Abstract
Treatment of pathological spinal discs in vivo by injection of protein crosslinking reagents to restore their mechanical properties is a new possible approach to the treatment of degenerative disc disease. In this study, the thermal stability of the collagen in disc annulus was measured by differential scanning calorimetry following treatment with six different crosslinking agents. The crosslinkers used were; L-threose (LT), genipin (GP), methylglyoxal (MG), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) glutaraldehyde (GA) and proanthrocyanidin (PA).
Untreated tissue displayed a prominent peak at approximately 66-68°C. Comparison of endothermal patterns of untreated and crosslinker treated disc annulus tissue samples showed that a new peak appeared at a higher temperature following treatment. The temperature of the new peak qualitatively depended on the crosslinker in the following order GA>MG>GP>PA=EDC>LT. This suggested that the enhanced thermal stability of collagen in the annulus tissue was related to the nature of the crosslinker used. In addition, the enthalpic ratios of the lower temperature (non-crosslinked) peaks in the treated and untreated tissue, and of the higher and lower temperature peaks in the treated tissue, both indicated that the various crosslinkers crosslinked the tissue with different efficiencies.
Our data suggest that the ability of GP to penetrate into the disc as well as form long and short range crosslinks may make it the most suitable candidate for clinical development. In addition, binary combinations of long- and short- range crosslinkers, such as PA with LT may also provide synergistic effects due to their substantially different physicochemical properties.
Keywords: Spinal Disc, Degenerative Disc Disease, crosslinking, DSC, calorimetry
Introduction
Degenerative disc disease (DDD) is a chronic pathology of the spinal disc resulting in the gradual deterioration of this tissue1,2, and remains among the most costly and prevalent health problems in the world today3. As the tissue degrades its mechanical properties alter to the point where they are unable to withstand the stresses and strains of physiological mechanical loading4, leading to bulging, tearing and eventual rupture. Nonsurgical exogenous crosslink therapy (NEXT) is an experimental technology for the treatment of DDD based on the injection of protein crosslinking agents into the affected tissue. The crosslinks thus formed serve to augment endogenous crosslinks in order to enhance the mechanical strength of the annulus5,6 and the stability of the intervertebral joint7. It is hoped that such treatment in vivo could both ameliorate the progress of and the symptoms of DDD.
Collagenous matrices stabilized by crosslinks are used widely8-10. A number of alternative physical procedures have been employed to crosslink collagen, including dehydrothermal treatment and ultraviolet irradiation11,12. Various chemical crosslinkers that have been used for this purpose, include; diisocyanates13, acyl azide14, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)13, genipin (GP)7,15, proanthrocyanidin (PA)16 and glutaraldehyde (GA)17.
In the context of DDD, two parameters are most likely to influence the efficacy of protein crosslinkers injected into the annulus fibrosis: reactivity and diffusion. Highly reactive reagents that could not diffuse within the disc and highly mobile reagents that reacted very slowly would likely be equally ineffective. We have previously studied a number of crosslinkers with regard to reaction kinetics and determined the conditions for their optimal reactivity18. These studies were conducted using homogenized annulus tissue in order to minimize the contribution of differential diffusion effects to the observed chemical reactivities. The crosslinkers studied were methylglyoxal (MG), L-threose (LT), EDC, GP, PA and GA. Not surprisingly, there were substantial differences in the reactivity of these reagents, both in terms of their reaction rates and saturating concentrations. Due to the differences in size and structure between the various crosslinkers, we suspected that there would also be differences between their diffusion rates. In order to develop a product with the greatest possibility of providing clinical efficacy, we therefore decided to also investigate the penetration of the crosslinkers into the disc to determine whether any such differences were evident and whether they might be substantial enough to affect performance.
It has been reported that the thermal stability of the collagen is correlated to the number of crosslinks19,20 and differential scanning calorimetry (DSC) has provided a powerful tool for examining changes in connective tissue collagen21-26. DSC is sensitive to both the amount of covalent crosslinking21,25, and to level of hydration of the collagen24. In the present study, we utilized DSC to analyze crosslinker diffusion within bovine disc annulus following treatment with various crosslinking reagents.
Methods
Genipin was obtained from Challenge Bioproducts Co., Ltd. (Taiwan) and proanthrocyanidin from Polyphenolics (Madera, CA). All other reagents were from Sigma. Statistical differences between groups were analyzed using a non-parametric Mann-Whitney test, with a significance level of α = 0.01. Discs were treated under conditions that we had previously optimized biochemically18.
Our crosslinker concentrations had been chosen to minimize possible cytotoxicity to fibroblasts within the annulus and nucleus during clinical use, while maximizing reaction rates based on our previous work18. Differences produced by the relative reaction rates of the crosslinkers were minimized by the 24 hour soaking time of the samples, which was twice as long as the amount of time required to complete crosslinking by the slowest reacting crosslinker tested (LT)18.
Eight bovine lumbar motion segments from 3 different 4-6 month old calves were transected through the mid-transverse plane to expose the entire disc. The two halves were soaked individually in either buffer or buffer containing crosslinker at 37°C for 24 hours. Each crosslinker was soaked in a different buffer based on our previous optimization study18. Crosslinker concentrations and buffers were 20mM GP (in 100mM Tris/100mM tri-sodium phosphate, pH9), 20mM MG (in 100mM EPPS, pH8), 2.65mM EDC (in 100mM MES, pH6), 0.1% (w/v) PA (in 100mM EPPS, pH8), 100mM L-threose (in 100mM EPPS, pH8) and 5mM GA (in 100mM EPPS, pH8).
To determine the effect of buffer osmolarity on tissue swelling, we soaked each half of a transected disc in either 100mM EPPS buffer or 100mM EPPS/100mM tri-sodium phosphate buffer as described above. Three biopsies from each disc were taken, weighed, dehydrated at 100°C overnight and reweighed to determine their water content.
Following incubation, segments were then rinsed with water and 8 biopsies excised from each annulus with a  -inch punch to the depth of the end plate and at 45° intervals around the circumference of the disc. One segment was treated with each crosslinking reagent, except for GA and PA where two segments were treated.
-inch punch to the depth of the end plate and at 45° intervals around the circumference of the disc. One segment was treated with each crosslinking reagent, except for GA and PA where two segments were treated.
Samples were frozen at −20°C and later analyzed using a Perkin-Elmer DSC 7. Samples were weighed accurately and scanned from 40-100°C at 5°C/min with an empty pan as a reference. A baseline, produced by scanning two empty pans, was subtracted from all thermograms.
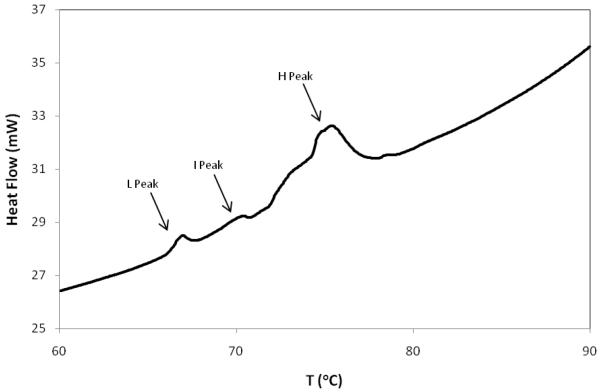
Only three peaks were observed in this temperature range during this study (although not all samples contained all three peaks). For the purposes of this paper, we have termed these the low (L), intermediate (I) and high (H) temperature peaks. The L-peak generally appeared below ~68°C while the H-peak appeared in the range of 73-85°C. The I peak appeared infrequently and did so at a temperature of between 70 and 72°C. An example thermogram depicting all three peaks in the same sample is depicted in figure 1.
Figure 1.
Example thermogram an EDC-treated disc annulus sample. A bovine spinal motion segment was bisected transversely and one half soaked in EDC solution (2.65 mM EDC in 100mM MES, pH6). The position of the L, I and H peaks are shown by the arrows.
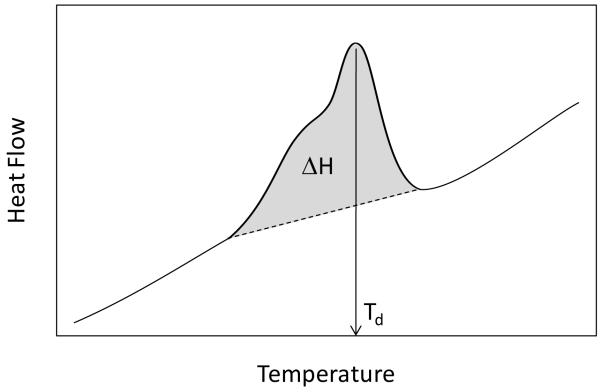
Peak denaturation temperature (Td) was determined numerically from thermograms for each endothermal process while transitional enthalpies (ΔH) were determined from the area under each peak and normalizing by dividing by the specimen mass (Fig. 2). In some cases H peaks were less symmetrical and contained shoulders and/or multiple merged peaks. In these cases, peak temperature was defined as the point of greatest amplitude. We were unable to discern any correlation between peak asymmetry and treatment or source of the specimens. For the purposes of this paper, all references to enthalpy (ΔH) refer only to peak transitional enthalpies, and not to the enthalpy of the entire denaturation process.
Figure 2.
Schematic diagram depicting determination of Td and ΔH. Td was determined from the position point of the greatest amplitude of the peak while ΔH was determined by linear baseline extrapolation and peak integration (grey area).
We sought to describe the extent of crosslinking for each crosslinker treatment using two different ratios defined by the following equations:
Where H and L are the averaged enthalpies of the H and L peaks respectively from the crosslinked samples and Ltreated and Luntreated are the averaged enthalpies of the L peaks in the crosslinked and control samples respectively. All averaged enthalpic peak values and the ratios derived from them are depicted in Table 2. R1, therefore, is related to the amount of collagen crosslinked as represented by the relative sizes of the L and H peaks in only the treated samples, while R2 gives a description of crosslinking extent only in terms of loss of the native L peak following treatment.
Table 2.
Peak enthalpies and enthalpic ratios of tissue treated with different crosslinkers. The average enthalpies (ΔH) of L and H peak transitions were determined in J/g and enthalpic ratios (R1 and R2) calculated as described in the text. The p values of the differences in the enthalpies of the L peaks of the treated and untreated samples are also shown.
| Untreated (L) | Crosslinked (L) | Crosslinked (H) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ΔH | SD | n | ΔH | SD | n | ΔH | SD | p | R1 | R2 | |
| GP | 8 | 2.99 | 1.48 | 8 | 0.40 | 0.30 | 8 | 2.60 | 2.82 | <0.001 | 0.87 | 0.87 |
| MG | 7 | 4.41 | 2.29 | 7 | 1.49 | 1.08 | 7 | 3.58 | 4.20 | 0.03 | 0.71 | 0.66 |
| EDC | 8 | 1.92 | 0.85 | 8 | 0.18 | 0.27 | 8 | 2.86 | 1.20 | <0.001 | 0.94 | 0.91 |
| PA | 16 | 2.71 | 2.21 | 11 | 1.30 | 1.30 | 11 | 1.14 | 1.89 | 0.049 | 0.47 | 0.52 |
| LT | 8 | 1.87 | 0.98 | 8 | 0 | 0 | 8 | 3.62 | 3.20 | <0.002 | 1 | 1 |
| GA | 16 | 4.07 | 2.48 | 6 | 1.43 | 0.76 | 6 | 1.20 | 0.97 | <0.002 | 0.46 | 0.42 |
During calculation of enthalpic ratios, 0 was used when determining the average enthalpy crosslinker-treated specimens that lacked an L peak. In the case of EDC treatments where an I peak was sometimes observed, the enthalpy of the I peak was added to that of the H peaks.
Results
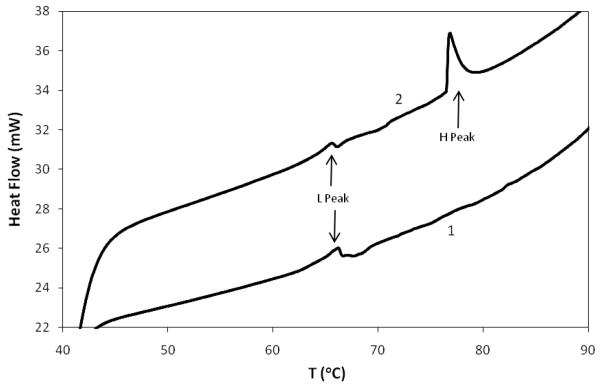
Figure 3 shows the temperature dependence of heat flow through tissue treated with either buffer or with GP. The buffer treated sample displayed an endothermic peak at ~68°C, representing the Td of native disc collagen, the major protein component of the annulus. The peak was reduced, but still detectable, in the GP treated sample indicating that not all the collagen had been crosslinked. The reduction in the 68°C peak area was accompanied by the appearance of a higher temperature peak at ~ 76.5°C, representing more thermally stable, crosslinked collagen.
Figure 3.
Example thermograms of bovine annulus tissue. Bovine spinal motion segments were bisected transversely and each half soaked in either 100mM Tris/100mM tri-sodium phosphate, pH 9 (1) or the same buffer containing 20mM genipin (2). Samples of tissue from each segment were analyzed by DSC The position of the L and H peaks are shown by the arrows.
DSC thermograms were qualitatively similar for samples treated with MG, PA, MG and GA. H peaks appeared between 73 and 84°C, depending on the crosslinker, and L peaks remained at approximately 67°C, which was the same as their corresponding untreated counterparts. The only exceptions were LT and EDC. LT treatment completely eliminated the original L peak and produced an H peak at ~73°C. EDC was unusual in that it produced two higher temperature peaks. The H peak appeared at ~75°C, while a lower I peak was also observed at ~71°C. All eight samples contained the H peak, while the I peak was observed in five of the eight samples and the L peak in three samples. One sample contained no L or I peaks and one contained both L and I peaks (Fig. 1).These results are summarized in Table 1.
Table 1.
Summary of DSC results obtained from tissue treated with various crosslinking agents. Samples from untreated and crosslinked bovine discs were analyzed by DSC. The average temperatures and standard deviations (SD) of the low- (L), intermediate- (I) and high- (H) temperature transition peaks were calculated. In some samples, certain peaks were not detected (nd).
| Untreated | Crosslinked | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | L (°C) | SD | n | L (°C) | SD | n | I (°C) | SD | n | H (°C) | SD | |
| GP | 8 | 68.3 | 1.4 | 5 | 68.9 | 3.1 | - | nd | - | 8 | 76.5 | 4.3 |
| MG | 7 | 67.6 | 1.3 | 7 | 67.1 | 1.9 | - | nd | - | 7 | 78.7 | 4.8 |
| EDC | 8 | 66.7 | 0.9 | 3 | 66.3 | 0.6 | 5 | 71.5 | 0.73 | 8 | 75.2 | 1.0 |
| PA | 16 | 66.7 | 1.0 | 11 | 66.1 | 1.2 | - | nd | - | 11 | 74.0 | 3.1 |
| LT | 8 | 67.2 | 0.8 | - | nd | - | - | nd | - | 8 | 73.5 | 3.1 |
| GA | 16 | 66.6 | 0.8 | 6 | 67.8 | 1.6 | - | nd | - | 6 | 84.5 | 1.3 |
In all cases the average Tds of the H peaks were significantly higher than the average L peak temperatures of the corresponding control specimens (p<0.0025). In addition, the temperature difference between the untreated L peaks and the EDC treated I peaks was also significant (p=0.003). There was no significant difference in the temperatures of the L peaks between any of the treated and untreated samples. The mean Td of the GA H peak (84.9 °C) was significantly higher than all the other crosslinkers’ H peaks (p<0.005). The differences being between 3.5 and 7 times the intradiscal and interdiscal variability of the control Td. In some cases (MG, PA and GA) some samples (9 out of a total of 120) did not yield interpretable thermograms and the data from these samples were discarded. We also often observed small shoulders in the L peak of untreated samples at a slightly lower temperature than the peak maximum (not shown), but never in samples treated with any of the crosslinking agents. No more than 30% of the untreated samples displayed the shoulder, and there was no apparent correlation of its appearance with respect to buffer treatment, biopsy location or specimen.
We calculated two ratios, R1 and R2, to describe the relative size of the area (enthalpy) under the H peak (Table 2). LT exhibited the highest ratio (1.0 for both R1 and R2), indicating that all of the collagen had been crosslinked, while GA displayed the lowest value (R1=0.46, R2=0.42) indicating that only 40-50% of the collagen had reacted. About 50% of the collagen was crosslinked in the PA-treated sample while the remaining reagents resulted in R values of between 0.66 and 0.94.
Discussion
The two most important parameters that could influence outcome from a crosslinking product for the treatment of DDD would be the reaction rate of the crosslinker and its ability to diffuse and access potential crosslinking sites. We have previously characterized a number of crosslinking reagents with regard to their optimal reaction conditions18, and in this study, we examined crosslinker diffusion within the tissue.
The osmolarity of the buffers used in these studies were essentially the same (since the concentrations of the crosslinking reagents were relatively small). The only exception was the GP buffer whose osmolarity was approximately 4-fold greater than the other buffers. In order to dispel the concern that the different osmolarity of the GP buffer might affect penetration due to differential tissue swelling during incubation, we showed that following soaking in the respective buffers, the water content of annulus tissue was was 78.2% ± 0.04 in EPPS/phosphate and 78.8% ± 0.04 in EPPS, suggesting that differential swelling was not a concern in these studies. Protease inhibitors were not used in order to exclude reagents that might not be present in a clinical formulation from the study. However, experiments on tissue soaked in the presence or absence of EDTA showed no evidence for protein hydrolysis by endogenous proteases during the incubation following subsequent analysis of the tissue by DSC or by monitoring the release of potential cleaved peptides into solution.
Collagen is the major component of the annulus fibrosis and endogenous collagen cross-links accumulate as the tissue ages, increasing its thermostablity20. We therefore used DSC to monitor the diffusion of crosslinking reagents within the tissue indirectly. Collagenous tissue exhibits a characteristic endothermal peak at a temperature of ~68°C. Crosslinking results its reduction accompanied by the appearance of new peaks at elevated temperature.
We considered using chemical analysis to monitor crosslinker diffusion, but this would be difficult since reacted crosslinker is rendered insoluble. In addition analysis of tissue extracts following exposure to crosslinker would also be problematic since some crosslinkers are either chemically unstable in solution (e.g. GP and EDC) or do not contain suitable chromophore. We therefore monitored penetration indirectly using DSC.
Our initial aim had been to simulate clinical treatment by monitoring crosslinker diffusion following injection into intact discs. We found, however, that there was a substantial amount of heterogeneity in the thermograms of numerous biopsies taken from the same untreated disc. In fact, intradiscal variability (standard deviation) of both Td and ΔH were on the same order of magnitude as interdiscal variability. Injection of crosslinker into the disc produced changes in thermograms that were too small to reliably and consistently detect over this background without implementing a stochastic experimental approach (repeated measurements for each disc). Our hypothesis was that chemical crosslinking would change the mean thermogram metrics for each disc based on the observation that similar crosslinker formulations did produce significant effects on tissue and joint mechanics6. In order to produce more crosslinks, and so detect their formation by DSC, we needed to introduce more crosslinker into the discs, i.e. by soaking the tissue.
Crosslinker diffusion is affected by both molecular weight and interaction and reaction with the tissue. Numerous high-temperature peaks were asymmetric, suggesting that crosslinking is a complex process and that unequivocal interpretation of our data is problematic. We simplified our interpretation by assuming that reduction in the 68°C peak represents the fraction of the molecular population that has been crosslinked to any degree, while the magnitude of the temperature change represents the degree to which the collagen has been crosslinked, i.e. the density and/or number of crosslinks per molecule. Crosslink density or number could be enhanced by agents that can either access more sterically obscured sites and/or span longer distances.
GA crosslinking displayed the largest shift at ~17°C which was significantly higher than the H peaks produced by the other crosslinkers (p<0.005). GA was the smallest molecule we tested (MW 100.12) and polymerizes during reaction27. This may have allowed it to access sterically less-accessible sites and also to crosslink more spatially distant sites than the other crosslinkers. GP can also polymerize28 while PA is itself a polymer29, but produced smaller shifts(~8°C) that were not significantly different from the H peaks formed by the other reagents excepting GA. GP and PA contain bulky aromatic structures, and may be unable to access sites available to the smaller GA, resulting in a lower crosslink density despite their ability to span larger distances. MG, which does not polymerize, exhibited the second highest peak shift (~11.5°C) but, excluding GA, this was only marginally different from the LT H peak (P=0.049), the difference being approximately 3.5 times the intradiscal or interdiscal variability of control Td. MG is the smallest of the crosslinkers tested (MW 72.02) and its ability to reach less accessible sites may have compensated for its inability to form longer crosslinks.
LT displayed the smallest shift (~6°C) but this was only significantly less than the GA H (p=0.0005) peak and marginally less than the MG H peak (p=0.049). LT is also small (MW 120.1), but was not as effective at increasing the Td as MG. LT crosslinking is the slowest of these reagents by an order of magnitude18. Thus the low shift may have been for kinetic reasons. EDC exhibited a peak shift of 9°C and also produced a unique third, intermediate (I) peak. The nature of this peak is unclear, but may be due to the unique chemistry which crosslinks amines with carboxyls, not other amines. EDC also forms the shortest crosslinks in this study and thus potential crosslinking sites must be spatially closer than for the other reagents. While EDC is also relatively small (MW 155.2) the requirement that potential targets be in close proximity, may limit the crosslink density compared to, for example, MG.
To obtain information on the fraction of collagen crosslinked, we utilized the enthalpic data from our experiments. We utilized two crosslinking ratios (CRs) to describe the modification of the native collagen population, one in terms of the relative enthalpies of the L and H peaks in treated samples (R1) and one in terms of the decrease in the L peak following treatment (R2). The values of both R1 and R2 were generally in good agreement (Table 2).
GA treated samples demonstrated the lowest CR (~45%). GA can polymerize extensively during reaction15 and is the fastest reacting reagent that we have tested18. Thus, while GA is an effective crosslinker, its diffusion into the annulus may be retarded by its rapid reaction with the tissue and also by its formation of a tight polymeric network that could act as a barrier for its continued penetration into the disc. PA displayed the second lowest CR (~50%). and also reacts very rapidly18 and is a polymer. These factors may limit its diffusion rate. Examination of treated samples showed that only the surface had been stained brown, indicating that diffusion was limiting. While PA and GA are excellent crosslinkers kinetically18, their low diffusion suggests that they may be unable to diffuse sufficiently within the disc following injection.
Although GP also polymerizes it displayed a much higher CR (87%) than GA and PA. Its reaction, however, is slower than that of GA18 and its diffusion may have been less retarded by interaction with the tissue. Also, while GA forms branches15, GP forms linear polymers which may retard its diffusion less than GA. Since GP-crosslinked samples turn dark blue, it was clearly visible that GP penetrated more deeply than PA.
LT is the only crosslinker that completely eliminated the L peak, and is also the slowest reacting of the reagents18. This slow rate may not have retarded diffusion as much as for the other crosslinkers, allowing LT to disperse throughout the tissue. LT, however, also produced the lowest temperature H peak, suggesting that while all of the collagen had reacted with LT, the crosslinking density was still low.
It should be noted that the absolute effects of these same crosslinkers on human annulus fibrosus may vary from the data presented here. It is our expectation that the same trends would carry over to human discs but that moderately degenerated human discs might have higher glycation-induced intrinsic crosslinking, and the matrix would be more obstructed by an accumulation of degraded matrix molecules30, posing an even higher barrier to penetration by the reagents.
Our data present intriguing possibilities with regard to the development of NEXT therapy. The data in this paper, coupled to our previous chemical characterization and mechanical testing6,18, suggest that GP is the most promising of the crosslinkers we have tested. However, since some crosslinkers appear to be able to react with sites that are inaccessible to others, it is possible that combinations of crosslinkers may produce synergies with regard to mechanical effects, although there may be both practical and regulatory limitations to the development of formulations containing more than 2 or 3 reagents. However, binary combinations of long chain (e.g. GA, PA or GP) and short chain (e.g. LT or EDC) crosslinkers, for example, may provide both a higher number and density of crosslinks due to their differing physicochemical properties. In addition, our observation that MG produces higher temperature peaks than GP, possibly due to steric considerations, coupled to our observation that these crosslinkers produce differential mechanical effects on annulus tissue6, opens the possibility that crosslinkers with different physicochemical properties may produce varied mechanical effects that can be tailored appropriately to treat a number of diverse joint pathologies. We are currently in the process of testing these possibilities.
Acknowledgments
This work was supported by the NIH (1R43 AR055014-01) and Orthopeutics, L.P.
References
- 1.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 2.Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus. 2002;13:1–6. doi: 10.3171/foc.2002.13.2.2. [DOI] [PubMed] [Google Scholar]
- 3.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 4.Acaroglu ER, Iatridis JC, Setton LA, et al. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20:2690–701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Chuang SY, Odono RM, Hedman TP. Effects of exogenous crosslinking on in vitro tensile and compressive moduli of lumbar intervertebral discs. Clin Biomech. 2007;22:14–20. doi: 10.1016/j.clinbiomech.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Slusarewicz P, Zhu K, Kirking B, et al. Optimization of Protein Crosslinking Formulations for the Treatment of Degenerative Disc Disease. Spine. 2010 doi: 10.1097/BRS.0b013e3181cc3de9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hedman TP, Saito H, Vo C, Chuang SY. Exogenous cross-linking increases the stability of spinal motion segments. Spine. 2006;31:480–5. doi: 10.1097/01.brs.0000224531.49174.ea. [DOI] [PubMed] [Google Scholar]
- 8.Yannas IV. Tissue regeneration by use of collagen-glycosaminoglycan copolymers. Clin Mater. 1992;9:179–87. doi: 10.1016/0267-6605(92)90098-e. [DOI] [PubMed] [Google Scholar]
- 9.Silver FH, Silver FH. Biomaterials medical devices and tissue engineering. An integral approach. Chapman and Hall; London: 1994. Wound dressing and skin replacement. [Google Scholar]
- 10.Schroeder-Tefft JA, Bentz H, Estridge TD. Collagen and heparin matrices for growth factor delivery. J Control Release. 1997;48:29–33. J Control Release. [Google Scholar]
- 11.Germain L, Auger FA, Grandbois E, et al. Reconstructed human cornea produced in vitro by tissue engineering. Pathobiology. 1999;67:140–7. doi: 10.1159/000028064. [DOI] [PubMed] [Google Scholar]
- 12.Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J Biomed Mater Res. 1988;22:939–57. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- 13.Rault I, Frei V, Herbage DA-M, Huc A. Evaluation of different chemical methods for crosslinking collagen gel, films and sponges. J Mater Sci Mater Med. 1996;7:221. [Google Scholar]
- 14.Petite H, Frei V, Huc A, Herbage D. Use of diphenylphosphorylazide for cross-linking collagen-based biomaterials. J Biomed Mater Res. 1994;28:159–65. doi: 10.1002/jbm.820280204. [DOI] [PubMed] [Google Scholar]
- 15.Sung HW, Liang IL, Chen CN, et al. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin) J Biomed Mater Res. 2001;55:538–46. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65:118–24. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann B, Seitz D, Mencke A, et al. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2009;20:1495–503. doi: 10.1007/s10856-009-3707-3. [DOI] [PubMed] [Google Scholar]
- 18.Slusarewicz P, Zhu K, Hedman TP. Kinetic Characterization and Comparison of Various Protein Crosslinking Reagents for Matrix Modification. J Mater Sci Mater Med. 2010 doi: 10.1007/s10856-010-3986-8. DOI: 10.1007/s10856-010-3986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judge MD, Reeves ES, Aberle ED. Effect of electrical stimulation on thermal shrinkage temperature of bovine muscle collagen. J Anim Sci. 1981;52:530–4. doi: 10.2527/jas1981.523530x. [DOI] [PubMed] [Google Scholar]
- 20.Judge MD, Aberle ED. Effects of chronological age and postmortem aging on thermal shrinkage temperature of bovine intramuscular collagen. J Anim Sci. 1982;54:68–71. [Google Scholar]
- 21.Kopp J, Bonnet M, Renou JP. Effect of collagen crosslinking on collagen-water interactions (a DSC investigation) Matrix. 1989;9:443–50. doi: 10.1016/s0934-8832(11)80013-2. [DOI] [PubMed] [Google Scholar]
- 22.Miles CA, Bailey AJ. Studies of the collagen-like peptide (Pro-Pro-Gly)(10) confirm that the shape and position of the type I collagen denaturation endotherm is governed by the rate of helix unfolding. J Mol Biol. 2004;337:917–31. doi: 10.1016/j.jmb.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Miles CA, Burjanadze TV, Bailey AJ. The kinetics of the thermal denaturation of collagen in unrestrained rat tail tendon determined by differential scanning calorimetry. J Mol Biol. 1995;245:437–46. doi: 10.1006/jmbi.1994.0035. [DOI] [PubMed] [Google Scholar]
- 24.Miles CA, Ghelashvili M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys J. 1999;76:3243–52. doi: 10.1016/S0006-3495(99)77476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigi A, Cojazzi G, Panzavolta S, et al. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials. 2001;22:763–8. doi: 10.1016/s0142-9612(00)00236-2. [DOI] [PubMed] [Google Scholar]
- 26.Kronick PL, Cooke P. Thermal stabilization of collagen fibers by calcification. Connect Tissue Res. 1996;33:275–82. doi: 10.3109/03008209609028885. [DOI] [PubMed] [Google Scholar]
- 27.Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 2003;24:759–67. doi: 10.1016/s0142-9612(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 28.Touyama R, Inoue K, Takeda Y, et al. Studies on the blue pigments produced from genipin and methylamine. II. On the formation mechanisms of brownish-red intermediates leading to the blue pigment formation. Chem Pharm Bull. 1994;42:1571–8. [Google Scholar]
- 29.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;26:781–4. [Google Scholar]
- 30.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]