Abstract
Objective
To characterize total body bone mineral content (BMC) and total body and spinal bone mineral density (BMD) in perinatally HIV-infected and uninfected children/youth across puberty.
Design
HIV-infected (7–24 years) were randomly selected from six strata based on Tanner stage/protease inhibitor use. HIV-uninfected were frequency-matched by Tanner group and sociodemographic background to the HIV-infected.
Methods
Dual-energy X-ray absorptiometry (DXA) measured BMC and BMD. Linear regression models tested differences in bone outcomes by HIV and the interaction of HIV by Tanner group (1–2, 3–4, 5). Models were performed separately by sex and adjusted for DXA scanner, race/ethnicity, height, age and lean body mass.
Results
HIV-infected (N = 236) and uninfected (N = 143) were comparable on sex and race/ethnicity. HIV-infected were slightly older (median 12.6 versus 11.9 years). In adjusted models, HIV-infected males had significantly lower total body BMC and total body and spinal BMD at Tanner 5, lower BMC at Tanner 3–4 and similar BMC and BMD at Tanner 1–2, compared to HIV-uninfected males. HIV-infected and uninfected girls did not differ significantly on any bone outcome, but there was a marginally significant interaction of HIV and Tanner group for spinal BMD. Kaletra/ritonavir was associated with lower BMC and total body BMD and nevirapine was associated with higher spinal BMD in a model with all HIV-infected.
Conclusions
Perinatally HIV-infected males showed more evidence of lower bone density especially in the final stage of pubertal development than HIV-infected girls and they may be at increased risk for bone disease during adulthood.
Keywords: antiretroviral, bone mass, children, DXA, perinatal HIV infection, Tanner stage
Introduction
Numerous studies have found lower bone mass in children with perinatally acquired HIV infection compared to healthy children of similar age and sex [1–5]. Cause and fracture risk are poorly understood. Many factors associated with bone loss in children with other chronic inflammatory diseases [6–8] are prevalent among HIV-infected children [9–11] including delayed growth and puberty, low lean body mass (LBM), chronic inflammation, hormonal dysregulation (IGF), vitamin D deficiency, malabsorption and physical inactivity. Finally, specific antiretroviral medications may be deleterious to bone [12,13].
Peak bone mass is achieved by age 30, but approximately 80% is attained by age 18 [14–16]. Healthy prepubertal boys and girls have similar rates of growth and bone accrual. During puberty, hormones stimulate rapid increases in height, weight and LBM, which stimulate rapid bone accrual [16,17]. The greatest gains in bone mass at the spine and femoral hip occur between ages 11 and 14 at Tanner stages 2–4 in girls and between ages 13 and 17 at Tanner stage 4 in boys [14,15]. Two years after menarche, girls gain minimal bone mass and bone accrual slows dramatically after age 17 in boys. [14,15,17]. Peak bone mass is primarily genetically predetermined, but calcium and vitamin D intake and physical activity can increase peak bone mass and clinical factors can compromise it [18]. Deficits in bone accrual during adolescence may remain through adulthood [19] and increase the risk of osteoporosis and fragility fractures later in life [20].
Most perinatally HIV-infected children in the United States today have not yet reached peak bone mass. Many are transitioning through puberty. We characterized bone mass across pubertal stages in perinatally HIV-infected compared to uninfected children/youth with comparable sociodemographic status, controlling for differences by age, race/ethnicity, height and total LBM between groups. Our results have important clinical implications concerning timing of bone acquisition in infected children and Tanner stage at which they begin to lag behind age-matched healthy peers.
Methods
Selection of participants
HIV-infected
For this cross-sectional study, we used a stratified design to select a representative sample of HIV-infected (HIV-pos) children/youth aged 7–24 years across six strata defined by protease inhibitor use/nonuse and Tanner stage (Tanner 1, Tanner 2–3, Tanner 4–5) [21].
HIV-uninfected
For comparison, we enrolled 50 HIV-uninfected children/youth (HIV-neg) aged 7–24 years into each of three Tanner strata with similar overall distribution of sex and race/ethnicity as the HIV-pos [21]. They were recruited at the same sites as the HIV-infected children/youth. The study was approved by the institutional review board at each site and informed consent was obtained from each participant (>18 years old) or their parent or guardian before enrollment.
Measurements
Anthropometry
Height and weight were measured according to standard techniques [21].
Tanner stage
Tanner stage was determined for breasts and/or pubic hair for females and genitalia and/or pubic hair for males by inspection. Tanner staging was assessed by certified pediatric medical practitioners who regularly assess growth and development in HIV-infected children. When both Tanner measurements were available, the most advanced level was used. Both measures were done on 76% of males and 72% of females. Growth velocity is greatest at Tanner 3 and 4, so Tanner stage was categorized into three groups (Tanner 1–2, Tanner 3–4 and Tanner 5) for this analysis, hereafter called ‘Tanner group’.
Laboratory
CD4 T-cell counts and plasma HIV-1 RNA levels (Monitor UltraSensitive Assay, Roche Molecular Diagnostics, Branchberg, New Jersey, USA) were measured at each site in laboratories certified by the Division of AIDS Virology and Immunology Quality Assurance Program.
Clinical history
Data were collected on clinical events, family history and current and lifetime use of individual antiretroviral drugs and CDC Disease Classification through chart review and interview.
Dietary intake and lifestyle
Average intake of specific foods and supplements over the past year was determined using the Block Food Frequency Questionnaire (NutritionQuest, Berkeley, CA). Vitamin D and calcium from food and supplements were categorized into ‘at or above’ or ‘below’ the dietary reference intake (DRI) for age and sex [22]. We assessed TV watching and cigarette smoking on the Block questionnaire and frequency of regular exercise in an additional questionnaire.
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) scans were performed on the whole body, to assess whole body bone mineral content (BMC), bone mineral density (BMD), lean body mass (LBM) and adiposity (total body, trunk, extremities), and on the lumbar spine (L1–L4). The Body Composition Analysis Center at Tufts University School of Medicine analyzed all scans to standardize measurements across sites. A phantom was circulated and scanned at each clinical site.
Statistical methods
The primary goal of this analysis was to compare each bone outcome (total body BMC and BMD, spinal BMD) in HIV-pos compared with HIV-neg children/youth in each Tanner group.
Comparison of baseline characteristics between HIV-pos and HIV-neg
Groups were compared using Fisher's exact test for categorical variables and Wilcoxon rank sum tests for continuous variables. Median age at puberty initiation was estimated using Turnbull's algorithm [23] and HIV-pos and HIV-neg were compared within each sex using an exponential survival model [24].
Comparison of bone outcomes between HIV-pos and HIV-neg across Tanner groups
For each outcome, we graphed observed (unadjusted) means [95% confidence intervals (CIs)] and calculated differences in means between HIV-pos and HIV-neg in each Tanner group, separately by sex. We then fit two sets of multiple linear regression models (‘partially adjusted’, ‘fully adjusted’) for each outcome to test for differences between HIV-pos and HIV-neg across Tanner group (Tanner by HIV interaction). Race/ethnicity and four groups of DXA scanners (Hologic Delphi, Hologic 1000, Hologic 4500/Discovery, Hologic Inc, Bedford, Massachusetts, USA) or Lunar (DPX IQ/XL/Prodigy/Prodigy DF/Prodigy PA, General Electric Healthcare, UK) were included in all models regardless of statistical significance because of strong associations with BMC and BMD. The ‘partially adjusted’ models included main effects for DXA scanner, race/ethnicity, HIV and Tanner group, and the interaction of HIV and Tanner group. The ‘fully adjusted’ models included all variables in the ‘partially adjusted’ models, and age, height, and total LBM. We screened potential confounders, including adiposity (total body fat, trunk fat, trunk-to-extremity fat ratio), calcium and vitamin D intake, daily exercise and TV hours. If the interaction term between HIV and Tanner was not significant, it was dropped from the model. Models for males and females were fit separately because of meaningful differences by sex across Tanner groups. For each model, residuals were evaluated for evidence of nonlinearity of the outcome with height, LBM, and age. One unduly influential data point (studentized residual >4) was deleted from final models. In ‘fully adjusted’ models we evaluated differences between HIV-pos and HIV-neg within each Tanner group by estimating the predicted mean outcome at the median age, height and LBM of HIV-pos and HIV-neg separately. We then calculated the difference (95% CI) between HIV-pos and HIV-neg within each Tanner group. We repeated the above analysis for the lumbar spine using bone mineral apparent density (spinal BMAD) calculated from the formula by Katzman et al. [25].
Antiretroviral use and bone outcomes in HIV-pos
To evaluate the influence of selected antiretroviral drugs on each outcome in the HIV-pos, we started with all variables in the ‘fully adjusted’ model described above, without the HIV term. We also included sex and current HIV-1 RNA level (log10 copies/ml) and lowest lifetime CD4 cell count (<200, 200–500, ≥500 cells/μl). We combined males and females because of limited power and no a priori hypothesis about differential effects of antiretroviral drugs by sex. We then fit two models for each outcome that included the above variables and 1) indicators for current use of each antiretroviral class (NRTI, NNRTI and protease inhibitor); or 2) current agents within each antiretroviral class [NRTI: zidovudine, didanosine, lamivudine, stavudine, and other NRTIs; NNRTI: nevirapine and efavirenz; protease inhibitor: nelfinavir, ritonavir (alone or Kaletra) and other protease inhibitors]. In the latter models each drug was evaluated individually, including an indicator for other drugs in that drug class. Forward stepwise regression was used starting with the most statistically significant individual drug and adding other individual drugs in its class and other classes one at a time, only keeping individual drugs in the model if significant at P <0.10. If no individual agents in a class were significant then an indicator for that class remained in the model. If no individual agent was significant in any class, the model would only contain indicators for NRTI, NNRTI or protease inhibitor use (i.e. the same as in set 1). We repeated the above analyses with cumulative time on each class of antiretroviral and individual antiretroviral drug. All analyses were performed in SAS Version 9.1 (SAS Institute, Cary, North Carolina, USA).
Results
Demographic, nutrition and body composition in HIV-pos and HIV-neg
Of 386 children/youth who completed P1045, 379 (143 HIV-neg and 236 HIV-pos) had readable DXA. Seven had no DXA [not ambulatory (4), too heavy for machine (2), no result (1)].
HIV-pos and HIV-neg participants did not differ by sex, race/ethnicity or Tanner group (Table 1). However, the HIV-pos were slightly older. HIV-pos were more likely to take calcium and vitamin D supplements and less likely to be below the DRI for these nutrients. HIV-pos children/youth had significantly lower sex-age adjusted height, weight and BMI Z-scores with greater impairment in HIV-pos males than HIV-pos females compared to their HIV-neg counterparts. There were no differences within HIV-pos and HIV-neg by sex in dietary intake, supplement use, or height, weight or CDC Z-scores (not shown).
Table 1. Baseline characteristics of HIV-infected and HIV-uninfected participants.
| Characteristic | Level | HIV-pos (n = 236) | HIV-neg (n = 143) | P value |
|---|---|---|---|---|
| Sex (N, %) | Male | 124 (52.5) | 83 (58.0) | 0.338a |
| Race/ethnicity (N, %) | White/Other | 31 (13.1) | 20 (14.0) | 0.977a |
| Black non-Hisp. | 129 (54.7) | 78 (54.5) | ||
| Hispanic | 76 (32.2) | 45 (31.5) | ||
| Tanner stage (N, %) | 1–2 | 117 (49.6) | 82 (57.3) | 0.270a |
| 3–4 | 71 (30.1) | 33 (23.1) | ||
| 5 | 48 (20.3) | 28 (19.6) | ||
| Age (median) | 12.6 | 11.9 | 0.028b | |
| Estimated median age (years) at initiation of puberty | Male | 11.2 | 10.3 | 0.080c |
| Female | 9.8 | 9.5 | 0.721c | |
| Exercise regularly (N, %) | No | 98 (41.9) | 57 (41.9) | 0.261b |
| 1–6 h/week | 54 (23.1) | 41 (30.1) | ||
| 1–3 h/day | 46 (19.7) | 31 (22.8) | ||
| >3 h/day | 36 (15.4) | 7 (5.1) | ||
| Hours TV (N, %) | 1–6 h/week | 24 (10.4) | 14 (10.0) | 0.661b |
| 1–3 h/day | 130 (56.5) | 77 (55.0) | ||
| >3 h/day | 76 (33.0) | 49 (35.0) | ||
| Smoking (N, %) | Yes | 7 (3.0) | 1 (0.7) | 0.268a |
| Calories (kcal/kg) (median) | 58.9 | 58.0 | 0.294b | |
| Protein (g/kg) (median) | 2.0 | 1.9 | 0.301b | |
| Fat (g/kg) (median) | 2.5 | 2.3 | 0.270b | |
| Carbohydrate (g/kg) (median) | 7.5 | 7.2 | 0.485b | |
| Calcium intake (mg) (median) | 1114 | 1017 | 0.109b | |
| % Below DRI for age and sex | 56.5 | 55.6 | 0.914a | |
| % Taking calcium supplements | 40.4 | 19.7 | <0.001a | |
| Vitamin D intake (IU) (median) | 345 | 229 | <0.001b | |
| % Below DRI for age and sex | 30.4 | 46.5 | 0.002a | |
| % Taking vitamin D supplements | 40.4 | 18.3 | <0.001a | |
| Height CDC z-score (median) (10th, 90th percentile) | ||||
| Male | −0.4 (−1.9, 0.7) | 0.3 (−1.0, 1.4) | <0.001b | |
| Female | −0.4 (−1.8, 1.0) | 0.0 (−1.3, 1.2) | 0.028b | |
| Weight CDC z-score (median) (10th, 90th percentile) | ||||
| Male | −0.1 (−1.3, 1.7) | 0.6 (−0.7, 2.4) | <0.001b | |
| Female | 0.2 (−1.1, 1.8) | 0.7 (−1.0, 2.1) | 0.029b | |
| BMI CDC z-score (median) (10th, 90th percentile) | ||||
| Male | 0.2 (−1.0, 1.8) | 0.6 (−0.5, 2.2) | 0.004b | |
| Female | 0.5 (−0.7, 1.7) | 0.8 (−0.8, 2.1) | 0.193b |
DRI, dietary reference intake.
Fisher's exact test excluding missing.
Wilcoxon rank sum test.
Exponential survival test.
Markers of disease severity and antiretroviral use in HIV-pos
Most HIV-pos were relatively healthy with CD4 cell counts at least 500 cells/μl (71%), CD4% at least 25% (73%) and HIV-1 RNA 400 copies/ml or less (56%) (Table 2). Some had past severe disease (40% CDC B and 9% CDC C).
Table 2. HIV disease severity and current use of individual antiretroviral drugs.
| Characteristic Disease severity |
Level | HIV-pos (N = 236) | |
|---|---|---|---|
| n | % | ||
| CD4 cell count (cells/μl) (N, %) | <200 | 6 | 2.6 |
| 200–<500 | 61 | 26.4 | |
| ≥500 | 164 | 71.0 | |
| Median (10th, 90th %le) | 665 | (339, 1034) | |
| CD4% (N, %) | <15% | 14 | 6.1 |
| 15–<25% | 49 | 21.2 | |
| ≥ 25% | 168 | 72.7 | |
| Median (10th, 90th %le) | 30 | (18, 41) | |
| HIV-1 RNA (copies/ml) (N, %) | ≤400 | 128 | 55.9 |
| Median (10th, 90th %le) | <400 | (<400, 12801) | |
| CDC disease stage (N, %) | N/A | 98 | 41.5 |
| B | 94 | 39.8 | |
| C | 44 | 18.6 | |
| Nadir CD4 cell count (cells/μl) | |||
| Median (10th, 90th %le) | 353 (56, 703) | ||
| Zenith HIV-1 RNA (copies/ml) | |||
| Median (10th, 90th %le) | 99518 (11900, >750000) | ||
| Antiretroviral medication | % | Median (years) | |
| Any NRTI | 98.3 | 8.1 | |
| Stavudine (d4T) | 47.5 | 5.8 | |
| Didanosine (ddI) | 32.6 | 5.1 | |
| Lamivudine (3TC) | 51.7 | 5.4 | |
| Zidovudine (ZDV) | 33.1 | 6.2 | |
| Tenofovir (TDF) | 5.9 | 1.6 | |
| Other NRTIa | 19.1 | 2.3 | |
| Any NNRTI | 36.9 | 4.4 | |
| Nevirapine (NVP) | 14.4 | 5.6 | |
| Efavirenz (EFZ) | 22.5 | 3.1 | |
| Any PI | 66.9 | 5.8 | |
| Kaletra (KAL) | 21.2 | 2.4 | |
| Nelfinavir (NFV) | 26.7 | 5.8 | |
| Ritonavir (RTV) | 17.8 | 5.6 | |
| Other PIb | 13.6 | 3.3 | |
Includes abacavir (n = 24), ddC (n = 1) and others (n = 9).
Includes saquinavir (n = 15), indinavir (n = 5), amprenavir (n = 7) and others (n = 8).
Table 2 shows percentage of HIV-pos currently using each antiretroviral drug and median years of continuous use to enrollment. Forty-two (18%) participants were currently receiving NRTIs alone, 36 (15%) NRTI/NNRTI combinations, 105 (44%) NRTI/protease inhibitor combinations, 47 (20%) NRTI/NNRTI/protease inhibitor combinations and six (2%) received other combinations including more than one protease inhibitor (not shown). All HIV-pos had received NRTIs, whereas 56% ever received NNRTIs and 67% ever received protease inhibitors, for a median of 9.1, 3.1 and 6.3 years of lifetime use, respectively (not shown).
Comparisons between HIV-pos and HIV-neg in bone outcomes
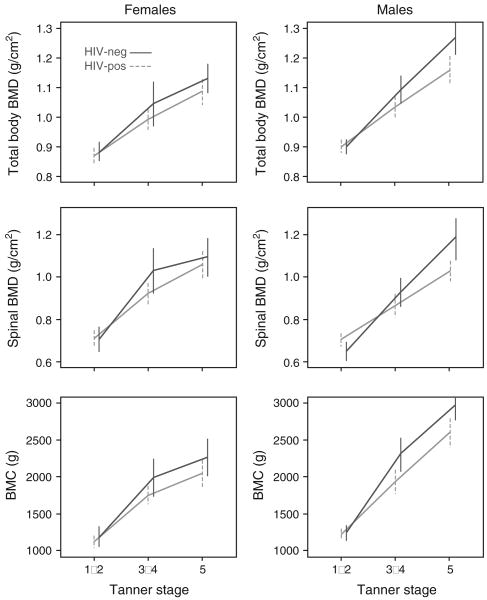
No potential confounders were significant at P <0.10 in ‘fully adjusted’ models for any outcomes in males or females and are not considered further. For each outcome, observed means (95% CI) for HIV-pos and HIV-neg in each sex and Tanner group are shown in Fig. 1 and observed (unadjusted) and adjusted differences (95% CI) in means are in Table 3.
Fig. 1. Observed (unadjusted) mean BMC and BMD (95% confidence interval) by HIV status and Tanner stage.
BMC, bone mineral content; BMD, bone mineral density.
Table 3. Differences between HIV-pos and HIV-neg by Tanner stage.
| Tanner | Observed (unadjusted) difference HIV-neg – HIV-pos mean (95% CI) | Model-predicted (adjusted) difference HIV-neg – HIV-pos mean (95% CI) | ||
|---|---|---|---|---|
| Malea | Total BMD (g/cm2) | 1–2 | −0.002 (−0.032, 0.028) | 0.001 (−0.047, 0.050) |
| 3–4 | 0.059 (−0.000, 0.117) | 0.047 (−0.010, 0.103) | ||
| 5 | 0.105 (0.035, 0.176)*b | 0.099 (0.038, 0.160)*c | ||
| Spinal BMD (g/cm2) | 1–2 | −0.055 (−0.111, 0.000) | −0.035 (−0.111, 0.042) | |
| 3–4 | 0.063 (−0.023, 0.148) | 0.015 (−0.074, 0.104) | ||
| 5 | 0.148 (0.036, 0.259)* | 0.133 (0.037, 0.228)* | ||
| BMC (g) | 1–2 | 3 (−116, 122) | 17 (−111, 145) | |
| 3–4 | 366 (92, 641)* | 310 (161, 459)* | ||
| 5 | 372 (75, 670)* | 352 (192, 512)* | ||
| Femaleb | Total BMD (g/cm2) | 1–2 | 0.012 (−0.028, 0.053) | 0.015 (−0.031, 0.060) |
| 3–4 | 0.051 (−0.029, 0.132) | 0.016 (−0.036, 0.069) | ||
| 5 | 0.044 (−0.024, 0.112) | 0.055 (0.002, 0.107)* | ||
| Spinal BMD (g/cm2) | 1–2 | −0.003 (−0.072, 0.067) | 0.004 (−0.065, 0.073) | |
| 3–4 | 0.102 (−0.015, 0.219) | 0.012 (−0.068, 0.091) | ||
| 5 | 0.038 (−0.072, 0.149) | 0.061 (−0.019, 0.141) | ||
| BMC (g) | 1–2 | 57 (−104, 218) | 56 (−79, 192) | |
| 3–4 | 231 (−46, 508) | 107 (−50, 263) | ||
| 5 | 208 (−105, 522) | 266 (109, 423)* |
Model: HIV, Tanner, HIV*Tanner, race/ethnicity, DXA machine, height, age, lean body mass.
Model: HIV, Tanner, race/ethnicity, DXA machine, height, age, lean body mass.
Asterisk (*) if 95% confidence interval does not cover ‘0’.
Males
At Tanner 1–2, there were no significant differences in any outcome between HIV-pos and HIV-neg. At Tanner 3–4, BMC was significantly lower in HIV-pos. At Tanner 5, total body and spinal BMD and total body BMC were all significantly lower in HIV-pos relative to HIV-neg (unadjusted). In the partially adjusted linear regression model, there were at least marginally significant interactions between HIV and Tanner group for all outcomes (total BMD: P = 0.065; spinal BMD: P = 0.018; total BMC: P = 0.068). In fully adjusted models, the interaction term between HIV and Tanner group was statistically significant (P < 0.011) for all outcomes. Similar to the unadjusted differences, HIV-pos males had significantly lower scores than HIV-neg males at Tanner 5 for total body and spinal BMD and total BMC in fully adjusted models. For BMC, HIV-pos also had significantly lower predicted mean outcomes than HIV-neg at Tanner 3–4. There were no differences at Tanner 1–2. For all three outcomes, the magnitude of the observed and estimated differences between HIV-pos and HIV-neg increased with Tanner group.
Females
No outcomes differed significantly between HIV-pos and HIV-neg in any Tanner group in unadjusted comparisons. In partially adjusted regression models there were no statistically significant interactions between HIV and Tanner group (P > 0.113) for any outcome. Total BMC was marginally significantly lower in HIV-pos (P = 0.045). In fully adjusted models, there was a marginally significant interaction of HIV and Tanner group for spinal BMD (P = 0.078), but no significant interaction in models for total BMC and BMD. In fully adjusted models with no interaction term, HIV was not a significant predictor of any outcome. As with males, the magnitude of the estimated differences between HIV-pos and HIV-neg increased with Tanner group, but the magnitude of predicted difference at Tanner 3–4 and 5 was greater in males than females.
Addition of variables for adiposity did not change the results among males or females and were not included in any of the final models. In addition, spinal BMAD yielded the same conclusions as spinal BMD. These results are not shown.
Influence of antiretroviral use in HIV-pos
In models with an indicator variable for each antiretroviral class, NNRTI use was significantly associated with higher BMC (62 g; 95% CI 1–122; P = 0.047) and higher spinal BMD (0.039 g/cm2; 95% CI 0.006–0.072; P = 0.021) compared to no NNRTI use. Protease inhibitors and NRTIs were not associated with any outcome at P < 0.10. In the final models (Table 4) evaluating the effect of individual antiretroviral drugs, Kaletra/ritonavir was associated with lower BMC and total body and spinal BMD. ZDV was also associated with marginally lower BMC but not other outcomes. In contrast, NVP was associated with higher BMC and spinal BMD but not total BMD. The results were consistent when antiretroviral class and individual agents were modeled as cumulative time on treatment. There was no strong trend for greater spinal BMD or total BMC with increasing time on NNRTIs.
Table 4. Antiretroviral medication and bone outcomesa,b.
| Antiretroviral type or specific agent Yes versus no | Total body BMC (g) | Total body BMD (g/cm2) | Spinal BMD (g/cm2) | |||
|---|---|---|---|---|---|---|
| All | All | All | ||||
| Est | P value | Est | P value | Est | P value | |
| PI | 0.8 (−55.3, 56.9) | 0.977 | 0.007 (−0.013, 0.278) | 0.475 | 0.013 (−0.017, 0.043) | 0.402 |
| Kaletra/ritonavir | −83.6 (−144.4, −22.8) | 0.008 | −0.033 (−0.056, −0.011) | 0.004 | −0.028 (−0.061, 0.005) | 0.094 |
| NNRTI | 0.009 (−0.013, 0.032) | 0.418 | ||||
| NVP | 67.6 (−11.9, 147.3) | 0.097 | 0.045 (0.003, 0.087) | 0.039 | ||
| EFV | 24.7 (−47.0, 96.4) | 0.501 | 0.022 (−0.017, 0.061) | 0.275 | ||
| NRTI | −28.9 (−199.2, 141.4) | 0.740 | −0.038 (−0.114, 0.038) | 0.329 | −0.070 (−0.181, 0.042) | 0.222 |
| Zidovudine | −52.7 (−109.8, 4.4) | 0.072 | ||||
Models adjusted for DXA machine type, sex, race/ethnicity, Tanner group, height, age and total lean body mass, lowest CD4 cell count, log10 HIV-1 RNA.
When only the class variable was included in the model, then there were no significant effects of individual drugs in that class.
Discussion
Highly active antiretroviral therapy (HAART) has improved the health and survival of perinatally HIV-infected children, thus many are now entering adolescence. Despite improved general health, HIV-infected children are likely to have persistent deficits in growth and be at risk for delayed puberty [9] and decreased bone mass [2,4,26,27]. The timing of these deficits during puberty has not been well studied, especially in the HAART era. We evaluated bone mass across stages of puberty in a randomly selected group of perinatally HIV-infected compared to uninfected children/youth of similar Tanner stage and sociodemographic status. Our most striking finding was that HIV-infected boys had significantly lower spinal and total body bone mass relative to uninfected boys and that difference was more pronounced with advancing puberty. The trend was similar in girls, but the difference between the infected and uninfected was smaller and not statistically significant. A secondary finding was that those receiving ritonavir, with or without lopinavir, had lower bone mass at the spine and total body and those receiving ZDV had lower total body bone mass. In contrast, nevirapine users tended to have higher bone mass at both sites.
To our knowledge, ours is the first study to report differences in bone mass between HIV-infected and uninfected children/youth across Tanner stage and by sex. Others adjusted for these factors. Our findings are consistent with dimorphic trends in bone density, structure and strength observed in healthy adolescent girls and boys [28]. As long bones increase in length, bone formation beneath the outer envelope (or periosteum) widens the skeletal shaft. Simultaneously, removal and replacement of bone along the inner envelope (endocortical component) establishes a medullary canal. Periosteal apposition generally exceeds endocortical resorption in young children and enlarging long bones thereby develop an increasingly thick cortex. This remodeling is similar in both sexes until puberty when sexual dimorphism occurs [28,29]. In girls, estrogen inhibits periosteal bone formation and limits growth of the skeletal diameter and promotes bone formation along the endocortical surface, ultimately narrowing a bone's inner diameter [30]. In boys, androgens secreted during puberty increase periosteal apposition, bone diameter, and cortical thickness. As a result, men have much larger, denser bones on average than women. Although speculative, in the current study, hormonal changes could have exaggerated the dimorphic changes in bone density that we observed.
Interestingly, dimorphic differences in bone loss are observed in HIV-infected adults. Two studies reported a higher prevalence of osteopenia in HIV-infected men compared with HIV-infected women [31,32]. Jacobson et al. [33] observed that HIV-infected adult men tended to have greater loss in bone mass over follow-up compared to premenopausal women, whereas postmenopausal women had greater losses than both men and premenopausal women. The reasons for this apparent sexual dimorphism have yet to be identified.
In our study, HIV-pos and HIV-neg boys and girls at Tanner stages 1–2 did not differ significantly for any skeletal outcome. This contrasts with the findings of Arpadi et al. [1] in which prepubertal perinatally HIV-infected children had lower total body BMC compared to uninfected children of similar age and sex, but different race/ethnicity. There are two potential reasons why our findings may differ. By design our comparison group was not matched on age but selected within the same Tanner groups as the HIV-pos. In fact, within Tanner 1–2, our HIV-infected were somewhat older than the uninfected (approximately 2 years for boys and more than 11/2 years older for girls) and may reflect delayed puberty [9,34]. Thus, prior to the adolescent growth spurt, our HIV-infected boys and girls may have acquired a similar amount of bone mass as the uninfected, but at a later age. Also, we enrolled participants in later years than Arpadi et al. (2004–2005 versus 1995–2000). Our HIV-infected children may have been treated with antiretroviral drugs younger, which may have had a positive impact on bone acquisition.
Whereas we did not observe differences in bone mass at Tanner 1–2 in either boys or girls, there were pronounced differences at later Tanner stages in boys. A few studies observed greater differences between HIV-infected and uninfected children/youth in older versus younger children while not specifically looking by sex or Tanner stage. Among HIV-infected girls aged 5–15 years old, O'Brien et al. [2] noted that total body BMC was further below the normal curve in older compared to younger children. Mora et al. [3] found lower spinal and total body BMD in the HIV-infected children compared to uninfected children aged 6.3–17.7 years. The groups were of similar age within each Tanner stage. Over a 12-month follow-up, the actual rate of increase in spinal BMD among the HIV-infected was similar to that estimated for HIV-uninfected. In contrast, the rate of increase in total body BMD was slower in the HIV-infected.
The effect of antiretroviral drugs on bone loss is inconsistent across studies. In our study, children receiving ritonavir had lower BMD and BMC. In a meta-analysis of adult cross-sectional studies [35], protease inhibitor-treated patients had a higher prevalence of low BMD (osteopenia or osteoporosis) than non-protease inhibitor-treated. In contrast, several longitudinal studies have not demonstrated greater losses in bone mass over time in protease inhibitor-treated patients. [36–40]. However, individual protease inhibitors may differ in their effect. In vitro, ritonavir was an inhibitor of osteoclast differentiation [41], which would be expected to protect bone mass [42]. We observed somewhat lower total body BMC among participants receiving ZDV. Jacobson et al. [33] observed greater total body bone loss in HIV-infected adults on ddI. Pan et al. [43] reported that ZDV was associated with increased osteoclastogenesis in vitro and decreased BMD in mice, and van Vonderen et al. [44] observed greater loss of BMD in the spine and femur in adult men receiving ZDV and 3TC compared to nevirapine, with a backbone of Kaletra/ritonavir in both groups. Other studies support our finding of NNRTI and especially nevirapine use and greater bone mass in perinatally infected children [4]. Nevirapine may have protective effects on bone, be a marker for better control of underlying disease or mitigate the effects of protease inhibitor treatment [44]. Only 5.9% of children/youth in our study were receiving tenofovir, a drug associated with bone loss in adults [33,45] and children [12].
Dual-energy X-ray absorptiometry is the standard clinical method to screen for low bone mass in children, adolescents, and adults [16]. DXA is preferred over axial computed tomography (CT) because it delivers lower radiation and well established normative data exist for children over 4 years up to adulthood [16]. DXA measures BMC and the projected surface area from which areal bone density, a two-dimensional measure, is calculated (in g/cm2), whereas CT provides a three-dimensional, volumetric bone density measure in g/cm3. A limitation of DXA is that measurements may be confounded by bone size as the anteroposterior diameter of bone is not evaluated, resulting in systemic underestimation of volumetric BMD for age in children with impaired growth and pubertal development who may have smaller bones [16]. By DXA, Pitukcheewanont et al. [46] found lower total body and spinal BMC and BMD in HIV-infected compared with matched uninfected children. In contrast, using CT these two groups did not differ on bone measures, but vertebral cross-sectional area, height and volume were lower in the HIV-infected. Thus, our finding of lower BMC and BMD during puberty in our HIV-infected compared to uninfected may be somewhat exaggerated on DXA because we could not account for bone size. However, we carefully adjusted for height and LBM which could partially explain body size and are positively correlated with bone mass. In addition, we evaluated differences across Tanner stages, not assuming a similar age between HIV groups at each Tanner stage. Finally, we repeated our analysis using spinal BMAD to account for bone size and our findings did not change [25].
Our HIV-infected and uninfected were well matched by sex, race and Tanner stage and sociodemographics which may decrease unmeasured confounding. However, our results may not represent all perinatally infected children and our estimates may be biased because some children/youth chose not to enroll, although we found no systematic reason for nonparticipation [21]. In the future, use of peripheral computed tomography (pQCT), which measures volumetric BMD and assesses skeletal dimensions [47], will improve our understanding of skeletal sequalae of this disease and associated therapies.
Future studies are needed to understand the cause of differences in HIV-infected compared to uninfected children/youth with measurement of hormones and other circulating mediators. Longitudinal studies that examine changes within individuals will help to clarify the effect of puberty and sex on bone acquisition and inform the design of interventions to improve bone accrual and prevent skeletal losses in HIV-infected patients.
Acknowledgments
All authors were involved in the design phase of the study and contributed intellectually to writing the manuscript. J.C.L., D.L.J., G.M.A. K.M. and C.M.G. participated during the analytic phase. This work was supported by 5U01A1068616 (D.L.J., J.C.L.) and 1 U01 AI068632-01 (G.M.A.).
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
The authors would like to thank the children who participated in this study, their families and the entire protocol 1045 team for their contributions and support. We would also like to thank Barb Heckman for outstanding support. The following sites and individuals have contributed to this study: Children's Hospital of Chicago; Tulane University School of Medicine, Charity Hospital of New Orleans: M. Silio, T. Alchediak, C. Borne, S. Bradford; SUNY Health Science Center, Stony Brook: S. Nachman, D. Ferraro, J. Perillo, S. Muniz; University of Puerto Rico; Harlem Hospital; NYU Medical Center/Bellevue; City Hospital at San Juan; New Jersey Medical School; Jacobi Medical Center; St. Jude Children's Hospital: M. Donohoe, N. Patel, S. Kaste, J. Utech; Boston Children's Hospital; University of North Carolina at Chapel Hill; University of South Florida Physicians Group; The Children's Hospital, University of Colorado, Denver: E. Barr, J. Maes, B. McFarland, S. Paul, Grant Number MO1 RR00069, GCRC Program, National Center for Research Resources, NIH; Medical College of Georgia; Duke University Medical Center: J. Hurwitz, J. Simonetti, M. Donnelly, C. Mathison; Texas Children's Hospital/Baylor; University of California San Francisco Medical Center and PCRC (RR001271); Yale University School of Medicine: W. Andiman, L. Hurst, S. Romano; Los Angeles County Medical/University of Southern California; Lincoln Medical and Mental Health; University of Rochester: G. Weinberg, B. Murante, S. Laverty; Metropolitan Hospital Center; Long Beach Memorial Medical Center; Johns Hopkins University Hospital; UCSD Medical Center; Children's Hospital at SUNY Downstate; Bronx-Lebanon Hospital; Harbor General – UCLA Medical Center; Children's National Medical Center – D.C.: D. Dobbins, T. Peron, D. Wimbley, H. Spiegel; Children's Diagnostic and Treatment Center of South Florida; Robert Wood Johnson University Hospital; Howard University Hospital; University of Florida at Gainesville; Mt. Sinai Hospital Medical Center, Women's and Children's HIV Program; SUNY Health Science Center, Syracuse; University of Alabama, Birmingham School of Medicine: R. Pass, M. Crain, N. Beatty, H. Charlton.
The study was supported by the National Institutes of Health (NIH).
Footnotes
The data were presented at the 10th Workshop on Adverse Drug Reactions and Lipodystrophy. London, England, November 2008 (oral abstract).
References
- 1.Arpadi SM, Horlick M, Thornton J, Cuff PA, Wang J, Kotler DP. Bone mineral content is lower in prepubertal HIV-infected children. J Acquir Immune Defic Syndr. 2002;29:450–454. doi: 10.1097/00126334-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien KO, Razavi M, Henderson RA, Caballero B, Ellis KJ. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr. 2001;73:821–826. doi: 10.1093/ajcn/73.4.821. [DOI] [PubMed] [Google Scholar]
- 3.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DL, Spiegelman D, Duggan C, Weinberg GA, Bechard L, Furuta L, et al. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr. 2005;41:339–346. doi: 10.1097/01.mpg.0000174468.75219.30. [DOI] [PubMed] [Google Scholar]
- 5.Amorosa V, Tebas P. Bone disease and HIV infection. Clin Infect Dis. 2006;42:108–114. doi: 10.1086/498511. [DOI] [PubMed] [Google Scholar]
- 6.Burnham JM, Shults J, Semeao E, Foster B, Zemel BS, Stallings VA, et al. Whole body BMC in pediatric Crohn disease: independent effects of altered growth, maturation, and body composition. J Bone Miner Res. 2004;19:1961–1968. doi: 10.1359/JBMR.040908. [DOI] [PubMed] [Google Scholar]
- 7.Burnham JM, Shults J, Dubner SE, Sembhi H, Zemel BS, Leonard MB. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum. 2008;58:2518–2527. doi: 10.1002/art.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Martino M, Tovo P, Galli L, Gabiano C, Chiarelli F, Zappa M, et al. Puberty in perinatal HIV-1 infection: a multicentre longitudinal study of 212 children. AIDS. 2001;15:1527–1534. doi: 10.1097/00002030-200108170-00010. [DOI] [PubMed] [Google Scholar]
- 10.Miller TL, Evans SJ, Orav EJ, McIntosh K, Winter HS. Growth and body composition in children infected with the human immunodeficiency virus-1. Am J Clin Nutr. 1993;57:588–592. doi: 10.1093/ajcn/57.4.588. [DOI] [PubMed] [Google Scholar]
- 11.Chantry CJ, Byrd RS, Englund JA, Baker CJ, McKinney RE. Pediatric AIDS Clinical Trials Group Protocol 152 Study Team. Growth, survival and viral load in symptomatic childhood human immunodeficiency virus infection. Pediatr Infect Dis J. 2003;22:1033–1038. doi: 10.1097/01.inf.0000100575.64298.bc. [DOI] [PubMed] [Google Scholar]
- 12.Gafni RI, Hazra R, Reynolds JC, Maldarelli F, Tullio A, DeCarlo E, et al. Decreased bone density (BMD) in HIV-infected children treated with tenofovir disoproxil fumarate (TDF)-containing HAART. Pediatr Res. 2004;55:329A–1329A. [Google Scholar]
- 13.Fernandez-Rivera J, Garcia R, Lozano F, Macias J, Garcia-Garcia JA, Mira JA, et al. Relationship between low bone mineral density and highly active antiretroviral therapy including protease inhibitors in HIV-infected patients. HIV Clin Trials. 2003;4:337–346. doi: 10.1310/4X0H-UVMJ-BHYW-CPFB. [DOI] [PubMed] [Google Scholar]
- 14.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 15.Rizzoli R, Bonjour JP. Determinants of peak bone mass and mechanisms of bone loss. Osteoporos Int. 1999;9(Suppl 2):S17–23. doi: 10.1007/pl00004155. [DOI] [PubMed] [Google Scholar]
- 16.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Saggese G, Baroncelli GI, Bertelloni S. Puberty and bone development. Best Pract Res Clin Endocrinol Metab. 2002;16:53–64. doi: 10.1053/beem.2001.0180. [DOI] [PubMed] [Google Scholar]
- 18.Loud KJ, Gordon CM. Adolescent bone health. Arch Pediatr Adolesc Med. 2006;160:1026–1032. doi: 10.1001/archpedi.160.10.1026. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein JS, Klibanski A, Neer RM. A longitudinal evaluation of bone mineral density in adult men with histories of delayed puberty. J Clin Endocrinol Metab. 1996;81:1152–1155. doi: 10.1210/jcem.81.3.8772591. [DOI] [PubMed] [Google Scholar]
- 20.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ. 1991;303:961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldrovandi GM, Lindsey JC, Jacobson DL, Zadzilka A, Sheeran E, Moye J, et al. Morphologic and metabolic abnormalities in vertically HIV-infected children and youth. AIDS. 2009;23:661–672. doi: 10.1097/QAD.0b013e3283269dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, D.C.: National Academy Press; 2000. Summary; pp. 1–20. [Google Scholar]
- 23.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. J Royal Statist Soc Series B. 1976;38:290–295. [Google Scholar]
- 24.Collett D. Modelling survival data in medical research. London: Chapman and Hall; 1994. [Google Scholar]
- 25.Katzman DK, Bachrach LK, Carter DR, Marcus R. Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab. 1991;73:1332–1339. doi: 10.1210/jcem-73-6-1332. [DOI] [PubMed] [Google Scholar]
- 26.Arpadi S, Horlick M, Shane E. Metabolic bone disease in human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:21–23. doi: 10.1210/jc.2003-031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan BM, Nelson RP, James-Yarish M, Emmanuel PJ, Schurman SJ. Bone metabolism in children with human immunodeficiency virus infection receiving highly active antiretroviral therapy including a protease inhibitor. J Pediatr. 2001;139:447–451. doi: 10.1067/mpd.2001.117005. [DOI] [PubMed] [Google Scholar]
- 28.Seeman E. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 29.Seeman E. The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clin North Am. 2003;32:25–38. doi: 10.1016/s0889-8529(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 30.Seeman E. Periosteal bone formation: a neglected determinant of bone strength. N Engl J Med. 2003;349:320–323. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 31.Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, et al. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22:395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- 32.Guaraldi G, Roverto A, Garlassi E, Zirilli L. Gender and gonadal function difference in the prevalence of bone mass reduction. CROI. 2009 [Google Scholar]
- 33.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49:298–308. doi: 10.1097/QAI.0b013e3181893e8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahoney EM, Donfield SM, Campbell H, Kaufman F, Gertner JM Hemophilia Growth Development Study. HIV-associated immune dysfunction and delayed pubertal development in a cohort of young hemophiliacs. J Acquir Immune Defic Syndr. 1999;21:333–337. doi: 10.1097/00126334-199908010-00012. [DOI] [PubMed] [Google Scholar]
- 35.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 36.Tebas P, Zhang J, Yarasheski K, Evans S, Fischl MA, Shevitz A, et al. Switching to a protease inhibitor-containing, nucleoside-sparing regimen (lopinavir/ritonavir plus efavirenz) increases limb fat but raises serum lipid levels: results of a prospective randomized trial (AIDS clinical trial group 5125 s) J Acquir Immune Defic Syndr. 2007;45:193–200. doi: 10.1097/QAI.0b013e318042e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan D, Upton R, McKinnon E, John M, James I, Adler B, et al. Stable or increasing bone mineral density in HIV-infected patients treated with nelfinavir or indinavir. AIDS. 2001;15:1275–1280. doi: 10.1097/00002030-200107060-00009. [DOI] [PubMed] [Google Scholar]
- 38.Dube MP, Qian DJ, Edmondson-Melancon H, Sattler FR, Goodwin D, Martinez C, et al. Prospective, intensive study of metabolic changes associated with 48 weeks of amprenavir-based antiretroviral therapy. Clin Infect Dis. 2002;35:475–481. doi: 10.1086/341489. [DOI] [PubMed] [Google Scholar]
- 39.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 40.Tebas P, Yarasheski K, Henry K, Claxton S, Kane E, Bordenave B, et al. Evaluation of the virological and metabolic effects of switching protease inhibitor combination antiretroviral therapy to nevirapine-based therapy for the treatment of HIV infection. AIDS Res Hum Retrovir. 2004;20:589–594. doi: 10.1089/0889222041217374. [DOI] [PubMed] [Google Scholar]
- 41.Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, et al. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest. 2004;114:206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Favus MJ. Primer on the metabolic bone diseases and disorders of mineral metabolism. Washington: Amercian Society for Bone and Mineral Research; 2003. [Google Scholar]
- 43.Pan G, Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM. AZT enhances osteoclastogenesis and bone loss. AIDS Res Hum Retroviruses. 2004;20:608–620. doi: 10.1089/0889222041217482. [DOI] [PubMed] [Google Scholar]
- 44.Van Vonderen MGA, Lips P, van Agtmael MA, Hassink EAM, Brinkman K, Geerlings SE, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 2009;23:1367–1376. doi: 10.1097/QAD.0b013e32832c4947. [DOI] [PubMed] [Google Scholar]
- 45.Gallant JE, Staszewski S, Pozniak AI, deJesus E, Suleiman JMAH, Miller MD, et al. Efficacy and safety of tenofovir DF vs. stavudine in combination therapy in antiretroviral-naive patients. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 46.Pitukcheewanont P, Safani D, Church J, Gilsanz V. Bone measures in HIV-1 infected children and adolescents: disparity between quantitative computed tomography and dual-energy X-ray absorptiometry measurements. Osteoporos Int. 2005;16:1393–1396. doi: 10.1007/s00198-005-1849-9. [DOI] [PubMed] [Google Scholar]
- 47.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, et al. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]