Abstract
Purpose of review
To review the recent advances in the epidemiology and pathophysiology of impulse control disorders (ICD) in Parkinson’s disease (PD).
Recent findings
Large cross-sectional and case-control multicentre studies show that ICDs in PD are common with a frequency of 13.6%. These behaviours are associated with impaired functioning and with depressive, anxiety and obsessive symptoms, novelty seeking and impulsivity. Behavioural subtypes demonstrate differences in novelty seeking and impulsivity suggesting pathophysiological differences. Observational and neurophysiological studies point towards a potential mechanistic overlap between the behavioural (ICDs) and motor (dyskinesias) dopaminergic sequelae. Converging data suggest dopamine agonists in ICDs appear to enhance learning from rewarding outcomes and impulsive choice. ICD patients also have enhanced risk preference and impaired working memory. Neuroimaging data points towards enhanced bottom-up ventral striatal dopamine release to incentive cues, gambling tasks and reward prediction, and possibly inhibition of top-down orbitofrontal influences. Dopamine agonist-related ventral striatal hypoactivity to risk is consistent with impaired risk evaluation.
Summary
Recent large scale studies and converging findings are beginning to provide an understanding of mechanisms underlying ICDs in PD which can guide prevention of these behaviours and optimize therapeutic approaches.
Keywords: Impulse control disorders, Parkinson’s disease, dopamine agonists, pathological gambling, impulsivity
INTRODUCTION
There has been much recent interest in the dopaminergic medication-related impulse control disorders (ICDs) including behaviours such as pathological gambling, compulsive shopping, hypersexuality and compulsive eating. Other behaviours include punding and dopaminergic medication use and more recently reported hoarding (1), kleptomania (2) and impulsive smoking (3). This review focuses only on recent publications and builds on a previous review in the same journal (4). Here, we review the recent advances focusing on epidemiology and pathophysiology of the ICDs. A separate review will focus on treatment issues in ICDs in the general population and in PD (5).
EPIDEMIOLOGY
The largest epidemiological evaluation of ICDs in PD is the multicentre North American DOMINION cross-sectional study (N=3090 patients) which reported a 6 month ICD prevalence of 13.6% (problem and pathological gambling 5.0%, compulsive sexual behaviour 3.5%, compulsive buying in 5.7%, and binge eating disorder 4.3%) (6). Single ICDs were more common with multiple ICDs present in >25%.
Treated PD patients were 25 times more likely to have pathological gambling than general-hospital controls in an Italian study (7) suggesting the behaviours in treated PD may be more common than the general population. Early untreated PD patients did not differ in ICD frequency from non-PD controls (8), suggesting that PD alone is neither causative nor protective, and that ICDs may be the result of interaction between predisposing factors and dopaminergic medication. Similarly, ICDs occur in a diverse range of non-PD patients treated with dopaminergic medication, such as restless legs syndrome (9), progressive supranuclear palsy (10) and multiple sclerosis (11). Furthermore, that PD patients have greater frequency of pathological gambling than amyotrophic lateral sclerosis suggests that ICDs are unlikely to be caused by a chronic neurological condition (12). Thus PD is neither protective nor necessary for the expression of DA-related ICDs, but may be facilitative.
ICDs and medication
The DOMINION study demonstrated a strong class association between ICDs and dopamine agonist (DA) use, and a weaker association with higher levodopa dose but not DA dose (6). The latter finding may have been obscured by the cross-sectional nature of the study; smaller studies have found an association with higher DA dose (13-15).
In a secondary DOMINION analysis, amantadine, used in the management of dyskinesia, was associated with ICDs (16). This contrasts with a randomised crossover controlled study in 17 patients demonstrating efficacy of amantadine in the treatment of pathological gambling in PD (17). The dual action of amantadine on glutamate and dopamine may explain the seemingly contradictory findings; further studies are required to address this issue.
Associated factors
Recent studies highlighting correlates of ICDs in PD might assist identifying patients at risk and have pathophysiological considerations. As the factors are cross-sectionally identified, whether some represent premorbid trait risk factors or state factors is not yet clear. The DOMINION study reported increased hypersexuality and pathological gambling in males and compulsive shopping and eating in females (6), consistent with findings in the general population. Younger PD patients are at greater risk, raising the possibility of different PD genotypic or phenotypic influences. PD patients with ICDs are more likely to be current or former smokers or have a family history of gambling problems (6, 18). Social factors are further emphasised by higher prevalence in the unmarried, and in the United States compared with Canada. The latter finding may also be partly caused by differing prescribing practices.
Consistent with associations observed with substance use disorders in the general population, novelty seeking and impulsivity have been associated in multiple studies with ICDs in PD patients (19-21) and confirmed in the case-control DOMINION study comparing 282 PD patients with ICDs with age-, gender- and medication matched PD controls (18). The same study demonstrated that PD patients with ICDs have higher scores on measures of depression, state and trait anxiety and obsessive-compulsive symptoms, highlighting the need to assess multiple mental health domains. The observation of greater functional impairment with activities of daily living stresses the impact of the disorder. Interestingly, general motivation was lower in ICD patients implying a focussing of motivation on ICD behaviours at the expense of other pursuits. The study uniquely demonstrated differences between behavioural subgroups, showing similarities between pathological gambling and compulsive shopping with higher novelty seeking and impulsive choice as compared to binge eating and hypersexuality suggesting potential pathophysiological differences (18).
PATHOPHYSIOLOGY
The concepts of the ‘overdose’ theory, the dopaminergic spectrum, and motor and behavioural side-effects of dopamine are relevant to both pathophysiology and clinical management.
The ‘overdose’ theory postulates that efficient function follows a eu-dopaminergic level, with both higher and lower dopamine levels associated with impaired function and a mid-range level associated with optimal function resulting in a Yerkes-Dodson-type ‘inverted-U’ shape function (22) (Figure 1). In PD, ventral striatal dopamine is preserved relative to dorsal striatal activity; thus, dopaminergic treatment titrated to alleviate motor dorsal striatal deficiencies may result in an ‘over-dosing’ in ventral cortico-striatal cognitive and limbic pathways.
Figure 1.
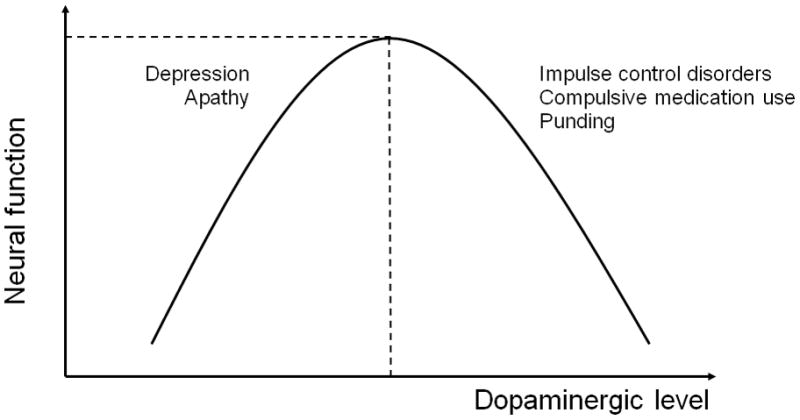
Dopamine level, function and behaviour
ICDs have also been postulated to exist on a spectrum of dopaminergic level with hyper-dopaminergic levels resulting in ICDs, compulsive medication use and punding behaviours, and hypo-dopaminergic levels resulting in apathy and depression (Figure 1). Depression (17/63) and apathy (34/63) were reported after subthalamic deep brain stimulation surgery for PD following marked dopaminergic medication withdrawal (23). Apathy correlated with mesolimbic dopaminergic denervation. ICDs or compulsive medication use behaviours (21/63) resolved in all patients following the marked decrease in dopaminergic medication. Furthermore, pramipexole has been shown to be an effective treatment for depression in PD in a large multicenter study (24). The symptoms of apathy and depression could thus be conceptualized as a mesolimbic hypodopaminergic syndrome and ICDs as a hyperdopaminergic syndrome.
A unifying view is that there is a common mechanism of action in the motor and non-motor domains of the corticostriatal circuitry as evidenced by similarities between ICDs and Levodopa-induced dyskinesias (reviewed in (25)). ICDs in PD are associated with oscillatory theta-alpha (mean 6.71 Hz) ventral subthalamic activity and coherence with non-motor prefrontal regions and are distinct from dyskinesias which are associated with a higher theta-alpha peak (mean 8.38 Hz), dorsal localisation and coherence with motor regions (26). PD patients with punding (27) or multiple ICDs (18) also have more severe dyskinesias relative to PD controls. Taken together, this evidence supports potentially unifying neurophysiological mechanisms linking motor and behavioural side-effects of dopaminergic treatment.
ICDs as a behavioural addiction
ICDs in the general population have epidemiological and phenomenological overlaps with substance addiction, leading to their classification as behavioural addictions (28). The association between ICDs and dopamine agonist withdrawal syndrome (DAWS) with physical and psychological symptoms unresponsive to levodopa provides evidence to support the association with withdrawal (29) and likely receptor supersensitivity. Although ICDs in PD are potentially an intriguing model for the study of ICDs in the general population, given the chronic exogenous dopamine stimulation, there may indeed be pathophysiological differences between the two groups.
The effects of chronic DA administration are relevant both pathophysiologically and in the interpretation of studies. Acute DA in rodents dose-dependently decreases dopaminergic firing related to D2 autoreceptor stimulation (30). In contrast, chronic DA administration normalizes dopaminergic and enhances serotonergic firing presumed related to D2/D3 autoreceptor downregulation and 5HT1A autoreceptor downregulation. These findings suggest that neuronal adaptation differences to chronic DA administration may be relevant to the pathophysiology of ICDs.
PD patients with pathological gambling on DA compared to PD controls have increased ventral striatal dopamine release to a gambling task in an [11C] raclopride positron emission tomography (PET) study (31). This finding is consistent with observations that different substances of abuse increase striatal dopamine release. What is not clear is whether this gambling-related increased dopamine release is secondary to its role in reward processing, incentive motivation, risk or ambiguity or hedonic tone; we parse these constructs in the following sections.
Dopamine and reward
Research on addiction has highlighted a role for dopamine in mediating reward-related processing leading to both the initial early substance experimentation and acquisition and the subsequent late stages of craving and compulsive use. Phasic release of ventral striatal dopamine is initially triggered by the unexpected receipt of reward but shifts to the cue predicting reward after associative learning (32) and along with glutamate, is necessary for the formation of conditioned responses. Converging primate and human functional magnetic resonance imaging (fMRI) studies demonstrate that dopamine responsiveness encodes discrepancies between rewards received and those predicted thus acting as a teaching signal signifying a reward prediction error (32).
Dopamine replacement therapy may influence physiological function either by exogenous tonic dopaminergic stimulation or interference with the endogenous, physiological, phasic striatal dopamine release. According to a proposed computational model, phasic dopamine activity after unexpected rewards exert a positive reinforcing effect by stimulating D1 receptors, whereas unexpected punishments lead to negative reinforcement by a phasic decrease in D2 receptor-mediated signalling (33). Persistent tonic stimulation could therefore simultaneously enhance D1-mediated effects and prevent pauses in D2 signalling, impairing negative feedback learning. This effect of DAs has been shown in new onset PD patients without ICDs: unmedicated patients were impaired at learning from positive outcomes and as a consequence of DA administration, were impaired at learning from negative outcomes (34). With respect to PD patients with ICDs, DAs have been shown in two studies to enhance learning from gain outcomes but the findings to negative outcomes were contradictory(35, 36).
DA in PD patients with either problem gambling or compulsive shopping were shown to enhance the rate of learning from gain-specific outcomes (35). Using a reinforcement learning computational approach that models reward prediction error activity to assess fMRI blood oxygen level-dependent (BOLD) response and indirectly assess phasic dopaminergic activity, DAs were shown to increase ventral striatal activity to prediction error in ICD, signifying a ‘better-than-expected outcome’ and enhanced reward prediction. These results are most consistent with the early acquisition stage and also relevant to forming learned associations with cues.
Dopamine and incentive salience
The incentive motivation theory hypothesises that dopamine alters nucleus accumbens sensitivity to incentive processing such that motivational value is assigned to cues associated with rewards making them desirable (37). Using [11C] raclopride PET imaging, PD patients with mixed ICDs were shown to have a heightened striatal dopamine release to heterogenous reward-related visual cues as compared to either neutral cues or to levodopa challenge (38). These findings were suggested to support an incentive salience process. Similarly, ventral striatal activation to gambling-related cues was demonstrated in a small fMRI study in PD patients with ICDs (39). These studies are consistent with studies in cocaine dependence demonstrating greater striatal dopamine release in response to cocaine cues (40). However, a separate study did not demonstrate differences in motivation as measured using response time to a reward incentive task (41).
Dopamine and risk and uncertainty
Pathological behavioural choices are associated with both positive and negative financial, social and occupational outcomes, thus consistent with definitions of risky (with known probabilities) or uncertain (with unknown probabilities) choices. Risk is encoded in the striatum and orbitofrontal cortex (42). Two studies focusing on risk demonstrate that DA increases risk-taking in PD patients with ICDs (36, 43). This risk-taking bias appears to be unrelated to loss aversion, and is accompanied by lower ventral striatal, orbitofrontal and anterior cingulate activity (43). The lower ventral striatal activity is consistent with an fMRI study of PD patients with ICDs using the Balloon Analogue Risk Task that examines uncertainty (44).
Dopamine, regulation of behaviour and impulsivity
The aforementioned studies focus on ‘bottom up’ striatal mechanism of reward and incentive. Some evidence for impaired ‘top down’ prefrontal regulation is beginning to emerge. Using H2O PET, in PD patients with pathological gambling engaged in a probabilistic gambling task, apomorphine challenge was associated with decreased activity in circuits involved in behavioural regulation including the lateral orbitofrontal cortex and rostral cingulate cortex (45). Similarly, resting state single photon emission tomography (SPECT) study in PD patients with pathological gambling demonstrated decreased functional connectivity between the striatum and anterior cingulate cortex, a region involved in negative feedback and conflict detection (8). These observations are consistent with lower orbitofrontal and anterior cingulate activity observed in subjects with substance use disorders in the general population.
Impulsivity, defined as a lack of behavioural inhibition, has multiple manifestations, including motor response inhibition, rapid decisions, impulsive action or premature responding, and impulsive choice. Impulsive choice is characterised by a preference for small, immediate, rewards, instead of larger, delayed, rewards and implicates the medial striatum, medial prefrontal and orbitofrontal cortex and subthalamic nucleus (reviewed in (46)). Several studies have demonstrated enhanced impulsive choice in PD patients with ICDs using delay discounting tasks with hypothetical long delayed monetary rewards (18, 41) and real-time short delay monetary rewards (47). In one study, impaired delay discounting with intact reward incentive performance in PD patients with ICDs was interpreted as a potential impairment in waiting for the delayed reward rather than an enhanced incentive towards the immediate reward. Alternatively, impulsive choice demonstrates a magnitude effect, whereby lower impulsive choices accompany increasing reward magnitude. This magnitude effect in delay discounting is less pronounced in PD patients with ICDs suggesting that DA may be associated with greater subjective devaluation of the delayed higher reward magnitude (18), resulting in greater impulsivity towards the smaller immediate choice.
With respect to other forms of impulsivity, DAs appear to enhance the rapidity of decision-making, also known as reflection impulsivity, in PD patients with ICDs, suggesting that the long term negative consequences may not be as carefully considered (47). That this form of impulsivity is also impaired by subthalamic stimulation (48) and that we show an impairment in patients with ICDs may explain impulsive behaviours observed in the postoperative period. Impulsive PD patients do not perform differently to non impulsive PD patients on the Stroop Colour Word test (36) that probes inhibition of prepotent responses and response selection associated with anterior cingulate function.
The question of whether PD patients with ICDs have lower D2/D3 receptor levels is potentially intriguing given rodent studies suggesting premorbid higher impulsivity and lower D2/D3 receptor levels predicts risk for cocaine addiction (49). The evidence from cross-sectional [11C] raclopride studies are mixed and may be limited by methodology. PD patients with pathological gambling have lower D2/D3 receptor binding on medication when performing a control task suggesting either lower D2/D3 receptor levels or enhanced dopamine release (31). Two other studies in PD patients with mixed ICDs and compulsive medication use did not detect group differences off medication (38, 50). Prospective studies may be useful to address these issues.
Of interest, DA appears to enhance altruistic punishment in PD patients with ICDs, where violators of social norms are punished when there is a personal cost association with their behaviour (51).
Dorsolateral prefrontal cortical function
Studies using the Frontal Assessment Battery have published disparate conclusions (21, 52, 53); however, the test is a brief screening instrument with limited specificity for dorsal or ventral prefrontal function. Emerging evidence suggests a potential working memory impairment associated with dorsolateral prefrontal function. Visuospatial working memory tested on medication was impaired in medicated PD patients with ICD compared with those without (47). Similarly, PD patients with ICD both on and off medications have a significantly reduced digit span compared with PD and control groups (36). These results suggest that dorsolateral cortico-striatal circuitry in PD with ICD might be similarly affected by ‘overdose’ from exogenous dopamine when on medication and possibly from endogenous dopamine when off medication.
Dopamine receptor subtypes
Dopamine D3 receptors are predominantly expressed in the ventral striatum and mediate reward, emotional and cognitive processes. Pramipexole and ropinirole, two widely used non-ergot DAs have greater D2/D3 selectivity relative to D1. That concurrent levodopa use with a DA increases the odds of an ICD (6) dovetails with a primate study demonstrating that levodopa administration resulted in ectopic induction of dorsal striatal D3 receptors (54).
Genetic polymorphisms may also contribute to ICD susceptibility. Evaluation of dopamine and glutamate receptors and serotonin transporter gene polymorphisms identified D3 dopamine receptor p.S9G and GRIN2B c.366C > G as a risk factor for ICDs in PD (14).
CLINICAL ISSUES
Patients and caregivers should be warned about the risk of development of ICDs at treatment onset and actively questioned on follow-up. Patients with a premorbid history of substance or behavioural addictions may be a greater risk for the development of these disorders. The validated Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP) has >80% sensitivity and specificity and can be completed in 5 minutes (55). Given the low positive predictive value (21-59%), clinical interview should follow a positive screen. The QUIP is valid also when completed by the patient’s informant (56).
Observational follow up studies suggest that a decrease or discontinuation of the dopamine agonist if tolerated may be efficacious for some patients (57). A recent cross-over randomized trial demonstrated efficacy of amantadine (17); however, a contradictory report of increased risk of ICDs associated with amantadine (6) suggest its role is not yet resolved. The efficacy of subthalamic stimulation in ICD patients, which allows a decrease in total dopaminergic dose and discontinuation of dopamine agonists, is not yet resolved with contradictory reports published (23, 58, 59). However, the recent demonstration of complete resolution of ICDs in a prospective study in which dopaminergic medications were dramatically decreased (23), suggests that retrospective studies reporting a post-operative worsening of symptoms may be related to inadequate postoperative titration of medications (59). Notably, ICD patients who are single and develop post-operative depression may be at greater risk of post-operative suicidal behaviours (60); careful pre-operative selection and postoperative follow-up are indicated.
CONCLUSION
Future studies should ideally be prospective, include larger sample sizes and confirm current early smaller reports, focussing not only on similarities but differences between behavioural subtypes including the relationship with punding and compulsive medication use and include post-mortem studies. Recent advances lead us to understand why a subgroup of patients develop these behaviours, how to prevent and to best manage these potentially debilitating behaviours.
KEY POINTS.
ICDs in PD are common with a frequency of 13.6%.
The behaviours impair activities of daily living and are associated with depressive, anxiety and obsessive symptoms. Behavioural subtypes demonstrate differences in novelty seeking and impulsivity.
Emerging evidence suggests a mechanistic overlap between ICDs and levodopa-induced dyskinesias.
Converging evidence suggests dopamine agonists in ICDs enhance learning from rewarding outcomes and increase impulsive choice. ICD patients also have enhanced risk preference and impaired working memory.
Neuroimaging evidence suggests enhanced bottom-up ventral striatal dopamine activity to incentive cues, gambling tasks and reward anticipation, and possibly impaired inhibition of top-down orbitofrontal influences. Ventral striatal hypoactivity to risk is consistent with impaired risk evaluation.
Acknowledgments
Dr. Voon is funded by the Wellcome Trust. Dr. Hallett is supported by the NINDS Intramural Program.
Footnotes
Financial disclosure: Valerie Voon is funded by the Wellcome Trust.
References
- 1.O’Sullivan SS, Djamshidian A, Evans AH, Loane CM, Lees AJ, Lawrence AD. Excessive hoarding in Parkinson’s disease. Mov Disord. 2010 Jun 15;25(8):1026–33. doi: 10.1002/mds.23016. [DOI] [PubMed] [Google Scholar]
- 2.Bonfanti AB, Gatto EM. Kleptomania, an unusual impulsive control disorder in Parkinson’s disease? Parkinsonism Relat Disord. 2010 Jun;16(5):358–9. doi: 10.1016/j.parkreldis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Bienfait KL, Menza M, Mark MH, Dobkin RD. Impulsive smoking in a patient with Parkinson’s disease treated with dopamine agonists. J Clin Neurosci. 2010 Apr;17(4):539–40. doi: 10.1016/j.jocn.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voon V, Potenza MN, Thomsen T. Medication-related impulse control and repetitive behaviors in Parkinson’s disease. Curr Opin Neurol. 2007 Aug;20(4):484–92. doi: 10.1097/WCO.0b013e32826fbc8f. [DOI] [PubMed] [Google Scholar]
- 5.Voon V. Treatment of impulse control disorders in the general population and Parkinson’s disease. Expert Opinion on Pharmacotherapy. in press. [Google Scholar]
- 6**.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010 May;67(5):589–95. doi: 10.1001/archneurol.2010.65. Cross-sectional study on ICD frequency. [DOI] [PubMed] [Google Scholar]
- 7.Avanzi M, Baratti M, Cabrini S, Uber E, Brighetti G, Bonfa F. Prevalence of pathological gambling in patients with Parkinson’s disease. Mov Disord. 2006 Dec;21(12):2068–72. doi: 10.1002/mds.21072. [DOI] [PubMed] [Google Scholar]
- 8.Cilia R, Cho SS, van Eimeren T, Marotta G, Siri C, Ko JH, et al. Pathological gambling in patients with Parkinson’s disease is associated with fronto-striatal disconnection: A path modeling analysis. Mov Disord. 2011 Feb 1;26(2):225–33. doi: 10.1002/mds.23480. [DOI] [PubMed] [Google Scholar]
- 9.Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010 Jan 1;33(1):81–7. [PMC free article] [PubMed] [Google Scholar]
- 10.O’Sullivan SS, Djamshidian A, Ahmed Z, Evans AH, Lawrence AD, Holton JL, et al. Impulsive-compulsive spectrum behaviors in pathologically confirmed progressive supranuclear palsy. Mov Disord. 2010 Apr 15;25(5):638–42. doi: 10.1002/mds.22902. [DOI] [PubMed] [Google Scholar]
- 11.Evans AH, Butzkueven H. Dopamine agonist-induced pathological gambling in restless legs syndrome due to multiple sclerosis. Mov Disord. 2007 Mar 15;22(4):590–1. doi: 10.1002/mds.21303. [DOI] [PubMed] [Google Scholar]
- 12.Wicks P, MacPhee GJ. Pathological gambling amongst Parkinson’s disease and ALS patients in an online community (PatientsLikeMe.com) Mov Disord. 2009 May 15;24(7):1085–8. doi: 10.1002/mds.22528. [DOI] [PubMed] [Google Scholar]
- 13.Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism Relat Disord. 2008;14(1):28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, et al. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord. 2009 Sep 15;24(12):1803–10. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord. 2007 Sep 15;22(12):1757–63. doi: 10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- 16.Weintraub D, Sohr M, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol. 2010 Dec;68(6):963–8. doi: 10.1002/ana.22164. [DOI] [PubMed] [Google Scholar]
- 17.Thomas A, Bonanni L, Gambi F, Di Iorio A, Onofrj M. Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol. 2010 Sep;68(3):400–4. doi: 10.1002/ana.22029. [DOI] [PubMed] [Google Scholar]
- 18**.Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, et al. Impulse control disorders in parkinson disease: A multicenter case-control study. Ann Neurol. 2011 Jan 10; doi: 10.1002/ana.22356. Case-control study on associated factors and behavioural subtype differences. [DOI] [PubMed] [Google Scholar]
- 19.Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006 Oct 10;67(7):1258–61. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- 20.Isaias IU, Siri C, Cilia R, De Gaspari D, Pezzoli G, Antonini A. The relationship between impulsivity and impulse control disorders in Parkinson’s disease. Mov Disord. 2008 Feb 15;23(3):411–5. doi: 10.1002/mds.21872. [DOI] [PubMed] [Google Scholar]
- 21.Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007 Feb;64(2):212–6. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- 22.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 23*.Thobois S, Ardouin C, Lhommee E, Klinger H, Lagrange C, Xie J, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010 Apr;133(Pt 4):1111–27. doi: 10.1093/brain/awq032. Subthalamic stimulation study addressing hyper- and hypo-dopaminergic spectrum and behavioural symptoms. [DOI] [PubMed] [Google Scholar]
- 24.Bxarone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010 Jun;9(6):573–80. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- 25.Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009 Dec;8(12):1140–9. doi: 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 26*.Rodriguez-Oroz MC, Lopez-Azcarate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, et al. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain. 2010 doi: 10.1093/brain/awq301. (in press). Neurophysiological study linking ICDs and levodopa-induced dyskinesia. [DOI] [PubMed] [Google Scholar]
- 27.Silveira-Moriyama L, Evans AH, Katzenschlager R, Lees AJ. Punding and dyskinesias. Mov Disord. 2006 Dec;21(12):2214–7. doi: 10.1002/mds.21118. [DOI] [PubMed] [Google Scholar]
- 28.Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008 Oct 12;363(1507):3181–9. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol. Jan;67(1):58–63. doi: 10.1001/archneurol.2009.294. Dopamine agonist withdrawal syndrome enhanced in ICD. [DOI] [PubMed] [Google Scholar]
- 30*.Chernoloz O, El Mansari M, Blier P. Sustained a8dministration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009 Feb;34(3):651–61. doi: 10.1038/npp.2008.114. Acute and chronic pramipexole effects on neurotransmitter firing rates. [DOI] [PubMed] [Google Scholar]
- 31.Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009 May;132(Pt 5):1376–85. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997 Mar 14;275(5306):1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 33.Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004 Dec 10;306(5703):1940–3. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 34.Bodi N, Keri S, Nagy H, Moustafa A, Myers CE, Daw N, et al. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009 Sep;132(Pt 9):2385–95. doi: 10.1093/brain/awp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010 Jan 14;65(1):135–42. doi: 10.1016/j.neuron.2009.12.027. Striatal activity enhanced in reward processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djamshidian A, Jha A, O’Sullivan SS, Silveira-Moriyama L, Jacobson C, Brown P, et al. Risk and learning in impulsive and nonimpulsive patients with Parkinson’s disease. Mov Disord. 2010 Oct 15;25(13):2203–10. doi: 10.1002/mds.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993 Sep-Dec;18(3):247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 38*.O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, et al. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain. 2011 Feb 24; doi: 10.1093/brain/awr003. Dopamine activity increased to cue incentive salience. [DOI] [PubMed] [Google Scholar]
- 39.Frosini D, Pesaresi I, Cosottini M, Belmonte G, Rossi C, Dell’Osso L, et al. Parkinson’s disease and pathological gambling: results from a functional MRI study. Mov Disord. 2010 Oct 30;25(14):2449–53. doi: 10.1002/mds.23369. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006 Jun 14;26(24):6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Housden CR, O’Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact Reward Learning but Elevated Delay Discounting in Parkinson’s Disease Patients With Impulsive-Compulsive Spectrum Behaviors. Neuropsychopharmacology. 2010 Jul 14; doi: 10.1038/npp.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006 Aug 3;51(3):381–90. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 43*.Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, et al. Dopamine agonists and risk: impulse control disorders in Parkinson’s disease. Brain. doi: 10.1093/brain/awr080. in press. Decreased risk preference and ventral striatal hypoactivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Mov Disord. 2010 doi: 10.1002/mds.23147. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TD, Houle S, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010 Nov 9;75(19):1711–6. doi: 10.1212/WNL.0b013e3181fc27fa. Dopamine agonists inihibit orbitofrontal activity in ICD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010 Jan;34(1):50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voon V, Reynolds B, Brezing C, Gallea C, Skaljic M, Ekanayake V, et al. Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 2010 Jan;207(4):645–59. doi: 10.1007/s00213-009-1697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007 Nov 23;318(5854):1309–12. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 49.Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007 Mar 2;315(5816):1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006 May;59(5):852–8. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 51.Djamshidian A, O’Sullivan SS, Doherty K, Lees AJ, Averbeck BB. Altruistic punishment in patients with Parkinson’s disease with and without impulsive behaviour. Neuropsychologia. 2010 Oct 19; doi: 10.1016/j.neuropsychologia.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santangelo G, Vitale C, Trojano L, Verde F, Grossi D, Barone P. Cognitive dysfunctions and pathological gambling in patients with Parkinson’s disease. Mov Disord. 2009 Apr 30;24(6):899–905. doi: 10.1002/mds.22472. [DOI] [PubMed] [Google Scholar]
- 53.Siri C, Cilia R, De Gaspari D, Canesi M, Meucci N, Zecchinelli AL, et al. Cognitive status of patients with Parkinson’s disease and pathological gambling. J Neurol. Feb;257(2):247–52. doi: 10.1007/s00415-009-5301-5. [DOI] [PubMed] [Google Scholar]
- 54.Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3363–7. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009 Jul 30;24(10):1461–7. doi: 10.1002/mds.22571. Validated screening instrument for ICDs in PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papay K, Mamikonyan E, Siderowf AD, Duda JE, Lyons KE, Pahwa R, et al. Patient versus informant reporting of ICD symptoms in Parkinson’s disease using the QUIP: validity and variability. Parkinsonism Relat Disord. 2011 Mar;17(3):153–5. doi: 10.1016/j.parkreldis.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB, et al. Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov Disord. 2008 Jan;23(1):75–80. doi: 10.1002/mds.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H, et al. Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Mov Disord. 2006 Nov;21(11):1941–6. doi: 10.1002/mds.21098. [DOI] [PubMed] [Google Scholar]
- 59.Lim SY, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, et al. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J Clin Neurosci. 2009 Sep;16(9):1148–52. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Voon V, Krack P, Lang AE, Lozano AM, Dujardin K, Schupbach M, et al. A multicentre study on suicide outcomes following subthalamic stimulation for Parkinson’s disease. Brain. 2008 Oct;131(Pt 10):2720–8. doi: 10.1093/brain/awn214. [DOI] [PMC free article] [PubMed] [Google Scholar]


