Abstract
Purpose
We investigated the feasibility and construct validity of the Dynamic Gait Index (DGI) in children and explored inter-rater and test-retest reliability. Methods: DGI performance of 10 children with Fetal Alcohol Spectrum Disorder (FASD) aged 8 - 15 years was compared to 10 age and sex matched children with typical development (TD). Inter-rater reliability was evaluated for 16 children (10 TD, 6 FASD); 11 children returned for a retest (5 TD, 6 FASD).
Results
The DGI is simple for raters to learn and easy to administer in children. A Mann-Whitney U test identified a significant difference on the DGI total score between children with FASD and TD (P=0.01). Inter-rater and test-retest reliability were promising but need to be further explored. Conclusions: The DGI was feasible and valid in a population of children aged 8-15 years with FASD and TD. Some modifications are suggested for administration of the DGI in children.
Keywords: adolescent, balance, child, construct validity, fetal alcohol spectrum disorder, feasibility, pediatrics, postural control
Introduction and Purpose
The Dynamic Gait Index (DGI) is a performance-based tool developed by Shumway-Cook and Woollacott to quantify dynamic balance abilities1 as well as to evaluate the individual's ability to modify gait in response to changing gait task demands.2 In adults, the DGI allows comparisons among groups of persons with various pathologies3 that affect balance abilities. The DGI has been shown to have excellent psychometric properties4-6 and takes approximately 10 minutes or less to complete.2 Despite the extensive use of the DGI in postural control research on older adults3, 5-7 the function of the DGI as a test of walking challenges in children has not been investigated.
Efficient balance and postural control in children are necessary prerequisites for many higher-level learning, play and social tasks such as navigating complex environments, controlling complex movements, and participating in childhood activities (e.g., playground activities and organized sports). Inadequate or inefficient postural control can contribute to inefficient motor performance and disorganized behavior. Among children with mild to moderate sensorimotor problems, such as children with Fetal Alcohol Spectrum Disorder (FASD),8 reduced efficiency and automaticity of postural control may interfere with the energy and attention that is needed to engage in and carry out more complex daily activities and learning tasks. Subsequently, children who have difficulties in maintaining their balance may demonstrate undesirable behaviors (e.g., clumsiness, bumping into other children, and inattention).9
The significance of postural control in children, in general, and dynamic balance, in particular, calls for sensitive and reliable measures of these constructs. Currently, measurement tools to assess dynamic balance in walking and coping with walking challenges for children are lacking. The DGI has been found to be a sensitive and efficient tool for adults2,3,5,6 therefore, it may be a useful tool with children. Our goal was to explore the use of the DGI for children with and without postural control dysfunction.
Children with Fetal Alcohol Spectrum Disorder (FASD) constitute one population of children with neurodevelopmental disorders who demonstrate balance and postural control deficits in which poor central processing and integration of sensory input are suspected.10 A clearer scientific understanding of balance impairments among children with FASD is missing and is required for adequate rehabilitation programs for this population.
In light of the need for sensitive assessment of dynamic balance for children with postural control dysfunction in general and children with FASD in particular, and considering the usefulness of the DGI in balance evaluation of adults the purposes of this study were multiple. The primary purposes were to: 1) investigate the feasibility of the Dynamic Gait Index in children with mild to moderate balance impairments as a result of FASD and children without balance impairments aged 8-15 years and 2) assess the construct validity of the DGI by comparing the dynamic balance performance of these 2 groups. Our secondary purpose was to explore the test-retest reliability and the inter-rater agreement of the DGI in a sample of children aged 8 to 15 years.
Methods
We completed a methodological study that compared the DGI performance of 10 children with fetal alcohol spectrum disorders (FASD) ages 8 to 15 years to 10 age and sex matched peers with typical development (TD) to examine construct validity, and explore inter-rater agreement and test-retest reliability of the DGI. This study was a part of a larger project investigating the sensory aspects of balance and postural control deficits in children with FASD.
Participants
To be included in this study, children could be male or female of any race and ethnic background, aged 8-15 who reside within the Seattle, Washington area. Participants with FASD were recruited though the Washington State Fetal Alcohol Syndrome Diagnostic and Prevention Network (FAS-DPN) clinical registry. All children with FASD had a diagnosis on the fetal alcohol spectrum based on the 4 Digit-Diagnostic code,11 confirmed prenatal alcohol exposure at any level, and evidence of a sensorimotor impairment based on a previous diagnostic assessment. Children with FASD who had other severe neuromotor conditions, a history of a serious head injury, an IQ < 60, or an unstable living situation were excluded from the study. A convenience sample of children with typical development (TD) was recruited through a university research participant pool, through posted flyers in the university community, and word-of-mouth. For each child with FASD enrolled in the study, we matched a child with typical development of the same age (± 6 months) and same sex. Children with typical development were excluded if they had sensorimotor or other developmental impairments based on an enrollment screen, or if they were enrolled in a special education program. Children in both groups were excluded if they had visual acuity impairment not correctable with glasses, a history of seizures, any serious medical or health condition, or a lower limb or back injury in the past 6 months. This study was approved by the University of Washington Institutional Review Board.
Instrumentation
The Dynamic Gait Index measures mobility function and dynamic balance in walking and stair climbing. There are 8 items on the DGI and each item is scored on a 4-point scale [(3) Normal; (2) Mild impairment; (1) Moderate impairment; (0) Severe impairment] with a maximal score of 24. The 8 items include walking, walking with speed changes, walking with vertical and then horizontal head turns, walking with a quick pivot stop, walking over objects, walking around objects and walking up and down stairs. Psychometric properties of the DGI have been established for adults. Test-retest reliability was found to be good (ICC3,1= .86, 95% CI .62 - .95) among 16 participants with peripheral vestibular disorders (ages 29 -78 years), who performed the DGI twice on the same day.5 Test-retest reliability and inter-rater reliability were also established among 25 participants with chronic stroke and mild to moderate disability who underwent two testing sessions 3 days apart (ages 26 – 75years) with 2 raters on the second testing session. The ICC for test-retest reliability was found to be .96 (95% CI .9 - .98) and for inter-rater reliability .96 (95% CI .83- .98).6 Furthermore to support construct validity, in a sample of 278 community-dwelling older adults, Herman et al. found significantly different DGI scores for participants who fell in the past 12 months and those who did not.1
Procedure
In this study, the DGI was administered as part of a standardized and experimental balance and coordination assessment battery. The test was always administered in the same quiet hallway, as the final measure within the 2 ½ hour test battery. Participants were generally given the standard verbal instructions of the DGI. An exception to that could be item no. 1 (“walk at your normal speed”) where the rater sometimes asked the child to “walk as if you are late to school” or “walk as if you are on your way to your soccer practice”. This was done if the rater felt that the child was walking slower than he/she had been previously observed to walk when not participating in the DGI test. In addition, in item 3 (gait with horizontal head turns) the child was instructed to look to 1 side and the other side, rather than to the right and then to the left, to minimize directional confusion. Items 2 through 7 were demonstrated once by the primary rater. Items 1 (normal walking speed) and 8 (normal stair climb) were not demonstrated because those items are scored based on a child's normal performance. We believed that observing the rater might bias the child's normal behavior.
Construct Validity
To assess the construct validity of the DGI in children with and without postural control dysfunction we compared the dynamic balance performance (expressed by total DGI score) of 10 children with TD to that of 10 children with FASD. A primary rater (‘Rater A’) instructed all children. The primary rater was either a Physical Therapist (PT) or an Occupational Therapist (OT) trained in the pediatric assessment battery.
Inter-rater agreement
To explore inter-rater agreement, 10 children with TD and 6 children with FASD from our original sample of 20 were rated simultaneously by 2 raters (4 of the children were not scored by 2 raters due to unavailability of the second rater.) The second rater (‘Rater B’) was a graduate research assistant, either a medical student or a PT PhD student. The raters did not consult each other during the test. All raters read the DGI instructions from Shumway-Cook and Woollacott2 and practiced test administration and scoring on adults.
Test-retest reliability
To assess test-retest reliability, we asked all children (with FASD or typical development) to come back for a second session within 2 weeks of the initial test session to re-administer the DGI. Eleven children (5 with TD and 6 with FASD) volunteered to participate in the retest assessment. Children who did not return could not do so due to other obligations and scheduling issues. None of the children refused to return due to fatigue or not enjoying the testing session. All children were males. The median age for the FASD group was 135.5 months (range 111 to 186) and for the TD group 120 months (range 113 to 187). The average time between the test and retest sessions was 12 days (range 7 to 21 days). The retest session lasted approximately 1 hour as the DGI was administered within a larger retest protocol. The scores of the primary rater (i.e. ‘Rater A’) were used for test-retest analysis.
Data Analysis
Descriptive statistics (means, SDs, median and range) were used to describe child and family demographic characteristics. We used Mann-Whitney U tests to compare parent level of education and household income between groups. Independent samples t tests were used to compare cognitive performance on the Kaufman Brief Intelligence Battery 2nd edition12 and balance performance on the Movement Assessment Battery for Children 2nd edition.13 A Mann-Whitney U test was also used to compare performance on the DGI total score. Because of the small sample size per group, we explored test-retest reliability and inter-rater reliability by plotting a visual display of individual scores and calculating inter-rater % agreement for each group (TD and FASD). Total scores of the groups combined were used to compute absolute agreement Intraclass Correlation Coefficients (ICCs), using a two-way, random effects model (ICC2,1). All calculations were done using the Statistical Package for the Social Sciences (SPSS) version 18.0 and significance was determined as P<.05.
Results
Descriptive statistics of the sample population appear in Table 1. There were statistically significant differences between the 2 groups on annual income (P <0.01) and child intellectual levels (P=.01) with the TD group having higher incomes and higher intellectual levels.
TABLE 1. Descriptive characteristics of the sample population.
| Characteristics | FASD | TD | P value |
|---|---|---|---|
| Age in months | |||
| Mean (SD) | 142.2 (30.4) | 144.5 (30.2) | |
| Median (Min, Max) | 130.5 (111,186) | 133.0 (113,187) | |
| Sex, number of females | 2 | 2 | |
| Parent highest level of education, % | .22c | ||
| High school diploma | 10.0 | 0.0 | |
| Some college | 20.0 | 10.0 | |
| College or professional Degree | 70.0 | 90.0 | |
| Annual income, % | <.01c | ||
| Less than $25,000 | 10.0 | 0.0 | |
| $25,000 to $50,000 | 20.0 | 0.0 | |
| $50,000 to $75,000 | 40.0 | 0.0 | |
| More than $75,000 | 30.0 | 100.0 | |
| MABC balance total scorea | |||
| Mean (SD) | 8.5 (2.7) | 11.3 (3.1) | .05d |
| Median (Min, Max) | 9.0 (5.0, 14.0) | 12.0 (6.0, 14.0) | |
| KBIT Matrices Standard Scoreb | |||
| Mean (SD) | 90.8 (19.2) | 112.7 (12.4) | .01d |
| Median (Min, Max) | 97.0 (56, 110) | 116.5 (93, 129) |
Movement Assessment Battery for Children 2nd edition (Mean = 10; SD =5)
Kaufman Brief Intelligence Battery 2nd edition (Mean = 100; SD =15)
Mann-Whitney U test
Independent samples t-tests
Feasibility
We found the DGI to be feasible, simple for raters to learn and easy to administer in our sample of children. Despite the need for demonstration of most tasks by the rater and the need for the children to repeat some test items based on misunderstanding the directions, the test administration took no longer than 10 minutes, consistent with previous reports for adults.2
Construct Validity
The DGI total score was significantly lower for the 10 children with FASD compared to 10 children with TD (P= .01). Children with typical development had a mean score of 23.3 (SD = 1.06) and a median score of 24 (range 21, 24), whereas children with FASD had a mean score of 21.4 (SD = 1.43) and a median score of 21 (range 19, 24). Comparison of the scores by item is displayed in Table 2.
TABLE 2. Mean (SD) DGI Scores Item by Item for Children with TD and FASD.
| TD | FASD | |
|---|---|---|
| Item 1 | 2.9 (0.3) | 2.6 (0.5) |
| Item 2 | 2.8 (0.4) | 2.9 (0.3) |
| Item 3 | 3.0 (0.0) | 2.4 (0.5) |
| Item 4 | 2.9 (0.3) | 2.6 (0.5) |
| Item 5 | 2.9 (0.3) | 2.5 (0.7) |
| Item 6 | 3.0 (0.0) | 2.9 (0.3) |
| Item 7 | 3.0 (0.0) | 2.8 (0.4) |
| Item 8 | 2.6 (0.5) | 2.7 (0.5) |
| Total DGI Score | 23.1 (1.1) | 21.4 (1.4) |
Inter-Rater Agreement
Inter-rater agreement for the DGI total scores for 10 children with TD was 90% as seen in Figure 1. For the single participant where the 2 raters did not agree, the total score difference was 2 points. The least reliable item for this group was item 4 (“gait with vertical head turns”) with 1 point difference twice. Comparison of DGI total scores by the 2 raters for 6 children with FASD is displayed in Figure 2. The total scores for 3 children differed by 1 point and the total score for 1 child differed by 3 points resulting in 50% overall agreement and 83% agreement for the score ± 1 point. The least reliable item for this group was item 3 (“gait with horizontal head turns”) with 1 point difference 4 times. ICC2,1 for both groups together (16 children) was found to be.82 (95% CI.49,.94, P= .001). The most reliable items for both groups together were item 6 (“Step over obstacle”) with no differences and items 1(“gait level surface”) and 8 (“stairs”) with1 point difference per group.
FIGURE 1.
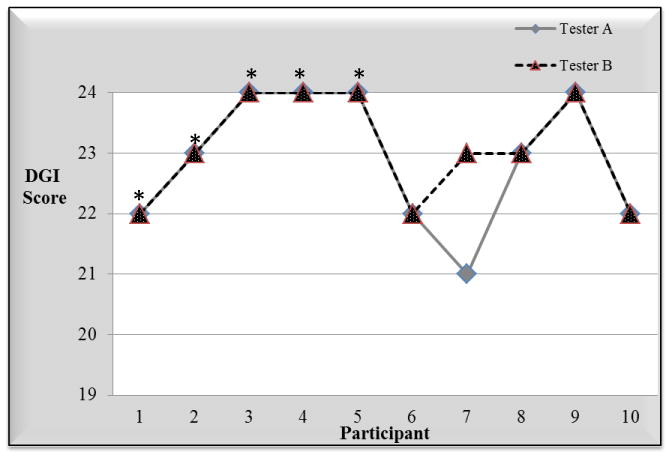
Inter-Rater Agreement for Children with Typical Development.
* The child also participated in test-retest analysis
FIGURE 2.
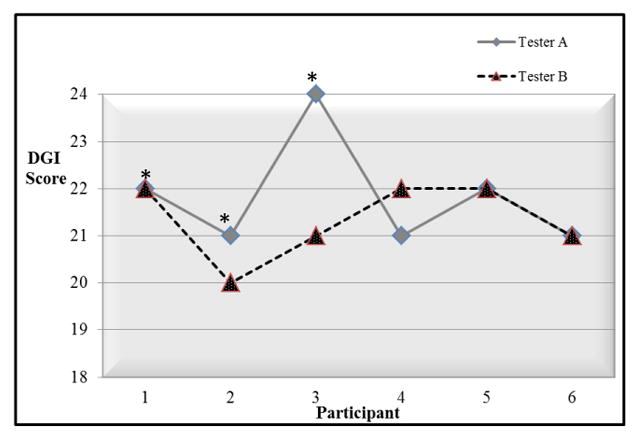
Inter-Rater Agreement for Children with Fetal Alcohol Spectrum Disorder.
* The child also participated in test-retest analysis
Test-Retest Reliability
Total scores for 5 children with TD were identical for 3 children and differed by 1 – 2 points for the other 2 (Figure 3). Total scores for children with FASD differed by 1 to 2 points for all 6 children (Figure 4). ICC2,1 for both groups together (11 children) was .71(95% CI .26,.91, P= .005).
FIGURE 3.
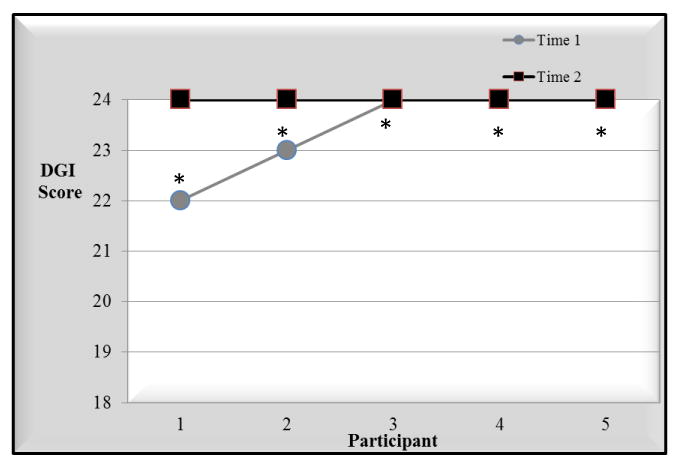
Test-Retest Reliability for Children with Typical Development.
* The child also participated in inter-rater reliability analysis
FIGURE 4.
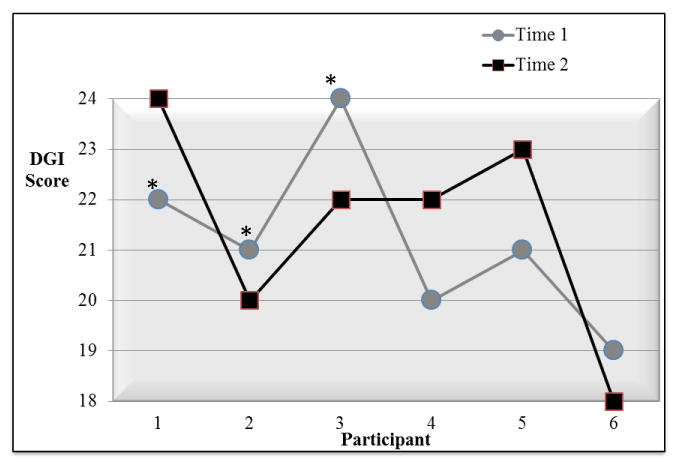
Test-Retest Reliability for Children with Fetal Alcohol Spectrum Disorder.
* The child also participated in inter-rater reliability analysis
Discussion
This study explored the feasibility and psychometric properties of the DGI in a sample of 10 children with FASD and 10 age and sex matched children with typical development. Overall, the DGI was found to be feasible and easy to administer to children taking no longer than 10 minutes to administer. The DGI total score showed significant group differences between children with and without postural control deficits. Agreement between raters and stability of performance over a 1-2 week time period was good for children with typical development but slightly lower for the children with FASD. Reliability needs to be further explored with a larger group of children with and without postural control impairments. Modifications for a pediatric version of the DGI are suggested below and should be evaluated in future research of the DGI in children.
We found a significant difference in our sample on the DGI total score despite the small sample size and the fact that most of the children with FASD presented with mild to moderate balance impairments. This suggests that the DGI may be sensitive to even subtle differences in balance abilities of children. The question remains whether this statistically significant difference (median difference of 3 points, P= .02) is clinically meaningful. In a study of adults with peripheral vestibular disorders, Hall and Herdman suggested that a change of 3 points or less in the DGI total score is considered within normal variability.5 Nevertheless, neither the minimal detectable change nor the minimal clinically important difference in the DGI total score has been established for children. Alsalaheen et al. investigated the effect of vestibular rehabilitation on children (ages 8-18 years) and adults (ages 19-73 years) post-concussion. They found a mean improvement of 3 points on the DGI total score (from 20 to 23) following treatment among 30 children and 18 adults.14 Whereas this score change indicated some improvements, the question of ‘clinically meaningful change’ requires further investigation.
Based on our experience, we suggest the following instruction modifications for use of the DGI with children to improve the feasibility, validity and reliability of the test in this population. First, demonstrate all items except for item 1 (‘normal walking’) and item 8 (‘normal stair climbing’). This will help clarify the test directions for the children. Minimizing the cognitive load will allow the score to reflect motor performance rather than a mix of motor and cognitive abilities. We suggest that items 1 and 8 should not be demonstrated to avoid bias and allow for observation of ‘normal’ performance. For example, a child may not use the railings while climbing the stairs if the rater did not demonstrate this even if the child normally uses the railings at home. Second, the walking task in item 1 should be given a concrete goal. Clinical observations of the children walking outside the test and during the test indicated that the children walked much faster outside of the test conditions. We believe that lack of understanding of the meaning of the test directive “walk as you normally do” interfered with the motor performance and may impact stability of performance over time as well as agreement between raters. Investigating the ‘Time Up and Go’ (TUG) in children Williams et al. concluded that a concrete task should be used in children (e.g. touch a target on a wall) as opposed to the more abstract instructions of the standard TUG.15 This conclusion is in agreement with Van der Weel et al. who suggested that abstract instructions limited performance in children with cerebral palsy.16 In our study, when the rater felt that the child was walking much slower than he/she normally would, the child was asked to “walk as if you are late to school” or “walk as if you are on your way to your soccer practice”. To make the task more concrete and engaging to all children we suggest placing an age-relevant toy or object several feet beyond the 20′ mark and use the directive “walk and pick up the toy”. Following the recommendations made by Bronfenbrenner et al., qualitative instructions such as ‘walk as fast as you can’ should be avoided to ensure natural performance.17 Third, on item 3, ask the child to ‘look to one side and then the other side’ rather than ‘look to the right and then to the left’. This should again help to minimize cognitive load and avoid directional confusion. Finally, item 8 for adults is instructed as follows: “Walk up these stairs as you would at home (i.e., using the rail if necessary). At the top, turn around and walk down”. We found that children, who were not using the rail outside the test, chose to use it because they were given the option. We believe that as a result, children with TD scored lower on this item than children with FASD. Therefore, when instructing children on this task we suggest not mentioning the rail.
We also suggest a few scoring modifications for children. In our sample, items 3 (“walking with horizontal head turns”) and 4 (“walking with vertical head turns”) were found to be the least reliable items. In adults, any decrease in gait speed requires a point deduction. In children, however, slight decrease in walking speed may be normal. As a result of the raters' attempt to follow the test instructions, together with a belief that ‘slight decrease’ in gait speed is normal in children, agreement between raters on those two items was lower than on other items. To improve inter-rater reliability on these items, future studies of the DGI in children should develop speed differences norms for different age groups in a large sample of children with typical development. Item 6 (step over an obstacle) should be demonstrated and performed again if the child did not step over the box. We found that errors on this item were mostly related to confusion and resolved once the child was given a second opportunity. Following this recommendation on our study we obtained 100% inter-rater agreement on this item. Likewise, the scoring instructions for item 8 (stair climbing) require the rater to take off a point if the participant is using the rail. Because some children ran up and down the stairs while holding the rail (especially children with TD), we believe that this instruction may have led to the result that children with TD scored lower on this item that children with FASD. Therefore, we suggest giving the children a second opportunity climb the stairs without the use the rail before determining the score. A full description of our suggested modified pediatric DGI appears in the Appendix (Supplemental Digital Content 1, available at http://links.lww.com/PPT/A23).
This study had several limitations. First, the sample size for this exploratory study was small. Because of the small sample size, the information regarding the test reliability in children is descriptive in nature and should be taken with caution. Second, the test was carried out at the end of a 2.5-hour testing session. While the DGI is not a strenuous test, fatigue might have affected the groups differently. In addition, there was limited variability in the performance between groups, as none of the children had severe balance or postural control impairments. Children with typical development appeared to ceiling out on the DGI and some children with FASD displayed only minor balance problems. Clearly, a larger sample of children with more variability in balance abilities is required to investigate the function of the DGI in children at all functional levels.
Conclusions
In conclusion, we found the DGI to be feasible, easy to administer, and valid in children with and without postural control dysfunction ages 8-15 years. We suggested several modifications for administration of the test in the clinic to improve its use with children (see the Appendix at http://links.lww.com/PPT/A23). These modifications include demonstration of the items, simplifying the instructions and providing a concrete goal to the ‘normal walking’ task. From a clinical standpoint, we believe that these modifications will allow for better agreement between raters and stability of performance over time. Given the length of the test (approximately 10 minutes), its simplicity and the ease of administration we believe that the DGI may be applicable for children younger than 8 years of age as well as children with varying cognitive levels. These are important directions for future research. Future research should also examine the psychometric properties of the pediatric modified DGI in a larger sample of children with diverse motor abilities as well as different neurodevelopmental disabilities.
Supplementary Material
Acknowledgments
The authors acknowledge the children and their families for participating in this study. In addition, we thank all faculty involved in the larger study: Deborah Kartin, PT, PhD, Bob Price, MSME, Beth Gendler, MSW, Susan Astley, PhD and Brian Dellon, PhD. We also thank the students who helped in data collection: Stephanie Griffith, Tyler Fostick, Rebecca Levens, and Ethan Jordt.
Grant Support: This study was funded by the National Institute on Alcohol Abuse and Alcoholism research grant R21AA019579-01.
References
- 1.Herman T, Inbar-Borovsky N, Brozgol M, Giladi N, Hausdorff JM. The Dynamic Gait Index in healthy older adults: The role of stair climbing, fear of falling and gender. Gait & Posture. 2009;29(2):237–241. doi: 10.1016/j.gaitpost.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shumway-Cook A, Wollacott M. Motor Control: Theory and Practical Applications. Baltimore: Williams and Wilkins; 1995. [Google Scholar]
- 3.Cattaneo D, Jonsdottir J, Repetti S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Taylor & Francis Ltd; 2007. [DOI] [PubMed] [Google Scholar]
- 4.Chiu YP, Fritz SL, Light KE, Velozo CA. Use of Item Response Analysis to Investigate Measurement Properties and Clinical Validity of Data for the Dynamic Gait Index. Phys Ther. 2006;86(6):778–787. [PubMed] [Google Scholar]
- 5.Hall CD, Herdman SJ. Reliability of Clinical Measures Used to with Peripheral Vestibular Disorders. J Neurol Phys Ther. 2006;30(2):74–81. doi: 10.1097/01.npt.0000282571.55673.ed. [DOI] [PubMed] [Google Scholar]
- 6.Johanna J, Davide C. Reliability and Validity of the Dynamic Gait Index in Persons With Chronic Stroke. Arch Phys Med Rehabil. 2007;88(11):1410–1415. doi: 10.1016/j.apmr.2007.08.109. [DOI] [PubMed] [Google Scholar]
- 7.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the Probability for Falls in Community-Dwelling Older Adults. Phys Ther. 1997;77(8):812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 8.Grove CR, Lazarus JA. Impaired re-weighting of sensory feedback for maintenance of postural control in children with developmental coordination disorder. Hum Mov Sci. 2007;26(3):457–476. doi: 10.1016/j.humov.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Chen HF, Cohn ES. Social participation for children with developmental coordination disorder: conceptual, evaluation and intervention considerations. Phys Occup Ther Pediatr. 2003;23(4):61–78. [PubMed] [Google Scholar]
- 10.Roebuck TM, Simmons RW, Richardson C, Mattson SN, Riley EP. Neuromuscular responses to disturbance of balance in children with prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(9):1992–1997. [PubMed] [Google Scholar]
- 11.Kaufman A, K N. Kaufman Brief Intelligence Test. 2nd. Los Angeles: Western Psychological Services; 2004. [Google Scholar]
- 12.Henderson SE, S D. Movement Assessment Battery for Children. 2nd. London, UK: Psychological Corporation; 2007. [Google Scholar]
- 13.Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: an illustration of appropriate statistical analyses. Clin Rehabil. 1998;12(3):187–199. doi: 10.1191/026921598672178340. [DOI] [PubMed] [Google Scholar]
- 14.Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34(2):87–93. doi: 10.1097/NPT.0b013e3181dde568. [DOI] [PubMed] [Google Scholar]
- 15.Williams EN, Carroll SG, Reddihough DS, Phillips BA, Galea MP. Investigation of the timed ‘up & go’ test in children. Dev Med Child Neurol. 2005;47(8):518–524. doi: 10.1017/s0012162205001027. [DOI] [PubMed] [Google Scholar]
- 16.Van der Weel FR, Van der Meer AL, Lee DN. Effect of task on movement control in cerebral palsy: implications for assessment and therapy. Dev Med Child Neurol. 1991;33(5):419–426. doi: 10.1111/j.1469-8749.1991.tb14902.x. [DOI] [PubMed] [Google Scholar]
- 17.Bronfenbrenner U. Towards an experimental ecology of human development. Am Psychol. 1977;32:513–521. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


