Abstract
Tang et al demonstrate a pivotal role of the cyclooxygenase (COX) enzyme pathway in the pathogenesis of basal cell carcinoma in mice, with elegant experiments in mice overexpressing COX-1 and COX-2 or in mice deficient in COX-1 and 2 showing an increase or decrease respectively in mice bearing the basal call carcinoma prone patch heterozygote background. They followed the murine findings with a study in humans with loss of patch and found a trend towards decreased tumor burden of BCC in humans. This has implications for public health.
Basal cell carcinoma remains the most common form of cancer in humans, with over 1 million new cases yearly in the United States. Despite the usual nonaggressive course of basal cell carcinoma, it is a major cause of morbidity, because the primary mode of treatments are surgical and destructive (1). Basal cell carcinoma is often locally invasive and can compromise vital structures, such as the eye and nose, and is occasionally metastatic (2). In addition, studies on the treatment of basal cell carcinoma have been hindered by a paucity of cell lines from human patients. Indeed, the number of basal cell carcinoma cell lines currently used are far less than those derived from far less common malignancies, such as melanoma (3).
Despite the paucity of these lines, much progress has been made in determining the pathogenesis of basal cell carcinoma. Using a reverse genetic approach, dysregulation of the sonic hedgehog pathway, most commonly through deletion of the negative regulator of sonic hedgehog signaling, has been found to underlie the majority of basal cell carcinomas (4). The major genetic cause of basal cell carcinomas in humans, basal cell nevus syndrome, is caused by hemizygosity for patch. The role of the sonic hedgehog pathway in basal cell carcinoma has recently been elegantly demonstrated by the effect of a small molecule inhibitor in patients with metastatic basal cell carcinomas. Dramatic responses were noted, although breakthrough resistance was observed (2).
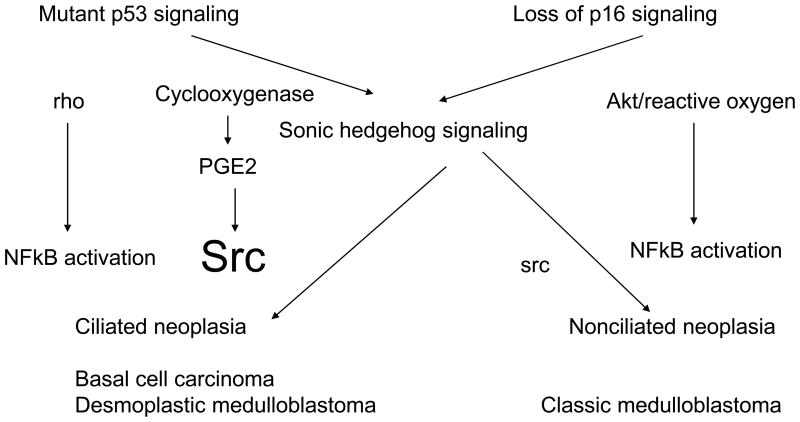
Overexpression of sonic hedgehog or loss of patch is by itself insufficient to cause the full blown spectrum of basal cell carcinoma (5, 6). Mice heterozygous for patch develop basal cell carcinomas after a course of radiation, and in humans, ultraviolet exposure or arsenic greatly enhances the development of basal cell carcinoma (7). Mutant p53 is extremely common in human basal cell carcinoma, and thus the combination of p53 dysfunction and sonic hedgehog activation accounts for some of the basal cell carcinoma phenotype. Sonic hedgehog does not require mutant p53, as it is often activated in medulloblastomas and pancreatic cancers that do not have mutant p53, but have loss of p16ink4a (8). Interestingly, two lines of evidence demonstrate that sonic hedgehog can signal through a p16ink4a/reactive oxygen pathway or a mutant p53/low reactive oxygen pathway (Figure 1). First, mice that constitutively express a dominant negative tuberin, when crossed into patched heterozygous mice, develop a high incidence of medulloblastoma, but do not develop basal cell carcinoma (9). The dominant negative allele of tuberin upregulates reactive oxygen, and the total lack of basal cell carcinoma supports the hypothesis that reactive oxygen may be protective against basal cell carcinoma (10). The second line of evidence is demonstrated by the effect of crossing mice defective in cilia into mice with activation of sonic hedgehog signaling. Loss of cilia prevents the development of basal cell carcinoma, and cilia are present in basal cell carcinoma of humans, but not in the types of medulloblastoma associated with elevated reactive oxygen (11).
Figure 1.
Differences in signaling in between tumors with mutant p53 vs loss of p16. Tumors with mutant p53 preferentially activate src through activation of PGE2-prostanoid signaling, while tumors with loss of p16 use reactive oxygen-NFkB signaling
In an elegant study, Tang and colleagues demonstrate the role of cyclooxygenase 1 and 2 in the development of basal cell carcinoma. They demonstrate that lack of either enzyme results in a very significant decrease in tumor size, but not in tumor number. Overexpression of cyclooxygenase conversely results in an increase in the size of basal cell carcinomas. Finally, treatment of patients with celecoxib, an oral inhibitor of cyclooxygenase 2, results in a trend towards decreased tumor burden in patients at high risk. Clearly, cyclooxygenase plays a role in basal cell carcinoma. However, two questions need to be answered. One, why is the suppression greater in mice than in humans. Second, given the cardiac risk factors of COX-2 inhibition, what is the optimal way to prevent and treat basal cell carcinoma in humans?
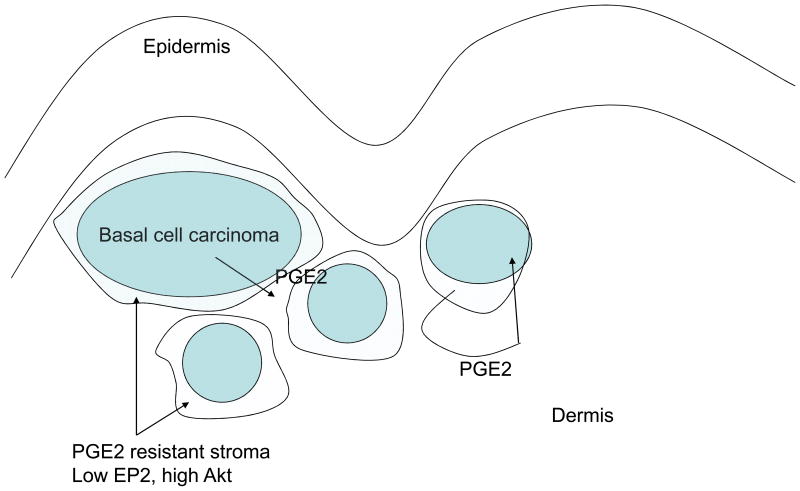
In examining the data, the tumor number appears to be similar in mice, regardless of the cyclooxygenase phenotype. This indicates that cyclooxygenase does not repair initial genetic mutations, but impairs tumor progression. It may do this by direct inhibition of tumor growth, increased apoptosis, or decreased angiogenesis. It is important to look at downstream events of cyclooxygenase to try to best use this information for prevention and treatment. Cyclooxygenases 1 and 2 both generate prostaglandin E2 (PGE2). PGE2 binds to one of 4 prostanoid receptors, and current data indicates that EP2 might play the most important role in cutaneous carcinogenesis. PGE2 signaling through prostanoid receptors activates a potentially pro-proliferative pathway, src, and an antiprolilferative pathway, cyclic AMP/CREB (12, 13). The ratio of src signaling to cyclic AMP/CREB signaling may differ between species and tissues. A recent study showed that normal fibroblasts undergo apoptosis in the presence of PGE2, but fibroblasts that are deficient in PTEN, and thus have increased activation of Akt, are resistant to PGE2 (14) (Figure 2). The implications of this is that continued PGE2 secretion from basal cell carcinomas could select for a stromal population having decreased PTEN/increased Akt that might provide support for basal cell carcinoma in vivo. Conversely, activation of cyclic AMP/CREB by PGE2 might limit the growth of neoplasias. Thus, the greater effect of cyclooxygenase inhibition in mice may be due to preferential inhibition of src mediated neoplasia in mice, while in humans, PGE2 mediated cyclic AMP/CREB signaling may play a greater role in EP receptor mediated signaling compared with mice.
Figure 2.
Model of role of PGE2 in basal cell carcinoma pathogenesis. Basal cell carcinoma cells produce PGE2, which selects for stromal cells that decrease expression of PTEN. These PTEN deficient stromal cells serve as support for the basal cell carcinoma. PGE2 also stimulates protumorigenic src and antitumorigenic cyclic AMP in the basal cell carcinoma itself.
What is the most rapid way to translate these findings to the clinic? The increased risk of cardiovascular events in patients taking cyclooxygenase makes us cautious about recommending widespread use of these drugs to prevent basal cell carcinoma in high risk patients. However, the studies of Tang lead us to two other possibilities that need further investigation. First, small molecules derived from plants have been shown to inhibit cyclooxygenase without cardiac toxicities. The reason for this is that these compounds inhibit other pathways that might compensate for the cardiac toxicities of pure cyclooxygenase inhibition. These compounds include curcumin, which has many activities, including angiogenesis inhibition, cyclooxygenase inhibition, and NFkB inhibition(15, 16). Another molecule recently shown to inhibit cyclooxygenase mRNA production is honokiol (17), which is systemically available, inhibits angiogenesis, and promotes apoptosis by inhibition of ras mediated phospholipase D activation, and promotion of mitochondrial apoptosis (18). Honokiol has been shown to have particular efficacy against tumors with mutant p53 (18).
The second approach is inhibition of src. As mentioned above, src is likely the major mediator of PGE2 mediated tumor promotion. One would predict that epidermal knockout of src, preferentially in an inducible fashion, would have a strong inhibitory effect on the development of basal cell carcinoma in mice. Src can be inhibited in two major ways. First, src has kinase activity, and src inhibitors are currently in clinical trial (19, 20). Second, src requires modification by the covalent addition of myristoyl moities by the enzyme N-myristoyltransferase 1, which permits membrane localization. Recently, we have discovered a small molecule, Tris (dibenzylideneacetone) dipalladium, which inhibits N-myristoyltransferase with submicromolar potency and inhibits the growth of melanoma in mice (21). Interestingly, mice heterozygous in N-myristoyltransferase 1 are viable and fertile, demonstrating that systemic inhibition of this pathway is a feasible target for chemoprevention (22).
Basal cell carcinoma remains a significant cause of morbidity and occasional mortality as the most common form of cancer in humans. Despite the inability to study these tumors in vitro using traditional NCI 60 cell line studies, a large amount of data has been obtained to point towards druggable targets. The findings from this study point towards combination therapy of cyclooxygenase-src activation and sonic hedgehog signaling, which may be synergistic in decreasing tumor burden in patients with or at high risk for basal cell carcinoma.
Acknowledgments
J.L. Arbiser was supported by a Veterans Administration Merit Award, NIH grant R01 AR02030, and grants from the Jamie Rabinowitch-Davis Foundation and the Minsk Foundation.
Footnotes
Conflicts of interest: Jack L Arbiser and Emory University are pursuing patents on honokiol and tris DBA palladium.
Reference List
- 1.Walling HW, Fosko SW, Geraminejad PA, Whitaker DC, Arpey CJ. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389–402. doi: 10.1023/B:CANC.0000031775.04618.30. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM, Lum BL, Darbonne WC, Marsters JC, Jr, de Sauvage FJ, Low JA. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 3.Grando SA, Schofield OM, Skubitz AP, Kist DA, Zelickson BD, Zachary CB. Nodular basal cell carcinoma in vivo vs in vitro. Establishment of pure cell cultures, cytomorphologic characteristics, ultrastructure, immunophenotype, biosynthetic activities, and generation of antisera. Arch Dermatol. 1996;132:1185–1193. doi: 10.1001/archderm.132.10.1185. [DOI] [PubMed] [Google Scholar]
- 4.Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH., Jr Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 5.Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3:788–792. doi: 10.1038/nm0797-788. [DOI] [PubMed] [Google Scholar]
- 6.Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Jr, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 7.Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–1291. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 8.Wetmore C, Eberhart DE, Curran T. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res. 2000;60:2239–2246. [PubMed] [Google Scholar]
- 9.Bhatia B, Northcott PA, Hambardzumyan D, Govindarajan B, Brat DJ, Arbiser JL, Holland EC, Taylor MD, Kenney AM. Tuberous sclerosis complex suppression in cerebellar development and medulloblastoma: separate regulation of mammalian target of rapamycin activity and p27 Kip1 localization. Cancer Res. 2009;69:7224–7234. doi: 10.1158/0008-5472.CAN-09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govindarajan B, Brat DJ, Csete M, Martin WD, Murad E, Litani K, Cohen C, Cerimele F, Nunnelley M, Lefkove B, Yamamoto T, Lee C, Arbiser JL. Transgenic expression of dominant negative tuberin through a strong constitutive promoter results in a tissue-specific tuberous sclerosis phenotype in the skin and brain. J Biol Chem. 2005;280:5870–5874. doi: 10.1074/jbc.M411768200. [DOI] [PubMed] [Google Scholar]
- 11.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnini S, Finetti F, Solito R, Terzuoli E, Sacchetti A, Morbidelli L, Patrignani P, Ziche M. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J. 2007;21:2418–2430. doi: 10.1096/fj.06-7581com. [DOI] [PubMed] [Google Scholar]
- 13.Brouxhon S, Kyrkanides S, O’Banion MK, Johnson R, Pearce DA, Centola GM, Miller JN, McGrath KH, Erdle B, Scott G, Schneider S, VanBuskirk J, Pentland AP. Sequential down-regulation of E-cadherin with squamous cell carcinoma progression: loss of E-cadherin via a prostaglandin E2-EP2 dependent posttranslational mechanism. Cancer Res. 2007;67:7654–7664. doi: 10.1158/0008-5472.CAN-06-4415. [DOI] [PubMed] [Google Scholar]
- 14.Sagana RL, Yan M, Cornett AM, Tsui JL, Stephenson DA, Huang SK, Moore BB, Ballinger MN, Melonakos J, Kontos CD, Aronoff DM, Peters-Golden M, White ES. Phosphatase and tensin homologue on chromosome ten (PTEN) directs prostaglandin E2-mediated fibroblast responses via regulation of E prostanoid 2 receptor expression. J Biol Chem. 2009 doi: 10.1074/jbc.M109.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arbiser JL, Klauber N, Rohan R, van LR, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 17.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-kappaB activation pathway. Mol Cancer Res. 2006;4:621–633. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 18.Garcia A, Zheng Y, Zhao C, Toschi A, Fan J, Shraibman N, Brown HA, Bar-Sagi D, Foster DA, Arbiser JL. Honokiol suppresses survival signals mediated by Ras-dependent phospholipase d activity in human cancer cells. Clin Cancer Res. 2008;14:4267–4274. doi: 10.1158/1078-0432.CCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 20.Sakamaki H, Ishizawa K, Taniwaki M, Fujisawa S, Morishima Y, Tobinai K, Okada M, Ando K, Usui N, Miyawaki S, Utsunomiya A, Uoshima N, Nagai T, Naoe T, Motoji T, Jinnai I, Tanimoto M, Miyazaki Y, Ohnishi K, Iida S, Okamoto S, Seriu T, Ohno R. Phase 1/2 clinical study of dasatinib in Japanese patients with chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol. 2009;89:332–341. doi: 10.1007/s12185-009-0260-2. [DOI] [PubMed] [Google Scholar]
- 21.Bhandarkar SS, Bromberg J, Carrillo C, Selvakumar P, Sharma RK, Perry BN, Govindarajan B, Fried L, Sohn A, Reddy K, Arbiser JL. Tris (dibenzylideneacetone) dipalladium, a N-myristoyltransferase-1 inhibitor, is effective against melanoma growth in vitro and in vivo. Clin Cancer Res. 2008;14:5743–5748. doi: 10.1158/1078-0432.CCR-08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrivastav A, Varma S, Lawman Z, Yang SH, Ritchie SA, Bonham K, Singh SM, Saxena A, Sharma RK. Requirement of N-myristoyltransferase 1 in the development of monocytic lineage. J Immunol. 2008;180:1019–1028. doi: 10.4049/jimmunol.180.2.1019. [DOI] [PubMed] [Google Scholar]