Abstract
Fourteen patients who developed B cell lymphomas or lymphoproliferative lesions after kidney, liver, heart, or heart-lung transplantation in Pittsburgh during 1981–1983 had active infection with Epstein-Barr virus (EBV) of the primary (six patients), reactivated (seven patients), or chronic (one patient) type. In transplant patients without tumors, the incidence of EBV infection was 30% (39 of 128). Only three of these patients had primary infections. Thus the frequency of active infection was significantly higher in patients with tumors, and patients with primary infections were at greater risk of developing tumors. Five of 13 tumors tested contained EBV nuclear antigen (EBNA) and nine of 11 contained EBV genomes detected by DNA-DNA hybridization with BamHI K, BamHI W, or EcoRI B cloned probes. All EBNA-positive tumors, except one, were also positive by hybridization. Only one tumor was negative for both EBNA and EBV DNA. These data suggest that EBV plays an etiologic role in the development of these lesions.
Epstein-Barr virus (EBV) is a human herpesvirus associated with an array of conditions that range from inapparent infection and infectious mononucleosis to lethal lymphoproliferative syndromes, nasopharyngeal carcinoma, Burkitt’s lymphoma, and B cell lymphomas in immunocompromised patients [1]. The precise role of the virus in carcinogenesis is unclear, although in Burkitt’s lymphoma the importance of viral transformation of infected B lymphocytes and chromosomal translocations has been emphasized [2]. It is even less clear in lymphomas and lymphoproliferative lesions arising in immuno-compromised patients, where the immunopathology may not be uniform and where chromosomal studies are largely lacking.
Recently, we reported on the reversibility of lymphomas and lymphoproliferative lesions in a series of 17 transplant patients after reduction of cyclosporine and steroid immunosuppression [3]. In that preliminary report we noted that seven of these patients had evidence of primary EBV infections and eight had evidence of reactivated infection. Six tumors had evidence of EBV nuclear antigen (EBNA) and seven had evidence of EBV DNA by nucleic acid hybridization.
This paper examines in detail the relation between infection with EBV and the tumors in 14 of these 17 patients. The three patients who were excluded had their original transplants in Colorado (patients 1, 2, and 3 [3]). New data provided here include (1) frequency of EBV infection in the general transplant population; (2) additional serological studies in patients with tumors; (3) correlation of laboratory evidence of EBV infection and clinical illnesses accompanying the appearance of tumors; and (4) details of hybridization studies with highly specific and sensitive probes consisting of cloned fragments of the EBV genome.
Calne et al. [4] and Thiru et al. [5] first observed three cases of lymphoma in 34 transplant patients receiving cyclosporine, of whom one had evidence of primary infection. Hanto et al. [6] reported the results of studies on 19 renal transplant patients who developed lymphoproliferative disorders and lymphomas after transplantation. All the patients except one were receiving azathioprine, prednisone, and antithymocyte globulin. Two of their patients developed primary EBV infection, in six the infection reactivated, and 12 had evidence of EBV DNA in their tumors by hybridization studies. Bieber et al. [7] reported that five of 39 heart transplant recipients receiving cyclosporine, prednisone, and antithymocyte globulin developed lymphomas. Four tumors were positive for EBV DNA by cRNA-DNA filter hybridization, and three patients had serological evidence of EBV infection.
We report here evidence for active EBV infection in all of our patients with tumors and a significantly higher frequency of primary infection than found in control transplant recipients. Antibody responses to all EBV antigens and details of hybridization studies have not been reported in previous studies of patients with tumors.
Materials and Methods
Surveillance of transplant patients and specimen collection
In 1981 cyclosporine was introduced at the University Health Center of Pittsburgh as the main immunosuppressant for transplant recipients. Liver, heart, and heart-lung transplantations were initiated, and the existing renal transplant program was greatly expanded. Since that time we have carried out a system of prospective surveillance for herpesvirus infection in all transplant patients. Serum specimens were collected when possible before or at time of transplantation and at least twice monthly for the first three months and monthly for six to 12 months after transplantation. Throat-wash, urine, and buffy coat specimens for cytomegalovirus (CMV) isolation were also collected at the same intervals. In many cases, when viral illness was suspected, additional specimens were obtained. Complete sets of sera were available for all 14 patients with EBV-related lymphomas or lymphoproliferative syndromes. Five to 24 serum samples from each patient were titrated for antibodies to EBV antigens five to 72 months after transplantation. Every transplant recipient was seen or followed up by one of us or by fellows of the Division of Infectious Diseases.
Viral syndrome
A viral syndrome was defined as an illness with fever of at least 37.7 C that persisted for four days or more and could not be attributed to nonviral agents, despite careful evaluation and culturing. The syndrome was assumed, although not proven, to be due to EBV, CMV, or both because of laboratory correlations. These illnesses were associated with one or more of the following manifestations: (1) persistent pharyngitis or tonsillitis, (2) cervical lymphadenopathy, (3) leukopenia (white cell count ≤4,000), and (4) atypical lymphocytosis (≥10% atypical cells).
Lymphoma and lymphoproliferative lesions
The definition of these two terms is based on anatomic pathology, as previously reported [3]. For convenience we refer to the combined groups as “tumors.”
EBV serology and serological diagnosis
Immunosuppressed patients may have atypical serological responses to EBV infection. For example, it is well known that antibodies to EBNA do not always develop in such patients after primary infection [8]. The sensitivity and specificity of IgM antibodies to viral capsid antigen (VCA) and IgG antibodies to early antigen (EA) are also unclear. Most authors have relied on changes in IgG antibody to VCA to diagnose EBV infection in such patients [1, 5–7].
For routine serological surveillance, primary EBV infection was diagnosed when an initially seronegative individual seroconverted and developed IgG antibody to VCA. The diagnosis of reactivated infection required a fourfold or greater rise in titer of this antibody in a previously seropositive patient. In both cases, unsustained antibody rises within the first postoperative month were excluded from consideration because of possible passive transfer of antibodies by transfusions.
To evaluate the occurrence of other antibodies in patients with tumors, we also measured titers of IgM antibody to VCA, IgG antibody to EA, either D or R, and antibody to EBNA. IgA antibodies to VCA and EA were qualitatively determined. Significant rises in titers of IgG antibodies to VCA and EA were used to diagnose infection.
The following tests were performed by indirect or anticomplement EBV-specific immunofluorescence tests. Titers of IgM and IgG antibodies to VCA were determined on acetone-fixed HR1-K cells by using fluorescein isothiocyanate (FITC)–labeled antibody to IgM and antibody to IgG, according to Schmitz and Scherer [9] and Henle and Henle [10], respectively. In order to eliminate rheumatoid factor as a cause of a false-positive IgM test, we adsorbed some sera with heat-aggregated human gammaglobulin, according to Shirodaria et al. [11]. IgG antibody to EA (D or R types) was determined according to the methods of Henle et al. [12], by using Raji cells induced with 5-iodo-2′-deoxyuridine. D antigen is detectable after both acetone and methanol fixation, whereas R antigen is denatured by methanol fixation. Titers of antibody to EBNA were determined on Raji cells by using the anticomplement immunofluorescence test of Reedman and Klein [13], with human C3 and FITC-labeled antibody to human C3. For the determination of IgA antibodies to VCA and IgA antibodies to EA, specific antibody to human IgA was used. In all these tests, positive and negative human sera against the appropriate EBV antigens, as well as heat-inactivated (56 C for 30 min) C3, and negative human lymphoblastoid (RAMOS) or leukemic myeloblastoid (K-562) cell lines were used as EBV specificity controls. Tests on selected samples for IgG antibody to VCA, IgM antibody to VCA, and IgG antibody to EA were confirmed in the laboratory of Dr. W. Henle (Joseph Stokes, Jr. Research Institute, Children’s Hospital, University of Pennsylvania, Philadelphia).
Tests for heterophil antibody
Selected sera from all patients with tumors were tested by using the Sylvana slide-test kit (differential slide test; GIBCO, Grand Island, NY). Briefly, two 20-µl aliquots of serum were adsorbed with either beef erythrocyte stroma (10%) or guinea pig kidney tissue (10%) before addition of citrated horse red blood cells.
CMV infection
As in previous studies [14, 15], CMV infection was diagnosed by recovery of CMV from throat, urine, or buffy coat specimens on human fibroblast monolayers, by conversion or elevation in titers of antibodies to CMV by anticomplement immunofluorescence [14], or by both. Infections were classified as either primary or reactivated depending upon whether the patient was seronegative or seropositive before transplantation. CMV serological and virological studies were performed on all patients.
Tests for EBNA in tumor tissue
The presence of EBNA in lesions was detected by anticomplement immunofluorescence by using the method of Reedman and Klein [13]. Cryostat sections or fresh-impression smears of tissue were fixed with acetone. Four sera that were positive for antibody to EBNA, of which one was free of antibody to CMV and one was free of antibody to herpes simplex virus, and two sera that were negative for antibody to EBNA were used as primary reagents.
DNA hybridization studies
Frozen (−70 C) tumor tissue specimens were examined for their EBV genome content by Southern blot hybridization analysis. One specimen was studied by Joseph Pagano (University of North Carolina, Chapel Hill, NC) by using a cloned probe consisting of a BamHI W fragment of EBV DNA. Two specimens were studied by Drs. M. L. Cleary and J. Sklar (Stanford University, Stanford, Calif) by using a dot blot method with an EcoRI B probe.
All other tests were performed in the laboratory of G. M. at Yale University by using a plasmid containing either the BamHI K or EcoRI B fragment of the EBV genome as described by Andiman et al. [16]. Briefly, 1 µg of tissue DNA was digested at 37 C for 2–3 hr with a sixfold excess of the restriction endonuclease BamHI. DNA samples were heated to 65 C for 5 min, placed on ice for 10 min, and then electrophoresed through horizontal 0.5%0 agarose gel for 18 hr at 40 V. The DNA was transferred to nitrocellulose by blotting overnight with 20 × SSC (SSC: 3 M NaCl and 0.3 M sodium citrate) according to the procedure of Southern [17]. Plasmids containing the respective fragments were labeled with 32P by nick translation. Southern blots were hybridized with labeled probes at 65 C for 18–64 hr in plastic bags in a shaking water bath. Blots were exposed to Kodak XAR5 or BB5 film (Eastman Kodak, Rochester, NY) with intensifying screens for various lengths of time. Every experiment contained, as a positive control, a titration of the plasmid pBR322 containing the EBV BamHI K fragment or the plasmid pACYC184 with the EBV EcoRI B fragment. Probes were prepared from these plasmids. Titrations of pBR-BamHI K from 100 to 1 pg represent the signal equivalent to 10, 1, and 0.1 copies per cell in a sample of 106 cells. Another positive control consisted of 500 ng and 50 ng (the equivalent of 105 and 104 cells) of BamHI-digested cellular DNA from a cell line (FF 467) known to contain 40 EBV genome copies per cell. The negative control was human placental DNA.
Statistics
To compare differences in proportions between two groups, we used the χ2 test with Yates’s correction.
Results
EBV infection in transplant patients
To illustrate the background frequency of EBV infections in our transplant groups, we present results of titrations of IgG antibody to VCA from 128 renal, liver, heart, and heart-lung recipients without lymphomas or lymphoproliferative disorders (table 1). Sera were collected as described in Materials and Methods. A pretransplant and at least one posttransplant serum sample, covering a period ranging from 1.5 to 32 months, were titrated from each patient. The mean duration of follow-up was seven months.
Table 1.
Primary and reactivated EBV infections in transplant recipients who did not develop lymphomas.
| Type of transplant | ||||
|---|---|---|---|---|
| Characteristic | Renal | Liver | Heart and heart-lung |
Total |
| Pretransplant IgG antibodies to VCA | ||||
| Negative* | 1/3 | 0/0 | 2/2 | 3/5 |
| Positive† | 5/34 | 14/41 | 17/48 | 36/123 |
| Total (%) | 6/37 (16) | 14/41 (34) | 19/50 (38) | 39/128 (30) |
| Duration of follow-up‡ | 5.9 ± 0.2 | 3.7 ± 0.4 | 10.5 ± 1.0 | 7.0 ± 0.5 |
No. with serological evidence of primary infection/total no. in category.
No. with serological evidence of reactivated infection/total no. in category.
In months, mean ± SE.
The mean frequency of infection in these 128 patients was 30%. Only five patients were seronegative before transplantation and were susceptible to primary infection. The frequency of primary infections among seronegative subjects was 60% (3 of 5), and 29% (36 of 123) of those who had experienced a primary infection in the past had a reactivated infection.
Lymphomas and lymphoproliferative lesions in Pittsburgh
To estimate the frequency of these diseases in the four major transplant groups as of June 1983, we calculated the number of patients with tumors and the total number of transplantations (table 2). Data on the renal transplant patients go back to December 1979. The other three types of transplants were begun in 1981, and such organ recipients were given cyclosporine and prednisone. There were 82 renal transplant recipients who were maintained on a regimen of azathioprine and prednisone until 1981. After that time they too were treated exclusively with cyclosporine and prednisone.
Table 2.
Lymphomas and lymphoproliferative lesions (LPL) in transplant patients in Pittsburgh (December 1979–June 1983).
| Type of transplant | No. of transplants |
No. with lymphoma and LPL (%) |
|---|---|---|
| Renal | 315 | 5 (1.6) |
| Liver | 129 | 3 (2.3) |
| Heart | 48 | 3 (6.3) |
| Heart and Lung | 6 | 2 (33.3) |
| Total | 498 | 13 (2.6) |
One (patient 10) of the 14 patients was not included in the table. She received a liver transplant in Colorado in 1977 but was followed up by T. E. S. in Pittsburgh from 1981 to September 1983, at which time her tumor was discovered. She died shortly after retransplantation surgery.
It is apparent that the heart and the heart-lung groups had a significantly higher frequency of tumors than did the other groups (P < .01). The same conclusion was reached in a summary of 5,550 transplant recipients receiving cyclosporine [18].
Development of antibodies to EBV antigens in patients with tumors
The antibody responses to EBV antigens of the 14 tumor patients before and after transplantation are summarized in tables 3 and 4. All 14 patients had serological evidence of active EBV infection. Thirteen patients had rises in their titers of antibody to VCA or EA, a finding indicating primary or reactivated infection (table 3). One renal transplant patient (patient 14), a 52-year-old man with polycystic kidneys, did not show a rise in any serological marker but had persistently elevated IgG antibodies to EA (R) for 16 months after his transplant. He developed a diffuse, noncleaved large-cell lymphoma in the left side of the neck three months after transplantation. This lymphoma was negative for EBNA and negative for EBV DNA by hybridization studies.
Table 3.
Serological changes during primary and reactivated EBV infections in patients with lymphoma.
| Significant changes in | |||||
|---|---|---|---|---|---|
| Type of infection (n) | IgG anti-VCA |
IgM anti-VCA |
IgG anti-EA |
IgA anti-VCA |
No. positive for heterophil agglutinin (%) |
| Primary (6) | 6 (100) | 3 (50) | 5 (83) | 3 (50) | 0 (0) |
| Reactivated (7) | 4 (57) | 3 (43) | 7 (100) | 5 (71) | 1 (14) |
NOTE. Primary infections are infections in patients whose pretransplant serology for EBV was negative. Reactivation occurred in seropositive patients. Results are no. of patients (%).
Table 4.
Characteristics of transplant patients with EBV and CMV infections and with lymphomas and lymphoproliferative lesions.
| Patient, sex, age* |
Type of transplant |
Type of EBV infection† |
Onset of antibody rise‡ |
Onset of viral syndrome‡ |
Type of CMV infection† |
Time of tumor diagnosis‡ |
Type of tumor§ |
Clonality‖ | Tumor EBNA |
DNA hybridization (probe)# |
Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, F, 62 | Kidney | P | 4 | None | R | 4.5 | DL: stomach | indet | − | ND | Alive |
| 2, M, 16 | Kidney | P | 3.5 | 2 | – | 3.5 | DHL: small bowel | λ-chain | − | + (Bam-K) | Alive |
| 3, M, 17 | Liver | P | 4.5 | 5 | P | 6 | DL: small bowel | poly | ND | ND | Alive |
| 4, M, 22 | Heart | P | 2 | 5 | R | 6 | DL: neck, lung | indet | + | + (Bam-W)** | Dead |
| 5, M, 22 | Heart-Lung | P | 1.2 | 3 | P | 2 | DL: tonsils, lung | poly | + | + (Eco-B) | Alive |
| 6, M, 20 | Heart-Lung | P | 2 | 3 | – | 4 | DL: neck nodes | x-chain | − | + (Eco-B)** | Dead |
| 7, M, 56 | Kidney | R | 6 | None | – | 6 | DHL: prostrate | indet | − | + (Bam-K) | Alive |
| 8, M, 30 | Kidney | R | 4 | None | P | 4.5 | DL: small bowel | λ-chain | − | + (Bam-K) | Alive |
| 9, M, 20 | Liver | R | 7 | 8 | R | 8 | B cell hyp: intestines | poly | − | + (Bam-K) | Dead |
| 10, F, 13 | Liver | R | 67 | None | – | 67 | DL: neck nodes | poly | + | − (Bam-K) | Dead |
| 11, F, 21 | Liver | R | 1 | 6 | – | 7 | DL: intestines | x-chain | + | + (Bam-K) | Alive |
| 12, M, 44 | Heart | R | 2 | None | – | 3 | B cell hyp: inguinal node | poly | − | ND | Alive |
| 13, M, 51 | Heart | R | 1.5 | 2 | P | 6.5 | DL: lung, adrenal | indet | + | + (Eco-B) | Dead |
| 14, M, 52 | Kidney | C | … | None | R | 3 | DL: neck nodes | indet | − | − (Bam-K) | Alive |
NOTE. + = positive; − = negative; ND = not done.
Age at time of transplant.
P = primary infection; R = reactivated; C = chronic.
Time in month, after transplantation. (The times of antibody rise represent maximums, as earlier serum may not have been available or a transfusion effect to be excluded.)
DL = diffuse, large-cell, noncleaved lymphoma; DHL = diffuse histocytic lymphoma; hyp = hyperplasia.
Indet = indeterminate; poly = polyclonal.
Bam-K = BamHI K; Bam-W = BamHI W; Eco-B = EcoRI B.
Tumor of patient 4 was tested by Southern blot by J. Pagano; tumor of patient 6 was tested by M. Cleary and was found positive, tests by G. M. were negative.
Six patients (patients 1–6) developed primary infections. These were documented by the absence of IgG antibodies to VCA before transplantation and a serological conversion one to five months after transplantation. Five patients had concomitant conversions of antibodies to EA (R) from negative titers to titers between 1:10 and 1:40. One (patient 2) did not develop antibodies to EA. Only three patients (patients 4, 5, and 6) developed positive titers of IgM antibodies to VCA of 1:5.
Seven patients who had IgG antibodies to VCA before transplantation had evidence of reactivated infection. All but one (patient 10) of these EBV reactivations occurred between one and seven months after transplantation. Four (patients 9–12) developed a fourfold or greater rise in titer of IgG antibody to VCA. All seven showed significant rises in IgG antibody to EA (R). One (patient 12) had a pretransplant titer of IgG antibody to EA (R) of 1:10 that, at about the time of elevation of titers of IgG antibody to VCA, shifted to a titer of IgG antibody to EA (D) of 1:20. This was the only patient in whom a shift from antibody to R to antibody to D was observed. Three patients had IgM antibody to VCA (patients 10, 12, and 13). Because rheumatoid factor may give a false-positive IgM test [19, 20], sera from these three patients were retitrated after adsorption with aggregated gammaglobulin. The titers remained unchanged, a result suggesting that IgM antibodies to VCA may truly be present in some reactivated EBV infections.
Table 3 also shows results of heterophil agglutinin tests that were performed on sera selected from all 14 patients about the time of significant serolgical changes. The test was positive in only one patient (patient 9), who had a reactivated infection. Heterophil antibody was notably absent in all primary infections.
EBV-specific IgA antibodies are a highly characteristic feature of nasopharyngeal carcinoma [21, 22] and can be found in 38%–86% of patients with EBV mononucleosis [21, 23]. IgA antibodies to VCA and IgA antibodies to EA were qualitatively determined in all serum samples. No clear-cut pattern was detected.
Antibodies to EBNA did not develop in four of the six patients who had primary infections during the two to 19 months of follow-up (data not shown). Two patients (patients 1 and 5) developed a low titer (1:5) that became negative again in one (patient 5). This differs from the usual situation in primary infection, in which antibody to EBNA routinely develops during convalescence [24]. All seven patients with reactivated infections had detectable though low titers of antibody to EBNA before transplantation. In two (patients 9 and 13), antibody to EBNA became undetectable during reactivated infection. The failure of immunosuppressed patients to develop antibody to EBNA is well described [8].
In summary, antibody responses in EBV infections in these patients with lymphoproliferative disorders were different from those seen in normal patients. In primary infections all patients seroconverted their IgG antibodies to VCA, and no additional cases were diagnosed on the basis of antibody to EA. Unlike primary infection in normal subjects, not all patients developed IgM antibodies to VCA early or antibodies to EBNA late in their course [24, 25], and no patient with primary infection developed a heterophil agglutination titer. It is possible, of course, that in some cases a transient IgM titer was missed [26].
Reactivated infections were less easy to diagnose and interpret. They were generally diagnosed by significant rises in titers of IgG antibody to VCA or EA in patients who were seropositive for IgG antibody to VCA before transplantation. A majority (four of seven patients) had significant rises in titers of IgG antibody to VCA, and all seven patients had significant rises in titers of antibody to EA. Changes in antibody to EA (R) in transplant patients indicative of reactivated infection were shown by Cheesman et al. [27]. In nonimmunosuppressed patients with persistent symptomatology, elevated titers of antibody to EA (D or R) have been correlated with active, persistent EBV infection [28, 29].
IgM antibody to VCA was not diagnostic of primary infection as it occurred in three reactivated infections. This has been described in normal subjects [30]. The appearance of IgM antibody to CMV has also been described in reactivated CMV infections in immunosuppressed heart transplant recipients [31].
Clinical association with EBV infection
Table 4 also lists data concerning the nature of the EBV infection and tumors in the 14 patients. Five of six patients with primary EBV infections developed a symptomatic “viral syndrome” two to five months after transplantation. The one patient with a primary EBV infection who did not have a viral syndrome (patient 1) was first noted to have gastrointestinal bleeding. A gastric ulcer was found that on biopsy showed a diffuse large-cell lymphoma.
Viral syndromes occurred less often in the patients with reactivated EBV infections (three of seven) but were similar to those seen in primary EBV infections.
It was not always possible to ascribe the viral syndrome to EBV infection, as eight of 14 patients also had primary or reactivated CMV infections (table 4). However, six patients did not have any evidence of CMV infection by culture or serology. Three (patients 2, 6, and 11) had viral syndromes that could only be accounted for by EBV.
These viral syndromes occurred either at or up to a few months before the diagnosis of a tumor. In 13 of 14 patients, the tumors developed within eight months of transplantation. The presence or absence of a viral syndrome did not predict the histological, biologic, and virological properties of the tumor.
Development of tumors and evidence of EBV activity
Table 4 lists the histological and immunopathological diagnoses of the tumors in these 14 patients. Except for two, who had B lymphocyte hyperplasia, all patients had malignant B cell lymphomas. As previously reported [3], the pathology and clonality of the tumor did not predict the clinical course, the outcome, or whether tumor progression was reversible.
No fresh tissue was available from patient 3. Tumors from the remaining 13 patients were examined for EBNA or EBV DNA or both by blot hybridization. Tissues from 11 patients were examined by both techniques. Ten (77%) of 13 were positive by one method or the other.
Results of seven blot hybridizations with either the EcoRI B or BamHI K fragment as probes are presented in figures 1 and 2. To summarize, the sensitivity of the EcoRI B probe is about four copies per cell (figure 1). About 30 copies per cell were detected in the lung of patient 13, and 30–40 copies per cell were detected in the two tonsil specimens from patient 5. None were detected in the lymph node of patient 6. Note that all positive samples contained a group of BamHI fragments that were expected to be present in EcoRI B and separated by Southern blot analysis, which makes the findings even more convincing. The sensitivity of the BamHI K probe was about one copy per cell (figure 2). The prostate specimen from patient 7 contained >10 copies per cell, the intestinal tumor of patient 11 had ~10 copies per cell, the mesenteric lymph node of patient 9 had about one copy per cell, and the intestinal tumor of patient 8 had >10 copies per cell. The weak positive result by blot analysis may possibly be related to the fact that patient 9 was diagnosed as having a lymphoproliferative syndrome.
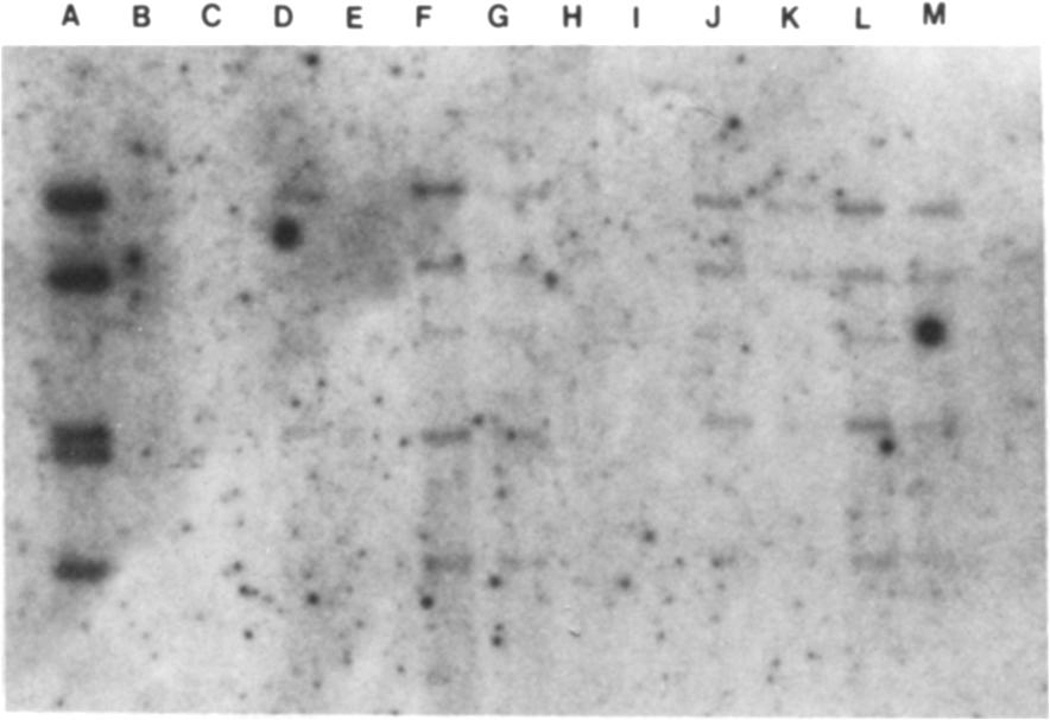
Figure 1.
Southern blot analysis of tumor tissue from organ transplant recipients with the EBV probe EcoRI B. Lane A contains the equivalent of 330 copies per cell of probe DNA in a sample of 106 cells. Lane D contains DNA from a lymphoid line containing 40 genome copies per cell; the DNA from 105 cells was loaded in this lane. Lane E contains DNA from an EBV-negative cell line. Lanes F and G contain 1 µg and 500 ng, respectively, of DNA from the lung biopsy of a heart transplant recipient (patient 13); lanes H and I contain 1 µg and 500 ng, respectively, of DNA from a lymph node of a heart-lung transplant recipient (patient 6); lanes J and K contain 1 µg and 500 ng of DNA from one tonsil, and lanes L and M contain 1 µg and 500 ng of DNA from the other tonsil of patient 5, a heart-lung transplant recipient. EBV DNA was found in tissues from patients 13 and 5 but not 6. Lanes G, K, and M each contain the DNA equivalents of 105 cells. The signals corresponding to these three samples represent the equivalent of ~40 copies per cell. Note that all samples contain a group of BamHI fragments expected to be present in EcoRI B probe.
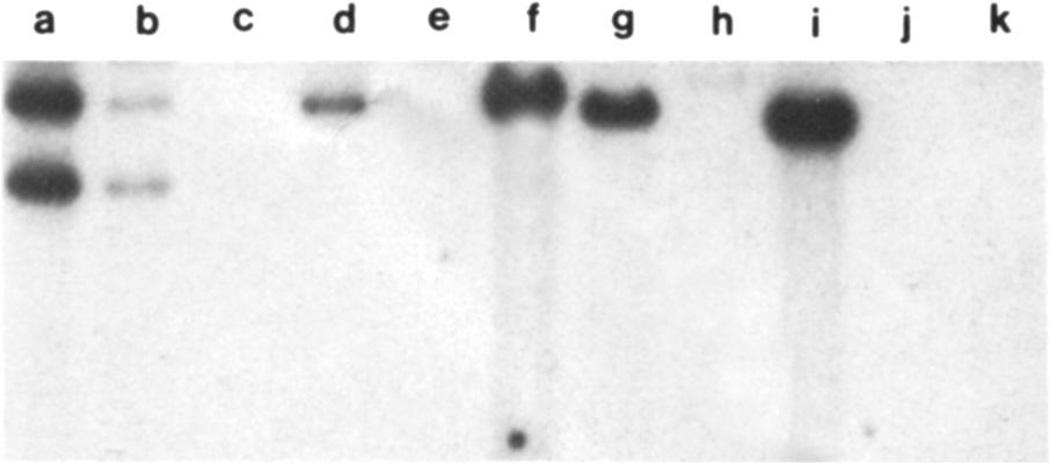
Figure 2.
Southern blot analysis with the EBV probe BamHI K. Titration of the probe is found in lanes a–c. Lane a contains 100 pg of the probe. Lane b contains 10 pg of BamHI K, the equivalent of one genome copy per cell in 106 cells. Lane c contains 1 pg. Lane d contains DNA from a lymphoid line containing 40 copies per cell; the DNA from 105 cells was loaded in this lane. Thus, the signal is equivalent to four copies per cell in a sample of 106 cells. Lane e is a negative control. Lanes f–i contain 5 µg of DNA from each of four patients (7, 11, 9, 8); this is ~ 106 cell equivalents of DNA per lane. Thus, samples f, g, and i have 10 copies per cell, and sample h has four copies per cell. Lanes j and k contain DNA from an unrelated patient. The BamHI K fragment is known to vary in size.
The lymph node of patient 6 was also tested by M. Cleary and J. Sklar at Stanford by dot hybridization with an EcoRI B probe and was found, in contrast to the above results, to be positive. This may be due to a difference in the sensitivity of the test in two laboratories, or the dot blot may give nonspecific hybridization. More likely, it represents the presence of more genome-positive cells at one site than at another.
The hybridization test seemed to be more sensitive than the test for EBNA. This would be expected, based on the size of the specimen that could be examined by the two methods. Also, EBNA is quite labile. The tumor of patient 10 was positive for EBNA but negative by hybridization. This patient’s tumor cells were cultured and passed in vitro. They were uniformly positive for EBNA and their karyotype was normal (R. W. A., unpublished observation).
There was only one patient whose tumor was negative for both EBV markers – patient 14. This was the only patient in the series who did not have serological evidence of a primary or reactivated EBV infection but had persistent antibody to EA, which we interpreted as indicating active chronic infection. The clinical course of this patient was not different from the others.
Discussion
The frequency of EBV infection in our transplant populations varied from 16% to 38% (mean, 30%). Renal transplant patients had a lower rate of EBV infection than did the other transplant groups, but this observation requires further substantiation.
In contrast, 13 of our 14 patients with tumors had evidence of primary or reactivated EBV infection. Patient 14 had evidence compatible with chronic infection. Excluding this patient, the rate of infection was 93 %. This was significantly higher than the rate of primary or reactivated EBV infection in the general transplant population (P < .0005). Not all reactivated EBV infections in patients with tumors were detected by the test for IgG antibody to VCA alone. By using rises in titers of IgG antibody to VCA as the sole criterion, 10 of 14 tumor patients had evidence of infection (table 3). This rate was still significantly higher than the rate of infection for the whole transplant group (P < .005). Another objection may be that in the general population, inadequate length of follow-up may have precluded detection of late infections. To address this point, we first note that EBV infection in most tumor patients was detected by 4.5 months after transplantation (10 of 13; table 4). Eliminating all patients with <4.5 months of follow-up from the cohort of table 1, we still have 97 patients and an infection rate of 39%. This is still lower than the infection rate in tumor patients with the test for IgG antibody to VCA alone (P < .05). Thus, there was a significant association between the development of tumors and the presence of EBV infection.
Patients with primary EBV infections seemed to be at greatest risk. Six (43 &) of 14 patients who developed tumors had primary EBV infections. This is significantly different from the proportion of primary EBV infections in a population of patients without tumors (table 1; 3 [8%] of 39, P < .01). This point is not evident from previous reports [4, 7].
The rates of EBV infection in our transplant populations were significantly lower than were the rates of CMV infection: they were, respectively, 77%, 66%, and 96% in renal, liver, and heart and heart-lung recipients [32]. Cheeseman et al. [27] found that all the renal transplant patients in their series were initially seropositive, and only those patients who received antithymocyte globulin reactivated. Marker et al. [33] reported that 30 (34%) of 88 transplant patients who also received antithymocyte globulin had serological changes indicative of EBV infection. Our own previous work [34] showed that 32% of renal patients on azathioprine and prednisone developed EBV infection, as detected serologically. It is possible that reported reactivation rates are falsely low since, as shown in this paper, some cases can only be detected by the demonstration of a rise in titers of antibody to EA.
Morbidity due to EBV infection after organ transplantation has not received as much attention as morbidity due to CMV infection. EBV mononucleosis and malignant lymphoproliferative syndromes have been reported [33, 35], and pneumonitis or pulmonary infiltrates due to EBV have been suspected [27]. It is quite clear from our data that a “viral syndrome” may accompany EBV infection, particularly if the infection is primary. CMV may have played a role in some of these viral illnesses, but we have patients in whom no CMV infection could be documented.
It is unclear whether the development of lymphomas was enhanced by the use of cyclosporine, as opposed to other immunosuppressants. None of the renal tranplant recipients who developed lymphomas were receviving azathioprine, but the total number of patients taking azathioprine was too small for adequate analysis. Experience from Minnesota, however, shows that such patients are also at risk [6]. There is no experience in Pittsburgh with liver or heart transplant patients using other immunosuppressants. The heart and heart-lung transplant patients seem to be at greatest risk (this paper, [18]). This may reflect the use of supplemental antithymocyte globulin therapy in four of the five heart and heart-lung transplant patients with tumors. However, we documented high rates of EBV and other herpesvirus infections in the heart transplant patients at our institution before the introduction of antithymocyte globulin therapy [36]. Thus, other factors, as yet undefined, related to the type of transplant may be important.
Bieber et al. [7] reported that 10 (5%) of 200 heart transplant recipients receiving azathioprine, prednisone, and antithymocyte globulin developed lymphomas, whereas five (13%) of 39 developed lymphomas when cyclosporine was substituted for azathioprine. It is likely that the occurrence of such tumors may be decreased by adjustment of cyclosporine doses guided by serum levels [18].
Evidence for EBV infection in patients with tumors was matched by evidence for EBV viral markers in their tumors. Ten (91%) of 11 specimens examined for both EBNA and the presence of EBV DNA by blot hybridization were positive for one or both markers. Nine were positive by hybridization alone. These findings should be viewed against the background that positive EBV hybridizations were not found in 48 lymphomas and leukemias in nontransplant patients [16]. It is possible that increased sensitivity of the hybridization techniques, which are now limited to ~104 genomes or more would have allowed us to detect EBV in all of our tumors.
The precise role that EBV infection plays in the genesis of these tumors is unknown. Hanto et al. [6] postulate a hierarchy of disorders: a nonmalignant lymphoproliferative disorder, polyclonal B cell hyperplasia, polyclonal B cell lymphoma, and finally, monoclonal B cell lymphoma. Patients were also divided into a younger or older group and a group with early or late onset. They suggest that the tumor may progress from polyclonality to monoclonality, although more direct evidence for such progression is not available. We did not see a difference in the clinical course according to age, time of onset, or clonality. In fact it was difficult to distinguish the two patients with B cell hyperplasia from the 12 with lymphomas, except by tissue examination. Possibly we are primarily describing what they would consider to be early tumors.
In addition to immortalization of B lymphocytes, oncogenesis by EBV in Burkitt’s lymphoma requires chromosomal transformation [2]. Hanto et al. [6] describe the karyotypes of six lymphomas. A number of these were abnormal, but the translocation from chromosome eight to 14, commonly seen in Burkitt’s lymphoma, was not found. We have examined the chromosomes from cells cultured from a diffuse large-cell lymphoma of patient 10. All of these cells were diploid, and no aberrations were observed (R. W. A. and S. Pan, unpublished data). Further chromosomal studies are clearly needed to define the complex biology of these tumors.
Finally, although reactivated EBV infection has already been clearly correlated with tumors, we have ascertained that patients with primary EBV infections are at much greater risk of developing lymphomas or the lymphproliferative syndrome. The question arises as to how these tumors may be prevented. Patients at risk for primary infection are easily identified by measuring pretransplant sera for antibodies to EBV. Should a vaccine against EBV be developed, seronegative transplant recipients would be candidates for immunization. The possible benefits of specific immunoglobulins, interferon, or antiviral agents administered prophylactically should also be considered. We have not observed a therapeutic effect with acyclovir in these conditions, and we reemphasize the importance of decreasing immunosuppression [3]. We suggest that the accumulation of more data of the type we have presented on EBV infections and lymphomas is necessary to ascertain the extent of this problem, particularly after routine monitoring of serum cyclosporine levels has become common practice.
Acknowledgments
This work was supported in part by grant 1-RO1-AI-19377 from the National Institute of Allergy and Infectious Diseases.
We thank Dr. M. Nalesnik for the use of anatomic and immunopathological data; Drs. J. Pagano, M. Cleary, and J. Sklar for hybridization studies; and P. Sowizral and L. T. White for technical assistance.
References
- 1.Klein G, Purtilo DT, editors. Symposium on Epstein-Barr virus-induced lymphoproliferative diseases in immunodeficient patients; Cancer Res; 1981. pp. 4209–4304. [PubMed] [Google Scholar]
- 2.Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc Natl Acad Sci USA. 1979;76:2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, Rosenthal JT, Hakala TR, Shaw BW, Jr, Hardesty RL, Atchison RW, Jaffe R, Bahnson HT. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calne RY, Rolles K, White DJG, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, Lewis P. Cyclosporine A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 5.Thiru S, Calne RY, Nagington J. Lymphoma in renal allograft patients treated with cyclosporin-A as one of the immunosuppressive agents. Transplant Proc. 1981;13:359–364. [PubMed] [Google Scholar]
- 6.Hanto DW, Gajl-Peczalska KJ, Frizzera G, Arthur DC, Balfour HH, Jr, McClain K, Simmons RL, Najarian JS. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative diseases occurring after renal transplantation. Ann Surg. 1983;198:356–369. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieber CP, Heberling RL, Jamieson SW, Oyer PE, Cleary M, Warnke R, Saemundsen A, Klein G, Henle W, Stinson E. Lymphoma in cardiac transplant recipients associated with cyclosporin A, prednisone and anti-thymocyte globulin (ATG) In: Purtilo DT, editor. Immune deficiency and cancer. New York: Plenum; 1984. pp. 309–320. [Google Scholar]
- 8.Henle W, Henle G. Epstein-Barr virus-specific serrology in immunologically compromised individuals. Cancer Res. 1981;41:4222–4225. [PubMed] [Google Scholar]
- 9.Schmitz J, Scherer M. IgM antibodies to Epstein-Barr virus in infectious mononucleosis. Archiv fürdie Gesamte Virus-forschung. 1972;37:332–339. doi: 10.1007/BF01241456. [DOI] [PubMed] [Google Scholar]
- 10.Henle W, Henle G. Immunofluorescence in cells derived from Burkitt’s lymphoma. J Bacteril. 1966;91:1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirodaria PV, Fraser KB, Stanford F. Secondary fluorescent staining of virus antigens by rheumatoid factor and fleuorescein-conjugated anti-IgM. Ann Rheum Dis. 1973;32:53–57. doi: 10.1136/ard.32.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henle W, Henle G, Zajac BA, Pearson G, Waubke R, Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970;169:188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- 13.Reedman BM, Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 973;11:499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- 14.Rao N, Waruszewski DT, Armstrong JA, Atchison RW, Ho M. Evaluation of anti-complement immunofluorescence test in cytomegalovirus infection. J Clin Microbiol. 1977;6:633–638. doi: 10.1128/jcm.6.6.633-638.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dummer JS, Hardy A, Poorsattar A, Ho M. Early infections in kidney, heart, and liver transplant recipients on cyclospsorin. Transplantation. 1983;36:259–267. doi: 10.1097/00007890-198309000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Andiman W, Gradoville L, Heston L, Neydorff R, Savage ME, Kitchingman G, Shedd D, Miller G. Use of cloned probes to detect Epstein-Barr viral DNA in tissues of patients with neoplastic and lymphoproliferative diseases. J Infect Dis. 1983;148:967–977. doi: 10.1093/infdis/148.6.967. [DOI] [PubMed] [Google Scholar]
- 17.Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 18.Beveridge T, Krupp P, McKibbin C. Lymphomas and lymphoproliferative lesions developing under cyclosporin therapy [letter] Lancet. 1984;1:788. doi: 10.1016/s0140-6736(84)91293-5. [DOI] [PubMed] [Google Scholar]
- 19.Nikoskelainen J, Klemola E, Leikola J. Epstein-Barr virus-IgM antibody test in infectious mononucleosis [letter] J Infect Dis. 1976;134:313–314. doi: 10.1093/infdis/134.3.313. [DOI] [PubMed] [Google Scholar]
- 20.Henle G, Lennette ET, Alspaugh MA, Henle W. Rheumatoid factor as a cause of positive reactions in tests for Epstein-Barr virus-specific IgM antibodies. Clin Exp Immunol. 1979;36:415–422. [PMC free article] [PubMed] [Google Scholar]
- 21.Henle G, Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976;17:1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 22.Ho HC, Ng MH, Kwan HC, Chan JCW. Epstein-Barr virus-specific IgA and IgG serum antibodies in nasopharyngeal carcinoma. Br J Cancer. 1976;34:655–660. doi: 10.1038/bjc.1976.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans AS, Niederman JC. EBV-IgA and new heterophile antibody tests in diagnosis of infectious mononucleosis. J Clin Pathol. 1982;77:555–560. doi: 10.1093/ajcp/77.5.555. [DOI] [PubMed] [Google Scholar]
- 24.Henle W, Henle G, Horwitz CA. Infectious mononucleosis and Epstein-Barr virus-associated malignancies. In: Lennette EH, Schmidt NJ, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 5th ed. Washington, DC: American Public Health Association; 1979. pp. 441–470. [Google Scholar]
- 25.Horwitz CA, Henle W, Henle G, Polesky H, Balfour HH, Jr, Siem RA, Borken S, Ward PCJ. Heterophil-negative infectious mononucleosis and mononucleosis-like illnesses. Am J Med. 1977;63:947–957. doi: 10.1016/0002-9343(77)90550-2. [DOI] [PubMed] [Google Scholar]
- 26.Nikoskelainen J, Leikola J, Klemola E. IgM antibodies specific for Epstein-Barr virus in infectious mononucleosis without heterophil antibodies. Br Med J. 1974;4:72–75. doi: 10.1136/bmj.4.5936.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheeseman SH, Henle W, Rubin RH, Tolkoff-Rubin NE, Cosimi B, Cantell K, Winkle S, Herrin JT, Black PH, Russell PS, Hirsch MS. Epstein-Barr virus infection in renal transplant recipients. Ann Intern Med. 1980;93:39–42. doi: 10.7326/0003-4819-93-1-39. [DOI] [PubMed] [Google Scholar]
- 28.Jones JF, Ray CG, Minnich LL, Hicks MJ, Kibler R, Lucas DO. Evidence for active Epstein-Barr virus infection in patients with persistent, unexplained illnesses: elevated anti–early antigen antibodies. Ann Intern Med. 1985;102:1–7. doi: 10.7326/0003-4819-102-1-. [DOI] [PubMed] [Google Scholar]
- 29.Straus SE, Tosato G, Armstrong G, Lawley T, Preble OT, Henle W, Davey R, Pearson G, Epstein J, Brus I, Blaese RM. Persisting illness and fatigue in adults with evidence of Epstein-Barr virus infection. Ann Intern Med. 1985;102:7–16. doi: 10.7326/0003-4819-102-1-7. [DOI] [PubMed] [Google Scholar]
- 30.Sumaya CV. Endogenous reactivation of Epstein-Barr virus infections. J Infect Dis. 1977;135:374–379. doi: 10.1093/infdis/135.3.374. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen L, Kelsall D, Nelson R, Carney W, Hirsch M, Winston D, Preiksaitis J, Merigan TC. Virus-specific IgG and IgM antibodies in normal and immunocompromised subjects infected with cytomegalovirus. J Infect Dis. 1982;145:191–199. doi: 10.1093/infdis/145.2.191. [DOI] [PubMed] [Google Scholar]
- 32.Ho M, Wajszczuk CP, Hardy A, Dummer JS, Starzl TE, Hakala TR, Bahnson HT. Infections in kidney, heart, and liver transplant recipients on cyclosporine. Transplant Proc. 1983;15:2768–2772. [Google Scholar]
- 33.Marker SC, Ascher NL, Kalis JM, Simmons RL, Najarian JS, Balfour HH., Jr Epstein-Barr virus antibody responses and clinical illness in renal transplant recipients. Surgery. 1979;85:433–440. [PubMed] [Google Scholar]
- 34.Armstrong JA, Evans AS, Rao N, Ho M. Viral infections in renal transplant recipients. Infect Immun. 1976;14:970–975. doi: 10.1128/iai.14.4.970-975.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grose C, Henle W, Horwitz MS. Primary Epstein-Barr virus infection in a renal transplant recipient. South Med J. 1977;70:1276–1278. doi: 10.1097/00007611-197711000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Dummer JS, Bahnson HT, Griffith BP, Hardesty RL, Thompson ME, Ho M. Infections in patients on cyclosporine and prednisone following cardiac transplantation. Transplant Proc. 1983;15:2779–2781. [Google Scholar]