Abstract
Diabetes is increasing in the world and causes severe cardiovascular complications. Diabetes-induced limb ischemia leads to foot amputation and therapeutic remedies are urgently needed. Here we report that local injection of mesenchymal stem cells (MSCs) prestimulated with epidermal growth factor (EGF) restored blood flow and vasculogenesis in the ischemic hind-limb of type II diabetic (db−/db−) mice. Bone marrow cells from db−/db− mice are altered as evidenced by increased oxidative stress and reduced Akt and adhesion molecules when compared with control (db−/db+). Femoral artery ligation-induced ischemia was performed in the hind-limb of db−/db− and db−/db+ mice for 28 days. Enhanced green fluorescent protein (EGFP)-MSCs stimulated±exogenous EGF for 24 h were injected locally into the ischemic muscle. Blood flow measured with MoorLDI-Laser and microangiography assessed with X-ray showed 100% recovery in db−/db+ compared to 50% recovery in db−/db− mice. Interestingly, db−/db− mice had 60 and 96% blood flow recovery and 61 and 98% of vasculogenesis when treated with MSCs alone or MSCs modified with EGF, respectively. Western blot analysis of hind-limb muscles revealed an increase in Akt and vascular endothelial growth factor receptor phosphorylation and hypoxia-inducible factor) expression in db−/db− mice injected with MSCs or MSCs +EGF compared to db−/db− mice. Fluorescent microscopic images show that EGFP-MSCs differentiate into new microvessels. Adhesion and migration of MSCs on cultured endothelial cells were ICAM1-, VCAM1- and Akt-dependent mechanism and elevated when MSCs were prestimulated with EGF compared with nonstimulated MSCs. Our novel study data provide evidence that in type II diabetes, stimulated MSCs with EGF enhance the recovery of blood flow and angiogenesis.
Keywords: type II diabetes, stem cells, vasculogenesis, EGF, ischemia
Diabetes mellitus is a serious risk factor leading to vascular complication and the development of atherosclerotic, end-stage renal failure, blindness and heart disease.1 In fact, cardiac morbidity and mortality in patients with diabetes mellitus are greatly enhanced,2 and up to 80% in diabetic patients, associated with vascular disease.3 Peripheral vascular disease (PVD) associated with impaired vasculogenesis is another major problem in diabetic patients. It affects 7.5% diabetic patients aged between 60 and 64 years in the USA, and PVD is responsible for 3900 lower limb amputation per million in a year.4
Recently, a growing number of studies reported abnormalities of neovascularization in diabetic patients with coronary or peripheral artery diseases,5 and abnormal enhanced angiogenesis.6 In addition, defective arteriogenesis, another form of vessel growth refers to the transformation of preexisting arteriolar pathways into large conductance arteries, has also been reported in patients with diabetes mellitus.7 The underlying mechanism of this neovascularization defects still remains unclear. Recent evidence indicates that endothelial progenitor cells isolated from diabetic patients show a marked defect in migration in response to vascular endothelial growth factor (VEGF).8 Interestingly, it has been advanced that multipotent stromal cells (MSCs) can differentiate into cardiomyocytes, endothelial cells (ECs) and smooth muscle cells.9 In addition, previous reports describe the beneficial effect of administration of MSCs into injury tissue and indicate that they exert a paracrine effect in the repair of tissue.10 The initial steps following injection of MSCs are homing to impaired tissue, adhesion, diapedesis and migration.11 All these events are critical in the beneficial effect of injected MSCs. Therefore, increasing the homing, adhesion and migration could significantly enhance the therapeutic effect of injected MSCs. Thus, in previous studies, growth factors were used to improve the therapeutic effect of MSCs.12 Interestingly, recent studies showed the importance of epidermal growth factor (EGF) or the EGF family member, HB-EGF (heparin-binding epidermal growth factor-like growth factor), in the differentiation of human-derived MSCs into ECs, adhesion of fibroblast13 and angiogenesis.14 Thus, we hypothesize that MSCs prestimulated with EGF can significantly facilitate the recovery of neovasculogenesis and subsequently improve tissue perfusion. The purpose of this study was to determine the potential therapeutic effect of locally injected stem cells alone or treated with EGF on vasculogenesis in the ischemic hind-limb of type II diabetic mouse (db−/db−).
MATERIALS AND METHODS
Antibodies
Phosphorylated VEGF receptor (Tyr845), VEGF, total Akt, phosphorylated Akt (Ser476), phosphorylated p40Phox (Thr154), Von Willebrand factor, total eNOS, hypoxia inducible factor (HIF), ICAM1 and VCAM1 were purchased from Cell Signaling and Promega.
Drugs
PI3-kinase inhibitor (LY-294002, 1 μM) and EGF were purchased from Sigma.
Bone Marrow Isolation
Bone marrow was isolated from db−/db− and their control as previously reported.15
Mice Multipotent Stromal Cells
Mice multipotent stromal cells were provided by the Gene Therapy Center, Tulane University. MSCs were collected from the bone marrow of the femurs and tibias of 8- to 10-week-old C57/Bl6 mice. mMSCs were obtained from the inbred transgenic strain C57Bl/6-TgN(ACTbEGFP)1Osb (GFPtg) that expressed enhanced green fluorescent protein (EGFP; Jackson Laboratories) for identification as previously described.16 The isolation and purification of MSCs was described in detail by Dobson et al.15
Animal Model and Surgery
A total of 56 male type II diabetic (db−/db−; with C57/Bl6 genetic background) and their control (db−/db+) between 8- and 10-week-old mice were used in this study. This study conformed to the principles of the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and was approved by the Tulane University Institutional Animal Care and Use Committee. The hind-limb ischemia model was produced in db−/db− and db−/db+ mice by ligation of the proximal segment of the right femoral artery as previously described.17
Treatment of db−/db− Mice with MSCs
The db−/db− mice were subjected to local injection, MSCs (200 000 per injection) stimulated±10 ng/ml of exogenous EGF for 24 h, in the three muscles (quadriceps ‘U’, gluteus ‘L’ and tibia ‘T’) of the right hind-limb just after femoral artery ligation. Then, mice received MSCs once a week for 3 weeks. As control, a group of db−/db− mice were subjected to local injection of PBS only.
Laser Doppler Measurement of Hind-Limb Blood Flow
Hind-limb blood flow measurement over the region of interest was performed at baseline before surgery, immediately postoperatively and serially over the 4-week period with laser Doppler perfusion imaging (Moor Instruments).
X-Ray Quantification of Hind-Limb Angiogenesis
Vessel density was assessed by high-definition microangiography at the end of the treatment period, as previously described.18
Western Blot Analysis
Western blot analysis of tissue and cultured cells was performed as previously described.19–21 The quantification of western blot was determined using Multi Gauge software (Fujifilm).
Immunohistochemistry
Immunohistochemistry was performed as previously described.19,21,22 MSCs are tagged with EGFP and do not have to be labeled for easy visualization.
Cultured Endothelial Cells from Microvessels
Endothelial cell isolation and culture were performed as previously described.19 Briefly, 6–8 hearts from C57/Bl6 were isolated and perfused with Hanks’ balanced salt solution supplemented with 175 U/ml of collagenase II (Worthington) and 0.25 mg/ml of elastase for 45 min. The collected ECs were then placed in a flask coated with fibronectin. ECs at passages 3–8 were used for adhesion, migration assay and western blot analysis.
Adhesion Assay of MSC
Endothelial cells were cultured to confluency and then serum starved for 48 h. EGFP-MSCs±exogenous EGF were placed on confluent ECs for 15 min, 2, 3, 4 and 24 h. Using another experimental design, we pretreated EGFP-MSCs with ICAM1 and/or VCAM1 antibodies and PI3-kinase inhibitor±EGF then placed on confluent cultured ECs for 24 h. At the end of experiment cells were washed, fixed and then adherent cells were counted under fluorescent microscope.
Migration Assay of MSC
Migration assay was performed using cell culture inserts (3 μm pore size; Millipore, MA, USA) coated with 2.5 μg/ml of fibronectin (BD Biosciences, Bedford, MA, USA). MSCs±EGF were collected, counted and then applied (500 cells per well) on endothelial monolayer. Cells were incubated for 0, 2, 4 and 8 h time points for real-time recording migration assay at 37°C and 5% CO2. Transmigration was monitored by phase video microscopy (Olympus 1X81) at ×20 magnification. Images were collected every 30 min for 8 h with a Hamamatsu C4880 charge-coupled device camera run by MetaMorph imaging software. For standard assay, we incubated cells for 24 h, then the upper surface of the filter was rapidly wiped with a cotton swab to remove nonmigratory cells and immediately the migrated cells were fixed in 10% formalin. EGFP-MSCs were counted under a phase-contrast microscope equipped with ocular grids.
Statistical Analysis
Results are expressed as mean±s.e.m. One-way or two-way ANOVA was used to compare each parameter when there were two independent groups. Comparisons between groups were performed with Bonferroni post hoc t-tests when the ANOVA was statistically significant. Values of P<0.05 were considered statistically significant.
RESULTS
Bone Marrow Stem Cell Alterations in Type II Diabetes
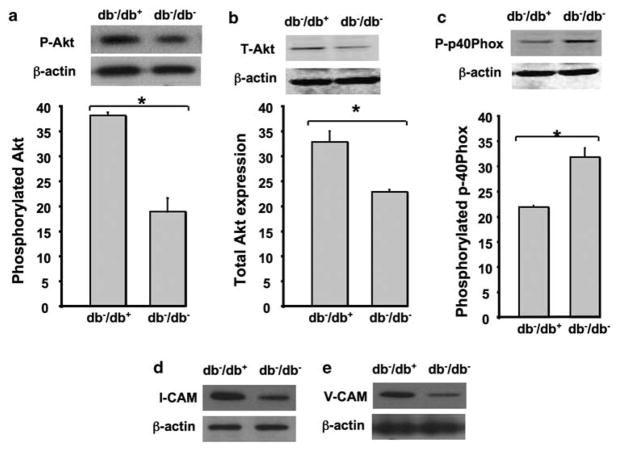
We isolated bone marrow from db−/db− and db−/db+ mice and then subjected to western blot analysis for phosphorylated and total Akt, and p-40Phox. The data revealed that bone morrow cells from db−/db− mice express less phosphorylated and total Akt compared with control (Figure 1a and b). In contrast, phosphorylated p-40Phox was significantly increased in bone morrow cells from db−/db− mice compared with db−/db+ (Figure 1c). Figure 1d and e illustrates that adhesion molecules (ICAM1 and VCAM1) were significantly reduced in bone marrow cells from db−/db− compared with db−/db+.
Figure 1.
Western blots analysis and quantitative data showing: (a, b) the phosphorylated (P-Akt) level and total Akt (T-Akt) expression in bone marrow isolated from type II diabetic mice (db−/db−) and their control (db−/db+) mice. (c) The phosphorylated p40Phox (P-p40Phox) level in bone marrow isolated from type II diabetic mice (db−/db−) and their control (db−/db−). (d, e) The expression of adhesion molecules (ICAM1 and VCAM1) in bone marrow isolated from type II diabetic mice (db−/db−) and their control (db−/db−); β-actin expression; n =5, *P<0.05 statistically significance between db−/db− and db−/db+.
Effects of Local Injection of MSCs±EGF on Blood Flow Recovery in Ischemic Hind-Limb in db−/db− Mice
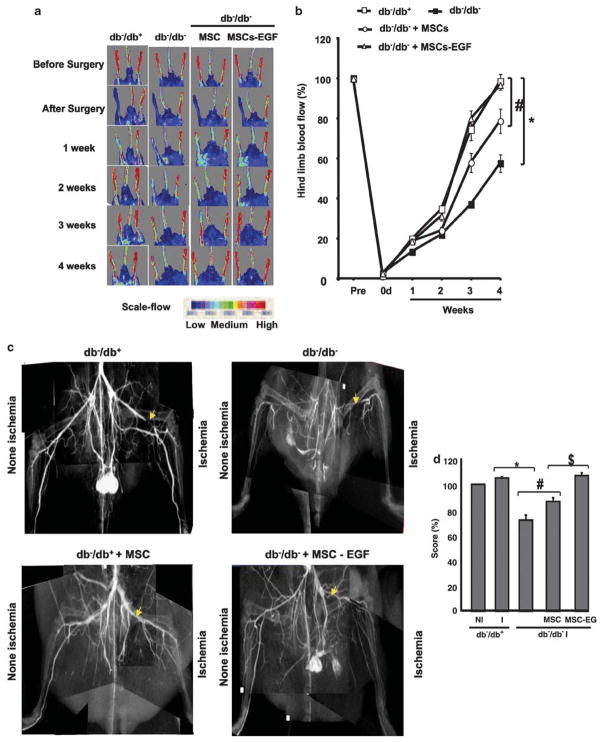
After femoral ligation, the blood flow was significantly reduced (less than 2% of the value before ligation) in all groups (Figure 2a and b). In db−/db− mice, blood flow recovered 50% in 4 postoperative weeks (Figure 2a and b). However, in db−/db+ mice, blood flow recovered 100% in 4 postoperative weeks (Figure 2a and b).
Figure 2.
Blood flow analysis of hind-limb of type II diabetic mice (db−/db−) and their control (db−/db−). (a) Blood flow was measured with MoorLDI-Laser in all groups (db−/db+, db−/db−, db−/db− injected with MSCs, and db−/db− injected with MSCs prestimulated with EGF) before surgery, just after surgery and once a week for 4 weeks. This figure is representative of n =6. (b) Blood flow measurement with MoorLDI-Laser in all groups (db−/db+, db−/db−, db−/db− + MSCs and db−/db− + MSCs prestimulated with EGF) before surgery, just after surgery and once a week for 4 weeks; n =6; *P<0.05 statistically significance between db−/db− vs db−/db+ and db−/db−+MSCs; #P<0.05 statistically significance between db−/db−+MSCs vs db−/db−+MSCs-EGF and db−/db+. (c) Microangiography performed at the end of the treatment period in all groups (db−/db+, db−/db−, db−/db− injected with MSCs and db−/db− injected with MSCs prestimulated with EGF). Contrast media (barium sulfate, 0.5 g/ml) were injected into the abdominal aorta, then angiography of the right and left hind-limbs was assessed with digital X-ray transducer acquired images. Images were then assembled to obtain a complete view of the hind-limb. The image is representative of n =6. (d) Quantitative data (score in percent) showing non ischemic (NI) and ischemic (I) vessel density using Multi Gauge (Fujifilm) by selecting the same area for measurement in all groups, n =6; *P<0.05 statistically significance between db−/db+ and db−/db−; #P<0.05 statistically significance between db−/db− and db−/db−+MSCs; $P<0.05 statistically significance between db−/db−+MSCs and db−/db−+MSCs-EGF.
Mice received intramuscular injection of MSCs (200 000 cells in 100 μl of sterile PBS) as a bolus into the upper, lower and tibia muscle of the ischemic hind-limb in db−/db− mice. Interestingly, in db−/db− mice, ischemic hind-limb injected with MSCs alone or prestimulated with EGF showed a significant increase in blood flow recovery compared with ischemic hind-limb of db−/db− mice (Figure 2a and b). Blood flow recovery was significantly higher with MSCs prestimulated with EGF (100%) compared with MSC alone (70%; Figure 2a and b).
Effects of Local Injection of MSCs±EGF on Vessel Density in Ischemic Hind-Limbs in db−/db− Mice
Vessel density was evaluated by high-definition microangiography at the end of the treatment period. We injected contrast media (barium sulfate, 0.5 g/ml) into the abdominal aorta. Angiography of the right and left hind-limbs was assessed and then a digital X-ray transducer acquired the images. After 4 weeks, vessel density was not different between the ischemic (right) leg and the nonischemic (left) legs in db−/db+. However, in db−/db− mice the ischemic hind-limb vessel density was significantly decreased compared to db−/db+. In db−/db− mice injected with MSCs, vessel density was significantly increased in ischemic hind-limb vs db−/db− mice untreated. Interestingly, the MSCs prestimulated with EGF increased further the vessel density in ischemic hind-limb of db−/db− to a level similar to that observed in db−/db+ mice (Figure 2c and d).
Effects of Local Injection of MSCs±EGF on Capillary Density in Ischemic Hind-Limb in db−/db− Mice
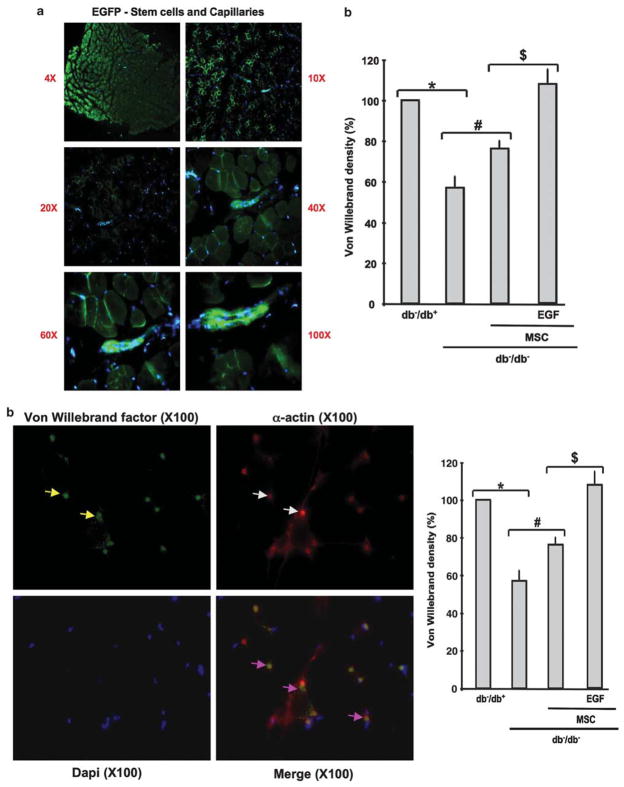
In db−/db− mice, capillary density determined by immunostaining was decreased by 40% in ischemic hind-limb compared with db−/db+ (100%). The number of capillaries reached 80 and 100% in ischemic hind-limb of db−/db− injected with MSCs and MSCs prestimulated with EGF, respectively (Figure 3b). By fluorescent microscopy, EGFP-MSCs were visualized at low and high power of magnification. Data revealed that EGF-MSCs were able to differentiate to neovessels when injected to ischemic hind-limb of db−/db− mice (Figure 3a).
Figure 3.
(a) Image with low- and high-power magnification showing the formation of new vessels from EGFP-MSCs injected into ischemic hind-limb of db−/db− mice; picture representative of n =5. (b) Example of immunostaining with specific antibodies for α-actin (Red staining, white arrows) and Von Willebrand factor (green staining yellow arrows; capillaries; yellow staining (purple arrows) represents arterioles ‘merge between green and red’ in ischemic hind-limb from db−/db− mice treated with MSCs prestimulated with EGF; and cumulative data of Von Willebrand factor staining in hind-limb from all groups (db−/db+, db−/db−, db−/db− injected with MSCs, and db−/db− injected with MSCs prestimulated with EGF), n =6; *P<0.05 statistically significance between db−/db+ and db−/db−; #P<0.05 statistically significance between db−/db− and db−/db−+MSCs; $P<0.05 statistically significance between db−/db−+MSCs and db−/db−+MSCs-EGF.
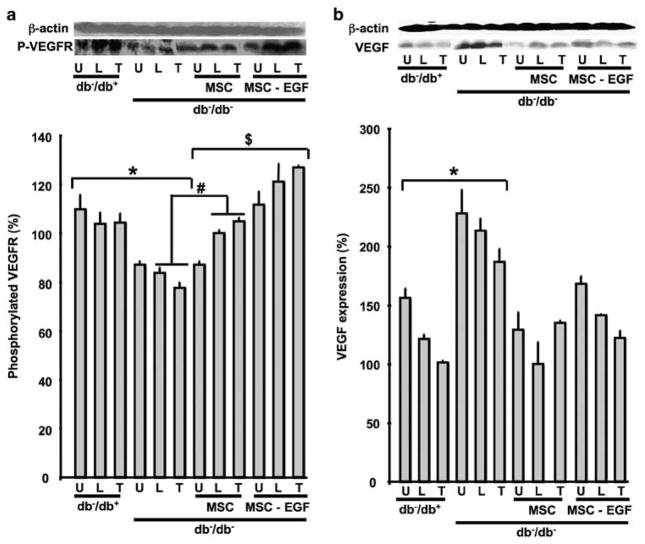
Effects of Local Injection of MSCs±EGF on VEGF Expression and VEGF Receptor Phosphorylation in Ischemic Hind-Limb in db−/db− Mice
Western blot analysis showed an increase in VEGF expression in U, L and T muscles in ischemic hind-limb of db−/db− mice compared with db−/db+ (Figure 4b). In contrast, VEGF receptor phosphorylation was significantly decreased in db−/db− mice compared with db−/db+, suggesting that VEGF signaling is impaired in db−/db− mice (Figure 4a). Interestingly, the injection of MSCs to ischemic hind-limb increased the phosphorylation of VEGF receptor in db−/db− mice. Furthermore, the prestimulation of MSCs with EGF increased further VEGF receptor phosphorylation in ischemic tissue in db−/db− and reduced VEGF expression in ischemic hind-limb of db−/db− mice (Figure 4a and b).
Figure 4.
Western blots analysis and quantitative data. (a) VEGF receptor (VEGFR) phosphorylation level and β-actin expression in hind-limb ischemic muscles (quadriceps ‘U’, gluteus ‘L’ and tibia muscles ‘T’) from all groups (db−/db+, db−/db−, db−/db− injected with MSCs and db−/db− injected with MSCs prestimulated with EGF), n =6; *P<0.05 statistically significance between db−/db+ vs db−/db−; #P<0.05 statistically significance between db−/db− and db−/db−+MSCs; $P<0.05 statistically significance between db−/db− + MSCs and db−/db−+MSCs-EGF. (b) VEGF (VEGF) and β-actin expression in hind-limb ischemic muscles (quadriceps ‘U’, gluteus ‘L’ and tibia muscles ‘T’) from all groups (db−/db+, db−/db−, db−/db−+MSCs and db−/db−+MSCs prestimulated with EGF), n =6; *P<0.05 statistically significance between db−/db+ and db−/db−.
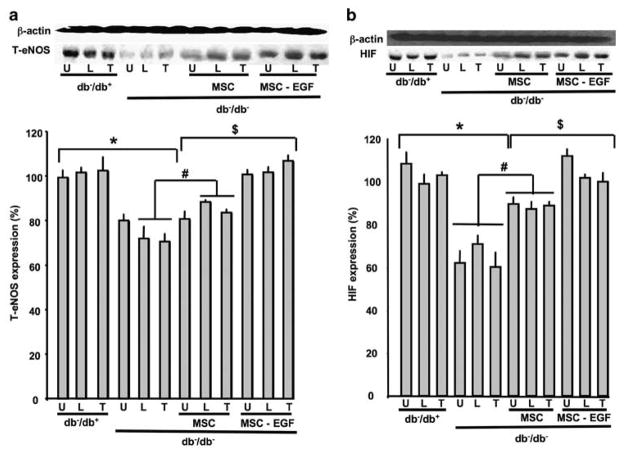
Effects of Local Injection of MSCs±EGF on eNOS and HIF Expression in Ischemic Hind-Limb in db−/db− Mice
Figure 5a shows the effect of MSCs on total eNOS expression. In db−/db−, eNOS expression was significantly reduced in all muscles of the ischemic hind-limb (Figure 5a). The injection of MSCs significantly increased eNOS expression, whereas MSCs prestimulated with EGF totally restored the expression of eNOS in the ischemic hind-limb of db−/db− mice (Figure 5a). In db−/db− mice, HIF expression was significantly decreased compared with db−/db+ in the three muscles (U, L and T) with hind-limb ischemia (Figure 5b). The administration of MSCs increased the expression of HIF in U, L and T in db−/db− mice (Figure 5b). The prestimulation of MSCs with EGF further increased HIF expression in db−/db− compared with untreated MSCs (Figure 5b). Our study data show that MSCs prestimulated with EGF increased the neovascularization in ischemic hind-limb through an increase in eNOS and HIF expression (Figure 5a and b).
Figure 5.
(a) Western blots analysis and quantitative data showing total eNOS (T-eNOS) and β-actin expression in hind-limb ischemic muscles (quadriceps ‘U’, gluteus ‘L’ and tibia muscles ‘T’) from all groups (db−/db+, db−/db−, db−/db− injected with MSCs and db−/db− injected with MSCs prestimulated with EGF), n =6; *P<0.05 statistically significance between db−/db+ and db−/db−; #P<0.05 statistically significance between db−/db− and db−/db−+MSCs; $P<0.05 statistically significance between db−/db− + MSCs and db−/db−+MSCs-EGF. (b) Western blots analysis and quantitative data showing hypoxia-inducible factor (HIF) and β-actin expression in hind-limb ischemic muscles (quadriceps ‘U’, gluteus ‘L’ and tibia muscles ‘T’) from all groups (db−/db+, db−/db−, db−/db− injected with MSCs and db−/db− injected with MSCs prestimulated with EGF), n =6; *P<0.05 statistically significance between db−/db+ and db−/db−.
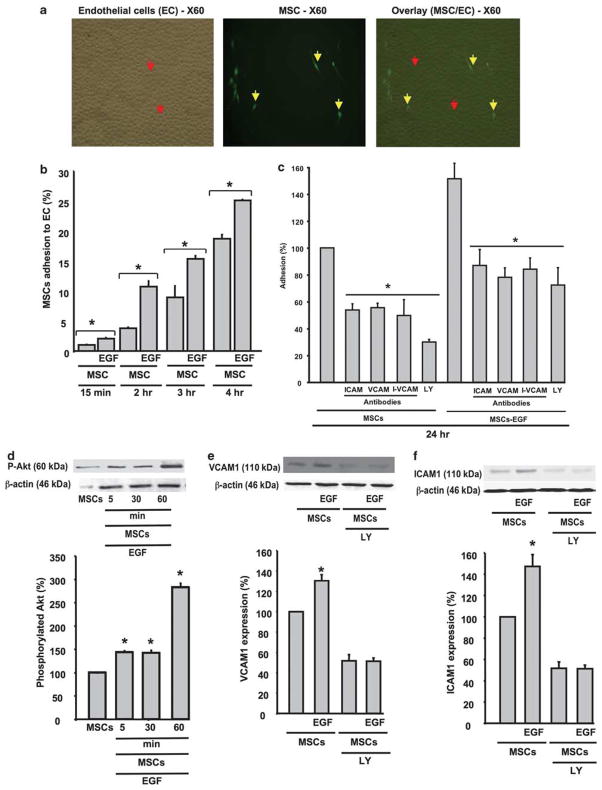
Effect of EGF on MSCs Adhesion to Endothelial Cells
The adhesion of MSCs to ECs was time dependent (Figure 6a and b). The prestimulation of MSCs with EGF for 24 h before incubation with ECs significantly increased the adhesion rate of MSCs to ECs in a time-dependent manner (Figure 6b).
Figure 6.
(a) Example of adhesion assay of MSCs (yellow arrows) on confluent cultured endothelial cells (Ecs; red arrows) at ×60 power of magnification. (b) Quantitative data representing the percent of MSCs, prestimulated or not with EGF, adhesion to ECs at different times—15 min, 2, 3 and 4 h; n =6; *P < 0.05 statistically significance between MSCs and MSCs +EGF. (c) Quantitative data representing the percent of adhesion to ECs at 24 h of pretreated MSCs±EGF with PI3-kinase-Akt inhibitor (LY, 1 μM) and specific antibodies for ICAM1 and VCAM1; n =6; *P<0.05 statistically significance between MSCs±EGF and MSCs±EGF +LY, or ICAM1 or VCAM1 antibodies. (d) Western blots analysis and cumulative data showing Akt phosphorylation (P-Akt) level in MSCs stimulated with EGF time-dependent manner (5, 60 and 60 min) and β-actin expression, n =6; *P<0.05 statistically significance between MSCs and MSCs +EGF. (e) Western blots analysis and cumulative data showing VCAM1 expression in MSCs stimulated with EGF±PI3-kinase-Akt inhibitor (LY, 1 μM) and β-actin expression, n =6; *P<0.05 statistically significance between MSCs stimulated with EGF vs MSCs and MSCs ±EGF +LY. (f) Western blots analysis and cumulative data showing ICAM1 expression in MSCs stimulated with EGF±PI3-kinase-Akt inhibitor (LY, 1 μM) and β-actin expression, n =6; *P<0.05 statistically significance between MSCs +EGF vs MSCs and MSCs±EGF +LY.
The adhesion of MSCs to ECs was significantly decreased by the blockade of ICAM1 and VCAM1 (Figure 6c). Similarly, specific antibodies of VCAM1 and ICAM1 significantly reduced the EGF MSC-induced adhesion to ECs (Figure 6b). When both specific antibodies of ICAM1 and VCAM1 were added to MSCs untreated or prestimulated with EGF before incubation with ECs, the adhesion to ECs was significantly reduced compared with MSCs±EGF (Figure 6c); however no additive effect was observed compared with ICAM-1 or VCAM-1 antibodies alone (Figure 6c). Pretreated MSCs with PI3-kinase inhibitor significantly reduced the adhesion of MSCs stimulated±EGF to ECs (Figure 6c).
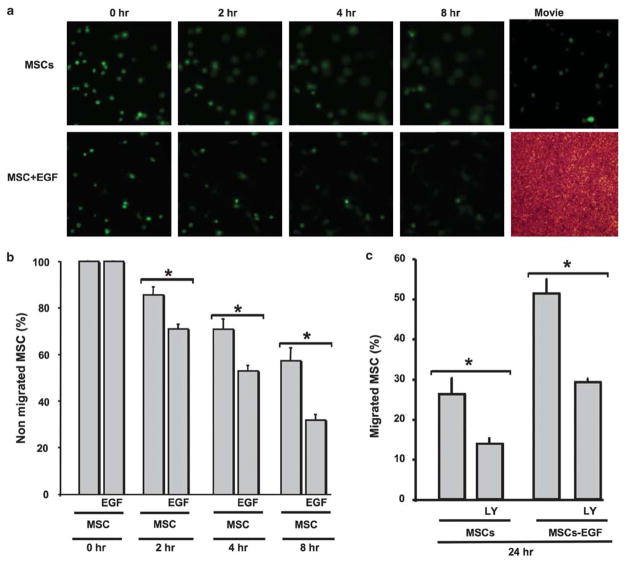
Effect of EGF and AKT on MSCs Migration
The migration of MSCs through ECs was time dependent (Figure 7a and b). The stimulation of MSCs with EGF before incubation with ECs significantly increased their migration in a time-dependent manner (Figure 7a and b). Moreover, the treatment of MSCs with PI3-kinase inhibitor (LY-294002, 1 μM) significantly decreased the migration through ECs of MSCs and MSCs stimulated with EGF (Figure 7c).
Figure 7.
(a) Life imaging migration of MSCs and MSCs +EGF at 0, 2, 4 and 8 h (see Supplementary Movie online). (b) Quantitative data showing percent of nonmigrated MSCs alone or stimulated with EGF at 0, 2, 4 and 8 h, n =6; *P<0.05 statistically significance between MSCs +EGF and MSCs. (c) Quantitative data of migrated MSCs alone or MSCs +EGF in the presence of PI3-kinase-Akt inhibitor (LY, 1 μM) after 24 h incubation time, n =6; *P<0.05 statistically significance between MSCs vs MSCs +LY; MSCs +EGF and MSCs +EGF +LY.
DISCUSSION
The major finding of this study is the efficacy of prestimulated MSCs with EGF as a therapy for enhanced recovery of angiogenesis and blood flow of the ischemic of hind-limb of type II diabetic mice. These results indicate that the effect of EGF-stimulated MSCs is mediated through the modulation of VEGF, VEGF receptor, and eNOS and Akt pathways. We have shown that weekly local injection of MSCs prestimulated with EGF to ischemic hind-limb muscles significantly restored vessel density and subsequently the perfusion of the ischemic hind-limb.
In this study, we found that bone marrow cells isolated from diabetic mice show a reduction in phosphorylated and total Akt expression and an increase in p-40Phox phosphorylation in db−/db− as compared with db−/db+ mice. We also demonstrated that bone marrow cells from db−/db− show less adhesion molecules expression (ICAM1 and VCAM1) indicating that the cell migration process is compromised. These data are in agreement with previous reports indicating that bone marrow cells are altered in type II diabetes.23,24 The hind-limbs of type II diabetic mice were subjected to ischemia for 4 weeks. Our study data revealed that db−/db− mice show reduced blood flow recovery when compared with db−/db+ mice, indicating that angiogenesis was compromised in type II diabetes. These data are in agreement with previous studies showing that neovascularization is altered in type II diabetic patients and in an animal model.25–27 Interestingly, the treatment of db−/db− mice with local injection of MSCs alone or MSCs stimulated with EGF show a significant enhancement in blood flow recovery reaching 100% with MSCs prestimulated with EGF. Our study data are in agreement with those of our previous study showing that MSCs prestimulated with EGF enhance collateral vessel growth in the rat heart after repetitive episodic serials of ischemia/reperfusion.22,28,29 The increased efficiency of modified stem cells was also reported in a previous studies showing that lentivirally mediated gene transfer in transplanted stem cells increases in vivo chemoprotection of progenitor cells.30 A number of reports show the angiogenic potential of EGF administration in ischemic tissue. We have previously shown that EGFR tyrosine kinase phosphorylation is increased in db−/db− mice as is associated with microvascular dysfunction.19 In addition, it has been shown that increased HB-EGF, an agonist of EGFR tyrosine kinase, failed to induce angiogenesis in ischemic tissue.31 On the basis of these observations, we did not treat db−/db− mice with exogenous EGF.
Using an alternative strategy, we evaluated the formation of new vessels in ischemic hind-limb of mice injected with contrast media to assess vascularization with X-ray. The data revealed that db−/db− mice displayed fewer vessels density compared with db−/db+ mice. Importantly, db−/db− injected with MSCs alone or MSCs prestimulated with EGF significantly increased vessel density compared with db−/db− mice. The effect of MSCs prestimulated with EGF was greater than the effect of MSCs alone. We also performed immunostaining using a specific antibody for Von Willebrand factor, which is an EC marker. The data showed a reduction in Von Willebrand factor capillary staining in db−/db− mice as compared with db−/db+. Interestingly, db−/db− mice injected with MSCs alone or MSCs stimulated with EGF showed an increase in Von Willebrand factor capillary staining compared with db−/db− mice. The effect of MSCs prestimulated with EGF was significantly greater compared with MSCs alone, indicating that the biologic effect of MSCs prestimulated with EGF is greater than with MSCs treatment alone. These data are in agreement with previous study showing the beneficial effect of modified stem cells.22,30 From these data, it is clear that the stimulation of MSCs with EGF before injection is in part to facilitate the integration of the MSCs and increase their availability in the ischemic tissue.
The mechanism of how MSCs improve vascularization and subsequently blood flow is unknown. It is more likely that MSCs exert paracrine effects32 and/or differentiate into vascular cells.33 In this study, we have shown that injected MSCs can differentiate into new vessels. These data are in agreement with previous studies showing the formation of new vessels from stem cells.33 It is also more likely that paracrine effects and differentiation coexist and function together for a beneficial response of the MSCs. The mechanism dictating stem cells differentiation to vascular cells is still unknown. Nevertheless, studies by Madeddu and co-workers34 showed that stem cells could release VEGF and interleukin-8,35 and differentiate into vascular cells involved in the formation of new vessels.33 In our study we focused on factors known to be involved in angiogenesis, such as HIF, VEGF, VEGF receptor phosphorylation, eNOS and Akt. Interestingly, western blot analysis of ischemic hind-limb muscles showed an increase in VEGF in db−/db− mice compared with db−/db+ mice. Conversely, VEGF receptor phosphorylation was significantly reduced in ischemic hind-limb of db−/db− mice compared to db−/db+ mice. These data indicate that the VEGF/VEGF receptor pathway is impaired in type II diabetes. Our findings are in agreement with a previous study showing an increase in VEGF in type II diabetes.36 Interestingly, in vivo local injection of MSCs alone or MSCs stimulated with EGF significantly reduced VEGF expression and increased VEGF receptor phosphorylation. The effect of MSCs prestimulated with EGF is greater than the effect of MSCs alone. Similarly, HIF and eNOS expression levels were significantly reduced in db−/db− mice compared with db−/db+ mice. Our study data are in agreement with a previous study showing reduction in eNOS in db−/db− mice.37 Interestingly, db−/db− mice injected with MSCs alone or MSCs stimulated with EGF significantly increased HIF and eNOS expression in the ischemic hind-limb of db−/db− mice. The effect of MSCs prestimulated with EGF was significant compared to the effect of MSCs alone. These data indicate that MSCs stimulated with EGF restore blood flow by increasing vessel formation more through the HIF, VEGF-VEGF receptor and eNOS pathways. Our study data are in agreement with previous studies showing an alteration in eNOS and the VEGF pathway in type II diabetes.19,36 It was also reported that treatment of ischemic tissue with stem cells (EP cells) restored eNOS expression and the VEGF pathway.38
The first step of injected MSCs to tissue is adhesion, migration and engraftment. To determine the intracellular pathway involved in adhesion and migration, we performed adhesion and migration assays of MSCs on cultured ECs isolated from microvessels. We have shown that the adhesion of MSCs to confluent cultured ECs was time dependent. Interestingly, adhesion of MSCs was significantly higher when MSCs were prestimulated with EGF. To determine the mechanism involved in the adhesion of MSCs to ECs, we pretreated MSCs with PI3-kinase/Akt pathway inhibitor and specific antibodies for the adhesion molecules (ICAM1 and VCAM1). Our study data revealed that the PI3-kinase/Akt pathway and adhesion molecules (ICAM1 and VCAM1) were important in the adhesion of MSCs to ECs. To strengthen our point we performed western blots analysis on MSCs stimulated with EGF and the data illustrated that phosphorylated Akt is increased in a time-dependent manner. We have also shown that MSCs stimulated with EGF for 24 h significantly increase ICAM1 and VCAM1 expression in an Akt-dependent-mechanism manner. Similarly, MSCs migration through ECs was time dependent and significantly enhanced when MSCs were stimulated with EGF. The inhibition of PI3-kinase/Akt pathway significantly reduced the migration of MSCs indicating the importance of the Akt pathway.
The use of stem cells for cellular therapy becomes a reality as new basic research and clinical trials show promise of efficiency for the treatment of diseases of ischemic organs. In this study we showed the effective use of modified stem cells in the recovery of blood flow in an ischemic hind-limb of type II diabetic animal models through the modulation of eNOS, HIF and VEGF/VEGF receptor pathways. These data offer a new direction in the understanding of the biological effects of administered stem cells prestimulated with EGF in the treatment of ischemic tissue diseases.
Supplementary Material
Acknowledgments
We acknowledge grant support from National Institutes of Health (P20RR017659, HL26371 NCRR); (R01HL095566, Dr Matrougui); Mansoura University, Egypt (Fellowship, Ali H Amin).
Footnotes
Supplementary Information accompanies the paper on the Laboratory Investigation website (http://www.laboratoryinvestigation.org)
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Khan MA, Collins AJ, Keane WF. Diabetes in the elderly population. Adv Ren Replace Ther. 2000;7:32–51. doi: 10.1016/s1073-4449(00)70004-5. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 3.Feener EP, King GL. Vascular dysfunction in diabetes mellitus. Lancet. 1997;350(Suppl 1):SI9–S13. doi: 10.1016/s0140-6736(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 4.Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care. 1983;6:87–91. doi: 10.2337/diacare.6.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson-Berka JL. Vasoactive factors and diabetic retinopathy: vascular endothelial growth factor, cycoloxygenase-2 and nitric oxide. Curr Pharm Design. 2004;10:3331–3348. doi: 10.2174/1381612043383142. [DOI] [PubMed] [Google Scholar]
- 7.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 8.Segal MS, Shah R, Afzal A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 9.Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 10.Hofstetter CP, Holmstrom NA, Lilja JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 11.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Wang MH, Singh RK, et al. Epidermal growth factor induces CD44 gene expression through a novel regulatory element in mouse fibroblasts. J Biol Chem. 1997;272:14139–14146. doi: 10.1074/jbc.272.22.14139. [DOI] [PubMed] [Google Scholar]
- 13.Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241–250. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 14.Javazon EH, Colter DC, Schwarz EJ, et al. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 15.Dobson KR, Reading L, Haberey M, et al. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int. 1999;65:411–413. doi: 10.1007/s002239900723. [DOI] [PubMed] [Google Scholar]
- 16.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D, Branch BG, Pattillo CB, et al. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci USA. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobi J, Tam BY, Wu G, et al. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110:2424–2429. doi: 10.1161/01.CIR.0000145142.85645.EA. [DOI] [PubMed] [Google Scholar]
- 19.Belmadani S, Palen DI, Gonzalez-Villalobos RA, et al. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belmadani S, Zerfaoui M, Boulares HA, et al. Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose. Am J Physiol Heart Circ Physiol. 2008;295:H69–H76. doi: 10.1152/ajpheart.00341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su J, Lucchesi PA, Gonzalez-Villalobos RA, et al. Role of advanced glycation end products with oxidative stress in resistance artery dysfunction in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1432–1438. doi: 10.1161/ATVBAHA.108.167205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belmadani S, Matrougui K, Kolz C, et al. Amplification of coronary arteriogenic capacity of multipotent stromal cells by epidermal growth factor. Arterioscler Thromb Vasc Biol. 2009;29:802–808. doi: 10.1161/ATVBAHA.109.186189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 24.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 25.Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arteriosc Thromb Vasc Biol. 2008;28:1606–1613. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiekofer S, Galasso G, Sato K, et al. Impaired revascularization in a mouse model of type 2 diabetes is associated with dysregulation of a complex angiogenic–regulatory network. Arterioscler Thromb Vasc Biol. 2005;25:1603–1609. doi: 10.1161/01.ATV.0000171994.89106.ca. [DOI] [PubMed] [Google Scholar]
- 27.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez A, Rota M, Nurzynska D, et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 29.Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larochelle A, Choi U, Shou Y, et al. In vivo selection of hematopoietic progenitor cells and temozolomide dose intensification in rhesus macaques through lentiviral transduction with a drug resistance gene. J Clin Investig. 2009;119:1952–1963. doi: 10.1172/JCI37506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chalothorn D, Moore SM, Zhang H, et al. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884–1890. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]
- 32.Stastna M, Abraham MR, Van Eyk JE. Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 2009;583:1800–1807. doi: 10.1016/j.febslet.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tillmanns J, Rota M, Hosoda T, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci USA. 2008;105:1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Invernici G, Emanueli C, Madeddu P, et al. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barcelos LS, Duplaa C, Krankel N, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen MP, Hud E, Shea E, et al. Vitreous fluid of db/db mice exhibits alterations in angiogenic and metabolic factors consistent with early diabetic retinopathy. Ophthalmic Res. 2008;40:5–9. doi: 10.1159/000111151. [DOI] [PubMed] [Google Scholar]
- 37.Emanueli C, Caporali A, Krankel N, et al. Type-2 diabetic Lepr(db/db) mice show a defective microvascular phenotype under basal conditions and an impaired response to angiogenesis gene therapy in the setting of limb ischemia. Front Biosci. 2007;12:2003–2012. doi: 10.2741/2205. [DOI] [PubMed] [Google Scholar]
- 38.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochimica Polonica. 2003;50:49–59. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.