Abstract
Alemtuzumab was used as an induction agent in 205 renal transplant recipients undergoing 207 living donor renal transplants. All donor kidneys were recovered laparoscopically. Postoperatively, patients were treated with tacrolimus monotherapy, and immunosuppression was weaned when possible. Forty-seven recipients of living donor renal transplants prior to the induction era who received conventional triple drug immunosuppression without antibody induction served as historic controls. The mean follow-up was 493 days in the alemtuzumab group and 2101 days in the historic control group. Actuarial 1-year patient and graft survival were 98.6% and 98.1% in the alemtuzumab group, compared to 93.6% and 91.5% in the control group, respectively. The incidence of acute cellular rejection (ACR) at 1 year was 6.8% in the alemtuzumab group and 17.0% (p < 0.05) in the historic control group. Most (81.3%) episodes of ACR in the alemtuzumab group were Banff 1 (a or b) and were sensitive to steroid pulses for the treatment of rejection. There was no cytomegalovirus disease or infection. The incidence of delayed graft function was 0%, and the incidence of posttransplant insulin-dependent diabetes mellitus was 0.5%. This study represents the largest series to date of live donor renal transplant recipients undergoing alemtuzumab induction, and confirms the short-term safety and efficacy of this approach.
Keywords: Acute cellular rejection, African American, alemtuzumab, Campath-1H, focal segmental glomerulosclerosis, HIV, steroid-free, tacrolimus monotherapy
Introduction
Alemtuzumab (Campath-1H) is a humanized anti-CD52 IgG monoclonal antibody that is associated with prolonged depletion of T cells from the peripheral circulation after systemic administration (1, 2). B cells, natural killer cells, and monocytes are also depleted by alemtuzumab to a lesser extent. While the Food and Drug Administration (FDA) initially approved it for the treatment of chronic B-cell lymphocytic leukemia in 2001 (3, 4), the ability of alemtuzumab to deplete lymphocytes from the circulation has generated interest in the use of this antibody in solid organ transplantation. While alemtuzumab has not been approved by the FDA for use in organ transplantation, the experiences with this monoclonal antibody in renal transplantation have been promising (2, 5–18).
We have implemented an immunosuppressive regimen based on two key principles: recipient pretransplant lymphoid depletion and minimal use of posttransplant steroid-free immunosuppression (19, 20). Immunosuppressive regimens based on these principles are intended to deplete pre-operatively pre-existing alloreactive donor-specific T cells to prevent acute rejection, while minimizing postoperative immunosuppression with a calcineurin inhibitor (CNI) permits interaction between donor and recipient leukocytes. We have previously observed that the use of preconditioning permits spaced weaning of immunosuppression (8–10, 21). The use of alemtuzumab, compared with thymoglobulin has been associated with a substantially lower incidence of acute cellular rejection (ACR), but both have been associated with a low incidence of infectious complications and other adverse events (8–10, 21). Here, we present the largest series to date of living donor renal transplantation performed with alemtuzumab induction and tacrolimus monotherapy.
Patients and Methods
Between January 15, 2003 and December 30, 2005, a total of 207 consecutive laparoscopic live donor nephrectomies resulting in 207 living donor renal transplantations in 205 kidney-only recipients (two re-transplants) were performed at the University of Pittsburgh Medical Center Starzl Transplantation Institute (UPMC STI). Forty-six living donor kidney recipients with previous extra-renal solid organ transplants (n = 43) or positive pretransplant crossmatches (n = 3) were excluded. Prospective data were collected on both donors and recipients. The immunosuppressive regimen was based upon pretreatment with intravenous 30 mg Campath-1H (alemtuzumab, Berlex, Seattle, WA, USA). Pre-medication was with 1 g of methylprednisolone, which was repeated prior to reperfusion to avoid intra-operative hypotension from cytokine release syndrome. Posttransplantation low-dose tacrolimus monotherapy (target trough of 10 ng/mL) for the first 100 days posttransplantation was given, starting on postoperative day 1. At approximately 100 days posttransplantation, the twice-daily dose of tacrolimus was consolidated to once a day in recipients with no clinical evidence of ACR (i.e. increase in creatinine confirmed by biopsy). Protocol biopsies were not performed. If the patient continued to do well, the dose was weaned to once every other day after another 1 to 4 months. Further weaning was continued to three times a week, twice a week or once a week at 2 to 6 months intervals if the patient continued to do well in the absence of a rising creatinine or clinical ACR. Episodes of biopsy-proven ACR were treated with a 1 g of intravenous methylprednisolone or 30 mg intravenous Campath-1H, for steroid-resistant ACR.
These protocols were approved by the UPMC Innovative Practices Committee and the UPMC Pharmacy and Therapeutics committee as previously described (8–10, 21). Data analysis was approved by the University of Pittsburgh Institutional Review Board. As an historical control group, retrospective data on 47 living donor renal transplants performed between 28 March, 1998 and 18 July, 2001 at UPMC STI prior to the thymoglobulin and alemtuzumab induction era (after excluding recipients with previous extra-renal solid organ transplants, positive pretransplant crossmatches and recipients who received any induction therapy) were collected. These recipients received no induction therapy and were treated with triple conventional immunosuppression consisting of tacrolimus, prednisone and mycophenolate mofetil.
Statistical analysis
Baseline demographic and laboratory factors were described as means (±standard deviation) for continuous variables and as frequency distributions for dichotomous variables. Fisher's exact χ2 was used to compare differences in ACR, human leukocyte antigen (HLA) mismatch, panel reactive antibodies (PRA)>20% and creatinine. Statistical significance of the differences between groups was tested using two-sample t-tests or analysis of variance (ANOVA) for continuous variables and chi square tests for categorical variables.
Actuarial recipient and graft survivals were calculated beginning at the time of transplantation. Kidney graft failure was defined as death of recipient, removal of the allograft or loss of function requiring return to dialysis. Actuarial Kaplan-Meier survivals were calculated using the Statistical Package for the Social Science (SPSS) software and the series was followed until 30 December, 2005. The magnitude of a factor's association with survival was estimated by the Breslow generalized Wilcoxon statistic, a test of the null hypothesis that there is no association between the factor and survival. We present cumulative recipient and graft survival curves using Kaplan-Meier methods. All statistical tests were two-tailed; statistical significance was defined by a p < 0.05.
Results
Recipient characteristics and survival
The mean follow-up for the 205 living donor renal transplant recipients that received alemtuzumab induction was 493 ± 282 days. Of the 205 recipients, 28 (13.7%) had previous failed transplants. Eighteen (8.7%) recipients had a 2nd and 10 (4.9%) recipients had a 3rd to 5th transplant. There were 4 (1.8%) HIV+, 13 (5.8%) pediatric and 31 (15.1%) African American recipients. The average HLA mismatch was 3.2 ± 1.6, and 6.5% of recipients had a pretransplant PRA ≥ 20%. See Table 1 for detailed recipient characteristics.
Table 1.
Characteristics of living donor renal transplant recipients. Two hundred five recipients who received alemtuzumab induction with tacrolimus monotherapy were compared to 47 historical control recipients who were transplanted prior to the induction era and are on standard triple immunosuppression (tacrolimus, mycophenolate mofetil, and prednisone)
| Alemtuzumab induction | Historical control | p-value | |
|---|---|---|---|
| Recipients (%) | 205 (100%) | 47 (100%) | |
| Transplants | 207* | 47 | |
| Age (years) | 44.1 ± 17.6 | 46.2 ± 16.9 | N.S.** |
| Primary graft | 177 (86.3%) | 40 (85.1%) | N.S. |
| Re-transplants | 28 (13.7%) | 7 (14.8.%) | N.S. |
| 2nd transplants | 18 (8.7%) | 7 (14.8%) | |
| 3rd to 5th transplants | 10 (4.9%) | 0 (0%) | |
| HIV+ recipients | 4 (1.8%) | 0 (0%) | |
| Pediatric recipients | 13 (5.8%) | 3 (6.4%) | N.S. |
| HLA mismatch | 3.2 ± 1.6 | 2.9 ± 1.5 | N.S. |
| PRA > 20% | 6.5% | 6.4% | N.S. |
| Mean recipient follow-up (days) | 493 ± 282 | 2101 ± 640 | |
| Actuarial 1-year recipient survival (%) | 98.6% | 93.6% | N.S. (p = 0.183) |
There were two re-transplants in the series.
N.S. = nonsignificant, p > 0.05.
By comparison, none of the patients in the historical control group was HIV+ at the time of transplantation. In the historic control group, HLA matching (mean HLA mismatch was 2.9 ± 1.5; HLA A, p = 0.36; HLA B, p = 0.36; HLA DR, p = 0.16) and pretransplant PRA ≥ 20% (6.4%, p > 0.05) were the same in alemtuzumab group. The mean follow-up for the historical control group was 2101 ± 640 days.
Actuarial 1-year recipient survival was 98.6% in the group receiving alemtuzumab induction and 93.6% (p = 0.187) in the historic control group, respectively (see Figure 1A). Recipient survival in the group that received alemtuzumab induction was 98.0% at 493 days mean follow-up. There were four deaths in this group (at 192, 345, 725 and 976 days posttransplantation); three had excellent graft function at the time of their death. Two of the deaths were related to iatrogenic causes. One patient died of sepsis secondary to bowel perforation during an allograft nephrectomy at an outside institution for allograft failure because of biopsy-proven recurrent focal segmental glomerulosclerosis (FSGS). Another patient died from sepsis with a functioning allograft after suffering an esophageal perforation during an esophagogastroduodenoscopy (EGD); immunosuppression was discontinued during sepsis. The 3rd and 4th deaths were from a myocardial infarction 24 months posttransplantation, and from a nonsmall cell lung cancer 32 months posttransplantation, respectively. These two late deaths (at 725 and 976 days posttransplantation) account for the drop in the tail end of the actuarial recipient and graft survival curves (see Figure 1 A,B). Only 16 recipients had follow-up time > 900 days. However, we strongly caution that the acute drop in the tail end may also represent a serious long-term concern. Clearly, long-term follow-up is required to demonstrate this.
Figure 1. Actuarial patient (A) and graft survival (B) of recipients of live donor kidney transplantation.
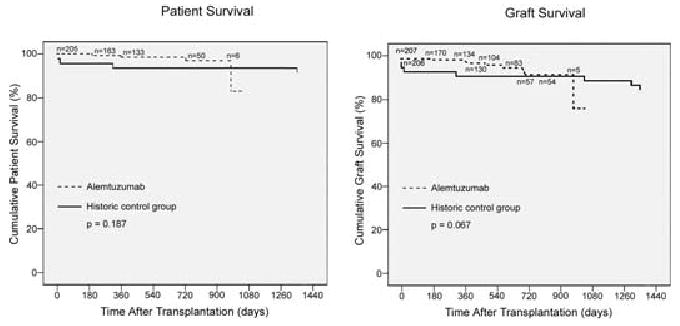
The drop at the tail end of the alemtuzumab curve is associated with a very small recipient population at the outer fringe of follow-up time with two deaths occurring at posttransplantation days 976 and 725. The longest patient and graft follow-up of the alemtuzumab group is 1028 days and mean follow-up 493 days.
Graft survival and function
Actuarial 1-year graft survival was 98.1% in the alemtuzumab and 91.2% in the historic control groups, respectively (see Figure 1B and Table 2). This was not statistically significant (p = 0.067) but the alemtuzumab group tended to have improved graft survival. There were a total of nine renal allograft losses at follow-up (at 3, 176, 345, 365, 482, 582, 690, 725 and 976 days posttransplantation). The incidence of delayed graft function (DGF) is 0%.
Table 2.
Living donor renal transplant recipients graft survival and function
| Alemtuzumab induction | Historical control | p-value | |
|---|---|---|---|
| No. of recipients | 205 | 47 | |
| No. of grafts | 207 | 47 | |
| Actuarial 1-year graft survival (%) | 98.1% | 91.2% | N.S. (p = 0.067) |
| Creatinine at 1 year (mg/dL) | 1.46 ±0.51 | 1.58 ± 1.23 | N.S |
| Creatinine at 493 days follow-up (mg/dL) | 1.47 ±0.67 | 1.48 ±0.54 | N.S. |
N.S. = nonsignificant, p > 0.05.
Of the patients in the alemtuzumab and the historic control groups with functioning grafts, the mean creatinine at 1 year was 1.46 ± 0.51 mg/dL and 1.58 ± 1.23 mg/dL (p > 0.05), respectively (see Table 2). At a mean of 493 days follow-up, the mean creatinine was 1.47 ± 0.67 mg/dL and 1.48 ± 0.54 mg/dL in the alemtuzumab and historic control groups, respectively.
Immunosuppression status and frequency of dosing
At mean 493 days follow-up, of the 205 patients that received alemtuzumab induction, 90 (43.9%) recipients were on daily (bid and qd) tacrolimus monotherapy, 87 (42.4%) recipients had been weaned to spaced-dose monotherapy and 21 (10.2%) recipients were on multi-immunosuppressive drug therapy (a combination of mycophenolate mofetil, prednisone or rapamune were added to tacrolimus monotherapy because of episodes of ACR) (see Table 3). The HLA mismatch for patients who received alemtuzumab induction on daily (twice daily or bid and once daily or qd) tacrolimus monotherapy versus Spaced-dose monotherapy was the same (2.98 ± 1.68 vs. 3.24 ± 1.61, p > 0.05). Of importance in this protocol, at 493 days mean follow-up, 90% of recipients were still completely steroid-free since the time of transplantation. The frequency of immunosuppression dosing for recipients receiving tacrolimus monotherapy is presented in Table 4. Of the 87 recipients who were weaned to spaced-dose monotherapy, 45 (22.0%) recipients were on every other day (qod), 35 (17.1%) recipients were on three times per week (3×/week), 4 (2.0%) recipients were on twice per week (2×/week), and 3 (1.5%) recipients were on once per week (1×/week) tacrolimus. A total of six recipients had HLA identical donors and their frequencies of tacrolimus dosing are as follows (see Table 4): bid (n = 2), qod (n = 2), 2×/week (n = 1), 1 ×/week (n = 1). Of the 167 alemtuzumab induction recipients who have ≥ 6 months follow-up, the majority of recipients (n = 87, 52.1%) are on spaced-dose monotherapy. We anticipate the number of recipients on spaced-dose monotherapy will continue to increase over time.
Table 3.
Immunosuppression regimen status and frequency of immunosuppression dosing in living donor renal transplant recipients
| Alemtuzumab induction | Historical control | |
|---|---|---|
| No. of recipients | 205 | 47 |
| Daily monotherapy | 90 (43.9%) | 15 (31.9%) |
| Spaced-dose monotherapy | 87 (42.4%) | N/A |
| Multi-immunosuppressive drug therapy | 21* (10.2%) | 20 (42.5%) |
On multiple immunosuppressive drugs (not just tacrolimus monotherapy) because of ACR episodes, a combination of mycophenolate mofetil, prednisone or rapamune were added.
Table 4.
Frequency of dosing in 205 recipients receiving alemtuzumab induction and tacrolimus monotherapy at mean follow-up of 493 days
| Alemtuzumab induction | HLA-identical transplants | |
|---|---|---|
| No. of recipients | 205 | 6 |
| Twice daily (bid) | 15 (7.3%) | 2 (33.3%) |
| Once daily (qd) | 75 (36.6%) | – |
| Every other day (qod) | 45 (22.0%) | 2 (33.3%) |
| Three times per week (3×/week) | 35 (17.1%) | – |
| Twice per week (2×/week) | 4 (2.0%) | 1 (16.7%) |
| Once per week (1×/week) | 3 (1.5%) | 1 (16.7%) |
| Multiple immunosuppressive drugs | 21 (10.2%) | – |
Incidence and severity of ACR
In the alemtuzumab group, the cumulative incidences of ACR at 1, 2, 3, 4, 6 and 12 months and at a mean follow-up of 493 days were 1.5% (n = 3), 1.5% (n = 3), 2.0% (n = 4), 2.4% (n = 5), 2.9% (n = 6), 6.8% (n = 14), and 10.7% (n = 22), respectively. In the historic control group, the cumulative incidences of ACR at 1, 2, 3, 4, 6 and 12 months and 493 days were 12.8% (p < 0.05), 12.8% (p < 0.05), 12.8% (p < 0.05), 17.0% (p < 0.05), 17.0% (p < 0.05), 17.0% (p < 0.05) and 21.3% (p < 0.05), respectively (see Table 5). The number of recipients with recurrent (>1 episode) ACR in the alemtuzumab versus historic control group was 45.5% (10 of 22) versus 50.0% (5 of 10).
Table 5.
Incidence of living donor renal transplant recipients with ACR* at mean follow-up of 493 days
| Alemtuzumab induction | Historical control | p-value | |
|---|---|---|---|
| Recipients | 205 | 47 | |
| Cumulative recipients with ACR* at | |||
| ≤1 month** | 1.5% (3) | 12.8% (6) | p < 0.05 |
| ≤2 months | 1.5% (3) | 12.8% (6) | p < 0.05 |
| ≤3 months | 2.0% (4) | 12.8% (6) | p < 0.05 |
| ≤4 months | 2.4% (5) | 17.0% (8) | p < 0.05 |
| ≤6 months | 2.9% (6) | 17.0% (8) | p < 0.05 |
| ≤12 months | 6.8% (14) | 17.0% (8) | p = 0.047 |
| At mean follow-up of 493 days, cumulative recipients with ACR were | 10.7% (22) | 21.3% (10) | p < 0.05 |
| ACR recipients with >1 ACR episode | 45.5% (10) | 50.0% (5) | – |
| Weaning attempted | 57.0% (118) | – | |
| Preweaning ACR incidence | 4.3% (9) | – | |
| Postweaning ACR incidence | 6.3% (13) | – |
All ACR were biopsy proven.
Table 6 detailed the characteristics of the 22 alemtuzumab recipients with 32 episodes of ACR. The presence of graft failure, and episodes, severity, timing and treatment of ACR in the alemtuzumab group is also depicted in Table 6. In the 22 alemtuzumab patients with ACR, 5 lost their renal allograft (patient #12 had immunosuppression reduced secondary to BK nephropathy with subsequent ACR; patient #17 died from a myocardial infarction with resultant graft loss; patient #22 died from sepsis secondary to an esophageal perforation from an EGD with immunosuppression withdrawn and resultant allograft loss from Banff 3 ACR; patient #21 and #19 had ACR at 282 and 478 days posttransplantation). Only one patient (#8) had antibody-mediated rejection (AMR) with evidence of positive diffuse peritubular C4d and donor-specific antibody (DSA) identification and titer. This patient responded to plasmapheresis and intravenous immunoglobulin (IVIg) with resolution of her DSA titer.
Table 6.
Recipients with ACR
| Pt | Graft failed | # ACR | <borderline | 1a | 1b | 2a | 2b | 3 | AMR | Days to 1st ACR | Prewean vs. postwean ACR | Wean time | Treatment of ACR | A MM | B MM | DR MM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | No | 1 | 1 | 1 | 0 | 0 | 0 | 112 | Prewean ACR at 112 days | Solu | 2 | 1 | 2 | |||
| #2 | No | 2 | 1 | 1 | 0 | 0 | 0 | 142 | Prewean ACR at 142 days | Solu/solu | 1 | 2 | 1 | |||
| #3 | No | 1 | 0 | 0 | 1 | 0 | 0 | 17 | Prewean ACR at 17 days | Thymo | 0 | 1 | 1 | |||
| #4 | No | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 62 | Prewean ACR at 62 days | None | 0 | 1 | 1 | ||
| #5 | No | 1* | 1 | 1 | 1 | 0 | 0 | 335 | Postwean ACR at 179 days | 156 | Solu/solu/solu | 0 | 1 | 1 | ||
| #6 | No | 1 | 0 | 1 | 0 | 0 | 0 | 356 | Prewean ACR at 356 days | None | 2 | 2 | 1 | |||
| #7 | No | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 541 | Postwean ACR at 211 days | 330 | Solu | 1 | 2 | 2 | |
| #8 | No | 2 | 1 | 0 | 0 | 0 | 0 | C4d+,DSA+ | 8 | Prewean ACR at 8 days | Solu/PP + IVIg | 1 | 1 | 1 | ||
| #9 | No | 2 | 1 | 1 | 0 | 0 | 0 | 220 | Prewean ACR at 220 days | Solu/solu | 1 | 2 | 2 | |||
| #10 | No | 1 | 0 | 0 | 1 | 0 | 0 | 541 | Postwean ACR at 283 days | 258 | Campath | 1 | 1 | 1 | ||
| #11 | No | 2 | 1 | 1 | 0 | 0 | 0 | 680 | Postwean ACR at 422 days | 258 | Solu | 1 | 2 | 2 | ||
| #12 | Yes, +BK virus | 2 | 1 | 1 | 0 | 0 | 0 | 303 | Postwean ACR at 175 days | 128 | Solu/IVIg | 2 | 2 | 2 | ||
| #13 | No | 2 | 1 | 1 | 0 | 0 | 0 | 435 | Prewean ACR at 435 days | Campath/solu | 2 | 2 | 1 | |||
| #14 | No | 1 | 1 | 0 | 0 | 0 | 0 | 18 | Prewean ACR at 18 days | Solu/solu | 1 | 1 | 1 | |||
| #15 | No | 2 | 1 | 1 | 0 | 0 | 0 | 387 | Postwean ACR at 171 days | 216 | Campath | 1 | 1 | 1 | ||
| #16 | No | 2 | 1 | 1 | 0 | 0 | 0 | 399 | Postwean ACR at 206 days | 193 | Solu/solu | 1 | 1 | 1 | ||
| #17 | Yes | 1 | 1 | 0 | 0 | 0 | 0 | 255 | Postwean ACR at 60 days | 195 | Solu | 1 | 1 | 1 | ||
| #18 | No | 2 | 0 | 1 | 1 | 0 | 0 | 345 | Postwean ACR at 138 days | 207 | Campath/solu | 0 | 1 | 2 | ||
| #19 | Yes | 1 | 1 | 0 | 0 | 0 | 0 | 478 | Postwean ACR at 289 days | 189 | Solu | 1 | 1 | 1 | ||
| #20 | No | 1 | 0 | 1 | 0 | 0 | 0 | 577 | Postwean ACR at 318 days | 259 | Campath | 1 | 0 | 1 | ||
| #21 | Yes | 2 | 1 | 0 | 0 | 1 | 0 | 282 | Postwean ACR at 93 days | 189 | Solu/campath | 2 | 2 | 2 | ||
| #22 | *&astYes, no immuno | 1 | 0 | 0 | 0 | 0 | 1 | 195 | Postwean ACR at 82 days | 113 | None, esophageal perf | 2 | 2 | 2 |
Pt = recipients.
ACR = number of episodes of acute cellular rejections (Banff 1a, 1b, 2a, 2b and 3 classification).
Days to 1 st ACR = days to 1 st episode of ACR.
Prewean vs. postwean ACR = 1st episode of preweaning (from posttransplant days) vs. postweaning ACR (days after weaning occurs).
Wean time = days to wean tacrolimus monotherapy from a consolidated daily dosing to less than that (e.g. qd to qod/3×/week).
AMR = antibody-mediated rejection with evidence of +C4d and +DSA (donor specific antibody). Pt underwent plasmapheresis + IVIg
MM = HLA mismatch at the A, B and DR loci.
Solu = solumedrol pulse.
Thymo = thymoglobulin.
pp = plasmapheresis.
Campath = 30 mg IV×1.
1 = one continuous episode of ACR from 2a to 1b to 1a and resolution with solumedrol.
Yes, no immuno = Pt died from sepsis secondary to an esophageal perforation from an EGD with immunosuppression withdrawn and resultant loss of allograft from ACR.
Of the recipients who received alemtuzumab induction and had weaning of their maintenance immunosuppression, 9 (4.4%) experienced pre-weaning ACR (ACR prior to weaning of immunosuppression) and another 13 (6.3%) patients developed ACR after weaning of daily monotherapy (postweaning ACR) (see Table 5). There were a total of 32 episodes of biopsy-proven ACR in the 22 alemtuzumab recipients with ACR (see Table 7). Most (81.3%) episodes of ACR were Banff 1 (a or b) and were sensitive to steroid pulses for treatment of rejection. There were 14 (43.8%) Banff 1a and 12 (37.5%) Banff 1b ACR episodes. Another four (12.5%) ACR episodes were graded as Banff 2a and one (3.1%) was graded as Banff 2b. There was one (3.1%) episode of Banff 3 rejection. Banff 2 ACRs were treated with a single dose of 30 mg alemtuzumab intravenously (22).
Table 7.
Severity of ACR episodes by Banff score
| Banff score | 32 ACR episodes in 22 alemtuzumab recipients | 19 ACR episodes in 10 historic control recipients |
|---|---|---|
| 32 (100%) | 19 (100%) | |
| Banff 1a | 14 (43.8%) | 6 (31.6%) |
| Banff 1b | 12 (37.5%) | 5 (26.3%) |
| Banff 2a | 4 (12.5%) | 3 (15.7%) |
| Banff 2b | 1 (3.1%) | 4 (21.1%) |
| Banff 3 | 1 (3.1%) | 1 (5.3%) |
There were 19 episodes of biopsy-proven ACR in the 10 historic control recipients with ACR (see Table 7). Fiftyeight percent (11 of 19 episodes) had Banff 1 ACR (6 of 19 or 31.6% had Banff 1a and 5 of 19 or 26.3% had Banff 1b), 36.8% (7 of 19 episodes) had Banff 2 (3 of 19 or 15.7% had Banff 2a and 4 of 19 or 21.1% had Banff 2b) and 5.3% (1 of 19 episodes) had Banff 3 ACR.
A total of six recipients had HLA identical donors in the alemtuzumab group. There was no episode of ACR in these HLA identical recipients. The incidence of ACR did not appear to be higher in African Americans [6.5% (2/31) at 1 year (p > 0.05), and 12.9% (4/31) at 493 days follow-up]. One of the African Americans who had ACR was noncompliant.
Complications
At follow-up, there was no recipient with cytomegalovirus (CMV) disease or infection. CMV disease was defined as the presence of signs and symptoms of tissue injury with isolation of virus and/or histopathologic or immunohistochemical evidence of CMV. CMV infection was defined by isolation of virus or detection of viral proteins or nucleic acids in any body fluid or tissue specimen. All recipients received prophylactic valganciclovir for 6 to 9 months. Three (1.5%)of the 205 recipients developed BK nephropathy and were treated with weaning of immunosuppression and cidofivir. One of these three BK nephropathy recipients eventually lost her renal allograft. There was no posttransplant lymphoproliferative disease (PTLD) in the group that received alemtuzumab induction. The incidence of recurrent FSGS was 5.6% (1/18).
In the alemtuzumab group, 26 (12.7%) recipients had diabetes prior to transplantation. But only one (0.5%) patient developed new onset insulin-dependent diabetes mellitus posttransplant. Twenty percent (48 of 205) of recipients had a pretransplant fasting glucose >120 mg/dL. In the historic control, 17.0% (8 of 47) of recipients had pretransplant diabetic nephropathy, and about 10% developed insulin-dependent diabetes mellitus posttransplantation.
With alemtuzumab induction, one vascular thrombosis occurred in this series (this was related to traumatic transection of the renal vein and artery at the hilum when the recipient woke up despite vascular repair). Ureteral stenosis occurred in one patient (0.5%); this was in a HIV+ recipient. The stenosis was revised with a uretero-ureteral anastomosis to the native ureter, and this recipient continues to have an excellent graft function. There also appears to be no higher incidence of pulmonary toxicity (<1%), with the vast majority of recipients extubated posttransplantation not requiring an intensive care unit.
Neutropenia was treated with subcutaneous injection(s) of Neupogen® (Filgrastim, Amgen Inc., Thousand Oaks, CA, USA) on an outpatient basis. There were a total of 40 episodes of neutropenia requiring Neupogen® in the 205 recipients; 15% of recipients received Neupogen®. Each dose of 300 μg subcutaneous Neupogen® costs about $140. The incidence of readmission to the hospital for any reason was<10% within the first 3 months posttransplant. The most common reasons were urinary tract infection, dehydration, a rising serum creatinine and the need to exclude ACR.
Discussion
This report, which represents the largest series to date of living donor renal transplantation recipients receiving alemtuzumab induction, confirms the short-term safety and efficacy of this drug as an induction agent. Despite the complexity of the recipient population, which included 28 re-transplants (8.7% had 2nd transplants, 4.9% had 3rd to 5th transplants), 4 HIV+ recipients and 13 pediatric recipients, the incidence of ACR at 1 year was 6.8%, and the actuarial 1-year patient and graft survivals were 98.6% and 98.1%, respectively. These short-term survivals are superior to those of the US Transplant-Scientific Registry of Transplant Recipients (SRTR) database; for year 2002, the 1-year unadjusted patient and graft survivals were 97.6%, and 90.5%, respectively (23). Furthermore, good graft function was also observed, with a mean creatinine of 1.46 ± 0.51 mg/dL at 1 year and 1.47 ± 0.67 mg/dL at mean 493 days follow-up. The group that received alemtuzumab induction had a significantly lower incidence of ACR (6.8%) than the historic controls (17%) at 1 year (p = 0.047). Most importantly, the incidence of recipient complications at 1 year, including PTLD (0%), CMV disease or infection (0%) and posttransplant insulin-dependent diabetes mellitus (0.5%), was exceptionally low.
Our findings support and extend those of Calne and colleagues in 1997 who first employed alemtuzumab as induction therapy in renal transplantation, followed by low-dose cyclosporine monotherapy (5). These initial results of 13 patients were promising and prompted further trials to study the use of alemtuzumab as an induction agent in renal transplantation. A 5-year follow-up study compared outcomes in 33 renal transplant recipients who received alemtuzumab induction therapy followed by low-dose cyclosporine monotherapy to 66 renal transplant contemporaneous control recipients who received conventional immunosuppression consisting of cyclosporine, azathioprine and prednisolone (6). While 14% of patients in the alemtuzumab group experienced ACR beyond 1-year posttransplant compared to none in the control group, the incidence of ACR was similar at 5 years (33.6% in the alemtuzumab group vs. 31.5% in the control group). The overall 5-year patient (88% vs. 83%) and graft (79% vs. 74%) survival rates, as well as the incidence of infectious complications, were similar in both alemtuzumab and control group, respectively. They concluded that alemtuzumab allowed satisfactory long-term patient and graft survivals equivalent to those seen with standard triple immunosuppression, while avoiding steroid therapy.
In addition, several recent studies have demonstrated that alemtuzumab is an effective induction agent in both living donor and deceased donor renal transplantation with few short-term side effects. Knechtle and colleagues from the University of Wisconsin utilized alemtuzumab induction therapy followed by sirolimus monotherapy in a pilot study involving 29 renal transplant recipients at 3 to 29 months of follow-up (11). In this study, there were no infectious complications and no evidence of malignancy. However, 8 (27.6%) of the 29 patients experienced rejection, and while the rejection was treated successfully in 7 patients, 1 patient experienced graft loss. Of the eight patients that experienced rejection, five had evidence that received alemtuzumab experienced a significantly of humoral rejection. More recently, Knechtle and colleagues reported on the results of 126 renal allograft recipients who received alemtuzumab induction therapy compared with other recipients who received either anti-CD25 antibody, thymoglobulin, or other induction therapy with a CNI, mycophenolate mofetil and prednisone for maintenance (12). The group that received alemtuzumab experienced a significantly lower incidence of ACR compared to the other groups, while the rates of infection and malignancy were not significantly different. Importantly, patients with DGF experienced less rejection with alemtuzumab than the control groups and had improved graft survival. Kaufman and colleagues (13) reported similar results with kidney transplant patients receiving alemtuzumab (n = 123) or basiliximab (n = 155) induction with a prednisone-free maintenance protocol using tacrolimus (6 to 8 ng/mL) and mycophenolate mofetil combination. A lower rate of early (<3 months) ACR was observed in the alemtuzumab (4.1%) versus the basiliximab (11.6%) group, but the actual ACR rates for both groups in living donor recipients were equivalent at 1 year (alemtuzumab = 14.3%, basiliximab = 12.4%). The 1-year actual recipient and graft survivals among living donor recipients treated with alemtuzumab were 96.7% and 98.9%, respectively.
Kirk et al. reported seven nonsensitized recipients of living donor kidneys treated perioperatively with alemtuzumab and followed them postoperatively without maintenance immunosuppression (14). All recipients developed reversible ACR within the first month that was characterized by predominantly monocytic infiltrates. These episodes were responsive to treatment with steroids or sirolimus or both. In a more recent pilot study of five recipients of live donor kidneys treated with perioperative alemtuzumab and deoxyspergualin (which has an inhibitory effect on monocytes and macrophages) without maintenance immunosuppression, Kirk et al. reported that all five recipients developed reversible ACR that was similar in timing, histology, and transcriptional profile to that seen in patients treated with alemtuzumab alone (15). T-cell depletion combined with deoxyspergualin induces tolerance in nonhuman primates but does not appear to induce tolerance in humans. It appears that chemokine production is not adequately suppressed with alemtuzumab induction and that some form of maintenance immunosuppression is required with this protocol.
Recently Flechner and colleagues (7), in a pilot study of 22 kidney transplant recipients receiving alemtuzumab induction, sirolimus, and mycophenolate mofetil maintenance without CNI and steroids, demonstrated a relatively high incidence of ACR (36% at 1 year) and possible pulmonary toxicity, raising concerns about the efficacy and safety of the protocol. This and other studies point to the importance of at least an initial period of CNI in recipients receiving alemtuzumab induction. We did not see a higher incidence of pulmonary toxicity with the vast majority of our recipients extubated posttransplantation not requiring an intensive care unit.
A recent randomized controlled clinical trial compared thymoglobulin (n = 30), alemtuzumab (n = 30) and daclizumab (n = 30) in deceased donor renal transplant recipients (16). Induction therapy with alemtuzumab and tacrolimus (trough of 4 to 7 ng/mL) and mycophenolate mofetil (1 g daily) combination maintenance therapy was associated with similar 1-year actuarial patient, graft survival and function (thymoglobulin = 92%, 88%, creatinine clearance 80; alemtuzumab = 100%, 100%, creatinine clearance 73; daclizumab = 88%, 88%, creatinine clearance 81), respectively. The majority (80%) of patients remained steroid-free at 1 year posttransplantation. In addition, encouraging excellent preliminary results have also been obtained with alemtuzumab induction in HIV+ recipients (10) and pediatric recipients (17, 18).
While alemtuzumab depletes both T- and B lymphocytes from the peripheral circulation, its effects on monocytes are less pronounced, and plasma cells are not effectively depleted from the circulation. Accordingly, recent reports have described monocyte-predominant ACR (14) as well as severe, early acute humoral rejection (24). We did not see a higher incidence of early severe acute humoral rejection in our patients with tacrolimus monotherapy. The importance of CNI requirement in the early posttransplant period with alemtuzumab induction was demonstrated in the pilot studies by Flechner and Knechtle et al. (7, 11, 12). The majority of ACR episodes in this current study was Banff 1a or 1b, and was successfully treated with a steroid bolus.
The findings in this report have several limitations. While this study confirms the short-term safety and efficacy of alemtuzumab, long-term follow-up is clearly needed. A mean follow-up of 493 ± 282 days in 205 recipients is relatively short. The acute drop in the tail end of the Kaplan-Meier patient and graft survival curves may be related to the small number of patients at long-term follow-up. However, it may also represent a serious long-term concern. More careful assessment of graft function with creatinine clearance at various time points may provide a more accurate measurement of renal function. Continued follow-up will clearly be required to assess long-term graft survival and function. The delayed incidence of ACR that occurs as lymphocytes return to baseline will need to be monitored closely, as will the rates of late chronic allograft nephropathy. Re-population of the profound depletion of peripheral blood lymphocytes, monocytes, and NK cells by alemtuzumab can take up to 1 year. Knechtle and colleagues (2) found lymphocytes reach at least 80% of baseline values 18 to 24 months post-alemtuzumab induction. It is also hoped that with a lower starting dose of tacrolimus progressively weaned over time, a decreased incidence of CNI nephrotoxicity will be observed compared to traditional triple immunosuppression.
The long-term benefits of alemtuzumab induction with tacrolimus monotherapy clearly warrant further investigation. The immunologic mechanisms that permit the ability to wean immunosuppression in these patients have yet to be elucidated. Additional studies directed at elucidating these mechanisms are currently under way (25). It is hoped that monitoring of class I and II antibodies by enzyme-linked immunoassay and identification of DSAs can potentially aid in weaning of immunosuppression and thus decrease the incidence of postweaning ACR. We are also in the process of assessing both the frequency and function of donor-reactive T cells, as well as identifying cytokine gene polymorphism and assaying alloantibody titers in recipients who have or have not experienced rejection after spaced-dose weaning. The advisability of empiric immunosuppression withdrawal in otherwise stable patients remains to be established. Given the increased, albeit low, rate of acute rejection seen post-weaning in previously stable patients, this approach remains investigational.
References
- 1.Hale G, Dyer MJ, Clark MR, et al. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988;2:1394–1399. doi: 10.1016/s0140-6736(88)90588-0. [DOI] [PubMed] [Google Scholar]
- 2.Knechtle SJ. Present experience with Campath-1H in organ transplantation and its potential use in pediatric recipients. Pediatr Transplant. 2004;8:106–112. doi: 10.1046/j.1399-3046.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Pangalis GA, Dimopoulou MN, Angelopoulou MK, et al. Campath-1H (anti-CD52) monoclonal antibody therapy in lymphoproliferative disorders. Med Oncol. 2001;18:99–107. doi: 10.1385/mo:18:2:99. [DOI] [PubMed] [Google Scholar]
- 4.Liu NS, O'Brien S. Monoclonal antibodies in the treatment of chronic lymphocytic leukemia. Med Oncol. 2004;21:297–304. doi: 10.1385/MO:21:4:297. [DOI] [PubMed] [Google Scholar]
- 5.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative Campath 1H, and low-dose cyclosporine monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 6.Watson CJ, Bradley JA, Friend PJ, et al. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation— efficacy and safety at five years. Am J Transplant. 2005;5:1347–1353. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 7.Flechner SM, Friend PJ, Brockmann J, et al. Alemtuzumab induction and sirolimus plus mycophenolate mofetil maintenance for CNI and steroid-free kidney transplant immunosuppression. Am J Transplant. 2005;5:3009–3014. doi: 10.1111/j.1600-6143.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro R, Basu A, Tan H, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg. 2005;200:505–515. doi: 10.1016/j.jamcollsurg.2004.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan HP, Kaczorowski DJ, Basu A, et al. Steroid-free tacrolimus monotherapy following pretransplant Thymoglobulin or Campath and laparoscopy in living donor renal transplantation. Transplant Proc. 2005;37:4235–4240. doi: 10.1016/j.transproceed.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Tan HP, Kaczorowski DJ, Basu A, et al. Living-related donor renal transplantation in HIV+ recipients using alemtuzumab preconditioning and steroid-free tacrolimus monotherapy: A single center preliminary experience. Transplantation. 2004;78:1683–1688. doi: 10.1097/01.tp.0000145880.38548.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knechtle SJ, Pirsch JD, H Fechner J, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: Results of a pilot study. Am J Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 12.Knechtle SJ, Fernandez LA, Pirsch JD, et al. Campath-1H in renal transplantation: The University of Wisconsin experience. Surgery. 2004;136:754–760. doi: 10.1016/j.surg.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman DB, Leventhal JR, Axelrod D, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in kidney transplantation: Comparison with basiliximab induction—Long-term results. Am J Transplant. 2005;5:2539–2548. doi: 10.1111/j.1600-6143.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 15.Kirk AD, Mannon RB, Kleiner DE, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation. 2005;80:1051–1059. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 16.Ciancio G, Burke GW, Gaynor JJ, et al. A randomized trial of three renal transplant induction antibodies: Early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80:457–465. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 17.Bartosh SM, Knechtle SJ, Sollinger HW. Campath-1H use in pediatric renal transplantation. Am J Transplant. 2005;5:1569–1573. doi: 10.1111/j.1600-6143.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro R, Ellis D, Tan HP, et al. Antilymphoid antibody preconditioning and tacrolimus monotherapy for pediatric kidney transplantation. J Pediatr. 2006;148:813–818. doi: 10.1016/j.jpeds.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. Natl Rev Immunol. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Murase N, Abu-Elmagd K, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro R, Jordan ML, Basu A, et al. Kidney transplantation under a tolerogenic regimen of recipient pretreatment and low-dose postoperative immunosuppression with subsequent weaning. Ann Surg. 2003;238:520–525. doi: 10.1097/01.sla.0000089853.11184.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu A, Ramkumar M, Tan HP, et al. Reversal of acute cellular rejection after renal transplantation with Campath-1H. Transplant Proc. 2005;37:923–926. doi: 10.1016/j.transproceed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Scientific Registry of Transplant Recipients Annual report. [Accessed March 4];2006 Available from: http://www.ustransplant.org.
- 24.Hill P, Gagliardini E, Ruggenenti P, Remuzzi G. Severe early acute humoral rejection resulting in allograft loss in a renal transplant recipient with Campath-1H induction therapy. Nephrol Dial Transplant. 2005;20:1741–1744. doi: 10.1093/ndt/gfh867. [DOI] [PubMed] [Google Scholar]
- 25.Murase N, Metes D, Zeevi A, et al. Immunomonitoring of liver recipients treated with tolerance-enhancing regimen of immunosuppression. Am J Transplant. 2004;4:400. [Google Scholar]


