Abstract
Hamster hearts transplanted into stable rat recipients of hamster livers (OLT rats) were hyperacutely rejected after transfer with unaltered rat antihamster hyperimmune serum (HS). This was followed by immediate liver xenograft rejection in 4 of 5 rats. In contrast, simple heat inactivation of the rat HS resulted in prolonged survival of hamster hearts to 25 days without deterioration effect in the liver xenografts. This effect was species-specific because third-party mouse heart grafts in OLT rats were hyperacutely rejected in minutes if either active or heat inactivated antimouse HS was given. In cytotoxicity experiments, the complement in OLT serum produced weak lysis of hamster lymphocytes, while efficiently doing so with mouse cell targets. Because normal hamster serum caused no lysis at all of hamster target cells, the residual low-grade lysis of OLT serum was possibly being mediated by extrahepatic sources of rat C. In conclusion, the homology of C and target cells represents a mechanism of protection that the liver confers to other organs, and that is most easily seen in xenografts but may be allospecifically operational with allografts as well within the limits of MHC restriction.
We recently reported that a hamster liver xenograft transplanted into a rat fosters the acceptance of skin and heart from the same or third-party hamsters while not affording protection to mouse organs from either humoral or cellular rejection (1). However, this effect was species-specific rather than having the individual or donor-strain specificity that has been demonstrated by Kamada et al. (2) in similar experiments with rat liver allografts. The hamster-to-rat heart xenotransplantation model was appropriate for experimental inquiries about complement because this organ is normally rejected in rats by mechanisms involving antibody and C activation (3, 4).
An explanation of the species-specific effect needs to accommodate the facts that in vitro erythrocyte lysis by C proteins is homologous species–restricted (5), and that antibody-coated target cells are resistant to lysis by homologous C but not by heterologous C (6). In addition, although in vitro culture studies have detected synthesis of C proteins by human and rodent mononuclear phagocytes (7, 8), the liver is the primary source of synthesis (9, 10). Finally, it has been established that like the liver allograft (11, 12), the liver xenograft retains its metabolic specificity, including the synthesis of albumin, clotting factors, and C3 (13, 14). Thus we postulated that the conversion of recipient C to that of the donor after liver xenotransplantation could result in a more conducive C environment for target cells not only of the liver, but also of companion organs from the donor species that are transplanted simultaneously or later.
To test this hypothesis, we transplanted hamster hearts into stable rat recipients of hamster xenografts and then performed serum transfer experiments with various hyperimmune sera (HS)* as the source of antibody and of activated and inactivated C. Additional in vitro experiments were designed to test the ability of the C in serum of stable liver xenograft recipients to support lysis of species-specific or third-party lymphocytes and of sheep red blood cells.
Materials and Methods
Animals
Male Syrian golden hamsters (100–120 g) and male Lewis (LEW) rats (250–270 g) purchased from Charles Rivers Laboratories (Wilmington, MA) were used as donors and recipients respectively. Male B10.BR mice weighing 28–32 g were purchased from Jackson Laboratories (Bar Harbor, ME) and used as third-party donors.
Surgical procedures
Orthotopic liver transplantation was performed according to Kamada's cuff technique (15) with modifications including cholecystectomy of the donor liver (16). Heterotopic cardiac transplants were performed in the abdominal cavity by the method of Ono and Lindsey (17).
Rejection of the heart xenografts was defined by cessation of the heartbeat on abdominal palpation and confirmed by histology. Liver xenograft rejection was suggested by the presence of signs of encephalopathy followed by death of the recipients. The diagnosis was confirmed by histology.
Protocol of immunosuppression
Intraperitoneal injections were given of cyclophosphamide (CyP) 8 mg/kg/day for 10 days, begun simultaneously with 1 mg/kg/day i.m. of FK506, which was continued for 30 days. No further treatment was given. In rats bearing hamster liver xenografts, test heart transplantation was performed 10–30 days after discontinuance of immunosuppression.
Preparation of hyperimmune serum
Ten LEW rats received hamster heart xenografts that were rejected in 3 days. Three days after this, the animals were exsanguinated for the serum collection. The antihamster lymphocytotoxic titer was approximately 1:4096 with little variation from animal to animal. The sera were divided into aliquots of 1 ml and stored at −70°C until used 1–3 weeks later. In preparation for injection, the sera were thawed on ice (4°C) or thawed and then warmed at 56°C for 30 min in order to inactivate the complement.
Ten other LEW rats received a B10.BR mouse heart xenograft that was rejected in 2 days. On day 4, the animals were sacrificed and their sera collected as described above. The antimouse lymphocytotoxic titer was 1:4096.
For in vitro experiments, samples of the foregoing antihamster and antimouse HS, as well as sera from normal rats or rats bearing liver xenografts, were absorbed with hamster or B10.BR mouse spleen cells. This provided an antibody-free source of species-specific C. The absorption using 5×108 hamster or B10.BR spleen cells per 2 ml serum was carried out by incubation for 1 hr at 4°C. The absorbed sera were then collected by centrifugation. The entire procedure was done twice. Afterwards, cytotoxicity was undetectable by the C-dependent cytotoxicity assay.
In vivo experimental design
The purpose of these experiments was to see if hyperacute rejection of hamster or mouse hearts was induced by serum transfer of their specific antisera. In 30 experiments, hamster heart grafts were transplanted into LEW rats belonging to the following groups: (1) LEW rats that received no immunosuppression (n=10); (2) LEW rats that were pretreated with the same 30-day protocol of immunosuppression used to induce liver xenograft acceptance but without liver transplantation (n=10); and (3) stable OLT recipients at 40–60 days after liver transplantation and 10 to 30 days after immunosuppression had been stopped (n=10).
At 10 min after revascularizing the heart xenograft, after it had a strong and regular beat, serum transfer was performed. Half the animals (n=5) in each of the 3 main groups were given 1 ml unaltered HS via the penile vein while the other half were given the same amount of inactivated serum (Table 1). As controls for groups 1 and 3, unaltered (n=5) and inactivated normal rat serum was given.
TABLE 1. Survival of hamster heart xenografts in liver xenograft recipients injected with active or decomplemented rat antihamster hyperimmune serum.
| Heart recipients (immunosuppression) | Transferred hyperimmune serum | |||
|---|---|---|---|---|
| Active | (MST±SD) | Inactive | (MST±SD) | |
| 1. Normal LEWa (none) | 2,2,2,3,3 min | (2.4±0.5) | 8,10,15,18,32 min | (16.6±9.4) |
| 2. LEW rat (CyP 8 mg/kg/day × 10 + FK506 1 mg/kg/day × 30)b | 2,2,3,3,4 min | (2.8±0.8) | 6,12,12,21,28 min | (15.8±8.6) |
| 3. OLT ratc,d (CyP + FK506) | 7,8,12,16,32 min | (15.1±10.1) | 23,23,25,27,28 days | (25.2±2.2) |
LEW rat recipients normally reject hamster hearts in a mean of 3.0 days (1).
Pretreatment (This kind of pretreatment normally extends survival of hamster hearts beyond 3 days).
LEW rats that received a hamster liver transplant 40–60 days before and were immunosuppressed with CyP and FK506 as in group 2 for 30 days, with no treatment thereafter. The preexisting liver xenografts were not adversely affected at the time of heart rejection using inactivated HS. With active HS, the liver of 4 of 5 rats underwent humoral rejection in less than 24 hr, the fifth rat survived another 50 days.
When unaltered (n=5) or decomplemented (n=5) normal rat serum was given instead of HS, heart xenograft survival was unaffected in group 1 (3 days), and in group 3 (25 days).
The same experiments were performed using B10.BR mouse hearts for transplantation to LEW recipient with injection of active or heat-inactivated rat antimouse HS (Table 2). After the serum transfer in both models, the time of rejection was recorded—defined as the cessation of heartbeat by direct observation or by daily palpation.
Table 2. Survival of third party B10.BR mice heart xenografts in liver xenograft recipients injected with active or decomplemented rat antimouse hyperimmune serum.
| Heart recipients (treatment) | Transferred hyperimmune serum | |||
|---|---|---|---|---|
| Active | (MST±SD) | Inactive | (MST±SD) | |
| 1. Normal LEWa (none) | 2,2,3,3,3 min | (2.6±0.5) | 5,5,11,19,23 min | (12.6±8.1) |
| 2. LEW rat (CyP 8 mg/kg/day × 10 + FK506 1 mg/kg/day × 30)b | 2,2,2,3,4 min | (2.6±0.8) | 6,9,15,21,30 min | (16.2±9.6) |
| 3. OLT ratc (CyP + FK506) | 2,3,3,8,10 min | (5.2±3.5) | 6,6,12,15,16 min | (11.0±4.7) |
LEW rat recipients normally reject B10.BR mice hearts in a mean of 2.7±1.7 days (unpublished observation).
Pretreatment.
LEW rats that received a hamster liver transplant 40–60 days before and were immunosuppressed with CyP and FK506 as in group 2 for 30 days, with no treatment thereafter. The liver xenografts were not adversely affected at the time of heart rejection.
To determine if the injected antihamster antibodies in the HS used for group 3 animals were rapidly removed by the previously transplanted hamster liver, serial blood samples were analyzed in the rats of group 3 before and every 5 min after the delayed heart xenotransplantation (n=2). Antibodies were determined by the CDC assay (see below).
In vitro studies
Two kinds of assays were performed, both designed to test the efficacy and specificity of C in the serum of the rat recipient of a hamster liver xenograft compared with C in the serum of the unaltered or sensitized rat or other species (mouse, hamster, rabbit). All tested sera were rendered cytotoxic antibody–free by prior absorption with the prospective species target cells.
CDC assay (hamster and mouse lymphocyte targets): These experiments are tabulated in Table 3. To each of the antibody-free sera (the complement source), a constant amount was added of the same decomplemented antihamster or antimouse antibody used in the in vivo experiments (titer 1:4096). After washing in RPMI medium and isolation, the cells were resuspended at a concentration of 5×l06/ml. Duplicate samples of 1 μl of decomplemented HS (rat antihamster or rat antimouse) and 1 μl of lymph node cell suspensions (hamster or B10.BR mice) were placed into 72 well tissue-typing trays (Robbins Scientific, Sunnyvale, CA). After incubation for 30 min at room temperature, 2-fold dilutions of the different sources of C were added to each well with reincubation for another 30 min at 37°C under 95% O2, and 5% CO2. Then 5 μl of 0.4% trypan blue was added to each well for staining. Dead cell percent was plotted against serum dilutions. The C titer was defined as the highest serum dilution with more than 25% cell lysis. RPMI medium served as negative controls.
Table 3. Summary of in vitro cytotoxicity experiments comparing heterologous and homologous sources of C with that in OLT recipients.
| Source of complement (noncytotoxic) | Lymphocyte target cell (lysis) | |
|---|---|---|
| Hamster | B10.BR mice | |
| 1. OLT recipient | Weak | Efficient |
| 2. Normal rat | Efficient | Efficient |
| 3. Rat antihamster HS | Efficient | ND |
| 4. Rat antimouse HS | ND | Efficient |
| 5. Normal hamster | No lysis | Modest |
| 6. Normal mouse | ND | No lysis |
| 7. Baby rabbit Ca | Efficient | Efficient |
Cederlane Laboratories Limited, Hornby, Ont. All other sera were prepared in our laboratory.
Hemolytic assay (target SRBC): With this modification of Mayer's method (18), the efficacy of SRBC-absorbed OLT serum was compared with that of normal rat, rat antihamster, and normal hamster in lysing of sheep red blood cells after the addition of a known amount of antibody. The tested sera were prepared by absorption with fixed SRBC for 15 min at 4°C. The constant antibody was rat antiserum to SRBC diluted 1:16, added to an equal volume of SRBC suspension (5×l06/ml) in PBS + 0.01 M EDTA and followed by incubation at 37°C for 30 min. The SRBC were washed three times with PBS + Ca 0.15 mM + Mg 0.5 mM (PBS2+). Then 100 μl of sensitized SRBC was distributed in a 96-well, U-bottomed Falcon plates (Becton Dickinson, Lincoln Park, NJ), and different amounts of C at the dilution of 1:2 were added. Additional amounts of PBS2+ were added to each well to reach a total amount of 200 μl/well. The plates were incubated for 9 hr at 37°C, then centrifuged for 2 min at 1000 rpm. Each well had a control with C but not SRBC to establish the background. The absorbance of the supernatant was evaluated at 405 nm.
Statistical analyses
Values were expressed as mean±SD. Student's t test was used to evaluate the differences observed between the mean values; differences were considered to be significant at P<0.05.
Results
In Vivo experiments
Hamster hearts: Hamster hearts, which are normally rejected in 3 days, were hyperacutely rejected in a few minutes if either unaltered or complement inactivated antihamster HS was given intraoperatively to untreated rats (group 1) or to rats pretreated with a 30-day course of immunosuppression (group 2) (Table 1).
Hyperacute rejection of hamster hearts also occurred after their transplantation to the rat recipients of liver xenografts if the rat HS was unaltered, and in addition the liver was promptly rejected in 4 of 5 such experiments (group 3). Inactivation of the rat HS prevented this humoral rejection of both organs (Table 1).
In control experiments for groups 1 and 3, absorbed normal unaltered and decomplemented rat serum did not cause hyperacute rejection, showing that the alternative pathway of complement activation was not responsible for the HS effect (Table 1).
In further controls for the group 3 experiments, serial antibody titers in the rat recipients were measured before and every 5 min after heart xenotransplantation. These were essentially the same for up to 60 min whether active or decomplemented HS was administered (data not shown), ruling out differences of antibody absorption as an explanation for the drastically different results with and without HS complement.
Mouse hearts
Mouse hearts underwent hyperacute rejection in untreated and previously immunosuppressed rats and in OLT recipients with the transfer of antimouse HS whether or not the serum was C-inactivated. The rejections did not adversely affect the previously placed hamster liver xenograft (Table 2).
In Vitro Experiments
Complement-dependent cytotoxicity (CDC): The serum complement from the rats bearing a hamster liver caused only weak lysis of hamster lymphocytes, while efficiently lysing mouse lymphocytes (Table 3). It was noteworthy that normal hamster serum caused no lysis at all of hamster target cells. These results and the control data outlined in Table 3 are shown in Figure 1.
Figure 1.
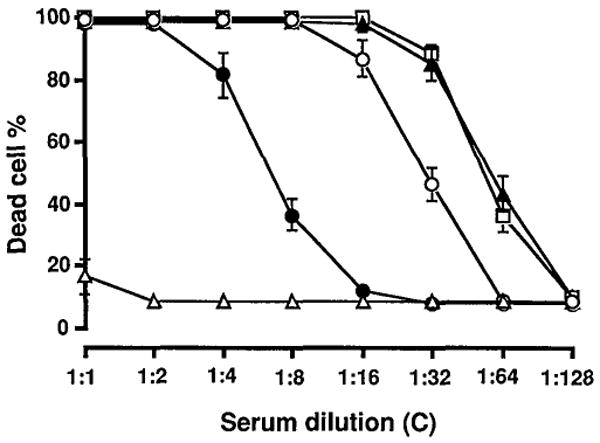
Hamster lymphocyte lysis produced by OLT serum as a source of C in the presence of a known amount of decomplemented rat antihamster HS. Lysis produced by OLT serum (closed circle) was compared with that caused by baby rabbit C (open square), rat antihamster HS (closed triangle), normal rat serum (open circle), and normal hamster serum (open triangle).
When the same OLT serum was used as a complement source for the mouse lymphocyte target (Table 3), lysis was at least as efficient as that caused by the serum or HS from rats, and serum from normal hamster or rabbit (Fig. 2). Mouse serum as a C source failed to cause lysis of mouse cells. This was analogous to the experiment in which hamster complement did not lyse hamster lymphocytes. Thus, in both experiments, the benign nature of homologous C was evident versus the vigorous reactivity of heterologous C.
Figure 2.
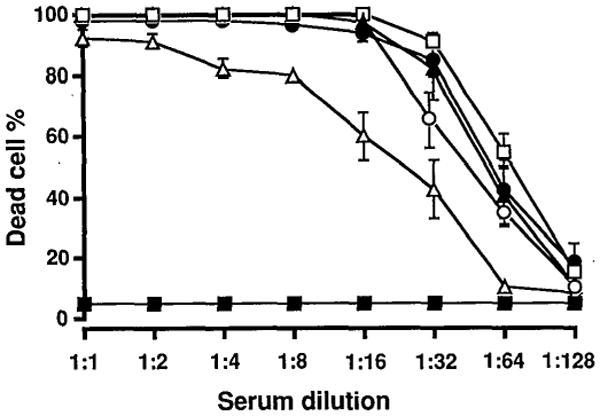
B10.BR mice lymphocyte lysis produced by OLT serum as a source of C in the presence of a known amount of decomplemented rat antimouse HS. Lysis caused by OLT serum (closed circle) was compared with that caused by baby rabbit C (open square), rat antimouse HS (closed triangle), normal rat serum (open circle), normal hamster serum (open triangle), and normal mouse serum (closed square).
Hemolytic assay
The hemolysis of SRBC caused by the OLT serum was equivalent to that produced by the serum of normal rats and greater than that of normal hamster serum. The most potent lysis was from rat HS (Fig. 3), a finding consistent with other research in nontransplant models demonstrating elevated C synthesis as part of the inflammatory response (19).
Figure 3.
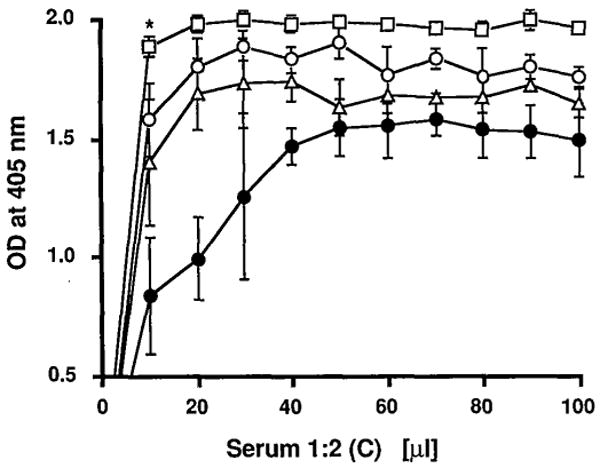
The hemolytic assay. C in OLT serum (open triangle) was tested in its ability to lyse sensitized SRBC. Lysis was compared with that produced by rat antihamster HS (open square), normal rat serum (open circle), and normal hamster serum (closed circle). Results are the mean of 3 experiments. * P<0.01 vs. normal rat serum.
Discussion
It is well known that the hepatocytes of a transplanted allograft (12) or xenograft (13, 14) retain their metabolic specificity, and that much of the body's complement (perhaps all of some components) is produced by the liver (9, 10). Notwithstanding the evidence that there is cumulatively significant extrahepatic synthesis of C (7, 8), we postulated that the new supply of donor specific C might be a survival advantage for the transplanted liver as well as for other organs from the same donor that are exposed to the recipient's altered metabolic environment. It is now realized that chimeric donor cells that have migrated ubiquitously from the graft contribute to this changed environment (20) including the monocyte/macrophage lineage that synthesize complement (7, 8).
The experiments reported herein support the C protection hypothesis, and strongly suggest but do not prove that the xenograft recipient has 2 C systems of which the dominant one is donor. The evidence for both propositions is compelling from both the in vivo and in vitro experiments. In essence, the in vivo experiments showed how the systemic introduction of active C in hyperimmune antihamster rat serum into the rat recipient of a stably functioning hamster liver caused prompt humoral rejection of a secondarily engrafted hamster heart that otherwise would have been protected. Coincidentally, the serum transfer caused prompt rejection of the liver itself. These lethal events were completely prevented by the simple expedient of decomplementing the serum by heating it at 56°C for 30 min. In experiments using rat antimouse serum, third-party mouse hearts in the OLT recipients were hyperacutely rejected with or without complement inactivation of the antimouse antiserum with no harm to the hamster liver xenograft.
This demonstration of the species specificity and efficacy of the dominant hamster complement system was confirmed by exhaustive in vitro assays, including complement dependent cytotoxicity. These tests also provided circumstantial evidence of significant amounts of rat complement in the rat recipients of hamster livers. Normal hamster serum caused no lysis at all of target hamster cells, confirming the findings of Van den Bogaerde et al. (21), whereas serum from the OLT recipient was moderately lytic when used as the complement source.
It remains to be studied in genetically controlled (inbred) models if the protection from humoral rejection endowed by the liver in the hamster to rat model is trans–species-specific without MHC restriction—or, more likely, if the generic umbrella for all hamsters is merely a reflection of intensive inbreeding and minimal genetic diversity in this animal as we suggested previously (1). If MHC restriction is found, the xenograft model should provide much needed insight into the mechanisms of hyperacute allograft rejection in sensitized recipients and how these can be altered. A frequently recorded probable example of exploitation of MHC restriction in the context of our studies was first reported by Fung et al. (22) and confirmed by others (23) who showed that a kidney allograft could be successfully transplanted across a positive lymphocytotoxic crossmatch, as long as it was preceded by the liver 12 hr or more earlier from the same donor.
The resistance to humoral rejection of the allograft liver itself can be explained by this mechanism. What must be accomplished for success with either a liver allograft or xenograft (and to organs to which they extend protection) is survival and function of the new liver long enough to allow the complement transition to begin. This objective has been readily achieved in crossmatch-positive liver allograft recipients with a drug cocktail that includes prostaglandin E1 and high doses of steroids (24). The soluble human complement receptor (type I) that inhibits the cleavage of complement components C3 and C5 and blocks the classical and alternative pathways of complement activation (25–27), affording significant protection from humoral rejection (28, 29) might buy enough time when combined with conventional immunosuppressants to make this objective attainable with xenografts.
Another implication of these studies that could have profound clinical significance is appreciation of the potential harm of infusing active human complement contained in the blood and blood products that are needed in large quantities when human liver xenotransplantation is performed. Without the precautions of complement inactivation, this could create conditions comparable to the in vivo animal experiments of the present study.
Footnotes
Presented at the 19th Annual Meeting of the American Society of Transplant Surgeons, May 19–21, 1993, Houston, TX.
This work was supported by Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations. CyP, cyclophosphamide; HS, hyperimmune serum.
References
- 1.Valdivia LA, Demetris AJ, Fung JJ, Celli S, Murase N, Starzl T. Successful hamster-to-rat liver xenotransplantation under FK506 immunosuppression induces unresponsiveness to hamster heart and skin. Transplantation. 1993;55:659. [PMC free article] [PubMed] [Google Scholar]
- 2.Kamada N, Brons G, Davies HffS. Fully allogeneic liver grafting in rats induces a state of systemic non-reactivity to donor transplantation antigens. Transplantation. 1980;29:429. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Valdivia LA, Monden M, Gotoh M, Nakano Y, Tono T, Mori T. Evidence that deoxyspergualin prevents sensitization and first-set cardiac xenograft rejection in rats by suppression of antibody formation. Transplantation. 1990;50:132. doi: 10.1097/00007890-199007000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Van den Bogaerde J, Aspinall R, et al. Induction of long-term survival of hamster heart xenografts in rats. Transplantation. 1991;52:15. doi: 10.1097/00007890-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Haensch GM, Hammer CH, Vanguri P, Shin ML. Homologous species restriction in lysis of erythrocytes by terminal complement proteins. Proc Natl Acad Sci USA. 1981;78:5118. doi: 10.1073/pnas.78.8.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson-Weller A. Decay accelerating factor (CD55) In: Parker CJ, editor. Membrane defenses against attack by complement and perforins. Berlin: Springer; 1992. [Google Scholar]
- 7.Colten HR. Biosynthesis of complement. Adv Immunol. 1976;22:67. doi: 10.1016/s0065-2776(08)60548-9. [DOI] [PubMed] [Google Scholar]
- 8.Cole FS, Colten HR. Complement biosynthesis. In: Till GO, Rother KO, editors. The complement system. Berlin: Springer; 1988. p. 44. [Google Scholar]
- 9.Alper CA, Johnson AM, Birtch AG, Moore FD. Human C3: evidence for liver as the primary site of synthesis. Science. 1969;163:286. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]
- 10.Wolpl A, Robin-Winn M, Pichlmayer R, Goldmann SS. Fourth component of complement (C4) polymorphism in human orthotopic liver transplantation. Transplantation. 1985;40:154. doi: 10.1097/00007890-198508000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwagi N, Groth CG, Starzl TE. Changes in serum haptoglobin and group specific component after orthotopic liver homotransplantation in humans. Proc Soc Exp Biol Med. 1968;128:247. doi: 10.3181/00379727-128-32988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Demetris AJ, Van Thiel DH. Medical progress: I. liver transplantation. N Engl J Med. 1989;321:1014. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdivia LA, Lewis JH, Celli S, et al. Hamster coagulation and serum proteins in rat recipients of hamster xenografts. Transplantation. 1993;56:489. doi: 10.1097/00007890-199308000-00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Fung J, Tzakis A, et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65. doi: 10.1016/0140-6736(93)92553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamada N, Calne RY. Orthotopic liver transplantation in the rat: technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47. [PubMed] [Google Scholar]
- 16.Valdivia LA, Monden M, Gotoh M, et al. Prolonged survival of liver xenografts from hamster to rat by splenectomy and cyclosporine administration. Transplantation. 1987;43:745. doi: 10.1097/00007890-198712000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ono K, Lindsey ES. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969;57:225. [PubMed] [Google Scholar]
- 18.Mayer MM. In: Experimental immunochemistry. Kabat L, Mayer MM, editors. Springfield, IL: Thomas; 1961. p. 133. [Google Scholar]
- 19.Ruddy S. Complement. In: Rose NR, Conway de Macario E, Fahey JL, Friedman H, Penn GM, editors. Manual of clinical laboratory Immunology. Washington, DC: American Society for Microbiology; 1992. p. 114. [Google Scholar]
- 20.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease. N Engl J Med. 1993;328:745. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van den Bogaerde J, Hassan R, White DG. An analysis of concordant xenografting. Transplant Proc. 1992;24:513. [PubMed] [Google Scholar]
- 22.Fung J, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20:88. [PMC free article] [PubMed] [Google Scholar]
- 23.Flye MW, Duffy BF, Phelan DL, Ratner LE, Mohanakumar T. Protective effects of liver transplantation on a simultaneously transplanted kidney in a highly sensitized patient. Transplantation. 1990;50:1051. [PubMed] [Google Scholar]
- 24.Takaya S, Iwaki Y, Starzl TE. Liver transplantation in positive cytotoxic crossmatch cases using FK506, high-dose steroids, and prostaglandin E1. Transplantation. 1992;54:927. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh CG, Marsch HC, Carson GR, et al. Recombinant soluble human complement receptor type I inhibits inflammation in the reversed passive arthus reaction in rats. J Immunol. 1991;146:250. [PubMed] [Google Scholar]
- 26.Rabinovich R, Yeh CG, Hillegass LM. Role of complement in endotoxin/platelet-activating factor–induced lung injury. J Immunol. 1992;149:1744. [PubMed] [Google Scholar]
- 27.Mulligan MS, Yeh CG, Rudolph AR, Ward PA. Protective effects of soluble CR1 in complement- and neutrophil-mediated tissue injury. J Immunol. 1992;148:1479. [PubMed] [Google Scholar]
- 28.Pruitt SK, Bollinger RR. The effects of soluble complement receptor type I on hyperacute allograft rejection. J Surg Res. 1991;50:350. doi: 10.1016/0022-4804(91)90202-w. [DOI] [PubMed] [Google Scholar]
- 29.Pruitt SK, Baldwin WM, Marsch HC, Jr, Lin SS, Yeh CG, Bollinger RR. The effect of soluble complement receptor type 1 on hyperacute xenograft rejection. Transplantation. 1991;52:868. doi: 10.1097/00007890-199111000-00022. [DOI] [PubMed] [Google Scholar]


