Abstract
Physiologic triggers and functional consequences of endogenous heat shock protein (HSP) responses in dendritic cells (DC) are poorly defined. In this study, we show that even in the absence of heat stress and infection, a specific cohort of DC/proinflammatory cytokines (IL-4-IL-13/IL-6/GM-CSF) institutes an enhanced inducible (i)HSP70 intracellular and extracellular response in human monocyte-derived DC, especially during the monocyte to DC transition. Interestingly, whereas heat stress alone initiated an intracellular iHSP70 response in monocyte DC precursors, it did not promote cell surface or secreted iHSP70 responses, both of which were induced by cytokines independently of heat. The cytokine-induced iHSP70 response, which did not occur in lymphocytes, or monocytes-macrophages generated with M-CSF, was instituted within 48 h of cytokine exposure, and peaked upon commitment to DC growth at 72 h. Although a return to baseline levels was noted after this period, a distinct rise in iHSP70 occurred again during terminal DC maturation. Chemical inhibition of the iHSP70 response with either triptolide or KNK-437 was coupled with inhibition of DC differentiation and yielded cells displaying features of monocytes-macrophages. Exogenously supplied riHSP70 amplified events associated with cytokine-advanced DC differentiation/maturation, most notably the up-regulation of antiapoptotic proteins (Bcl-xL). Engaging the HSP receptor CD40 with CD40L produced identical results as extracellular riHSP70, and, moreover, an enhanced iHSP70 response. Thus, distinct iHSP70 and HSP receptor-mediated responses are triggered by cytokines irrespective of heat stress and infection in monocyte-derived DC and may function to positively regulate monocyte-derived DC, especially during critical periods of their growth.
Heat shock proteins (HSP)3 are an ancient family of molecules promoting protein stability and transport, and immune activity (1, 2). They function inside cells to secure the proper folding of proteins during synthesis and to direct proteins to the appropriate intracellular compartment. Classic studies using heat as cellular stress have shown that de novo synthesis of certain inducible HSP (iHSP) ensures ample HSP to facilitate the refolding of unfolded or misfolded proteins (1, 2). In adaptive immunity, extracellular HSP, presumably released from either viable or damaged cells, chaperone extracellular immunogens into APCs such as dendritic cells (DC) (2–5). HSP enter immature DC via specific receptors; once inside the cell, they facilitate movement of the chaperoned Ag along the intracellular pathways associated with Ag presentation, i.e., class I and II MHC compartments (2, 6). The chaperoned Ag is delivered to the peptide-binding groove of the MHC molecule; Ag-loaded MHC molecules are then translocated to the DC surface, allowing for MHC-restricted Ag recognition by T cells. In the process called cross-priming, immature DC taking up the HSP-chaperoned Ag develop into fully matured DC (immunogenic DC) capable of potent T cell stimulation. Because HSP exhibit a peptide-binding potential that is similar to that of MHC molecules, cross-priming is an important mechanism by which to achieve active adaptive immunity (2). In a distinct (Ag-independent?) response, the engagement of extracellular HSP by immature APC-expressing HSP receptors has been suggested to initiate signal transduction events that promote inflammation and immune cytokine activity, including the production of cytokines driving DC maturation (2, 7–9).
There is little information concerning the immunologic significance of endogenous iHSP activity in DC. Notwithstanding the evolving role of extracellular HSP (especially that of iHSP70) as a positive regulator of DC growth, physiologic triggers of endogenous (extracellular and secreted) HSP activity in DC are poorly defined. Moreover, the intriguing possibility that DC themselves are a significant source of extracellular iHSP70 has not been fully explored. Although necrotic cells are an obvious source of extracellular HSP that may contribute to cross-priming events, extracellular iHSP70 has been shown to be secreted by distinct cell types, including tumor cells, B cells, and glial cells independently of cell death in response to heat stress and certain cytokines (7). Whether the intracellular, surface, and secreted production of endogenous iHSP70 in viable DC is regulated by similar or discrete mechanisms has not been studied. Interestingly, exposure of DC to heat stress has been reported to accelerate DC maturation and function, but the role of endogenous iHSP in this response is unclear (10–12).
In prior work, we demonstrated that extracellular iHSP70 is expressed on the cell surface of monocyte-derived DC present in the rheumatoid arthritis (RA) joint, which aside from containing high levels of proinflammatory cytokines contains very high levels of iHSP70 and is frequently subjected to hyperthermic stress (13–15). Based on the important role of DC in driving Th1 inflammatory-type responses, in that study we speculated that iHSP70 located on the immature DC surface and/or released by immature DC contributes to DC cross-priming events presumably occurring in the inflamed rheumatoid joint and driving autoimmunity (13, 16). Given the powerful cytoprotective role of HSP70 (17–19), including against TNF-induced lethal inflammatory shock (20, 21), in this study we extend our observations to postulate that endogenous iHSP70/HSP receptor activity in DC promotes positive effects on DC growth. Our objectives included distinguishing physiologic agents leading to enhanced intracellular and extracellular iHSP70 responses in inflammatory monocyte-derived DC in the presence/absence of heat stress (and in the absence of direct pathogenic stimuli), determining whether the endogenous iHSP70 response is associated with functionally distinct phases of DC growth (i.e., immature DC engaged in Ag processing vs mature DC engaged in Ag presentation), gaining insight into whether particular HSP receptors may participate in positive growth effects, and determining whether extracellular iHSP70-mediated positive effects on DC growth include up-regulation of antiapoptotic proteins.
Our results demonstrate that increased intracellular and extra-cellular iHSP70 expression is triggered in monocyte-derived DC by a cohort of cytokines (IL-4-IL-13/IL-6/GM-CSF) even in the absence of heat stress, infection, or cell death, and especially during the monocyte to DC transition. Our findings also suggest that an extracellular iHSP70/HSP receptor pathway may be involved in a positive DC growth response that includes the up-regulation of antiapoptotic proteins. Conceivably, such iHSP70-mediated cytoprotective responses would contribute to the survival of monocyte-derived DC developing in an inflammatory setting containing potential inducers of apoptosis such as TNF.
Materials and Methods
Isolation of cells
This study was conducted according to institutional guidelines. Blood from healthy volunteers was collected into vacutainer tubes containing sodium heparin after Institutional Review Board-approved signed informed consent. Mononuclear cells (MNC) were then prepared using density centrifugation (Accuprep; Accurate Chemical & Scientific) and resuspended in complete medium consisting of RPMI 1640 medium (Life Technologies) with 2 mM L-glutamine, 10 mM HEPES, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 5% pooled normal human serum (NHS/RPMI 1640).
DC growth and culture conditions
DC were generated with cytokines known to sustain monocyte-derived DC from enriched monocyte precursors after adherence of MNC on plastic and from MNC in suspension cultures, as previously described and routinely performed by us (13, 16). For suspension cultures, MNC were adjusted to 1 × 106 cells/ml in NHS/RPMI 1640 and placed in Teflon vials (Scientific Specialties Service). As previously reported (13, 16), and routinely performed by us, this suspension culture system coupled with flow cytometry-assisted multiparametric analysis allows accurate assessment of the effects of the various stimuli on developing DC, monocyte, and lymphoid sub-populations. Monocyte/DC subset gates were calculated on the basis of forward and side light scatter profiles and expression patterns of myeloid cell/DC-associated markers (class II MHC-DR, CD86, CD83, CD14, CD16, CD11c); lymphocytes were characterized by distinct light scatter patterns and lack of myeloid/DC markers. Comparisons between monocytes-macrophages and monocyte-derived DC and studies of fully matured DC were performed under adherent conditions.
Cells were incubated at 37°C or, as reported by others (10), exposed to heat stress in the febrile range. Unless otherwise stated, heat stress consisted of incubating cells at 39°C for 24 h, followed by incubation at 37°C for 24 h and then incubation at 39°C for 24 h in a 5% CO2 humidified incubator. Like others studying the effects of mild heat stress on DC, we found a period of recovery after the initial heat stress to be an accurate measure of the iHSP70 response (10). Although increases in iHSP70 levels were evident after 4 h at 39°C (data not shown), statistically significant responses to stimuli were consistently observed after 72 h of 39°C/37°C cycling. Cultures were supplemented with various combinations of cytokines, including proinflammatory cytokines known to sustain monocyte-DC development as indicated. Human rGM-CSF (Genzyme) was used at 50 U/ml; human rIL-4 (PeproTech) at 50 ng/ml; human rIL-13 (PeproTech) at 10 ng/ml; human rIL-6 at 200 U/ml; and human rTNF-α (Knoll Pharmaceuticals) at 500 U/ml. For the generation of monocytes-macrophages, human rM-CSF (Genzyme) was used at 50 ng/ml. Where indicated, terminal monocyte-DC differentiation was induced by the addition of TNF-α or LPS (Sigma-Aldrich) at 10 ng/ml on day 4. All culture conditions and culture vessels were low in endotoxin, as previously described by us (22). Chemical inhibition of the iHSP70 response was achieved with either triptolide (Sigma-Aldrich) or KNK-437 (Calbiochem). Both chemicals were dissolved in DMSO and stored at −20°C in a dessicated chamber protected from light. Triptolide was stored as a 10−2 M stock and KNK-437 as a 10 mM stock. Freshly prepared dilutions of either chemical were added to cells, as indicated. Low endotoxin CD40L (Cell Sciences) and riHSP70 (Assay Design) were added at 100–200 ng/ml at the indicated times.
Immunofluorescence (IF) studies
For detection of intracellular proteins (iHSP70, heat shock factor (HSF)-1, Bcl-2, Bcl-xL), cells were treated with Fix and Perm Kit (Caltag Laboratories), according to the manufacturer’s instructions, and then incubated at room temperature with the primary Ab, followed by incubation with the appropriate secondary Ab linked to Alexa Fluor 488 (Molecular Probes). After fixation in 1% PBS-buffered formalin, cells were immediately analyzed by flow cytometry (FACSCalibur; BD Biosciences). iHSP70 was detected using an anti-HSP70 mAb that is specific for iHSP70 and that does not cross-react with constitutive HSP70 (SPA-810; Assay Design). A polyclonal Ab (SPA-901; Assay Design) was used to detect the iHSP70 transcription factor HSF-1; a mAb to Bcl-2 (clone 124) was purchased from DakoCytomation; Bcl-xL mAbs (clone H-5) were purchased from Santa Cruz Biotechnology. For analysis of surface markers, cells were resuspended in staining buffer (PBS/0.1% sodium azide/1% albumin) and incubated at 4°C with Abs or isotype controls directly linked to either PE or FITC. Abs to CD11c, CD40, CD91, and CD16 were obtained from BD Pharmingen; anti-CD83 was obtained from Caltag Laboratories; and anti-CD14 was obtained from Sigma-Aldrich. The flow cytometer was calibrated using Calibrite beads (BD Biosciences), and ≥10,000 events were acquired. Results were obtained using CellQuest analysis software (BD Biosciences) and are expressed as percentage of positive cells or mean fluorescence intensity (MFI) after subtracting negative control values (iso-type-matched control Abs). Analysis was performed on gated monocyte/DC subsets using comparable gates for adherent and suspension cultures. Gates were calculated on the basis of forward and side light scatter profiles and expression patterns of myeloid cell/DC-associated markers, as indicated above. Analysis of cells within these gated areas produced similar patterns of iHSP70 responses for adherent and suspension conditions.
Cell surface protein biotinylation and iHSP70 immunoblot analysis
In preliminary experiments, we determined that surface HSP expression may not be consistently detected by IF analysis due to epitope masking (possibly resulting from partnering with other molecules at the cell surface). We therefore assessed surface iHSP70 expression by immunoblot analysis after biotinylation of cell surface membrane proteins. Briefly, cells were adjusted to a density of 20 × 106/ml and incubated with 2 mM NHS-LC-LC-biotin (Pierce) in PBS for 30 min on ice. After this time, cells were washed twice with PBS plus 100 mM glycine to quench the reaction and remove excess biotin reagent. Viability was >98% after the biotinylation procedure, as measured by exclusion of trypan blue. Cells were lysed in modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 0.5% sodium deoxycholate, and 1% Nonidet P-40) containing protease inhibitors (1 mM PMSF, 10 μg/ml apoprotinin, leupeptin, and trypsin inhibitor). Biotinylated surface proteins were then affinity purified with streptavidin-agarose (Pierce) at 4°C for 1–2 h, eluted by boiling in sample buffer for SDS-PAGE. Immunoblot analysis was performed using Abs to iHSP70 (StressGen Biotechnologies), followed by 2° Ab linked to HRP. Equivalent amounts of total protein were loaded for each sample, as calculated by starting cell counts, total protein values (Pierce BCA), and GelCode Blue staining of protein in gels (Pierce). Immunoreactivity was revealed by chemiluminescence (West Femto substrate; Pierce). Densitometric analysis was performed using a ChemiImager 4400 and software from Alpha Innotech. Lysates prepared from nonbiotinylated cells and subjected to streptavidin-agarose purification schemes did not produce reactivity in the immunoblot analysis with iHSP70 (data not shown).
ELISA
iHSP70 content in cell-free supernatants was measured using a commercial quantitative sandwich ELISA (Assay Design), with a sensitivity of 200 pg/ml. Experiments were performed exactly as recommended; samples were assayed in triplicate. Results were detected using a microplate ELISA reader at 450 nm and are expressed as nanograms of iHSP70 per milliliter. The Abs used in the ELISA are specific for iHSP70 and do not cross-react with constitutive HSP70.
Apoptosis
Cells were removed from culture, centrifuged (300 × g for 5 min), resuspended in cation-free low endotoxin PBS, and then placed in 1× annexin V binding buffer (1 mM HEPES (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2) at a concentration of 1 × 106 cells/ml. A total of 100 μl of the solution (1 × 105 cells) was then combined with 5 μl of annexin V-FITC (BD Pharmingen) for 15 min at room temperature in the dark, followed by the addition of 400 μl of binding buffer. Propidium iodide (Sigma-Aldrich) was added at 10–15 μg/ml after annexin V staining. Samples were acquired for flow cytometry analysis within 1 h and analyzed with CellQuest software. Apoptotic events were also assessed by light scatter patterns and morphological analysis of Wright-stained cells using light microscopy after examining >500 cells per condition.
Statistical analysis
Student’s t test and Mann-Whitney rank tests were performed using statistical software (SigmaStat; Jandel). A value of p ≤ 0.05 was considered to be a significant difference.
Results
A heat stress-independent iHSP70 response is initiated in monocyte-DC precursors in response to proinflammatory/DC growth cytokines
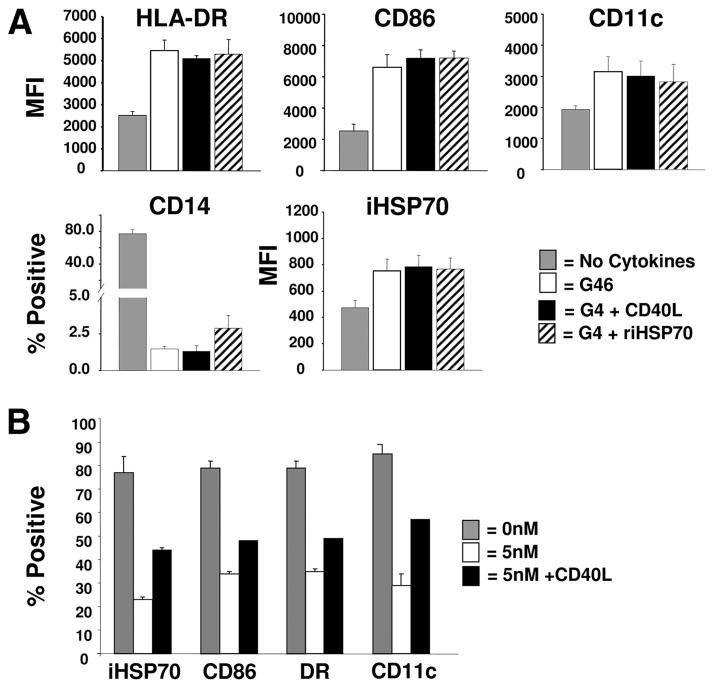
It has been shown that heat-induced expression of HSP promotes the synthesis of proinflammatory cytokines that may sustain DC growth, and that exogenous HSP directly binds to the DC surface to signal maturation (9, 23). However, the identification of physiologic mediators such as inflammatory cytokines/DC growth factors that may promote/sustain an endogenous HSP response in DC has not been established. Flow cytometric analysis of monocyte-DC precursors cultured in the absence of cytokines revealed that heat stress (39°C/37°C; 24-h cycling for 72 h) triggered increases in iHSP70 levels vs 37°C (Fig. 1, A and B). Intriguingly, culturing monocyte-DC precursors at 37°C in GM-CSF plus IL-4 also yielded increases in iHSP70 vs no cytokines at 37°C (Fig. 1B; p = 0.02, n = 4). Inclusion of IL-6 (an accessory DC/proinflammatory cytokine) enhanced the GM-CSF plus IL-4-mediated HSP response at either temperature; however, iHSP70 levels were highest with GM-CSF plus IL-4 plus IL-6 at 39°C vs 37°C (p = 0.04, n = 5). Thus, whereas heat stress and DC/proinflammatory cytokines cooperate to yield maximal levels of iHSP70 in the system tested, hyperthermia is not required for increasing iHSP70 levels in DC. In fact, cells cultured in GM-CSF plus IL-4 plus IL-6 at 37°C exhibited comparable levels of iHSP70 as cells exposed to hyperthermia alone (Fig. 1B; p = 0.16, n = 6). Inclusion of TNF-α from the outset did not yield increases in iHSP70 levels above GM-CSF plus IL-4 plus IL-6, indicating that TNF-α is not involved in promoting the cytokine-induced iHSP70 response in monocyte-DC precursors (Fig. 1B). IFN-γ also failed to amplify the GM-CSF/IL-4/IL-6 iHSP70 response (data not shown). In Fig. 1C, individual and combined effects of GM-CSF, IL-4, and IL-6 on iHSP70 induction were compared. Addition of GM-CSF alone, IL-6 alone, GM-CSF plus IL-6, or IL-4 plus IL-6 did not yield increases above no cytokine controls (p > 0.05). Based on these results, we conclude that GM-CSF, IL-4, and IL-6 act cooperatively to initiate an iHSP70 response in monocyte-DC precursors. In further support of cytokine-induced up-regulated iHSP70 activity, the total level of the iHSP70 transcription factor HSF-1 increased with GM-CSF, IL-4, and IL-6 treatment on day 3 compared with baseline values obtained on day 0 (Fig. 1D, upper section) and to no cytokine controls on day 3 (supplementary Fig. 1).4 Immunoblot analysis (Fig. 1D, lower section) and fluorescence microscopic analysis (supplementary Fig. 2)4 also demonstrated increased molecular mass corresponding to activated (hyperphosphorylated) HSF-1 and translocation of HSF-1 from the cytoplasm to the nucleus, respectively, with GM-CSF plus IL-4 plus IL-6.
FIGURE 1.
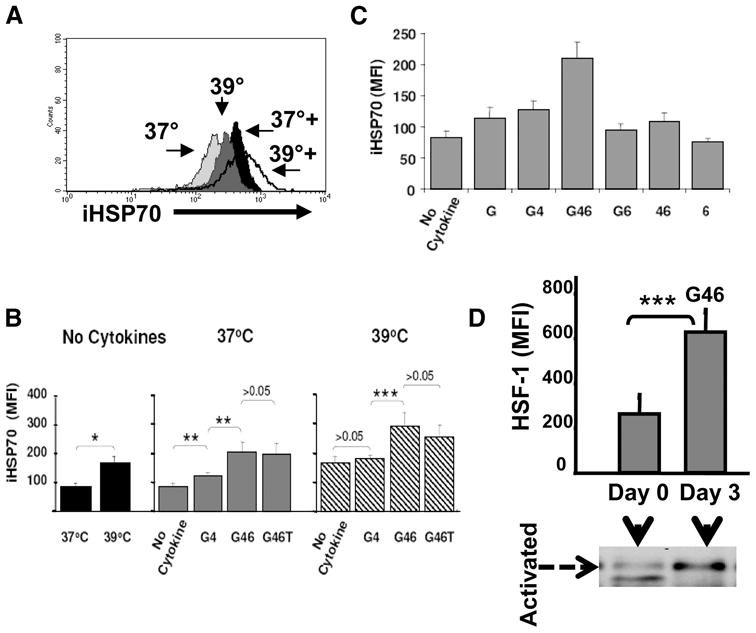
Heat-independent up-regulation of iHSP70 expression in maturing monocyte-derived DC by a discrete group of inflammatory/DC cytokines. iHSP70 levels are denoted as MFI in monocyte/DC and were analyzed by flow cytometry in permeabilized cells after 72 h of culture in the presence/absence of cytokines, with or without exposure to hyperthermia (39°C/37°C cycling for 72 h). GM-CSF = G; IL-4 = 4; IL-6 = 6; TNF = T. A, Overlay histograms depicting relative iHSP70 levels with/without heat in the absence/presence of cytokines (+ = G + 4+6+T). A typical experiment is shown (n ≥3). B, The effects of hyperthermia and GM-CSF plus IL-4 ± IL-6 ± TNF on iHSP70 expression (MFI). *, p < 0.0005; **, p < 0.02; ***, p < 0.05. For no cytokine, n = 11; for 37°C, n = 4–7; for 39°C, n = 4–11. Results represent the mean ± SE. C, Dissecting the cytokine response at 37°C: individual and combined effects of cytokines on iHSP70 induction. For all conditions tested except GM-CSF plus IL-4 and GM-CSF plus IL-4 plus IL-6, p > 0.05 vs no cytokine controls. n = 3–11. For GM-CSF plus IL-4 and GM-CSF plus IL-4 plus IL-6 vs no cytokine controls, p < 0.05. Results represent the mean ± SE. D, Increased HSF-1 activity with G46 as indicated by increases in the total levels of HSF-1 (bar graph, n = 3–5, p = 0.026) and increased levels of activated (hyperphosphorylated) HSF-1 by immunoblot analysis (n = 3).
IL-13 also exhibits the ability to initiate the iHSP70 response
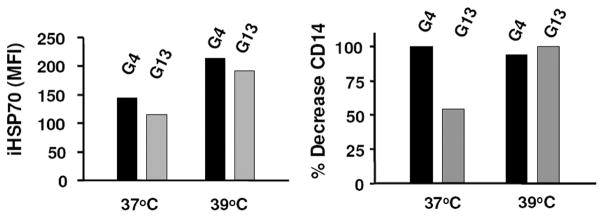
By sustaining DC growth, the production of proinflammatory cytokines such as IL-6 and Ab synthesis, IL-13 may be involved in inflammatory immunity (24–27). Because IL-13 and IL-4 induce many of the same biological responses, including the ability to promote DC differentiation from monocyte-DC precursors (27), we were interested in determining whether IL-13 could replace IL-4 in the cytokine-induced iHSP70 response. As shown in Fig. 2, GM-CSF plus IL-13 produced comparable iHSP70 levels to GM-CSF plus IL-4 by 72 h, and heat stress amplified the iHSP70 response generated with either GM-CSF plus IL-4 or GM-CSF plus IL-13 (heat vs no heat; p = 0.03, n = 6). Thus, the ability to increase iHSP70 levels in monocyte DC is shared by IL-4 and IL-13. At either temperature, GM-CSF plus IL-13 promoted DC maturation, as determined by loss of the monocyte marker CD14 (Fig. 2), increased MHC class II (DR) and costimulatory molecule (CD86) levels, and typical DC morphology (data not shown). However, even though GM-CSF plus IL-13 significantly reduced CD14 expression at 37°C (57% decreases vs no cytokine controls; p < 0.001, n = 6), GM-CSF plus IL-4 consistently yielded greater reductions in CD14 than GM-CSF plus IL-13 at 37°C (89% decrease vs no cytokine controls; p = 0.019 for GM-CSF plus IL-13 vs GM-CSF plus IL-4; n = 6). Incubation at 39°C yielded equally reduced CD14 levels on maturing DC (Fig. 2) with both cytokine combinations, indicating that heat amplifies the DC maturation effects of IL-13. These data are also consistent with previous observations that heat may accelerate DC maturation (10, 11).
FIGURE 2.
IL-13, which may substitute for IL-4 as a DC growth factor, also induces a coupled DC maturation/endogenous iHSP70 response. iHSP70 levels were determined in DC cultures instituted with GM-CSF plus IL-4 (G4) and GM-CSF plus IL-13 (G13) after 72 h by flow cytometry. To further assess differences between the two conditions, expression of the monocyte marker CD14 (shown as percentage of decrease in CD14+ cells) was also included on day 3. A typical experiment is shown; n ≥ 6 for each condition; for all conditions vs no cytokine controls, p < 0.005.
Up-regulation of iHSP70 occurs in monocyte DC during the monocyte to DC transition, but not in M-CSF-treated monocytes-macrophages, proinflammatory/DC cytokine-treated lymphocytes, or heat-stressed fully matured DC
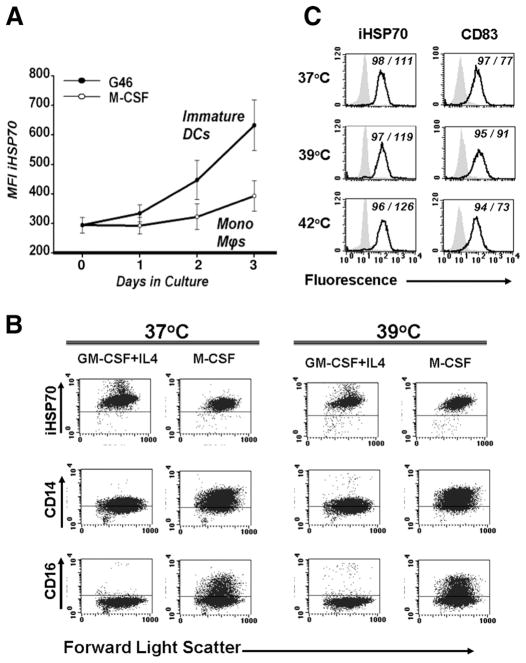
To date, our results indicate that by day 3, the combined effects of certain DC cytokines produce enhanced iHSP70 responses in monocyte-derived DC irrespective of heat stress. As determined by the down-regulation of monocyte-associated markers and up-regulation of DC-associated markers and function, these cells represent newly committed monocyte-derived DC (28). A temporal analysis of iHSP70 expression during the monocyte to DC transition period (days 0–3) (28) revealed that the DC cytokines induced a precipitous rise in iHSP70 levels between days 1 and 3, precisely the time corresponding to DC differentiation (Fig. 3A). In marked contrast, treatment with M-CSF, a noninflammatory cytokine sustaining the growth of monocytes-macrophages instead of DC from circulating monocyte precursors, did not produce increased iHSP70 responses above baseline levels during a similar 3-day period (Fig. 3A). Direct comparisons of iHSP70 expression during DC growth vs monocyte-macrophage growth further demonstrated significantly increased iHSP70 levels with DC cytokines on days 2 and 3 (Fig. 3A). As expected, monocyte precursors treated with M-CSF or DC cytokines exposed to heat stress underwent increases in iHSP70 levels (Fig. 3B). However, unlike the strong iHSP70 response induced by DC cytokines in the absence of hyperthermia (37°C), the M-CSF response at 37°C was no different from the no cytokine response at 37°C (p = 0.57, n = 3–4; data not shown). The M-CSF-driven growth of monocytes-macrophages over DC from peripheral blood monocyte precursors was confirmed by sustained expression of CD14 and CD16 (Fc receptors) (Fig. 3B) and Wright stain microscopic analysis (data not shown). DC-associated differentiation/maturation events during this period were similarly confirmed by flow cytometry-assisted analysis using a battery of DC-associated markers, including class II MHC-DR, CD86, CD83, CD11c, and CD40 (data not shown).
FIGURE 3.
The iHSP70 response is distinctly triggered during the monocyte to DC transition. A, Temporal analysis of iHSP70 expression in permeabilized cells during the first 3 days of DC growth (the monocyte to DC transition period) with GM-CSF plus IL-4 plus IL-6 (G46) vs monocyte-macrophage growth with M-CSF at 37°C. Note the precipitous rise of iHSP70 levels in DC cultures vs the relatively consistent levels in monocyte-macrophage cultures. n = 7–8. For G46 vs M-CSF, p = 0.003 and 0.031 for days 2 and 3, respectively; p = 0.08 for day 1. For G46, day 0 vs day 1, p = 0.39; for day 0 vs day 2, p = 0.054; for day 0 vs day 3, p = 0.001. For M-CSF, day 0 vs all days, p = 0.05. B, Cells cultured with DC-directed cytokines GM-CSF plus IL-4 or monocyte/macrophage growth factor M-CSF, in the absence/presence of heat stress, were analyzed after 72 h. The prevalence of CD14/CD16 markers with M-CSF confirms a monocyte/macrophage vs DC phenotype. For iHSP70, M-CSF-treated cells vs no cytokine controls at 37°C, p > 0.05; for GM-CSF plus IL-4 vs no cytokine controls, p < 0.05. Horizontal lines within each graph represent negative control values. Results represent one of two to four experiments conducted in parallel for each marker. C, The HSP response is only marginal in mature (CD83+) inflammatory DC after heat stress. Mature DC were generated with GM-CSF plus IL-4 on day 0, then TNF added on day 4. On day 7, cells were left at 37°C for 24 h, transferred to 39°C for 24 h, or pulsed for 45 min at 42°C, and then allowed to recover at 37°C for a total of 24 h. Enhanced levels of DC maturation markers and Wright stain microscopy (data not shown) confirmed a mature DC phenotype. The ↑CD83 MFI levels at 39°C vs 37°C are consistent with heat-induced DC maturation. A typical experiment performed in parallel is shown. n = 5 for 37°C and 39°C; n =3 for 42°C. Shaded histograms represent isotype controls. Numbers within graphs represent percentage of positive cells/MFI.
Terminally differentiated (fully matured) monocyte-derived DC generated with GM-CSF plus IL-4 plus TNF and subjected to either 39°C/37°C cycling or a 45-min pulse at 42°C (Fig. 3C) and analyzed on day 8 demonstrated only a marginal increase in iHSP70 (both conditions vs 37°C; p > 0.05, n = 3–5). Consistent with previous results demonstrating a lack of iHSP70 in lymphocytes present in the inflamed rheumatoid joint (13), normal peripheral blood lymphocytes cultured in the presence of inflammatory/DC cytokines ± heat stress expressed low levels of iHSP70 (Table I).
Table I.
Lymphocytes do not undergo an inflammatory cytokine-driven iHSP70 response in the absence of heata
| 37°C No Cytokine | 37°C G46 | 39°C No Cytokine | 39°C G46 | |
|---|---|---|---|---|
| MFI mean ± SE | 4.5 ± 1.0 | 5.3 ± 1.0 | 5.6 ± 0.7 | 5.4 ± 1.0 |
| Range | 3–7 | 3–8 | 4–8 | 3–8 |
| n | 4 | 4 | 5 | 5 |
Flow cytometric-assisted analysis of iHSP70 on permeabilized cells was performed after gating on lymphocytes (identified by forward and light scatter properties). In contrast to the iHSP70 MFI levels achieved in immature DC responding to GM-CSF plus IL-4 plus IL-6 (G46), lymphocytes responded poorly to GM-CSF plus IL-4 plus IL-6 and heat (<6.0 MFI). For all comparisons, p > 0.05 vs 37°C, no cytokine; analysis was performed on day 3.
Analysis of iHSP70 levels following the monocyte to DC transition period
To determine whether the cytokine-induced increase in iHSP70 was sustained in immature DC beyond the point of firm commitment to DC growth, we also studied iHSP70 levels after day 3. Included in the study was determination of iHSP70 expression in DC undergoing terminal maturation induced by either TNF or LPS (added on day 4). Flow-assisted analysis of permeabilized cells revealed significantly reduced levels of iHSP70 in day 7 GM-CSF plus IL-4 vs day 3 GM-CSF plus IL-4 cultures (Fig. 4A; p = 0.026, n = 3). A separate analysis performed between days 4 and 7 demonstrated that, with GM-CSF plus IL-4, iHSP70 levels were not increased beyond day 3 (data not shown). Actually, in the absence of a terminal differentiating agent, the iHSP70 response declines after peak expression on day 3. Terminal maturation initiated with TNF-α (representing sterile inflammation) (Fig. 4A) did not result in significantly increased iHSP70 levels vs GM-CSF plus IL-4 on day 7 (p > 0.05, n = 3), which is in line with our observation that TNF may not participate in prompting the iHSP70 response in DC (Fig. 1B). However, LPS-treated DC (representing pathogen-induced effects) exhibited higher levels of iHSP70 on day 7 than GM-CSF plus IL-4 on day 7 (p = 0.029, n = 3; Fig. 4A). Interestingly, the higher levels of endogenous iHSP70 achieved with LPS correlated with advanced DC maturation, especially as reflected by increased expression of CD83 and CD86 molecules (Fig. 4B). Collectively, these results demonstrate that once DC growth is established, iHSP70 levels return to baseline in immature DC. Nonetheless, terminal maturation signals may prompt a distinct schedule of up-regulated endogenous iHSP70 activity that is closely linked to the final stages of DC growth.
FIGURE 4.
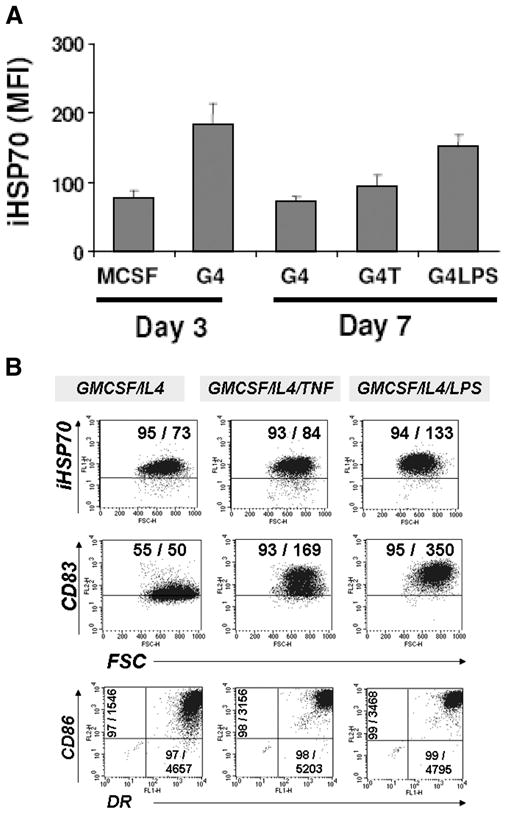
The expression of iHSP70 varies according to the stage of DC growth. Newly committed DC were studied on day 3; immature DC were studied on day 7; terminally matured DC were studied on day 7 (after the addition of TNF-α or LPS on day 4). Cells were cultured at 37°C and analyzed by flow cytometry. Comparisons were performed in parallel; n = 3 for all. A, Pooled results are shown; iHSP70 levels with M-CSF (similar to baseline levels) are shown for comparison. B, A representative dot plot analysis for day 7. Quadrants were set based on isotype controls. Numbers within graphs represent percentage of total positive cells/MFI of total events for each marker. Note decreased iHSP70 levels after day 3 and that higher iHSP70 levels prompted by LPS on day 7 correlate with increased expression of DC maturation markers. For days 3 vs 7 G4, p = 0.03.
An endogenous extracellular iHSP70 response is prompted in immature DC by inflammatory cytokines independently of heat stress
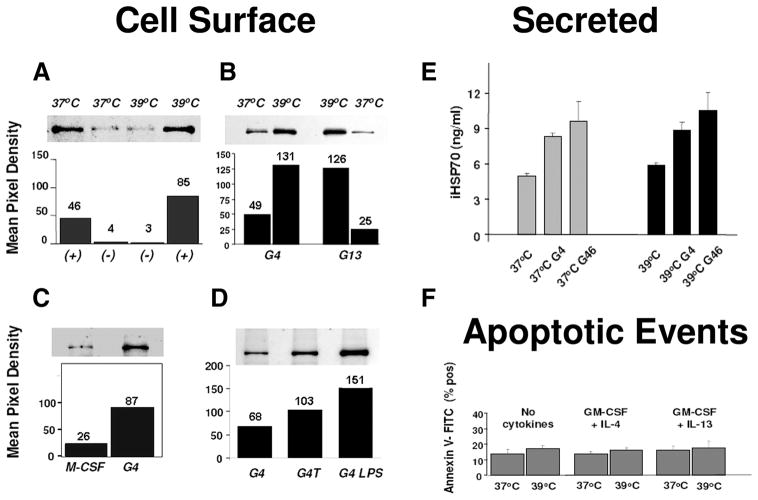
One of the least understood distribution patterns of HSP is extra-cellular expression occurring in the absence of necrotic cell death. In the next set of experiments, we determined that extracellular iHSP70 expression in monocyte-derived DC does not occur with heat stress alone and is fundamentally dependent on inflammatory/DC cytokines (Fig. 5). Surface protein biotinylation followed by immunoblot analysis revealed that on day 3, cells newly committed to DC growth under the influence of GM-CSF plus IL-4 plus IL-6 plus TNF at 37°C (37°+) expressed elevated levels of surface iHSP70 vs 37°C and 39°C no cytokine conditions (−); hyperthermia (39°+) furthered cytokine-induced effects (Fig. 5A). GM-CSF plus IL-4 and GM-CSF plus IL-13 also promoted surface iHSP70 expression independently of heat stress on day 3, although the surface response occurring with IL-13 (Fig. 5B) appeared to be more heat dependent. Consistent with the data depicted in Fig. 3 showing reduced iHSP70 levels with M-CSF vs GM-CSF plus IL-4 in permeabilized cells, surface expression on M-CSF-treated monocytes was reduced vs GM-CSF plus IL-4-treated DC (Fig. 5C). Thus, whereas heat stress increases intracellular levels of iHSP70, in our system, translocation to the DC surface is strictly inflammatory cytokine dependent. Nonetheless, hyperthermia may enhance cytokine-induced surface expression by increasing the available iHSP70 pool. Further comparisons of immature and mature DC cultured at 37°C and studied on day 7 indicated sustained expression of iHSP70 on the DC surface, especially when terminal DC maturation was achieved with LPS (Fig. 5D).
FIGURE 5.
Extracellular (cell surface and secreted) iHSP70 responses associated with monocyte-derived DC are heat stress independent. A–D, Cell surface iHSP70 protein was revealed after biotinylation and streptavidin selection, SDS-PAGE, and immunoblot analysis. Cells were cultured with combinations of GM-CSF (G)/IL-4 (4)/IL-13 (13), TNF-α, or M-CSF. A–C, Represent analysis on day 3. In A, + = G/4/6/TNF; − = no cytokines. B and C, Cells were cultured with combinations of G/4/13 or M-CSF. D, Cells were cultured for 7 days with/without TNF-α or LPS (added on day 4) to induce terminal DC maturation. A–D, The mean pixel density number shown above bars was obtained by densitometric analysis. A and B, Represent DC cultured in suspension; C and D, represent results obtained from monocyte precursors enriched by adherence. A–D, A representative comparison performed in parallel using cells prepared from the same donor is shown. For no cytokine cultures at 37°C and 39°C, n = 5; for all cytokine combinations, n = 2–4. E and F, Release of iHSP70 occurs independently of heat and apoptosis. E, Cell-free supernatants were collected after 72 h and immediately stored at −80°C until the time of the iHSP70-specific ELISA. G4 = GM-CSF plus IL-4; G46 = GM-CSF plus IL-4 plus IL-6. Results represent the mean ± SE. For all conditions tested, n ≥ 4. F, Apoptosis was measured after 72 h by flow cytometry-assisted analysis of annexin V-FITC-labeled cells. Results represent percentage of annexin V-FITC-positive cells, expressed as the mean ± SE (n = 3–6). For all comparisons, there is no significant difference (p > 0.05) in the percentage of apoptotic cells. Morphological examination of the cells by Wright stain analysis and light scatter patterns also showed a lack of apoptotic events between the conditions (data not shown).
Despite mounting evidence that extracellular HSP may target DC to promote cross-priming and adjuvant activity (3, 4, 9, 29, 30), the release of HSP from viable DC has not yet been well characterized. To determine whether iHSP70 was released from viable monocyte DC under the conditions studied, we analyzed cell-free supernatants obtained from cells cultured with inflammatory/DC cytokines ± heat stress (Fig. 5E). Results from an anti-iHSP70-specific ELISA of cell-free supernatants acquired on day 3 showed that iHSP70 secretion was markedly increased in cultures established with GM-CSF plus IL-4 ± IL-6 vs no cytokine controls (p < 0.05, n = 4–6). GM-CSF plus IL-13 also promoted secretion of iHSP70 in maturing cells (data not shown). Like the inability to promote surface iHSP70 expression, heat stress alone did not induce significant extracellular release of iHSP70 vs 37°C controls (p = 0.11, n = 4). Moreover, although hyperthermia increases the intracellular pool of iHSP70 in maturing DC, it is insufficient for promoting extracellular iHSP70 release from these cells. Consistent with the inability of M-CSF to promote intracellular and surface iHSP70 expression (Figs. 3, A and B, and 5C), release of iHSP70 from M-CSF-treated monocytes was also not increased above 37°C controls (<1 ng/ml vs 2 ng/ml, n = 4) and significantly decreased vs GM-CSF plus IL-4 at 37°C (p = 0.01, n = 4). The lack of translocation of phosphatidylserine to the cell surface membrane revealed that, with GM-CSF plus IL-4 (Fig. 5F) and GM-CSF plus IL-4 plus IL-6 (data not shown), extracellular iHSP70 originated from viable cells on day 3 and was not the result of increased apoptosis. Assessment of cell death between days 0 and 3 by flow cytometry-assisted multiparametric analysis demonstrated light scatter patterns consistent with viable cells and the absence of necrotic events (assessed as propidium iodide+ annexin V− cells) and, by Wright stain-assisted morphological examination showed no increases in apoptotic events above baseline (data not shown). Thus, inflammatory/DC cytokines promote up-regulated expression of surface-associated and secreted iHSP70 independently of heat stress and cell death.
Chemical inhibition of the endogenous iHSP70 response is coupled to DC differentiation/maturation arrest
Because of the novelty of the cytokine-induced endogenous iHSP70 response in DC, and the fact that heat stress alone does not sustain monocyte DC differentiation/maturation events, most of our subsequent focus was placed on elucidating the functional significance of the cytokine-induced iHSP70 response. To further study the link between the cytokine-triggered iHSP70 response and DC growth during the monocyte to DC transition, we tested the effects of two known inhibitors of the HSP response, KNK-437, a synthesized benzylidene lactam compound, and triptolide (PG490), a diterpene triepoxide extracted from the Chinese herb Tripterygium wilfordii. In line with its long-recognized immunosuppressive activity, recent studies indicate that pharmacologic concentrations of triptolide potently inhibit human and murine DC maturation, differentiation, and function (31–33). Although both triptolide and KNK-437 have been reported to inhibit the HSP70 response in cancer cells (34–38), their effects on the DC HSP response are unknown. Because triptolide has been shown to induce concentration-dependent apoptosis, we first conducted a careful dose analysis to ensure that any suppression of DC growth was not due to apoptosis. In agreement with prior reports (32, 33), DC cultures containing less than 10 nM triptolide did not display increased apoptosis. In marked contrast, with 100 nM triptolide, virtually all cells were undergoing cell death by day 3, as evidenced by flow cytometry-assisted analysis of light scatter patterns, trypan blue-assisted viability counts, and Wright stain morphological analysis (data not shown). Unlike triptolide, and consistent with its lower toxicity (36), KNK-437 similarly tested at a range of doses (10–100 nM) did not induce cell death in the DC cultures (data not shown).
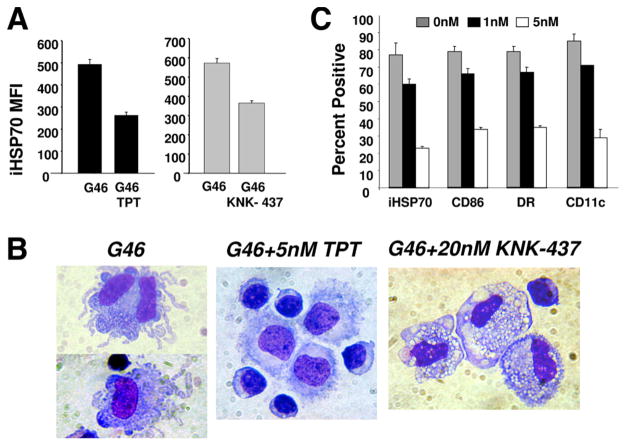
Fig. 6A demonstrates statistically significant inhibition of the DC cytokine-induced iHSP70 response when either triptolide or KNK-437 was added to the cultures (37°C) during the monocyte to DC transition period. Comparisons made between cells cultured in the presence/absence of each chemical were performed in parallel; results represent doses yielding optimal inhibition of the iHSP70 response. Wright stain-assisted microscopic analysis of cells displaying a reduced iHSP70 response with either chemical revealed a dramatically altered phenotype that was suggestive of disrupted DC growth (Fig. 6B). Instead of the irregularly shaped immature DC generated with DC cytokines in the absence of triptolide or KNK-437, the inclusion of either chemical in the culture yielded a predominance of uniformly shaped displaying various features of monocyte/macrophage-like cells. Further investigation revealed that chemical inhibition of the iHSP70 response with triptolide (Fig. 6C) and KNK-437 (data not shown) coincidently decreased the expression of DC-associated markers during the monocyte to DC transition period and confirmed DC growth arrest. With triptolide, optimal inhibition of the iHSP70 response was achieved with 5 nM, which also yielded optimal DC maturation arrest, assessed by the decreased distribution of HLA-DR, CD86, and CD11c (3- to 4-fold decreases vs cytokines no triptolide; n = 3, p < 0.019 for all markers). In further support of inhibition of the cytokine-induced iHSP70 response, decreases in the total level of HSF-1 were also noted with 5 nM triptolide (data not shown).
FIGURE 6.
Chemical inhibition of the cytokine-triggered iHSP70 response with triptolide or KNK-437 is coupled with DC maturation arrest. A, Triptolide (5 nM) or KNK-437 (20 nM) added to DC cultures (GM-CSF plus IL-4 plus IL-6 = G46) on day 1 yields statistically decreased levels of iHSP70 (measured by flow cytometry). Results represent mean ± SE, n = 3. For G46 no triptolide (TPT) vs G46 plus TPT, p = 0.002; for G46 no KNK-437 vs G46 plus KNK-437, p = 0.006. B, Photomicrographs taken on day 3 depicting typical immature DC morphology with G46 and monocyte/macrophage-like morphology with either TPT or KNK-437. Original magnification, ×60. C, Coincident inhibition of the iHSP70 (measured in permeabilized cells) and DC growth response (assessed by decreased expression of DC maturation markers) occurring with increasing physiological doses of TPT. Data were analyzed by flow cytometry; results represent the mean ± SE; n = 3. For G46 vs 5 nm TPT all markers, p ≤ 0.019.
HSP receptors, especially CD40, are expressed on monocyte-derived DC that also express extracellular iHSP70
Engagement of surface HSP receptors on DC by extracellular HSP may lead to initiation of signaling cascades associated with DC maturation and function (30, 39, 40). Before investigating the effects of extracellular iHSP70 in our system, we assessed the distribution of certain HSP receptors on DC exhibiting extracellular iHSP70 on day 3 and on DC induced to undergo terminal maturation (day 7). One of the earliest events accompanying monocyte-derived DC growth under the conditions tested is the rapid decline of CD14 on monocyte DC precursors (Figs. 2 and 3) (27). Thus, CD14 is not a HSP receptor that is likely to participate in the iHSP70 response at the stages of DC maturation studied. However, CD40 and CD91, which are additional receptors for HSP70, are abundantly expressed on the surface of DC on day 3 (Fig. 7A). Analysis of GM-CSF plus IL-4 ± TNF or LPS-treated cells on day 7also revealed persistence of these receptors, especially CD40, on DC (Fig. 7B). Studies with GM-CSF plus IL-4 plus IL-6 revealed similar patterns of HSP receptors (data not shown). Thus, the biological outcome resulting from engagement of extracellular iHSP70 by HSP receptors on the DC surface is likely to reflect the variable expression of distinct HSP receptors during different phases of DC growth.
FIGURE 7.
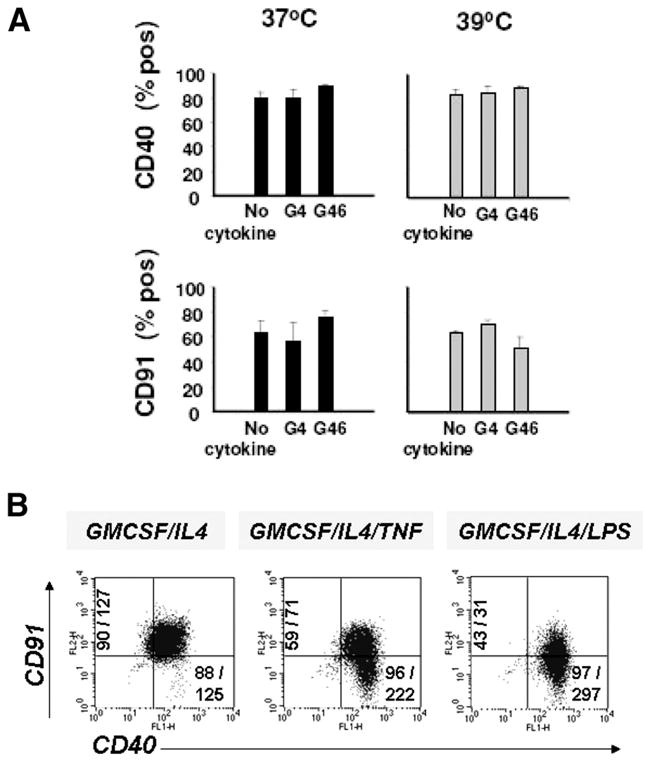
The HSP receptor CD40 is strongly expressed on newly committed DC on day 3 (A), immature DC on day 7, and fully matured DC on day 7 (B). Receptors were detected by flow cytometry-assisted IF analysis. Percentage of positive values is shown after subtracting isotype control values. Results represent the mean ± SE for A and a typical experiment for B. B, TNF-α or LPS was added on day 4 to DC cultures (37°C) to induce terminal DC maturation, numbers on the corresponding axis for CD91, and CD40 represent percentage of positive cells/MFI. For CD40, n = 3–8; for CD91, n = 3–7.
Extracellular iHSP70 cooperates with GM-CSF plus IL-4 to promote a coupled endogenous iHSP70/DC growth response
To study the biological response of cells engaged in the monocyte to DC transition to extracellular iHSP70, we added riHSP70 to the cultures. Because up-regulation of CD40 and extracellular iHSP70 occurred during similar time frames (Figs. 5 and 7) and CD40L and iHSP70 both signal via CD40 (39, 41–43), we also compared the effects of CD40L. In Fig. 1, we had determined that even though GM-CSF plus IL-4 prompted statistically significant increases in iHSP70 during the monocyte to DC transition, even higher levels were observed when IL-6 was included in the mixture. In Fig. 8A, we investigated whether exogenously supplied iHSP70 or CD40L would also amplify the effects of GM-CSF plus IL-4 to yield a coupled DC growth/iHSP70 response (at 37°C). Our results demonstrate that the inclusion of riHSP70 or CD40L to GM-CSF plus IL-4 cultures produced both DC differentiation/maturation and iHSP70 responses that were virtually identical with those produced by GM-CSF plus IL-4 plus IL-6 and statistically increased above the no cytokine controls (Fig. 8A). Recombinant iHSP70 or CD40L added to the cultures in the absence of cytokines, or GM-CSF combined with either riHSP70 or CD40L did not increase the iHSP70 response or DC growth (data not shown). These results indicate that signaling though the CD40 receptor may yield a coupled DC growth/iHSP70 response during the monocyte to DC transition period and are consistent with previous observations that signaling via CD40 advances monocyte-derived DC growth within 48 h of in vitro culture (28). Although it is possible that exogenous rHSP70 might have interfered with our subsequent measurement of endogenous HSP70, the increased expression of HSF-1 with riHSP70 plus GM-CSF plus IL-4 vs GM-CSF plus IL-4 plus IL-6 further indicated that the inclusion of riHSP70 during the monocyte to DC transition contributed to an endogenous HSP response (supplementary Fig. 1).4
FIGURE 8.
Extracellular riHSP70 and CD40L advance a coupled iHSP70/DC growth response during the monocyte to DC transition period. A, Recombinant iHSP70 or CD40L included in the GM-CSF plus IL-4 cultures produced virtually identical results as GM-CSF plus IL-4 plus IL-6. Results represent flow cytometry-assisted analysis performed on day 3, n ≥ 3 for all comparisons. For all cytokine conditions vs no cytokine controls, p < 0.05. B, Signaling via CD40 restores the chemically inhibited coupled iHSP70/DC growth response. The distribution of iHSP70 and DC maturation markers with increasing doses of TPT (added on day 1) and rescue effects of CD40L (added 1 h after the addition of TPT) was assessed by flow cytometry on day 3. Results represent pooled data from experiments performed in parallel; depicted as mean ± SE. DC growth was instituted from MNC with GM-CSF/IL-4/IL-6. n = 3, except for CD40L (n = 2).
Signaling through CD40 reverses the chemically inhibited iHSP70/DC growth response
The iHSP70 response appeared to be a required process during the monocyte to DC transition; consequently, we expected that reinstatement of DC maturation after reversal of triptolide effects would be associated with a renewed iHSP70 response. Because signaling though the CD40 receptor produced a coupled DC growth/iHSP70 response (Fig. 8A), we determined whether DC could be rescued from the inhibitory effects of triptolide via CD40. In Fig. 8B, we show that with CD40L (100–200 ng/ml) there was increased iHSP70 expression (~2- and 1.4-fold increases in percentage of positive and MFI values, respectively, over 5 nM triptolide; p = 0.019) and concurrent increased expression of DC maturation markers. Thus, reinstatement of the chemically inhibited iHSP70 response and DC maturation may be achieved simultaneously through CD40 signaling.
Cytokine-triggered iHSP70 activity during the critical monocyte to DC transition may include protection from apoptosis via Bcl-2 family members
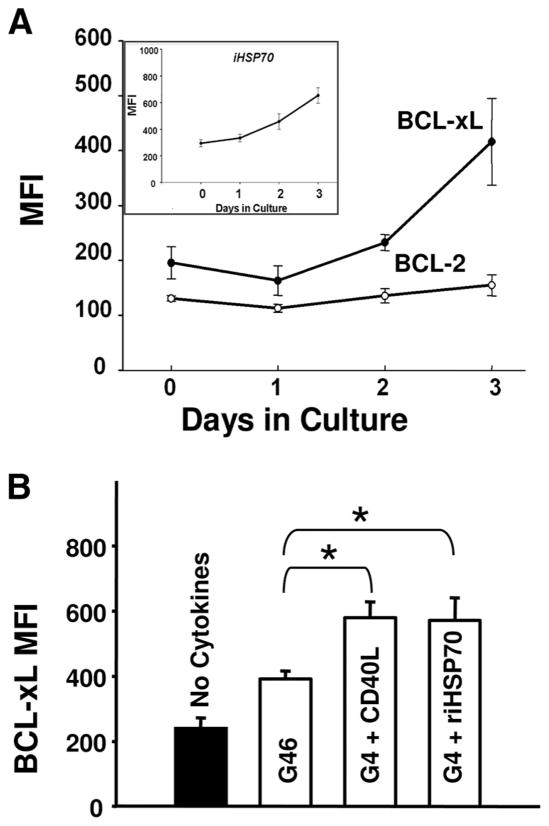
Because both iHSP70 and CD40L are prime inducers of antiapoptotic activity (17–19, 43, 44) and Bcl-2 family members govern DC survival during the entire DC lifespan (45–49), we contemplated the idea that enhanced iHSP70 activity triggered by cytokines (at 37°C) might be linked to DC survival responses involving antiapoptotic proteins. In support of this idea, Fig. 9A demonstrates coincident up-regulation of Bcl-xL (but not Bcl-2) and endogenous iHSP70 during the monocyte to DC transition. In further support of coupled regulatory processes involving iHSP70 and Bcl-xL, we also noted coincident down-regulation of endogenous iHSP70, Bcl-xL, and HSF-1 when DC maturation was arrested with triptolide or KNK (data not shown). In strong support of the idea that extracellular iHSP70 may trigger particular cytoprotective events in DC, Fig. 9B demonstrates that riHSP70 cooperates with GM-CSF plus IL-4 to up-regulate Bcl-xL in newly committed DC. Like the effects on the DC growth/iHSP70 response, CD40L produced virtually identical effects on Bcl-xL expression as riHSP70. Notably, the increases in Bcl-xL achieved with both extracellular iHSP70 and CD40L were even greater than those achieved with GM-CSF plus IL-4 plus IL-6 (p ≤ 0.019 for iHSP70 and CD40L vs GM-CSF plus IL-4 plus IL-6; n ≥ 5). These novel findings suggest that the immunologic significance of an iHSP70/CD40 signaling pathway on DC growth and function includes increased resistance to apoptosis. Although it is well established that members of the Bcl-2 family govern survival during the entire DC lifespan, our results establish that HSP70 may actively participate in this process by enhancing Bcl-xL expression during the critical monocyte to DC transition.
FIGURE 9.
Positive effects of iHSP70 during the monocyte to DC transition include enhanced expression of antiapoptotic Bcl-xL proteins. A, Up-regulated expression of endogenous iHSP70 and Bcl-xL, but not Bcl-2, occurs coincidently during the monocyte to DC transition. For iHSP70, day 0 vs day 3, p < 0.05; for Bcl-xL, day 0 vs day 3, p = 0.026; for Bcl-2, day 0 vs day 3, p = 0.5. n ≥ 4 for all conditions. B, Extracellular riHSP70 and CD40L (added on day 1) similarly enhance cytokine-triggered expression of Bcl-xL (measured on day 3). For no cytokines vs all cytokine conditions, p ≤ 0.003; for G46 vs G46 ± riHSP70 or CD40L, *, p ≤ 0.019. Data are depicted as the mean ± SE, n ≥ 5 for all. DC growth was instituted from MNC with GM-CSF/IL-4/IL-6 (at 37°C) for A and B.
Discussion
In this study, we identify physiologic triggers of endogenous iHSP70 activity in monocyte-derived DC and lend new insight into the immunological significance of an iHSP70/HSP receptor pathway in monocyte-derived DC. Our results demonstrate that endogenous (intracellular and extracellular) iHSP70 expression is triggered in monocyte-derived DC by a cohort of cytokines independently of heat stress, infection, or cell death, especially during the monocyte to DC transition. Our findings also suggest that an extracellular iHSP70/HSP receptor pathway may be involved in an autocrine-positive DC growth response that includes the up-regulation of distinct antiapoptotic proteins.
Although there is substantial evidence in support of the idea that exogenously supplied HSP70 mediates DC cross-priming and maturation, the endogenous production and/or physiologic triggering of iHSP70 in DC have been scarcely studied. To characterize physiologic factors capable of promoting endogenous iHSP70 responses in DC, normal human peripheral blood monocyte-DC precursors were cultured under inflammatory stress conditions (represented as proinflammatory/DC-directed cytokines) in the absence/presence of heat stress (39°/37°C; 24-h cycling for 72 h). Notably, whereas the combination of hyperthermia and a specific cohort of DC/proinflammatory cytokines (IL-4 or IL-13/IL-6 and GM-CSF) produced enhanced intracellular levels of iHSP70 during the monocyte to DC transition, levels of iHSP70 were substantially increased even in the absence of heat stress (Figs. 1 and 3, A and B). Moreover, whereas heat stress alone initiated an intracellular iHSP70 response, it did not promote increases in cell surface or secreted iHSP70, both of which were induced by IL–4/IL-6/GM-CSF in the absence of hyperthermia. Thus, the extracellular release and surface expression of iHSP70 promoted by inflammatory cytokines on maturing DC are synchronized events that are distinctly regulated from the intracellular iHSP70 response promoted by heat stress.
Even though GM-CSF plus IL-4 increased the iHSP70 response above control values in the absence of heat stress, further increases occurred when IL-6 was included in the mixture (Fig. 1). In support of augmented iHSP70 transcriptional activity with inflammatory/DC cytokines, increases in total levels, hyperphosphorylation, and nuclear translocation of the transcription factor HSF-1 occurred during the monocyte to DC transition (Fig. 1; supplementary Figs. 1 and 2).4 IL-6 and GM-CSF are known to induce STAT transcription factors that contribute to the development of inflammatory DC (50, 51). STAT-1 has also been shown to interact with HSF-1 to produce increased HSP70 transcription (50). Therefore, the ability of IL-6 to amplify the iHSP70 response in DC (Fig. 1, A and B) most likely includes enhanced STAT-1/HSF-1 interactions signaling up-regulation of iHSP70. The inclusion of TNF in the cytokine mix did not promote increased iHSP70 expression during the monocyte to DC transition (Fig. 1), which is consistent with previous observations that TNF-α may not positively regulate iHSP70 (52). Because HSF-1/HSP70 activity has been suggested to protect cells against TNF-α-mediated cytotoxicity (20, 21) conceivably in our system, the HSF-1/iHSP70 response provoked by IL-6/GM-CSF/IL-4 includes protection from the lethal effects of TNF-α, which in addition to promoting inflammation, is a hallmark proapoptotic molecule.
Cytokine-triggered production of iHSP70 was initiated as cells commit to monocyte-derived DC growth, as evidenced by the loss of monocyte markers (CD14, CD16), the increased distribution of DC maturation markers (DR, CD86, CD11c), and the capacity to stimulate allogeneic T cells (data not shown). Analysis of iHSP70 levels following the monocyte to DC transition period (day 3) revealed that in the absence of a terminal maturation agent (LPS or TNF-α), levels of iHSP70 in permeabilized cells returned to baseline by day 7 (Fig. 4A). However, induction of final DC maturation events was accompanied by both increased intracellular and surface expression of iHSP70, especially when LPS was used (Figs. 4 and 5). Whether the differences noted between TNF and LPS induction of iHSP70 during terminal stages of maturation are linked to differences in exogenous inflammation (represented by LPS as pathogen) vs endogenous inflammation (TNF-α representing nonpathogen, sterile inflammation) remains to be determined. In any case, these results demonstrate that up-regulated iHSP70 expression accompanies two critical phases of monocyte-DC growth; i.e., the monocyte to DC transition and terminal DC maturation. Because DC engaged in terminal maturation are specialized in Ag presentation rather than Ag uptake, it is unlikely that the increased iHSP70 response noted during terminal DC maturation reflects major cross-priming activity. Instead, as discussed below, the sustained expression of CD40 on the DC surface at this stage suggests an association with prosurvival and/or maturation activity of endogenous iHSP70.
We studied biological consequences resulting from chemically inhibiting the iHSP70 response during the monocyte to DC transition using triptolide or KNK-437. With both these agents, inhibition of the iHSP70 response and DC growth were coupled events that produced monocyte/macrophage-like cells lacking DC maturation markers (Fig. 6). In further support of a closely associated iHSP70/DC growth response, signaling through the heat shock protein receptor CD40 released DC from triptolide-induced maturation arrest to yield maturing DC exhibiting enhanced iHSP70 expression (Fig. 8B). Collectively, these results substantiate that increased endogenous iHSP70 activity and progression toward the monocyte-derived DC lineage are strictly coupled events.
In agreement with the growing impression that a HSP/CD40 pathway involving extracellular HSP70 may participate in driving DC maturation independently of CD40L expressed on T cells (47, 53), we noted that exogenously supplied riHSP70 cooperated with DC cytokines to enhance DC differentiation/maturation events during the monocyte to DC transition (Fig. 8A). Suggestive of an autocrine response, we also noted that signaling through CD40 stimulated endogenous iHSP70 activity during the monocyte to DC transition. In direct support of our premise that extracellular iHSP70 promotes cytoprotection during critical stages of DC growth, exogenously supplied riHSP70 (and CD40L) strongly enhanced the expression of the antiapoptotic protein Bcl-xL during the monocyte to DC transition (Fig. 9). In further support of an associated Bcl-xL and iHSP70 response, endogenous levels of Bcl-xL and iHSP70 were increased in parallel during the monocyte to DC transition period (Fig. 9A), and down-regulation of Bcl-xL and iHSP70 expression with triptolide treatment were coupled events (data not shown). Others have recently reported that activation of HSF-1 and up-regulation of iHSP70 are critical for the induction and molecular stabilization of Bcl-xL (by iHSP70) in a carcinoma cell line displaying resistance to apoptosis (54). Although we have yet to examine molecular interactions between Bcl-xL and iHSP70 in our DC model, we did establish that activation/up-regulation of HSF-1 accompanies the coupled iHSP70/Bcl-xL/DC growth response occurring during the monocyte to DC transition (Fig. 1 and supplementary Figs. 1 and 2).4
Apart from acting as an immunoadjuvant promoting DC maturation and the production of autocrine DC growth factors such as IL-12 as shown by others (42, 53), our results demonstrate that iHSP70 triggers a key survival response in monocyte-derived DC. Unlike HSP-mediated cross-priming activity, which may be restricted to the Ag-processing stages of DC growth, our studies suggest that cytoprotective functions mediated by iHSP70, like antiapoptotic activity mediated by Bcl-2 family members, are operative during different stages of DC growth, such as the monocyte to DC transition and final stages of DC maturation. Although DC maturing on days 3 and 7 in the absence of a terminal maturation agent commonly coexpressed CD40 and CD91, the distribution of these receptors changed during terminal maturation (Figs. 4B and 7). In difference to CD91 levels, which declined as DC approached terminal maturation, CD40 levels rose steadily until terminal DC maturation was achieved (Fig. 7B). Thus, at advanced maturation DC stages, extracellular HSP-HSP receptor interactions would conceivably facilitate terminal DC maturation/survival through engagement of CD40, rather than CD91. Such functional segregation would be in agreement with previous suggestions that DC triggering through CD40 primarily signals immunoadjuvant activity and that CD91 facilitates the cross-priming process (2, 40). Possibly, endogenous iHSP70 responses are also important for the survival of plasmacytoid DC, which are activated by signaling through CD40. Besides HSP70, other members of the HSP family, such as HSP90 and HSP60, and more recently, HSP70L1, have been implicated in DC cross-priming activities (10, 55). It remains to be determined whether or not these HSP members and/or other species of HSP receptors participate in regulating DC survival.
In addition to an important role in promoting normal DC activity, the physiological relevance of our observations includes understanding the regulation and involvement of endogenous HSP/DC interactions in pathological settings such as the rheumatoid joint. Conceivably, in the hyperthermic rheumatoid joint (containing an abundance of DC/proinflammatory cytokines), heat may function to amplify the cytokine-induced iHSP70 response. In support of this idea, we noted that unlike IL-4, the effects of IL-13 were most pronounced with heat stress (Fig. 5). Moreover, IL-13, not IL-4, has been associated with disease activity and DC growth in the autoimmune RA joint (24). Because prior studies show that short exposure of DCs to fever-like temperatures increases the secretion of IL-12p70 (11, 42), the enhanced effects of IL-13 noted by us with heat stress may also include the contribution of IL-12p70 toward DC growth activities in addition to a heat-induced increased intracellular iHSP70 pool. Considering that the RA joint contains a plethora of potentially lethal cellular stresses (as well as an abundance of monocyte/DC precursors), an endogenous HSP/HSP receptor pathway may function to secure the viability of monocyte-derived DC during distinct phases of growth, and hence, adaptive immune responses associated with autoimmunity (supplementary Fig. 3).4
We noted reduced levels of CD40 and CD91 with M-CSF vs GM-CSF/IL-4/IL-6 treatment (data not shown). Because M-CSF-treated cells also lacked surface iHSP70 (Fig. 5) and did not secrete iHSP70, the iHSP70/HSP receptor interactions discussed above would not be triggered by M-CSF. The lack of extracellular iHSP70, DC activity, proinflammatory/DC cytokines, and heat and the prevalence of M-CSF in the osteoarthritic joint (13–16) is further indication that a local HSP/HSP receptor/DC pathway may not operate in a nonautoimmune rheumatological condition. In other disease settings such as a local tumor environment characterized by excess secretion of immunosuppressive cytokines (M-CSF, IL-10), diminished iHSP70 activity driving DC survival/maturation may contribute to tumor escape of adaptive immunity.
Supplementary Material
Acknowledgments
We thank Elyse Clark for assistance in preparing the manuscript.
This work was supported in part by grants from the National Arthritis Foundation, National Institutes of Health Grant R03AR05396601 (awarded to F.S.-S.), and resources from the Department of Veterans Affairs Medical Center (Northport, NY).
Footnotes
Abbreviations used in this paper: HSP, heat shock protein; DC, dendritic cell; IF, immunofluorescence; iHSP, inducible HSP; MFI, mean fluorescence intensity; MNC, mononuclear cell; NHS, normal human serum; RA, rheumatoid arthritis; HSF, heat shock factor.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2002;2:185–194. doi: 10.1038/nri749. [DOI] [PubMed] [Google Scholar]
- 3.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol Immunother. 2006;55:329–338. doi: 10.1007/s00262-005-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol. 2003;170:3037–3045. doi: 10.4049/jimmunol.170.6.3037. [DOI] [PubMed] [Google Scholar]
- 6.Mycko MP, Cwiklinska H, Szymanski J, Szymanska B, Kudla G, Kilianek L, Odyniec A, Brosnan CF, Selmaj KW. Inducible heat shock protein 70 promotes myelin autoantigen presentation by the HLA class II. J Immunol. 2004;172:202–213. doi: 10.4049/jimmunol.172.1.202. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukocyte Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- 8.Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182:1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- 9.Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptide-bearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5:425–431. doi: 10.1379/1466-1268(2000)005<0425:hpbapn>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother. 2006;55:292–298. doi: 10.1007/s00262-005-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tournier JN, Hellmann AQ, Lesca G, Jouan A, Drouet E, Mathieu J. Fever-like thermal conditions regulate the activation of maturing dendritic cells. J Leukocyte Biol. 2003;73:493–501. doi: 10.1189/jlb.1002506. [DOI] [PubMed] [Google Scholar]
- 12.Hatzfeld-Charbonnier AS, Lasek A, Castera L, Gosset P, Velu T, Formstecher P, Mortier L, Marchetti P. Influence of heat stress on human monocyte-derived dendritic cell functions with immunotherapeutic potential for antitumor vaccines. J Leukocyte Biol. 2007;81:1179–1187. doi: 10.1189/jlb.0506347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171:5736–5742. doi: 10.4049/jimmunol.171.11.5736. [DOI] [PubMed] [Google Scholar]
- 14.Devereaux MD, Parr GR, Thomas DP, Hazleman BL. Disease activity indexes in rheumatoid arthritis; a prospective, comparative study with thermography. Ann Rheum Dis. 1985;44:434–437. doi: 10.1136/ard.44.7.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oosterveld FG, Rasker JJ. Effects of local heat and cold treatment on surface and articular temperature of arthritic knees. Arthritis Rheum. 1994;37:1578–1582. doi: 10.1002/art.1780371104. [DOI] [PubMed] [Google Scholar]
- 16.Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J Immunol. 2001;167:1758–1768. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]
- 17.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 18.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 19.Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–695. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- 21.Jaattela M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993;177:231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santiago-Schwarz F, Fleit HB. Identification of nonadherent mononuclear cells in human cord blood that differentiate into macrophages. J Leukocyte Biol. 1988;43:51–59. doi: 10.1002/jlb.43.1.51. [DOI] [PubMed] [Google Scholar]
- 23.Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, Issels RD. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602–1609. doi: 10.1002/1521-4141(200105)31:5<1602::AID-IMMU1602>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Tokayer A, Carsons SE, Chokshi B, Santiago-Schwarz F. High levels of interleukin 13 in rheumatoid arthritis sera are modulated by tumor necrosis factor antagonist therapy: association with dendritic cell growth activity. J Rheumatol. 2002;29:454–461. [PubMed] [Google Scholar]
- 25.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Interleukin-13 is involved in functional maturation of human peripheral blood monocyte-derived dendritic cells. Exp Hematol. 1999;27:326–336. doi: 10.1016/s0301-472x(98)00046-0. [DOI] [PubMed] [Google Scholar]
- 26.Silfversward CJ, Frost A, Brandstrom H, Nilsson O, Ljunggren O. Interleukin-4 and interleukin-13 potentiate interleukin-1 induced secretion of interleukin-6 in human osteoblast-like cells. J Orthop Res. 2004;22:1058–1062. doi: 10.1016/j.orthres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 28.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 29.Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev. 2005;11:17–33. [PubMed] [Google Scholar]
- 30.Valentinis B, Capobianco A, Esposito F, Bianchi A, Rovere-Querini P, Manfredi AA, Traversari C. Human recombinant heat shock protein 70 affects the maturation pathways of dendritic cells in vitro and has an in vivo adjuvant activity. J Leukocyte Biol. 2008;84:199–206. doi: 10.1189/jlb.0807548. [DOI] [PubMed] [Google Scholar]
- 31.Zhu KJ, Shen QY, Cheng H, Mao XH, Lao LM, Hao GL. Triptolide affects the differentiation, maturation and function of human dendritic cells. Int Immunopharmacol. 2005;5:1415–1426. doi: 10.1016/j.intimp.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Chen Y, Lamb JR, Tam PK. Triptolide, a component of Chinese herbal medicine, modulates the functional phenotype of dendritic cells. Transplantation. 2007;84:1517–1526. doi: 10.1097/01.tp.0000289990.55668.0d. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Murakami T, Oppenheim JJ, Howard OM. Triptolide, a constituent of immunosuppressive Chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood. 2005;106:2409–2416. doi: 10.1182/blood-2005-03-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 35.Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000;60:2942–2948. [PubMed] [Google Scholar]
- 36.Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res. 2001;7:215–219. [PubMed] [Google Scholar]
- 37.Guo F, Rocha K, Bali P, Pranpat M, Fiskus W, Boyapalle S, Kumaraswamy S, Balasis M, Greedy B, Armitage ES, et al. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res. 2005;65:10536–10544. doi: 10.1158/0008-5472.CAN-05-1799. [DOI] [PubMed] [Google Scholar]
- 38.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 39.Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Whittall T, McGowan E, Younson J, Kelly C, Bergmeier LA, Singh M, Lehner T. Identification of stimulating and inhibitory epitopes within the heat shock protein 70 molecule that modulate cytokine production and maturation of dendritic cells. J Immunol. 2005;174:3306–3316. doi: 10.4049/jimmunol.174.6.3306. [DOI] [PubMed] [Google Scholar]
- 41.Whittall T, Wang Y, Kelly CG, Thompson R, Sanderson J, Lomer M, Soon SY, Bergmeier LA, Singh M, Lehner T. Tumor necrosis factor-α production stimulated by heat shock protein 70 and its inhibition in circulating dendritic cells and cells eluted from mucosal tissues in Crohn’s disease. Clin Exp Immunol. 2006;143:550–559. doi: 10.1111/j.1365-2249.2006.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng JC, Hyde C, Pai S, O’Sullivan BJ, Nielsen LK, Thomas R. Monocyte-derived DC primed with TLR agonists secrete IL-12p70 in a CD40-dependent manner under hyperthermic conditions. J Immunother. 2006;29:606–615. doi: 10.1097/01.cji.0000211308.82997.4e. [DOI] [PubMed] [Google Scholar]
- 43.Bjorck P, Banchereau J, Flores-Romo L. CD40 ligation counteracts Fas-induced apoptosis of human dendritic cells. Int Immunol. 1997;9:365–372. doi: 10.1093/intimm/9.3.365. [DOI] [PubMed] [Google Scholar]
- 44.Koppi TA, Tough-Bement T, Lewinsohn DM, Lynch DH, Alderson MR. CD40 ligand inhibits Fas/CD95-mediated apoptosis of human blood-derived dendritic cells. Eur J Immunol. 1997;27:3161–3165. doi: 10.1002/eji.1830271212. [DOI] [PubMed] [Google Scholar]
- 45.Santiago-Schwarz F, Borrero M, Tucci J, Palaia T, Carsons SE. In vitro expansion of CD13+CD33+ dendritic cell precursors from multipotent progenitors is regulated by a discrete fas-mediated apoptotic schedule. J Leukocyte Biol. 1997;62:493–502. doi: 10.1002/jlb.62.4.493. [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Huang L, Shabier Z, Wang J. Regulation of the lifespan in dendritic cell subsets. Mol Immunol. 2007;44:2558–2565. doi: 10.1016/j.molimm.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haenssle H, Buhl T, Knudsen S, Krueger U, Rosenberger A, Reich K, Neumann C. CD40 ligation during dendritic cell maturation reduces cell death and prevents interleukin-10-induced regression to macrophage-like monocytes. Exp Dermatol. 2008;17:177–187. doi: 10.1111/j.1600-0625.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 48.Nopora A, Brocker T. Bcl-2 controls dendritic cell longevity in vivo. J Immunol. 2002;169:3006–3014. doi: 10.4049/jimmunol.169.6.3006. [DOI] [PubMed] [Google Scholar]
- 49.Hou WS, Van Parijs L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat Immunol. 2004;5:583–589. doi: 10.1038/ni1071. [DOI] [PubMed] [Google Scholar]
- 50.Stephanou A, Isenberg DA, Nakajima K, Latchman DS. Signal transducer and activator of transcription-1 and heat shock factor-1 interact and activate the transcription of the Hsp-70 and Hsp-90β gene promoters. J Biol Chem. 1999;274:1723–1728. doi: 10.1074/jbc.274.3.1723. [DOI] [PubMed] [Google Scholar]
- 51.Welte T, Koch F, Schuler G, Lechner J, Doppler W, Heufler C. Granulocyte-macrophage colony-stimulating factor induces a unique set of STAT factors in murine dendritic cells. Eur J Immunol. 1997;27:2737–2740. doi: 10.1002/eji.1830271038. [DOI] [PubMed] [Google Scholar]
- 52.Schett G, Steiner CW, Xu Q, Smolen JS, Steiner G. TNFα mediates susceptibility to heat-induced apoptosis by protein phosphatase-mediated inhibition of the HSF1/hsp70 stress response. Cell Death Differ. 2003;10:1126–1136. doi: 10.1038/sj.cdd.4401276. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–2429. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 54.Jacobs AT, Marnett LJ. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal treated colon cancer cells via stabilization of antiapoptotic Bcl-2 proteins. J Biol Chem. 2009;284:9176–9183. doi: 10.1074/jbc.M808656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.