Abstract
Cannabis dependence is a substantial public health problem. Behavioral treatments have shown promise, but there are no effective medications for cannabis dependence. The purpose of this study was to evaluate the safety and efficacy of dronabinol, a synthetic form of delta-9-tetrahydrocannabinol, a naturally occurring pharmacologically active component of marijuana, in treating cannabis dependence. 156 cannabis-dependent adults were enrolled in a randomized, double-blind, placebo-controlled, 12-week trial. After a 1-week placebo lead-in phase, participants were randomized to receive dronabinol 20 mg twice a day or placebo. Doses were maintained until the end of week 8 and then tapered off over 2 weeks. All participants received weekly motivational enhancement and relapse prevention therapy. Marijuana use was assessed using the timeline followback method. There was no significant difference between treatment groups in the proportion of participants who achieved 2 weeks of abstinence at the end of the maintenance phase (dronabinol: 17.7%; placebo: 15.6%). Although both groups showed a reduction in marijuana use over time, there were no differences between the groups. Treatment retention was significantly higher at the end of the maintenance phase on dronabinol (77%), compared to placebo (61%) (P = .02), and withdrawal symptoms were significantly lower on dronabinol than placebo (P= .02). This is the first trial using an agonist substitution strategy for treatment of cannabis dependence. Dronabinol showed promise, it was well-tolerated, and improved treatment retention and withdrawal symptoms. Future trials might test higher doses, combinations of dronabinol with other medications with complementary mechanisms, or with more potent behavioral interventions.
Keywords: Marinol, Dronabinol, cannabis dependence, marijuana dependence, cannabis withdrawal
1. Introduction
Marijuana is the most commonly used illicit drug in the United States (National Survey on Drug Use and Health, NSDUH, 2008). More that 95 million Americans 12 years or older have tried marijuana at least once and almost 25 million have used it in the past year (NSDUH, 2008). Most marijuana users discontinue use by their mid-20s, but a subset maintains daily, long-term use (Compton et al., 2004; Substance Abuse and Mental Health Services Administration, SAMHSA, 2009). After nicotine and alcohol, cannabis dependence is the most prevalent substance dependence syndrome in the general population (NSDUH, 2008; SAMHSA, 2009). Marijuana has surpassed heroin and cocaine as the most common illicit drug listed as the primary problem among patients seeking substance abuse treatment, with nearly 300,000 marijuana-related treatment admissions per year (Treatment Episode Date Set, 2007).
Despite considerable progress in the development of cannabis-dependence-specific psychotherapy interventions, the results of several psychotherapy trials indicate that most dependent patients in treatment do not achieve abstinence (Copeland et al., 2001; Dennis et al., 2004; MTPRG, 2004; Stephens et al., 2000). To date, most of the work conducted evaluating pharmacotherapies for cannabis dependence have been carried out with non-treatment seeking marijuana users in laboratory settings. Several medications have shown potential benefit in reducing some withdrawal symptoms (e.g. mitrazapine, nefazadone) whereas other medications have not shown any clear benefit in mitigating withdrawal symptoms (e.g., bupropion, divalproex) or in reducing the likelihood of relapse (e.g., baclofen) in the laboratory setting (Haney et al. 2001; 2003; 2004; 2010). Unexpectedly, naltrexone increased, rather than decreased, the intoxicating effects of smoked marijuana when administered to non-treatment seekers (Cooper and Haney, 2010). Similarly, outpatient double-blind, randomized treatment trials evaluating nefazadone, bupropion, or divalproex sodium have not shown superiority over placebo in reducing marijuana use (Carpenter et al., 2009; Levin et al., 2004). Compared to other addictive substances, the investigation of pharmacotherapies for cannabis dependence remains limited and no effective medication has yet been identified (McRae et al., 2009; Nordstrom and Levin, 2007; Vandrey and Haney, 2009).
The agonist substitution strategy has been effective for other substance use disorders, mainly nicotine (nicotine patch, other nicotine replacement products, varenicline) and opioid dependence (methadone, buprenorphine). Therefore, dronabinol, an orally bioavailable synthetic form of delta-9-tetrahydrocannabinol (THC), the main psychoactive component of marijuana acting at the cannabinoid 1 (CB1) receptor, seems a logical candidate medication for cannabis dependence. An ideal agonist medication has low abuse potential, reduces withdrawal symptoms and craving, and decreases the reinforcing effects of the target drug, thereby facilitating abstinence. Dronabinol has been shown to reduce cannabis withdrawal symptoms in laboratory settings among non-treatment seeking cannabis users (Budney et al., 2007; Haney et al., 2004). Although dronabinol produced modest positive subjective effects among cannabis users in the laboratory (Hart et al., 2005), there is little evidence of abuse or diversion of dronabinol in community settings (Calhoun et al., 1998). We conducted a randomized, placebo-controlled trial to evaluate the safety and efficacy of dronabinol for patients seeking treatment for cannabis dependence. This is, to our knowledge, the largest clinical trial to date to evaluate a pharmacologic intervention for cannabis dependence, and the first to attempt agonist substitution.
2. Methods
2.1 Study Participants
All participants were seeking outpatient treatment for problems related to marijuana use and were recruited by local advertising (print, radio, television, and subway) or by clinical referrals in the New York City metropolitan area. The advertisements asked “Is Marijuana a Problem for You?” and informed potential participants that they might qualify for a free and confidential research treatment study at Columbia University Medical Center. The medical screening included a complete history and physical exam, an electrocardiogram, and comprehensive laboratory testing. The psychiatric evaluation included the Structured Clinical Interview (SCID) for Diagnostic and Statistical Manual of Mental Disorders-Axis I disorders DSM-IV (American Psychiatric Association, 1994; First et al., 1995). Patients were queried on their current and past use of cannabis and other substances (i.e. alcohol, cocaine, opiates, sedatives, amphetamines, hallucinogens). Substance use assessment included the age of first use and age of regular use. Moreover, a Timeline Followback (TLFB) (Sobell and Sobell, 1992), for the past 30 days was also conducted for alcohol and other drugs of abuse. Participants were treated at the Substance Treatment and Research Service (STARS) of Columbia University/ New York State Psychiatric Institute.
Study inclusion required that participants were between the ages of 18-60, met DSM-IV-TR criteria for current cannabis dependence, reported using marijuana at least 5 days a week during the prior 28 days to study entry, and had a marijuana-positive urine drug screen on the day of study entry. Individuals who were stable and currently being treated for Axis I disorders with pharmacotherapy were not excluded from participating. Participants were excluded if they: 1) met DSM-IV-TR criteria for a current significant and unstable Axis I psychiatric condition which required psychiatric intervention or in the investigators opinion would be disrupted by study medication; 2) were physiological dependent on any substances (other than nicotine) that would require a medical intervention; 3) had significant current suicidal risk or a history of suicidal or homicidal behavior during the last two years; 4) had a history of seizures; 5) had an unstable physical condition (e.g. hypertension, elevated transaminase levels as evidenced by aspartate aminotransferase or alanine aminotransferase levels greater than 3 times the upper end of the laboratory reference range, uncontrolled diabetes); 6) had clinically significant coronary vascular disease as indicated by history or suspected by abnormal ECG or history of cardiac symptoms; 7) had a history of an allergic reaction to dronabinol; 8) were nursing and/or pregnant and/or females unwilling to use an effective method of birth control; 9) were in professions in which even mild intoxication would be hazardous (e.g., police officer, bus driver, firefighter); or 10) were court mandated to treatment. We have not taken court mandated patients at the clinic because generally court-mandated patients enter treatment under an arrangement between the court and treatment program where both urine toxicology and treatment attendance are reported to the court regularly. As a research clinic we cannot fulfill this reporting function, as participation in research treatment cannot be subject to coercive influences. Further, enrolling participants that are court mandated may provide other confounding variables (i.e. patients may be sent to jail if they do not follow through with treatment recommendations), such that this led us to exclude this specific cannabis-dependent population.
The study was approved by the Institutional Review Board of the New York State Psychiatric Institute and all participants provided written informed consent. The study was registered with clinicaltrials.gov: Identifier NCT00217971.
2.2 Study Design
The study was a single-site, randomized, double-blind, parallel-group, 12-week clinical trial comparing placebo to dronabinol. The trial included a one-week placebo lead-in phase, a one week medication titration phase, a 6-week medication maintenance phase, a 2-week dose taper phase, followed by a 2- week placebo lead-out phase. Participants were scheduled to attend the research clinic twice per week. Patients were randomized at the end of the placebo lead-in phase using a fixed block size of 4, stratified by joints used per week [<21 (n=69) versus ≥ 21 (n=87)] and whether or not they were receiving a psychotropic medication (n=18 versus n=138, respectively).
2.2.1 Medication
Dronabinol (DRO) or matching placebo (PBO) was prepared by the pharmacy at the New York State Psychiatric Institute, packaged in matching gelatin capsules with lactose filler and an equal amount of riboflavin. All capsules looked identical for both treatment conditions. Participants ingested two capsules, twice daily (a total of 4 capsules per day), for the duration of the trial; this included the placebo lead-in and lead-out phases as well as the titration and taper weeks. Participants were instructed to take the medication twice per day on a flexible schedule, either late afternoon and in the evening or twice in the evening. Medication was administered in individual vials identified by the participant’s initials and study day. Study medication was provided to participants on a weekly basis. Each week, participants were asked to return all bottles and unused medication. The study staff documented any unused or missed medication.
The placebo lead-in phase allowed us to randomize only those participants who demonstrated a commitment to the study by attending the required study visits during the first week of treatment. Additionally, the placebo lead-in phase allowed us to assess if some participants were able to significantly decrease their marijuana use during the first week of the study without receiving active medication. Participants who reported marijuana use less than twice a week during the placebo lead-in phase were considered placebo responders (n=9) and were not randomized but were followed clinically. They were eligible for the entire course of concurrent relapse prevention therapy.
Following the placebo lead-in phase, participants were randomized into either the DRO or PBO group. A research pharmacist, who was independent of the research team, conducted the randomization. DRO (or matching PBO) was titrated to the target dose over the first week after randomization, beginning at 10 mg per day in the evening, and increasing gradually to 20 mg twice daily, or the maximum dose the participant was able to tolerate. The dose (20 mg bid) was chosen based on laboratory evidence suggesting that this daily amount reduced withdrawal symptoms similar to a higher dose (Hart et al., 2002), would be well-tolerated, and perhaps be associated with less intoxication. If a participant could not tolerate at least 10 mg/day, the medication was discontinued. While there was ample evidence in the laboratory studies that chronic marijuana users tolerated high doses of dronabinol, we were concerned that it might impact on job functioning outside the laboratory. Specifically, we were concerned that patients might describe feeling too “high” or feel that they would be unable to carry out their duties at work or at home. Therefore, we closely monitored all patients that were enrolled in the study and asked if they felt any untoward side effects. If a patient reported that they felt too high or that it was impacting their functioning, then their dose were either lowered, the time of day the medication was given was changed, or the medication was discontinued. During weeks 9 and 10, a gradual taper was conducted, and finally, for weeks 11 and 12, all patients received placebo.
Riboflavin (approximately 100 mg/day) was added to all capsules to track compliance. The riboflavin marker procedure is a standard method to measure adherence to study medication in a clincal trial (Del Boca, et al., 1996). At each clinic visit urine samples were examined under an ultraviolet (UV) lamp in order to observe fluorescence signifying ingestion of the study capsules.
2.2.2 Manualized Psychotherapy
All participants received a weekly coping skills based individual psychosocial intervention along with a motivational enhancement therapy (MET) component similar in scope to more recently developed cannabis specific treatments (Steinberg et al., 2005). While there might be some concern that the psychotherapy platform might “overwhelm” a medication treatment effect, earlier psychotherapy studies have obtained modest rates of abstinence (Dennis et al., 2004; MTPRG, 2004). Moreover, Carroll and Rounsaville (2004) provide a convincing rationale for the utility of psychotherapy platforms in double-blind, placebo-controlled treatment trials including reducing attrition and promoting abstinence.
Patients were encouraged to consider the goal of abstinence and set a “quit date.” However, if a patient waivered and verbalized wanting to cut-down, rather than stop, then the therapist would not try to convince the patient otherwise, but rather, would work with them towards that goal. When appropriate, the therapist would revisit the possibility of setting a “quit date” or at least trying to “sample” abstinence during the trial. This approach was used to minimize disengagement and drop-out.
Therapy sessions were provided by doctoral or master’s level therapists who had received extensive training in the delivery of coping skills based treatments for substance dependence. Sessions were audio-taped and randomly selected to be reviewed during weekly supervision with a senior licensed clinical psychologist.
2.2.3 Voucher Incentives
Progressive incentive payments were provided for attendance and completion of study assessments to enhance compliance with study procedures and retention. Participants earned vouchers with a specific monetary value for attending their study appointments. Participants earned a $1.50 voucher on the first treatment visit. The value of the voucher for each subsequent consecutive visit increased by $1.50. Failure to attend study appointments reset the value of vouchers back to their initial $1.50 from which the voucher value escalated again according to the same schedule. If the participant attended three consecutive clinic visits following non-attendance, this returned the value of the voucher to the level achieved prior to the missed visit. Moreover, to improve pill bottle return and better assess medication compliance participants earned an additional $10 voucher for each consecutive pair of visits if they remembered to return their pill bottles and any remaining medication. Perfect attendance and compliance with returning pill bottles would result in a participant earning $570 in vouchers over the 3-month study period. Voucher earnings were redeemable for retail goods or services designated by the participant. Clinic staff maintained veto power over all purchases that were not considered in line with the treatment goals of increasing drug-free pro-social activities. Participants were also compensated $5.00-$20.00 in cash for transportation costs per visit.
2.3 Treatment Assessments
At each clinic visit (twice weekly), participants were asked to provide a urine specimen, complete self-report instruments, have their vital signs and side effects assessed, and complete a TLFB (Sobell and Sobell, 1992) assessment modified for marijuana at each of the two weekly visits. The initial phase of the TLFB interview asked participants their preferred method of use (e.g., joints, blunts, pipe, or ingestion), to estimate the amount of marijuana used in the preferred method, by using a surrogate substance (oregano), and to estimate the dollar value they would assign to the amount of marijuana measured. Because tobacco may be mixed with marijuana, we asked individuals to show us how much tobacco was mixed with the marijuana when arriving at our marijuana use estimates. The amount of surrogate substance, oregano, was weighed to the hundredth gram and recorded. The second phase of the interview was to complete a calendar procedure for the 30 days prior to study entry where an amount in grams was entered for each day. As part of the structured interview, if marijuana was shared with others, the amount estimated was divided by the number of individuals in order to attribute only the proportion used by the study participant. Our modified TLFB approach for marijuana use is a novel approach developed by our group (Mariani et al., 2010) to address shortcomings in quantification in self report data. While this quantification approach has not been used by other research groups, we have gained experience with this modification of the TLFB method and it is not dissimilar from asking participants to pour “drinks”, which is usually water, when quantifying alcohol use. Most psychotherapy trials simply assess for frequency of use rather than amount of use (Nordstrom and Levin, 2007) and our method was a way to obtain better quantification of use.
At each subsequent 2x/week clinic visit cannabis use was assessed using the TLFB and the use on days between clinic visits was measured. All clinical assessments of drug use were conducted on a weekly basis. Serum pregnancy tests for women were performed monthly.
2.3.1 Cannabis Use
The primary outcome measure was based on self-reported cannabis use. While quantitative urine drug screens (UDS) were conducted at each visit (2x/week), these could not be used as a main outcome measure because oral DRO produces a positive urine drug screen for THC. The urine drug screens from the placebo group were used to evaluate the correspondence with self report; a log linear regression found a strong association between urine drug screens and self-reported cannabis use (P = .003). This is consistent with findings from prior drug abuse treatment trials showing that self-report has strong correspondence with UDS (Carpenter et al., 2009; Carroll et al., 1994; Nunes et al., 1995; Nunes et al., 1998), suggesting that when elicited in research settings in a non-judgmental fashion, self-reports of drug use are accurate.
2.3.2 Marijuana Withdrawal
Marijuana withdrawal symptoms were assessed twice a week using 10 symptoms from the Withdrawal Checklist and identified by Budney et al. (1999) as the withdrawal discomfort score (WDS). The WDS includes symptoms of depressed mood, decreased appetite, irritability, sleep difficulty, craving to use marijuana, restlessness, nervousness/anxiety, headaches, strange/wild dreams and increased anger. This score ranges from 0-30.
2.3.3 Marijuana Craving
The Marijuana Craving Questionnaire (MCQ) is a self report instrument (Heishman et al., 2001) and draws 45 of its 47 items from the Cocaine Craving Questionnaire (Tiffany et al., 1993). Each item is rated on a seven point Likert scale from “strongly disagree” to “strongly agree”. The MCQ contains 4 factors termed compulsivity, emotionality, expectancy and purposefulness which were termed “subscales” for our secondary analyses. The MCQ-47 was completed once per month. A modified 12 item version was collected weekly. The MCQ-12 was constructed by selecting the three items from each factor that exhibited optimal within-factor reliability and inter-item correlation (Heishman et al., 2009). The 4 factor scores on the MCQ-12 range from 3-21.
2.3.4 Side Effects
Side effects were recorded using the Modified Systematic Assessment for Treatment and Emergent Events (SAFTEE) (Johnson et al., 2005; Levine and Schooler, 1986). Side effects were assessed twice a week by the research nurse and reviewed weekly by the study psychiatrist.
2.4 Data Analysis
The primary aim of the study was to compare the proportions of patients who achieved abstinence (defined as no marijuana use based on TLFB self-report) in the last two weeks of the medication phase of the trial (Weeks 7 and 8). Secondary outcomes were time to drop out of treatment, maximum number of consecutive days of abstinence (continuous), daily average amount of marijuana use (assessed weekly in dollars; continuous), days per week of marijuana use (categorical), and per-visit withdrawal discomfort score (continuous). The dichotomous primary outcome was analyzed using mixed effect model (MEM) with logistic link function with independent predictors: treatment (DRO vs. PBO) and baseline amount of marijuana used (dollars spent). Retention rates were compared using Kaplan-Meier curves and log-rank statistics. Maximum number of consecutive days of abstinence was analyzed using MEM with negative binomial link function. Longitudinal secondary outcomes were analyzed using MEM with appropriate link functions, while controlling for their respective baseline. The two-way interaction between treatment and time (i.e. week or visit), and the three-way interactions between treatment, time, and baseline values were assessed and included in the final models if found significant using an alpha significance level of .15. PROC GLIMMIX in SAS was used to conduct these analyses (SAS, 2000).
All analyses were conducted on the intent-to-treat population. All statistical tests were 2-tailed and employed an alpha significance level of .05, unless otherwise stated.
The safety of the treatment was evaluated based on reports of adverse events (AEs), vital signs, and serious adverse events. The incidence of treatment-emergent AEs was defined as AEs that occurred after the first administration of study medication. The overall incidence of treatment-emergent AEs described as moderate or severe were compared across treatment arms using chi-square tests or Fisher’s exact tests. The AEs that resulted in study discontinuation were tabulated by treatment group and listed individually.
A Data and Safety Monitoring Board met yearly to review study enrollment, overall medication tolerability, adverse and serious adverse events. Throughout the trial, no recommendations were made to alter the protocol or cease study enrollment.
3. Results
3.1 Participant Progress in Study and Demographics
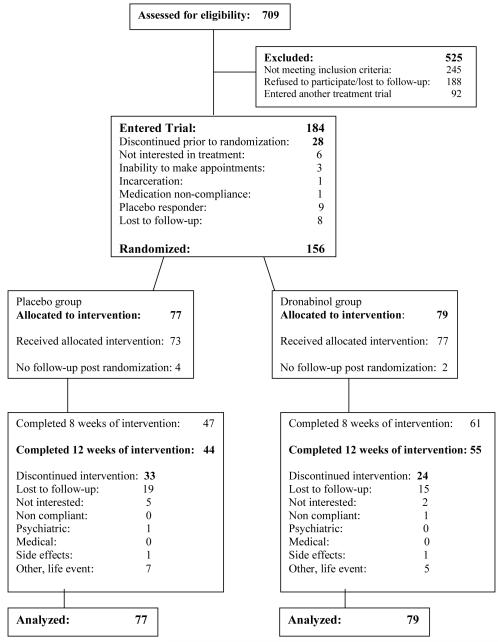
The CONSORT diagram showing participant flow through the study is provided in Figure 1. 709 participants were screened and a total of 156 participants were randomized between DRO (n=79; 50.6%) and PBO (n=77; 49.4%). The most common reason for screen failure was that the person was no longer interested in screening or was lost to follow-up (n=188). The other two most common reasons for screen failure was participation in another clinical trial (n=92) and exclusionary psychiatric diagnoses (n=90). Demographic and baseline clinical characteristics of randomized participants are shown in Table 1 and were not significantly different between the two treatment arms.
Figure 1.
Flow diagram of participants recruited to trial.
Table 1.
Demographic, baseline clinical characteristics and patterns of use of the patients randomized to placebo and dronabinol (N=156)*
| Characteristic | Placebo ** (n=77) |
Dronabinol (n=79) |
|---|---|---|
| Demographic characteristics | mean (SD) or n (%) | |
| Age (years) | 38.4 (9.2) | 36.9 (10.8) |
| Male | 61 (79.2%) | 67 (84.8%) |
| Race/Ethnicity*** | ||
| Hispanic | 16 (20.8%) | 22 (27.9%) |
| Black | 12 (15.6%) | 19 (24.1%) |
| White | 43 (55.8%) | 32 (40.5%) |
| Other | 6 (7.8%) | 6 (7.6%) |
| Education | ||
| High school | 19 (25.0%) | 24 (30.8%) |
| Some college | 17 (22.4%) | 15 (19.2%) |
| College | 27 (35.5%) | 29 (37.2%) |
| Graduate school | 13 (17.1%) | 10 (12.8%) |
| Employment status | ||
| Full-time | 49 (64.5) | 45 (57.0%) |
| Part-time | 11 (14.5) | 10 (12.7%) |
| Unemployed/others | 16 (21.1) | 24 (30.4%) |
| Currently married | 25 (32.5) | 24 (30.4%) |
| Clinical characteristics | ||
| Marijuana Withdrawal Checklist | ||
| Withdrawal Discomfort Score (WDS)-10 | 8.0 (5.4) | 7.1 (4.7) |
| Total | 14.6 (0.9) | 13.2 (9.0) |
| Marijuana Craving Questionnaire (MCQ) | ||
| MCQ: Compulsion | 3.5 (1.3) | 3.4 (1.2) |
| MCQ: Emotion | 4.5 (1.5) | 5.0 (1.4) |
| MCQ: Expectancy | 4.0 (1.3) | 4.2 (1.5) |
| MCQ: Purposefulness | 4.4 (1.6) | 4.5 (1.3) |
| MCQ: Total score | 4.0 (1.0) | 4.1 (1.0) |
| Hamilton-Anxiety | 4.7 (3.9) | 4.5 (3.8) |
| Hamilton-Depression (21-item) | 5.3 (3.5) | 5.3 (4.1) |
| Patterns of drug use | ||
| Age at first use | 15.4 (3.0) | 15.6 (4.0) |
| Age at regular use | 18.6 (5.2) | 18.6 (5.0) |
| median (IQR) | ||
| Days of use in last 7 days | 7 (7-7) | 7 (7-7) |
| Days of use in last 30 days | 30 (29-30) | 30 (29-30) |
| Amount spent per using day ($) | 5 (3-10) | 5 (3-10) |
| Amount per using day (g) | 0.5 (0.3-0.9) | 0.6 (0.4-0.9) |
Frequencies may not sum to N=156 due to missing values. Two patients did not report their education, and one patient did not report his employment status. Percentages may not add up to 100 due to rounding.
There were no statistically significant differences except MCQ: Emotion was p=.05
Participants classified their race based on options defined by the investigator
3.2 Primary Outcome: Two consecutive weeks of abstinence in Weeks 7 and 8
Table 2 presents the results for the primary and secondary outcomes. The primary outcome, the proportion of patients who achieved two-weeks of continuous abstinence in Weeks 7 and 8, was not significantly different between the DRO (17.7%) and PBO (15.6%) groups (P=.69). In defining the primary outcome, missing data in weeks 7 and 8 were scored as indicating cannabis use—a reasonable assumption given the overall rate of abstinence during treatment was low, and missing data in weeks 7 and 8 usually indicated dropout from treatment. A sensitivity analysis showed that for the study to be positive (i.e. significantly higher proportion of abstinence in DRO), two conditions must be true: 1) In the DRO group, missing data were at least 10x more likely to be abstinent than non-missing; 2) In the PBO group, missing data were at least 5x less likely to be abstinent than non-missing. In order to assess the robustness of the primary outcome, the proportion of patients achieving two consecutive weeks of abstinence in their last two weeks of study participation was analyzed. Abstinence rates were similarly low as for the primary outcome and did not significantly differ between DRO (21.3%, 16/76) and PBO (19%, 14/72).
Table 2.
Observed and modeled effect of treatment on primary and secondary outcomes
| Primary outcome | Secondary outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Two week abstinence in Weeks 7 and 8 |
Retention | Maximum number of consecutive days of abstinence |
Average dollars per day of use | Days of use per week |
Withdrawal Scale |
|||||||
| Observed | PBO | DRO | PBO | DRO | PBO | DRO | PBO | DRO | PBO | DRO | PBO | DRO |
| Count(%) or median (IQR) |
12/77 (15.6) |
14/79 (17.7) |
47/77 (61.0) |
61/79 (77.2) |
5 (2-16) |
6 (1-13) |
NA | NA | NA | NA | NA | NA |
| Modeled | Wald X2 | P Value | Log-rank X2 | P Value | F Value | P Value | F Value | P Value | Wald X2 | P Value | F Value | P Value |
| Baseline value | 0.51 | 0.47 | NA | - | 2.03 | 0.16 | NM | NM | 0.14 | 0.71 | NM | NM |
| Treatment | 0.16 | 0.69 | 5.03 | 0.02 | 0.07 | 0.79 | NM | NM | 0.38 | 0.54 | NM | NM |
| Time | NA | - | NA | - | NA | - | NM | NM | 78.72 | <.001 | NM | NM |
| Time * Treatment | NA | - | NA | - | NA | - | NM | NM | NA | - | 1.91 | 0.02 |
| Time * Trx * Baseline | NA | - | NA | - | NA | - | 4.71 | <.001 | NA | - | NA | - |
Abbreviations: PBO, placebo; DRO, dronabinol; Trx, treatment; NA, not applicable; NM, not meaningful.
Given that our primary outcome is two weeks of consecutive abstinence in weeks 7 and 8, the possible number of therapy sessions the patient could attend is dependent on this outcome. Of note, there was no significant interaction between the treatment arms and total number of therapy sessions attended.
3.3 Secondary outcomes
3.3.1 Retention in Treatment (Time to dropout)
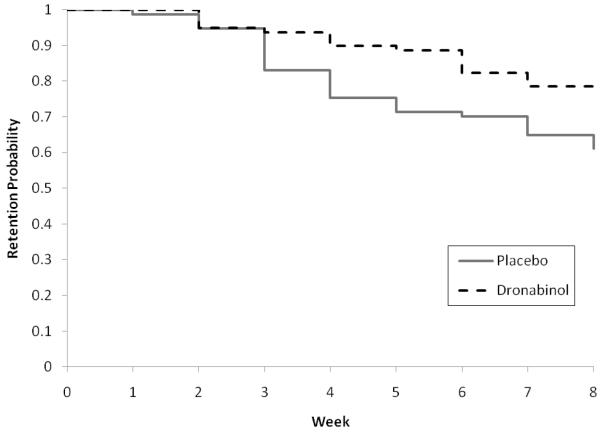
The survival curves describing retention in treatment for the DRO and PBO groups are shown in Figure 2. Retention was significantly better on DRO (77% retained to week 8), compared to PBO (61% retained to week 8) group (P=.02). Participants attended an average of 7.4 ± 3.7 therapy sessions (6.8 ± 3.8 PBO versus 8.0 ± 3.6 DRO, P=.06).
Figure 2.
Retention rates were found significantly different between the treatment groups based on log-rank statistics (P=.02).
3.3.2 Maximum number of consecutive days of abstinence
The median maximum number of consecutive days of abstinence was 6 days (Interquartile range: 1-13 days) for DRO, and 5 days (Interquartile range: 2-16 days) for PBO, resulting in not significantly different effects of treatment (P=.79).
3.3.3 Average amount of marijuana use
The daily average amount of marijuana was measured in dollars and a weekly average was obtained. For the change over time in this weekly average, a more complicated pattern emerged; there was a significant three-way interaction across treatment, week and baseline amount (P<.001) indicating that the effect of treatment over time was different for different levels of baseline use. The interaction plot suggested that for patients with high marijuana use at baseline, cannabis use was greater on DRO than PBO during some of the early weeks of the trial, with PBO experiencing a greater decline in cannabis use. This difference diminished toward the end of the trial as cannabis use and PBO were found to be comparable.
3.3.4 Days of use
The days per week of use was close to daily and did not differ between groups (P=.54); there was a significant effect of time (P<.001), indicating frequency of cannabis use decreased over weeks in the trial, but no significant interaction of treatment by time.
3.3.5 Marijuana withdrawal
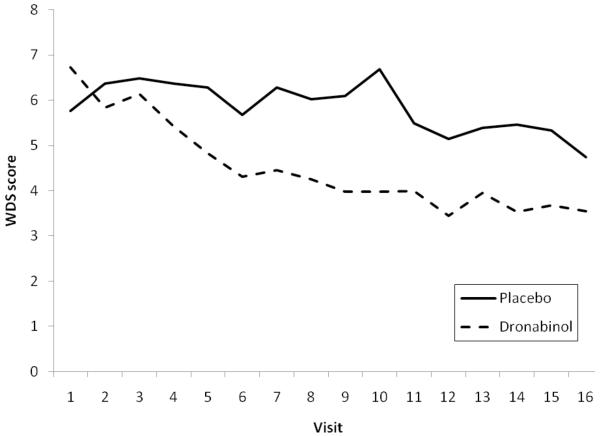
As can be seen in Table 2 and Figure 3, for the WDS there was a significant treatment by time interaction (P=.02), suggesting that WDS decreased over time for both treatment groups, but patients on DRO experienced significantly greater drop in WDS over time.
Figure 3.
Modeled withdrawal discomfort scores (WDS) between the treatment groups over time display a significant two-way interaction between time and treatment (P=.02). Results shown are based on an analysis using a mixed effect model.
3.3.6 Abstinence during 2-week lead-out phase
One question of interest was for those who achieved abstinence in the final weeks of the medication phase (weeks 7 and 8), whether abstinence was maintained during the 2-week medication taper and 2-week lead out phase when both groups were on PBO. The majority maintained their abstinence for the last 2 weeks of the study: 92.9% (13/14) for the DRO group and 91.7% (11/12) for the PBO group.
3.4 Medication Adherence, Safety and Tolerability
The median rate of compliance in both treatment arms was 92% (Interquartile range: 83%-99%). The median (intraquartile range) percentage of samples that flouresced (as a result of ingesting riboflavin) was 100% (88%-100%) for PBO and 94% (83%-100%) for DRO (P=.26). Eighty-nine percent of the DRO group tolerated the maximum dose (with a mean dose of 38.5 mg/day ± 4.7 mg/day) and 96% in the PBO arm tolerated the full dose prescribed. Thirteen patient required dose reductions: 4 experienced drowsiness/grogginess (2 on DRO and 2 on PBO), 4 reported feeling uncomfortably intoxicated (3 on DRO and 1 on PBO), 2 due to increased blood pressure (both on DRO), 2 reported having nightmares and sleep disturbances (1 on DRO and 1 on PBO), and 1 experienced lightheadedness (on DRO). Thus, only a small minority of patients required dose reductions due to side effects, suggesting that the medication was well-tolerated. 67% of the DRO group and 58% of the PBO group reported any adverse side effect. There were no significant differences across treatment arms and none were of sustained duration. There were 4 serious adverse events (3 in the DRO arm and 1 in the PBO arm) during the study. One patient required hospitalization for worsening diabetes after study completion during the follow-up period. One patient was involved in an altercation with the police resulting in a hospitalization with eventual discharge. One patient had worsening of chronic asthma that was previously well-controlled, resulting in a brief hospitalization. The fourth patient had a stomach virus and was hospitalized for dehydration and was released after several days. None of the 4 serious adverse events were deemed to be study-related.
4. Discussion
To our knowledge, this is the first controlled clinical trial to evaluate agonist substitution pharmacotherapy for cannabis dependence. Dronabinol was superior to placebo in promoting retention in treatment and reducing withdrawal symptoms. However, the overall proportion of patients achieving sustained abstinence was low, and there was no evidence for an advantage of dronabinol over placebo on the outcome of marijuana use. The low overall abstinence rate is similar to low rates found in other observational studies and clinical trials for cannabis dependence (Budney et al., 2006; Carpenter et al., 2009; Dennis et al., 2004; Hughes et al., 2008; Levin et al., 2004; McRae et al., 2009; Nordstrom and Levin, 2007) and highlights the need to develop medications. Effective agonist treatments for other drug problems (e.g. nicotine replacement, varenicline, methadone, buprenorphine) reduce withdrawal symptoms, improve treatment retention, reduce the reinforcing effects of the addictive drug, and promote abstinence (Comer et al., 2001; Garrison and Dugan, 2009; Mattick et al., 2009; Schottenfeld et al., 2008; Stead et al., 2008). Thus, dronabinol displayed some of the characteristics of an effective agonist treatment and represents a promising first step.
The findings suggest several future directions for the development of medication treatments for cannabis dependence. Among agonist treatments, dronabinol seems most analogous to nicotine replacement, in that it provides the primary pharmacologically active plant compound, delivered with slow pharmacokinetics--slow absorption compared to the spike in nicotine or THC blood level after smoking, and the ability to sustain a steady blood level, opposing withdrawal. Nicotine replacement has only a modest effect size for sustaining abstinence in nicotine dependence, but its effect can be augmented by combination with other medications, such as bupropion (Shah et al., 2008) or behavioral treatments (Reus and Smith, 2008). This suggests that future studies should test combinations of dronabinol with other treatments with complementary mechanisms.
In human laboratory models developed to test candidate medications for cannabis dependence, dronabinol reduced withdrawal symptoms (Budney et al., 2007; Haney et al., 2004), but failed to reduce cannabis self-administration (Hart et al., 2002). In the same laboratory model, the combination of dronabinol and the alpha-2 receptor agonist lofexidine reduced both withdrawal symptoms and cannabis self-administration (Haney et al., 2008). The correspondence between the findings with dronabinol alone (reduced withdrawal, but not cannabis taking behavior) and the results of the present clinical trial suggests the predictive validity and promise of the laboratory models, and suggests these models should continue to be used to rapidly screen medications or combinations for treatment of cannabis dependence. The findings also suggest the dronabinol/lofexidine combination has promise and should be tested in clinical trials for cannabis dependence.
The more powerful agonist treatments block the subjective and reinforcing effects of their target addictive drug, either through high receptor affinity, as in the case of varenicline for nicotine dependence and buprenorphine for opioid dependence--both are high affinity partial agonists (Lutfy and Cowan, 2004; Rollema et al., 2007) --or through induction of tolerance as in the case of methadone maintenance (Martin et al., 2009). Dronabinol, being in essence orally bioavailable THC, has the same affinity at the CB1 receptor as smoked THC. One laboratory study suggested dronabinol did modestly attenuate the positive effects of smoked marijuana (Hart et al., 2002), perhaps through induction of tolerance. Agonist treatments, particularly methadone, depend on achieving a sufficiently high dose to induce tolerance (Donny et al., 2005; Martin et al., 2009). The dose of dronabinol used in the present study, 20 mg twice a day, was well tolerated, and future clinical trials might consider using higher doses. An outpatient study in non-treatment seekers compared 2 dronabinol doses (30 and 90 mg/day in divided doses) to placebo and found that both doses mitigated withdrawal symptoms, but the higher dose produced additional suppression of withdrawal such that symptom ratings were no different than a smoking-as-usual condition (Budney et al., 2007). Minimal side effects were noted at either dose. Moreover, in the laboratory setting, higher doses than those used in our clinical trial were well-tolerated, although they did not reduce self-administration (Haney et al., 2008; Hart et al., 2002). Thus, we cannot be completely sure that “more would be better” but would be worthy of further investigation. Also, the field should seek to develop alternative high affinity CB1 receptor partial agonists.
Another possible explanation for the lack of an effect of dronabinol on cannabis use is a low motivation to quit. Budney et al. (2006) noted that marijuana-abusing treatment seekers report dissatisfaction with multiple areas of functioning and concerns about future health, but no immediate or dramatic socioeconomic or psychosocial problems as often seen with cocaine, heroin, or alcohol dependence. Consequences of use are often long-term and more subtle (Budney et al., 1998; Levin et al., 2006). Thus, trying to initiate change over a relatively short period (i.e. patients in the present trial were maintained on the maximum dronabinol dose for only 6 weeks), may have been inadequate. Instead a longer maintenance period may be required. For some individuals they may initially want to cut down first and if they are maintained on an agonist treatment for a prolonged period they may eventually choose or be able to quit. This has been demonstrated in nicotine dependent populations with nicotine replacements (Wang et al., 2008). However, since there are no empirical data to support this in cannabis-dependent populations, this possibility is highly speculative.
Motivational enhancement therapy was incorporated into the behavioral platform of the present trial, but more powerful behavioral approaches to enhance motivation might be needed. Voucher-incentives contingent on abstinence have been applied in adolescent and adult populations in cannabis-dependent patients with notable success (Budney et al., 2006; Stanger et al., 2009). Moreover, several pharmacologic trials in cocaine-dependent patients have found a synergy between abstinence-based vouchers combined with various medications (Kosten et al., 2003; Poling et al., 2006; Schmitz et al., 2008).
An impediment to combining voucher incentives contingent on abstinence with dronabinol is the fact that dronabinol tests positive on urine drug screens needed to confirm abstinence. In the present study, tetrahydrocannabivirin, a cannabinoid found in marijuana but not in dronabinol, was explored as an objective urine marker for marijuana use. However, urine tetrahyrdocannabavirin displayed poor sensitivity for cannabis use, perhaps because it is often not present in strains of marijuana now commonly consumed (Levin et al., 2010).
While low motivation to quit is a possible reason for the low rates of abstinence, even with dronabinol administration, the craving and withdrawal symptoms associated with marijuana cessation may impede success unless the pharmacologic agent is potent enough to mitigate these symptoms. Budney et al. (2008) found that cannabis and nicotine withdrawal share several similarities in terms of withdrawal symptoms. Thus, the physiologic and psychologic symptoms associated with cannabis withdrawal may be underappreciated. Supporting this, Hughes et al. (2008) found that marijuana users who are interested in quitting make numerous unsuccessful quit attempts.
Strengths of the present study included good protocol adherence, supported by voucher incentives contingent on adherence, good medication compliance assessed with the riboflavin method, a manual-guided platform treatment, and a sample size sufficient to detect clinically meaningful effects sizes in the range between small and medium. Reliance on self-report for measurement of cannabis use is a potential limitation. However, self-report using the timeline followback method (Sobell and Sobell, 1992) has been the gold-standard for numerous large clinical trials in alcohol-dependent samples (Johnson et al., 2005; Anton et al., 2006), and has shown good correspondence with urine tests in other clinical trials for drug dependence (Nunes et al., 1995, 1998). We adapted the timeline followback for precise measurement of quantities of cannabis (Mariani et al., 2010), and the strong association between self-report cannabis use and urine THC levels within the placebo group supported the validity of the self-reports using this approach. While these data are encouraging, replication and extension of these findings are needed to further validate this approach.
In conclusion, agonist substitution pharmacotherapy with dronabinol, a synthetic form of THC, showed promise for treatment of cannabis dependence, reducing withdrawal symptoms and improving retention in treatment, although it failed to improve abstinence. The trial showed that among adult cannabis-dependent patients, dronabinol was well accepted, with good adherence and few adverse events. Future studies should consider testing higher doses of dronabinol, with longer trial lengths, combining dronabinol with other medications acting through complementary mechanisms (Haney et al., 2008) or more potent behavioral interventions. Moreover, the field should particularly seek to develop high affinity CB1 partial agonists.
REFERENCES
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. Adults seeking treatment for marijuana dependence: a comparison with cocaine-dependent treatment seekers. Exp. Clin. Psychopharmacol. 1998;6:419–426. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J. Consult. Clin. Psychol. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J. Subst. Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol) J. Psychoactive Drugs. 1998;30:187–196. doi: 10.1080/02791072.1998.10399689. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am. J. Addict. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Arch. Gen. Psychiatry. 1994;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy of substance users. Drug Alcohol Depend. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacology (Berl) 2001;154:28–37. doi: 10.1007/s002130000623. [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA. 2004;291:2114–2121. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Opioid antagonism enhances marijuana’s effects in heavy marijuana smokers. Psychopharmacology (Berl) 2010;211:141–148. doi: 10.1007/s00213-010-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. J. Subst. Abuse Treat. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin. Exp. Res. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, et al. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. J. Subst. Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Donny EC, Brasser SM, Bigelow GE, Stitzer ML, Walsh SL. Methadone doses of 100 mg or greater are more effective than lower doses at suppressing heroin self-administration in opioid-dependent volunteers. Addiction. 2005;100:1496–1509. doi: 10.1111/j.1360-0443.2005.01232.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- Patient Edition (SCID-I/P, Patient Verision 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin. Ther. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197:157–68. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155:171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology (Berl) 2003;165:157–165. doi: 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, Foltin RW. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2010;211:233–244. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology (Berl) 2005;181:237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Peters EN, Callas PW, Budney AJ, Livingston AE. Attempts to stop or reduce marijuana use in non-treatment seekers. Drug Alcohol Depend. 2008;97:180–184. doi: 10.1016/j.drugalcdep.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J. Stud. Alcohol Suppl. 2005;(15):157–67. doi: 10.15288/jsas.2005.s15.157. discussion 40. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, Javors MA. Use of oral topiramate to promote smoking abstinence among alcohol-dependent smokers: a randomized controlled trial. Arch. Intern. Med. 2005;165:1600–1605. doi: 10.1001/archinte.165.14.1600. [DOI] [PubMed] [Google Scholar]
- Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am. J. Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Levin FR, Brooks DJ, Bisaga A, Raby W, Rubin E, Aharonovich E, Nunes EV. Severity of dependence and motivation for treatment: comparison of marijuana- and cocaine-dependent treatment seekers. J. Addict. Dis. 2006;25:33–41. doi: 10.1300/J069v25n01_06. [DOI] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Xie S, Murray KA. Delta9-tetrahydrocannabivarin testing may not have the sensitivity to detect marijuana use among individuals ingesting dronabinol. Drug Alcohol Depend. 2010;106:65–68. doi: 10.1016/j.drugalcdep.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol. Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr. Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani JJ, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: Blunts, joints, and pipes. Drug Alcohol Depend. 2010 Sep 20; doi: 10.1016/j.drugalcdep.2010.08.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Zweben JE, Thomas P. Opioid Maintenance Treatment. In: Reis RK, Fiellin DA, Miller SC, Saitz R, editors. Principles of Addiction Medicine - 4th Edition. Lippincott Williams & Wilkins; Philadelphia: 2009. pp. 671–688. [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, Brady KT. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105:132–138. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijuana Treatment Project Research Group Brief treatments for cannabis dependence: Findings from a randomized multisite trial. J. Consult. Clin. Psychol. 2004;72:455–466. doi: 10.1037/0022-006X.72.3.455. MTPRG. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am. J. Addict. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Nunes EV, McGrath PJ, Quitkin FM, Ocepek-Welikson K, Stewart JW, Koenig T, Wager S, Klein DF. Imipramine treatment of cocaine abuse: possible boundaries of efficacy. Drug Alcohol Depend. 1995;39:185–195. doi: 10.1016/0376-8716(95)01161-6. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Quitkin FM, Donovan SJ, Deliyannides D, Ocepek-Welikson K, Koenig T, Brady R, McGrath PJ, Woody G. Imipramine treatment of opiate-dependent patients with depressive disorders. A placebo-controlled trial. Arch. Gen. Psychiatry. 1998;55:153–60. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- NSDUH. Substance Abuse and Mental Health Services Administration . Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: [Accessed on September 2009]. 2009. (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293) Available online at http://oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.pdf. [Google Scholar]
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, Martell B, Kosten TR. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch. Gen. Psychiatry. 2006;63:219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Reus VI, Smith BJ. Multimodal techniques for smoking cessation: a review of their efficacy and utilisation and clinical practice guidelines. Int. J. Clin. Pract. 2008;62:1753–1768. doi: 10.1111/j.1742-1241.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies [Accessed on September. 2009]; SAMHSA. http://oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.pdf.
- SAS . SAS PROC GENMOD. SAS Institute; Cary, NC: 2000. [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Mazlan M. Maintenance treatment with buprenorphine and naltrexone for heroin dependence in Malaysia: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2192–2200. doi: 10.1016/S0140-6736(08)60954-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SD, Wilken LA, Winkler SR, Lin SJ. Systematic review and meta-analysis of combination therapy for smoking cessation. J. Am. Pharm. Assoc. 2008;48(2003):659–665. doi: 10.1331/JAPhA.2008.07063. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption in Measuring Alcohol Consumption. Humana Press; Totowa, New Jersey: 1992. [Google Scholar]
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug Alcohol Depend. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Steinberg KL, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M. Brief Counseling for Marijuana Dependence. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2005. Vol DHHS Publication No. (SMA) 05-4022. [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J. Consult. Clin. Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- TEDS. Substance Abuse and Mental Health Services Administration. Office of Applied Studies . Treatment Episode Data Set (TEDS). Highlights. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-45. Rockville, MD: 2007. http://wwwdasissamhsagov/teds07/tedshigh2k7pdf DHHS Publication No. (SMA) 09-4360. 2009. [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Connock N, Barton P, Fry-Smith A, Aveyard P, Moore D. Cut down to quit with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol. Assess. 2008;12:1–135. doi: 10.3310/hta12020. [DOI] [PubMed] [Google Scholar]