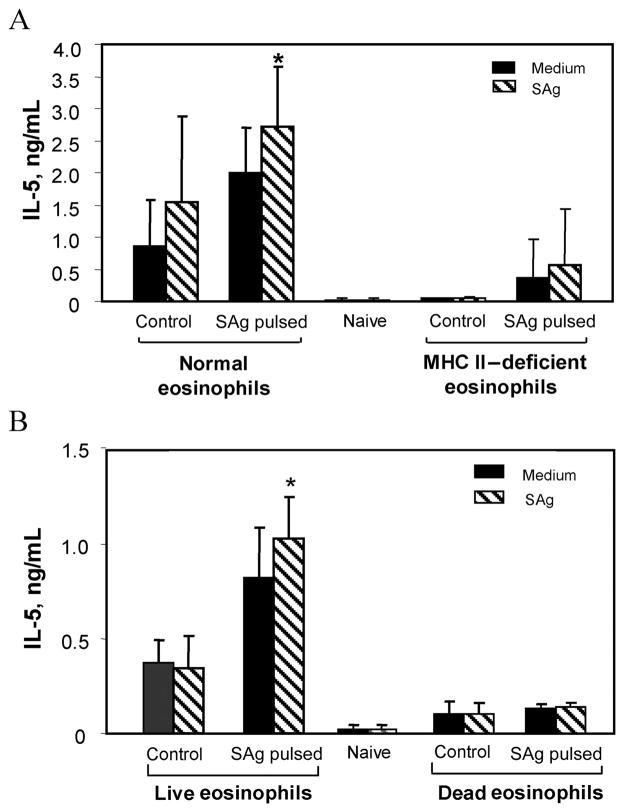
Requirement of MHC class II molecules and live cells for induction of IL-5 production by antigen-pulsed eosinophils
To confirm that eosinophils act as APCs in vivo, MHC class II– deficient mice were bred with IL-5 transgenic mice to provide a source of MHC class II– deficient eosinophils. Antigen-pulsed MHC class II– deficient eosinophils were unable to prime naive mice to produce IL-5, compared with normal antigen-pulsed eosinophils (figure 5A). To further confirm that eosinophils communicate directly with T cells, killed antigen-pulsed eosinophils were transferred into naive mice. Cells from mice that received the killed antigen-pulsed eosinophils did not show an increase in IL-5 production, compared with cells from mice inoculated with a similar number of live antigen-pulsed eosinophils (figure 5B). Therefore, the induction of IL-5 production by spleen cells was due to the presentation of antigen by eosinophils in conjunction with MHC class II molecules, and live eosinophils are required for the stimulation of T cells.
Figure 5.
Inducement of interleukin (IL)–5 production in conjunction with major histocompatibility complex (MHC) class II molecules and direct interaction with T cells by eosinophils. Mice were inoculated with 5 × 105 MHC class II– deficient eosinophils (A) or 5 × 105 dead eosinophils pulsed with antigen (B). Spleen cells from C57BL/6J mice inoculated with antigen-pulsed or untreated eosinophils were cultured in the presence of Strongyloides stercoralis antigen (SAg), and supernatants were screened for IL-5 production. Data are mean ± SD values from 5 mice per group. The asterisk indicates statistically significant (P ≤.05) production of IL-5 in mice receiving normal eosinophils, compared with that in mice receiving MHC class II– deficient or dead eosinophils.
Priming of naive mice to produce antigen-specific IgG and IgM by antigen-pulsed eosinophils
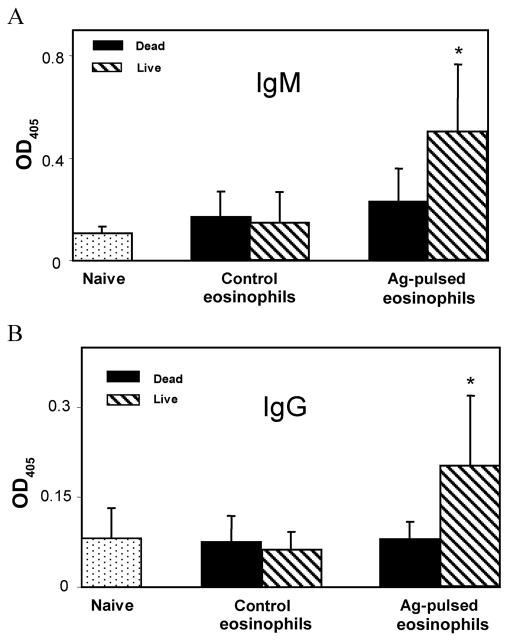
Antigen-pulsed eosinophils were inoculated 3 times into naive mice to determine whether the eosinophils could initiate antigen-specific antibody production. Serum levels of antigen-specific IgM (figure 6A) and IgG (figure 6B) antibodies were significantly higher in mice inoculated with live antigen-pulsed eosinophils than in mice injected with untreated control eosinophils or killed antigen-pulsed eosinophils.
Figure 6.
Priming of naive mice to produce antigen (Ag)–specific IgG and IgM antibody by Ag-pulsed eosinophils. Naive C57BL/6J mice were inoculated 3 times with Strongyloides stercoralis Ag-pulsed live or dead eosinophils for 3 weeks at 1-week intervals. Serum samples (1:400 dilution) were analyzed for Ag-specific IgM (A) and IgG (B) antibody levels. Data are mean ± SD values from 5 mice per group and are representative of results from 2 separate experiments. The asterisk indicates a statistically significant (P ≤.05) difference between antibody responses in mice exposed to dead vs. live Ag-pulsed eosinophils.
DISCUSSION
In vitro studies have demonstrated that S. stercoralis antigens activate eosinophils and induce expression of MHC class II and T cell costimulatory molecules. Furthermore, these activated eosinophils can stimulate naive and primed CD4+ T cells to produce antigen-specific Th2 cytokine responses [12]. The goal of the present study was to confirm the ability of eosinophils to present antigen in vivo, thereby priming naive mice and restimulating immunized mice to induce T and B cell responses against infection with S. stercoralis. In the initial experiments, which used naive mice, it was determined that eosinophils transferred into the peritoneal cavity migrated into the spleen and stimulated T cell proliferation. This finding is consistent with those of previous studies involving a mouse model of allergy, in which sensitized eosinophils instilled into the trachea of sensitized mice processed inhaled antigens, trafficked to regional lymph nodes, and functioned in vivo as APCs to stimulate responses of CD4+ T cells [26].
A single inoculation of S. stercoralis antigen–pulsed eosinophils was sufficient to prime naive mice and initiate the antigen-specific Th2 immune response. In addition, antigen-pulsed eosinophils also stimulated immunized mice to increase IL-4 and IL-5 production, thereby confirming the contribution of eosinophils as APCs for stimulating memory immune responses. These observations confirm the previously reported in vitro findings that S. stercoralis antigen–pulsed eosinophils are capable of priming naive T cells to initiate Th2 responses and activating antigen-specific T cells for increased IL-5 production [12]. The present data also extend the findings of earlier studies with ovalbumin-pulsed eosinophils, which were shown to stimulate memory T cells to increase proliferation and induce Th2 cytokine production [5, 26].
Experiments were performed to determine whether S. stercoralis antigen–pulsed eosinophils would induce antigen-specific B cell responses in naive mice. Both IgM and IgG function during adaptive protective immunity to S. stercoralis in mice [27]. Mice inoculated 3 times with antigen-pulsed eosinophils had levels of IgM and IgG in serum that were significantly higher than those in mice that received untreated control eosinophils. These experiments therefore suggest that eosinophils can actively participate in priming the immune system for the required CD4+ Th2 response as well as for the antibody-mediated humoral immune responses against infection.
It was possible that the induction of the immune response after transfer of antigen-pulsed eosinophils was caused by the contaminating macrophages and dendritic cells present in the eosinophil preparations. However, our present experiments demonstrated that the small number of contaminating dendritic cells and macrophages in the eosinophil preparations cannot account for the observed T cell stimulation and Th2 response in naive mice. This is in agreement with previous studies demonstrating that small numbers of APCs fail to stimulate the proliferation of T cells [12, 28].
Although these experiments demonstrated that antigen-pulsed eosinophils could prime naive mice for Th2 immune responses, it is possible that the stimulation of T cells and the induction of cytokine production occurred after dumping of antigens by the pulsed eosinophils. The antigens could then be presented to T cells by professional APCs present in the recipient mice. Alternatively, antigen-pulsed eosinophils may have died after injection into the peritoneum and were then phagocytosed by dendritic cells or macrophages. The antigens released from the antigen-pulsed eosinophils may then be presented by the dendritic cells or macrophages to T cells. To prove that eosinophils communicate directly to T cells, experiments were performed wherein antigen-pulsed MHC class II– deficient eosinophils were transferred into naive mice. These experiments demonstrated that the induction of IL-5 production by spleen cells from these mice was due to the presentation of antigen by transferred eosinophils in conjunction with MHC class II molecules and that the eosinophils were directly involved in the stimulation of T cells. This conclusion was based on the observation that eosinophils deficient in MHC class II molecules failed to initiate immune responses, compared with MHC class II-sufficient antigen-pulsed eosinophils. To further confirm this conclusion, dead antigen-pulsed eosinophils were inoculated into mice. Mice receiving dead antigen-pulsed eosinophils did not have an increase in IL-5 production, compared with mice that received live antigen-pulsed eosinophils, thereby showing that eosinophils direct interact with T cells and do not simply transport antigen to the professional APCs. In addition, levels of IgM and IgG antibodies were unchanged in mice inoculated 3 times with dead antigen-pulsed eosinophils, providing further support for the conclusion that eosinophils are directly involved in communication with T cells.
These experiments demonstrate that eosinophils function as APCs that stimulate T cell proliferation, Th2 cytokine production, and production of antibody by B cells. Although the model used in the present study does not entirely mimic natural infection, in which antigen is presented via the skin, it clearly supports the hypothesis that eosinophils, which come into direct contact with parasites during the innate immune response, participate in the induction of the adaptive immune response to infection with S. stercoralis. Eosinophils can kill larval S. stercoralis during the innate immune response [13] and through this process may capture parasite antigens to present to T cells and thereby act as the interface between the innate and adaptive immune responses to parasitic infections.
Acknowledgments
Financial support: National Institutes of Health (grants R01 AI47189, R01 A1 22662, and R01 AI50668); Mayo Foundation.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 53rd annual meeting of the American Society of Tropical Medicine and Hygiene, Miami, 7–11 November 2004 (abstract 520).
References
- 1.Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202–9. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 3.Shi HZ. Eosinophils function as antigen-presenting cells. J Leukoc Biol. 2004;76:520–7. doi: 10.1189/jlb.0404228. [DOI] [PubMed] [Google Scholar]
- 4.Xie ZF, Shi HZ, Qin XJ, Kang LF, Huang CP, Chen YQ. Effects of antigen presentation of eosinophils on lung Th1/Th2 imbalance. Chin Med J (Engl) 2005;118:6–11. [PubMed] [Google Scholar]
- 5.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4+ Th2 lymphocyte function. J Immunol. 2001;167:3146–55. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 6.Herbert DR, Lee JJ, Lee NA, Nolan TJ, Schad GA, Abraham D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2000;165:4544–51. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 7.Meeusen EN, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–54. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 9.Korenaga M, Hitoshi Y, Takatsu K, Tada I. Regulatory effect of antiinterleukin-5 monoclonal antibody on intestinal worm burden in a primary infection with Strongyloides venezuelensis in mice. Int J Parasitol. 1994;24:951–7. doi: 10.1016/0020-7519(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 10.Mawhorter SD, Pearlman E, Kazura JW, Boom WH. Class II major histocompatibility complex molecule expression on murine eosinophils activated in vivo by Brugia malayi. Infect Immun. 1993;61:5410–2. doi: 10.1128/iai.61.12.5410-5412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Pozo V, De Andres B, Martin E, et al. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992;22:1919–25. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 12.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–8. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galioto AM, Hess JA, Nolan TJ, Schad GA, Lee JJ, Abraham D. Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2006;74:5730–8. doi: 10.1128/IAI.01958-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerepesi LA, Hess JA, Nolan TJ, Schad GA, Abraham D. Complement component C3 is required for protective innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2006;176:4315–22. doi: 10.4049/jimmunol.176.7.4315. [DOI] [PubMed] [Google Scholar]
- 15.Herbert DR, Nolan TJ, Schad GA, Abraham D. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 2002;24:95–101. doi: 10.1046/j.0141-9838.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- 16.Rotman HL, Schnyder-Candrian S, Scott P, Nolan TJ, Schad GA, Abraham D. IL-12 eliminates the Th-2 dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol. 1997;19:29–39. doi: 10.1046/j.1365-3024.1997.d01-142.x. [DOI] [PubMed] [Google Scholar]
- 17.Rotman HL, Yutanawiboonchai W, Brigandi RA, et al. Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol. 1996;82:267–78. doi: 10.1006/expr.1996.0034. [DOI] [PubMed] [Google Scholar]
- 18.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–44. [PubMed] [Google Scholar]
- 19.Abraham D, Rotman HL, Haberstroh HF, et al. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByJ mice. Exp Parasitol. 1995;80:297–307. doi: 10.1006/expr.1995.1036. [DOI] [PubMed] [Google Scholar]
- 20.Herbert DR, Nolan TJ, Schad GA, Lustigman S, Abraham D. Immunoaffinity-isolated antigens induce protective immunity against larval Strongyloides stercoralis in mice. Exp Parasitol. 2002;100:112–20. doi: 10.1016/S0014-4894(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 21.Borchers MT, Justice PJ, Ansay T, et al. Gq signaling is required for allergen-induced pulmonary eosinophilia. J Immunol. 2002;168:3543–9. doi: 10.4049/jimmunol.168.7.3543. [DOI] [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 23.Stanley ER, Heard PM. Factors regulating macrophage production and growth: purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977;252:4305–12. [PubMed] [Google Scholar]
- 24.Del Prete A, Vermi W, Dander E, et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J. 2004;23:3505–15. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–54. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 26.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–53. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligas JA, Kerepesi LA, Galioto AM, et al. Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice. Infect Immun. 2003;71:6835– 43. doi: 10.1128/IAI.71.12.6835-6843.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handzel ZT, Busse WW, Sedgwick JB, et al. Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol. 1998;160:1279–84. [PubMed] [Google Scholar]