Abstract
In the nervous system, a perfect balance of excitation and inhibition is required, for example, to enable coordinated locomotion. In Caenorhabditis elegans, cholinergic and GABAergic motor neurons (MNs) effect waves of contralateral muscle contraction and relaxation. Cholinergic MNs innervate muscle as well as GABAergic MNs, projecting to the opposite side of the body, at dyadic synapses. Only a few connections exist from GABAergic to cholinergic MNs, emphasizing that GABA signaling is mainly directed toward muscle. Yet, a GABAB receptor comprising GBB-1 and GBB-2 subunits, expressed in cholinergic MNs, was shown to affect locomotion, likely by feedback inhibition of cholinergic MNs in response to spillover GABA. In the present study, we examined whether the GBB-1/2 receptor could also affect short-term plasticity in cholinergic MNs with the use of channelrhodopsin-2-mediated photostimulation of GABAergic and cholinergic neurons. The GBB-1/2 receptor contributes to acute body relaxation, evoked by photoactivation of GABAergic MNs, and to effects of GABA on locomotion behavior. Loss of the plasma membrane GABA transporter SNF-11, as well as acute photoevoked GABA release, affected cholinergic MN function in opposite directions. Prolonged stimulation of GABA MNs had subtle effects on cholinergic MNs, depending on stimulus duration and gbb-2. Thus GBB-1/2 receptors serve mainly for linear feedback inhibition of cholinergic MNs but also evoke minor plastic changes.
Keywords: locomotion, metabotropic GABA receptor, plasticity, channelrhodopsin-2, excitatory-inhibitory balance
in mammals, GABAB receptors are extrasynaptic, high-affinity G protein-coupled receptors (GPCRs) that either act as presynaptic autoreceptors on GABAergic neurons or detect spillover GABA, released at nearby synapses (Bettler et al. 2004). GABAB receptors are obligate heterodimers of B1 and B2 subunits (Jones et al. 1998; White et al. 1998) that together with auxiliary subunits (KCTD proteins) appear to form tetramers or even higher order oligomers (Schwenk et al. 2010). GABAB receptors can modulate the function of excitatory neurons by heterosynaptic inhibition, via signaling through heterotrimeric G proteins (through “released” Gβγ subunits), to inhibit presynaptic voltage-gated Ca2+-channels (Herlitze et al. 1996; Ikeda 1996). Alternatively, they can trigger postsynaptic G protein-activated inward-rectifying potassium (GIRK) channels, again via Gβγ subunits, to induce a slow inhibitory current (Luscher et al. 1997; Schwenk et al. 2010). Furthermore, via Gαo/Gαi pathways, mammalian GABAB receptors can activate or inhibit adenylyl cyclase. At glutamatergic synapses, GABAB receptors were implicated in synaptic plasticity (Davies et al. 1991; Mott and Lewis 1991), lowering cAMP levels and thus blocking stimulatory effects of increased Ca2+ on synaptic vesicle recruitment from the reserve pool (Sakaba and Neher 2003).
Cholinergic motor neurons (MNs) in the Caenorhabditis elegans ventral nerve cord activate muscles and GABAergic neurons at dyadic synapses/neuromuscular junctions (NMJs) to coevoke a contralateral inhibition of muscles, thus allowing a bend of the body to occur (Schuske et al. 2004; White et al. 1986). Very few “reverse” connections have been found from GABAergic to cholinergic MNs (White et al. 1986), making it unlikely that these connections contribute much to the excitatory-inhibitory balance. However, a heterodimeric GABAB receptor, comprising GBB-1 and GBB-2 subunits, was reported to be widely expressed in the nervous system; yet, among MNs, it was exclusively found in cholinergic cells (Dittman and Kaplan 2008). The gbb-1 or gbb-2 deletion mutants exhibit alterations in locomotion as well as increased paralysis induced by aldicarb, an inhibitor of acetylcholine (ACh) esterase. Since these effects are not exacerbated in gbb-1; gbb-2 double mutants, it is very likely that these receptors also form hetero(di)mers in C. elegans. Because of the aldicarb hypersensitivity of gbb-1 or gbb-2 mutants, it is thought that spillover GABA, sensed by the GBB-1/2 receptor, may cause heterosynaptic inhibition of cholinergic MNs. The identity of the G protein that the GBB-1/2 receptor couples to is unknown, although it has been suggested that GBB-1/2 receptors signal through the Gαo pathway, which inhibits cholinergic transmission by negatively regulating phospholipase C (Lackner et al. 1999). The GBB-1/2 receptor may directly influence cholinergic transmission, i.e., under “steady-state” conditions of GABA transmission, as triggered by ACh release stimulating GABA MNs. In this case, the amount of ACh transmission should linearly feed back on the activity of the cholinergic MNs, since more ACh release would also evoke more GABA release. Alternatively, spillover GABA could induce short-term synaptic plasticity in cholinergic synapses, causing nonlinear feedback regulation of ACh release.
To distinguish between these possibilities, one would ideally measure postsynaptic currents in muscle in response to constant or repeated stimulation of cholinergic MNs. However, the preparation of the C. elegans NMJ does not permit such experiments to be performed in a meaningful way (Richmond and Jorgensen 1999), because 1) commissural connections between cholinergic and GABAergic MNs are cut; 2) basal membranes surrounding the NMJs, which would certainly affect the diffusion of spillover GABA, are digested by collagenase treatment; and 3) the recording is done under buffer flow, which strongly dilutes any spillover transmitter. However, prolonged activation of C. elegans neurons in live animals and an indirect analysis of synaptic transmission can be achieved noninvasively by using optogenetic techniques (Nagel et al. 2005; Zhang et al. 2007). Channelrhodopsin-2 (ChR2), expressed and photoactivated in cholinergic cells, causes a simultaneous contraction of all body wall muscles, which depends on the efficacy of synaptic transmission and can easily be measured by automated video analysis (Liewald et al. 2008). Likewise, GABAergic neurons (expressing ChR2) also can be triggered by photostimulation, evoking body relaxation due to simultaneous inhibition of all body wall muscles, an effect that can be macroscopically measured to deduce defects or alterations in GABAergic signaling.
We were able to show that GBB-1/2 receptors contribute to the behavioral effects of photoinduced GABA release. The relaxation effects were completely abolished only if GBB subunits as well as the ionotropic GABAA receptor UNC-49 were eliminated. Deletion of gbb-2 had effects on locomotion that could be rescued or even overcompensated by expressing GBB-2(A484V; V572A) specifically in cholinergic MNs, indicating that these cells are the focus of GBB-2 activity. Furthermore, depending on photostimulus strength, duration, and frequency, we observed subtle influences of gbb-2 deletion on the effects of photoinduced ACh release. Thus GABAB receptor signaling in C. elegans mainly serves as a feedback control mechanism for cholinergic transmission, yet it also effects subtle plastic alterations in cholinergic MN function.
MATERIALS AND METHODS
Genetics.
C. elegans strains were cultivated using standard methods on nematode growth medium (NGM) and fed Escherichia coli strain OP50-1 (Brenner 1974). For optogenetic experiments, all-trans retinal (0.25 μl of a 100 mM stock in ethanol; Sigma) was added to 300 μl of OP50 culture and spread onto 5.5-cm culture dishes containing 10 ml of NGM. About 18 h before experiments, L4 larvae, grown on all-trans retinal plates, were placed on fresh all-trans retinal plates. Strains used (outcrossed 4–7 times, where appropriate) were as follows: N2: wild type (Bristol isolate), RM2710: snf-11(ok156), ZX426: N2; zxIs3[punc-47::ChR2(H134R)::YFP; lin-15+]I, ZX460: N2; zxIs6[punc-17::ChR2(H134R)::YFP; lin-15+]V, ZX464: unc-49(e407); zxIs3, ZX551: gbb-2(tm1165), ZX558: gbb-1(tm1406), ZX572: gbb-2(tm1165); zxIs3, ZX585: gbb-1(tm1406); zxIs3, ZX586: gbb-1(tm1406); unc-49(e407); zxIs3, ZX587: gbb-2(tm1165); unc-49(e407); zxIs3, ZX635: gbb-2(tm1165); zxIs6, ZX675: N2; zxIs3; zxIs6, ZX808: gbb-2(tm1165); zxIs3; zxIs6, ZX973: gbb-2(tm1165); snf-11(ok156); zxIs6, ZX974: snf-11(ok156); zxIs6, ZX1052: gbb-2(tm1165); zxEx455[punc-47::GBB-2(A484V; V572A); pmyo-2::mCherry], ZX1053: gbb-2(tm1165); zxEx456[punc-17::GBB-2(A484V; V572A); pmyo-2::mCherry], ZX1054: gbb-2(tm1165); unc-49(e407); zxIs3; zxEx457[punc-47::GBB-2(A484V; V572A); pmyo-2::mCherry], ZX1055: gbb-2(tm1165); unc-49(e407); zxIs3; zxEx458[punc-17::GBB-2(A484V; V572A); pmyo-2::mCherry], ZX1103: N2; zxEx465[punc-47::GBB-2(A484V; V572A); pmyo-2::mCherry], and ZX1104: N2; zxEx466[punc-17::GBB-2(A484V; V572A); pmyo-2::mCherry].
Molecular biology.
The plasmid encoding pmyo-2::mCherry (pCFJ90) was a kind gift of E. Jorgensen. Construction of plasmids used to generate zxIs3 and zxIs6 integrated transgenes was described previously (Liewald et al. 2008). The GBB-2(A484V; V572A) construct was generated as follows. The full-length GBB-2 cDNA with additional restriction sites at both ends was commercially synthesized (Eurofins MWG Operon) and subcloned into the punc-47::ChR2(H134R)::YFP plasmid (Liewald et al. 2008) using Tth111I and EcoRI. Toxicity of this sequence in various plasmid backbones and E. coli strains promoted random mutations in the GBB-2 sequence, which after transformation could be reduced by introducing an artificial intron near the 5′-end of the cDNA. However, the most promising clone of GBB-2 still contained two missense mutations resulting in A484V and V572A changes of the GBB-2 amino acid sequence [pCS150NT: punc-47::GBB-2(A484V; V572A)]. With the use of NheI and PvuI, the GBB-2 fragment was then subcloned into punc-17::ChR2(H134R)::YFP (Liewald et al. 2008) to generate pCS152NT [punc-47::GBB-2(A484V; V572A)].
Behavioral assays and data analysis.
Optogenetic/behavioral assays and automated video analysis for the extraction of worm body length were described previously (Liewald et al. 2008; Schultheis et al. 2011; Stirman et al. 2011; Weissenberger et al. 2011). In brief, for body length measurements, animals were transferred onto plain NGM plates and recorded with a PowerShot G5 or G9 digital camera (Canon) while blue light from a 50-W HBO lamp [450- to 490-nm green fluorescent protein excitation filter; intensity adjusted using neutral density filters (AHF Analysetechnik)] was applied. Light intensities were measured in the focal plane using a light power meter (Thorlabs). Light application was controlled using a computer-controlled shutter (Sutter Instruments). Velocity, bending angles, and trajectories were recorded using tracking software (Stirman et al. 2011) that controls an x,y-translational stage and allows photoactivation of ChR2 via a LCD projector (450–490 nm; ∼4 mW/mm2). Ten minutes before the start of these assays, animals were transferred to plain NGM plates. Swimming assays were performed in 96-well plates containing 80 μl of NGM and 80 μl of M9 saline per well. Animals were recorded under ×25 magnification with a PowerShot G9 digital camera (Canon) for 1 min, and swimming cycles were counted.
Electrophysiology.
Recordings from dissected C. elegans body muscle were performed as described previously (Nagel et al. 2005). After dissection, cells were treated for 8 s with 0.5 mg/ml collagenase (Sigma) in modified Ascaris Ringer's (AR; 150 mM NaCl, 5 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 15 mM HEPES, pH 7.35, 340 mosM) and washed with AR. Cells were clamped to −60 mV using an EPC10 amplifier with head stage and Pulse software (HEKA). The bath solution was AR; the pipette solution was 120 mM KCl, 20 mM KOH, 4 mM MgCl2, 5 mM Tris·HCl, pH 7.2, 0.25 mM CaCl2, 4 mM ATP, 36 mM sucrose, and 5 mM EGTA (315 mosM). Light activation was performed using an LED lamp (KSL-70; Rapp OptoElectronic, Hamburg, Germany) at a wavelength of 470 nm (maximum: 8 mW/mm2) and controlled by the HEKA software. Where appropriate, the light intensity of the LED lamp was reduced using the control unit.
RESULTS
GBB-1/2 receptors contribute to GABA effects at the NMJ.
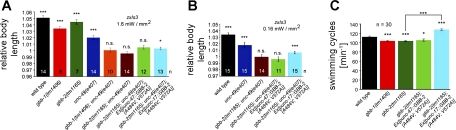
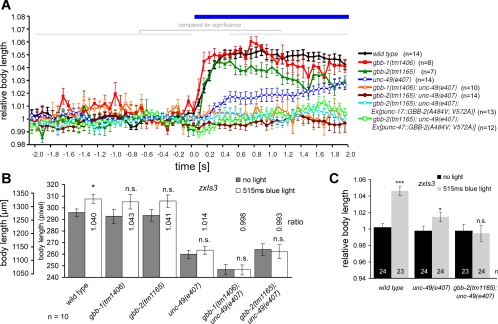
We previously analyzed the effects of photoevoked GABA release in animals expressing ChR2 in GABAergic neurons, i.e., containing transgene zxIs3[punc-47::ChR2(H134R)::YFP; lin-15+] (Liewald et al. 2008). Animals in which GABA MNs were photostimulated showed a body elongation of up to 5%, depending on photostimulus strength (1.6 vs. 0.16 mW/mm2), due to simultaneous, flaccid paralysis of all muscles (Fig. 1, A and B; Supplementary Video 1). (Supplemental material for this article is available online at the Journal of Neurophysiology website.) Body length measurements, obtained either by averaging body length over several video frames before and during photostimulation or, when higher numbers of animals were analyzed, in single frames, showed distinguishing significant differences when both absolute and normalized length were compared (Fig. 2, A–C). Interestingly, when this was repeated in mutants lacking the sole postsynaptic ionotropic GABAA receptor UNC-49 (Bamber et al. 1999), the elongation effects were not completely abolished but worms still elongated by up to 2% (Figs. 1, A and B, and 2, A–C; Supplementary Video 2). This indicated that an additional GABA receptor may affect this behavioral response, and we wondered whether this could be the GBB-1/2 receptor (Dittman and Kaplan 2008). Genomic deletion of the GBB-1/2 GABAB receptor on its own did not have any effects on the photoevoked elongation of zxIs3 animals, indicating that release of GABA was not affected by the gbb-1/2 mutations (Figs. 1, A and B, and 2, A–C). However, when we analyzed photoevoked GABA-mediated elongation in gbb-1(tm1406); unc-49(e407) or gbb-2(tm1165); unc-49(e407) double mutants, no effect whatsoever could be detected (Figs. 1, A and B, and 2, A–C). This demonstrated that the slight elongation effects remaining in unc-49 single mutants were mediated by the GBB-1/2 receptor.
Fig. 1.
GABAB receptors contribute to GABA photoevoked body elongation and affect swimming cycles in cholinergic motor neurons. A and B: channelrhodopsin-2 (ChR2), expressed in GABAergic motor neurons (MNs) (transgene zxIs3; Liewald et al. 2008), was photostimulated to evoke body elongation. Animals of the indicated genotypes were filmed, and the body length during 2 s before and from 0.5 to 2 s during the photostimulation was measured, normalized, and averaged. For an alternative method of analysis, see Fig. 2. Blue light intensities of 1.6 (A) and 0.16 mW/mm2 (B) were used. See text for list of strains used. C: swimming cycles in M9 buffer were compared in wild-type and gbb-2(tm1165) mutant animals in thrashing assays and could be rescued by expression of GBB-2(A484V; V572A) selectively in cholinergic MNs. Values are means ± SE; n = no. of animals assayed. Statistically significant differences (*P < 0.05; **P < 0.01; ***P < 0.001; 1-way ANOVA) were assessed with respect to body length before blue light stimulation (A and B) or to the wild-type level (C).
Fig. 2.
Analysis of light-evoked elongation in animals expressing ChR2 (transgene zxIs3) in GABAergic MNs by averaging over time periods or by comparing single time points and absolute lengths. A: animals of the indicated genotypes were filmed before and during a continuous light stimulus (15 frames/s), and body length was deduced from single video frames. The mean length of each animal during the first 30 frames (−2–0 s) was averaged and used to normalize the elongation during the light stimulus. The mean body length before (−2–0 s) and during the stimulus, after full elongation was reached (0.5–2 s), was then averaged for all animals of a genotype and compared, to analyze statistically significant differences (as indicated in Fig. 1A). B: data for animals from an experiment similar to that in A are shown in absolute values (pixels) as obtained from single movie frames (at 515 ms into the continuous stimulus) and compared with the mean length of the 15 frames before illumination onset. The ratio obtained corresponds to the type of data shown in Fig. 1A. For n = 10 animals each, statistical significance is obtained only for larger size differences. C: when larger numbers of animals are analyzed, normalized data for single time points (i.e., not averaged over time periods) allows statistically significant data to be obtained, despite small absolute changes (maximally 4–5% of the initial body length). Values are means ± SE; n = no. of animals assayed. *P < 0.05; **P < 0.01; ***P < 0.001; 1-way ANOVA.
Since the focus of expression in the motor neuron system is cholinergic neurons (Dittman and Kaplan 2008), is it likely that the GBB-1/2 receptor acts by heterosynaptic feedback inhibition of cholinergic neurons. To test this more directly, we turned to cell-specific rescue experiments. The gbb-2 cDNA was chemically synthesized, verified by sequencing, and then cloned into different promoterless vectors. However, handling any of the constructs containing this cDNA in various E. coli strains was problematic, because the sequence was apparently toxic in bacteria. Thus only clones with sequence errors were obtained. To reduce potential toxicity in E. coli, we introduced a C. elegans intron in the 5′ region. However, even then, the best clone we obtained contained two point mutations, introducing two amino acid changes: A484V, at the beginning of transmembrane helix 2 (TM2), and V572A, at the end of TM4 (Fig. 3). Since these amino acids likely are embedded in the membrane, and the changes are conservative, we do not expect major effects on the protein. This clone was then introduced into C. elegans expression vectors containing promoters for GABAergic (punc-47) or cholinergic neurons (punc-17) and injected into gbb-2(tm1165) and gbb-2(tm1165); unc-49(e407) animals containing the zxIs3 transgene. We obtained several transgenic lines in gbb-2 single-mutant background. However, despite injecting >100 animals and obtaining several hundred transgenic F1 progeny, we could not obtain any transgenic line in the gbb-2(tm1165); unc-49(e407) background. We thus could only test F1 progeny in this genetic background.
Fig. 3.
Amino acid alignment of GBB-1 and GBB-2 subunits with mouse GABAB1 and GABAB2 subunits. Transmembrane domains are indicated by shaded bars above the sequence, as well as the point mutations obtained in the cDNA clone of GBB-2 (circled, with mutated residue indicated below the sequence). gbb-1 is gene Y41G9A.4b, gbb-2 is gene ZK180.1, and mouse GABAB1 and GABAB2 subunits have accession numbers NM_019439.3 and NM_001081141.1, respectively. Alignment was done with ClustalX.
Expression of GBB-2(A484V; V572A) in cholinergic neurons slightly, but in a statistically significant manner, restored the zxIs3-dependent photoevoked body relaxation in gbb-2(tm1165); unc-49(e407) animals, particularly at low photostimulus intensity, whereas expression in GABAergic neurons did not show any statistically significant rescue (Fig. 1, A and B). Furthermore, we analyzed locomotion of strains lacking gbb-1 or gbb-2, without or with expression of GBB-2(A484V; V572A) in cholinergic or GABAergic neurons, by counting swimming cycles of the animals in M9 buffer (Fig. 1C). Whereas gbb-1 and gbb-2 mutants exhibited significantly fewer swimming cycles, GBB-2(A484V; V572A) expressed in cholinergic but not in GABAergic neurons rescued or even overcompensated the locomotion deficits of gbb-2(tm1165) mutants. Although we are cautious in overinterpreting our results due to the mutations in GBB-2 and the analysis of F1 rescue animals in gbb-2; unc-49 background, the data suggest that the focus of GBB-2 function in locomotion and NMJ function is in cholinergic MNs.
GBB-1/2 receptors affect locomotion in response to light-evoked GABA transmission.
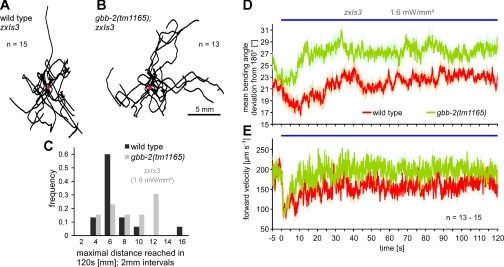
Dittman and Kaplan (2008) showed that the GBB-1/2 receptor has an influence on locomotion behavior (e.g., mean speed, directional turns, and area explored per unit time). Confirming these findings, we also observed that gbb-1 and gbb-2 mutants performed fewer directional turns per unit time compared with wild type (data not shown). We were interested in analyzing locomotion while GABA transmission was photostimulated, because this might further emphasize effects of the GBB-1/2 receptor. As we previously showed, prolonged photostimulation of GABA neurons via the zxIs3 transgene causes 4–5% body elongation that declines to 1–2% within 10–20 s, likely due to desensitization of the UNC-49 GABAA receptor (Liewald et al. 2008), but then, however, GABA effects sustain for several minutes (Schultheis et al. 2011). We thus used a recently developed tracking system, capable of selective photostimulation of freely behaving animals (Stirman et al. 2011), to track locomotion trajectories, speed, and mean bending angles of wild-type and gbb-2(tm1165) animals, both containing zxIs3. When analyzing animal trajectories, we observed that gbb-2; zxIs3 animals, while being photostimulated, reached larger maximal distances from the starting point [“Rmax” as defined by Dittman and Kaplan (2008); note that this is not the absolute distance traveled] during a 120-s period compared with wild type (Fig. 4, A–C). When we analyzed the mean bending angles (i.e., the deviation from 180°, averaged over 11 evenly distributed points along the “spine” of the animal), gbb-2 mutants exhibited much deeper bending angles (∼27 vs. ∼21° for the wild type) and gbb-2 animals moved generally faster than wild type (Fig. 4, D and E). This indicates that the function of the GBB-1/2 receptor may contribute to shaping the body curvature during sinusoidal locomotion, i.e., “smoothening” it. The lack of the receptor, which causes “loopier” locomotion, may thus directly contribute to the overall locomotion speed and, as a consequence of apparently less curved trajectories, to longer Rmax distances traveled.
Fig. 4.
Locomotion behavior is altered by GBB-2 function when GABA transmission is photostimulated. A and B: locomotion trajectories of wild-type (A) and gbb-2 mutant animals (B) expressing ChR2 in GABAergic MNs were tracked for 120 s under blue light exposure, and the data are superimposed at the origin (red circle). C: the maximal distance reached within 120 s [Rmax as defined by Dittman and Kaplan (2008), i.e., before the animal again moves closer toward the origin] is plotted in a histogram. D: mean bending angles were measured between 13 equidistant points along the animals' “backbones” (i.e., 11 angles) at 25 Hz and are expressed as deviation from 180°. E: forward velocities of the animals' centroids were measured at 25 Hz and averaged for each genotype. Values are means ± SE, n = no. of animals analyzed. Periods of ChR2 photostimulation are indicated by blue bars.
Continuous or pulsed photoactivation of cholinergic MNs at different stimulus strength.
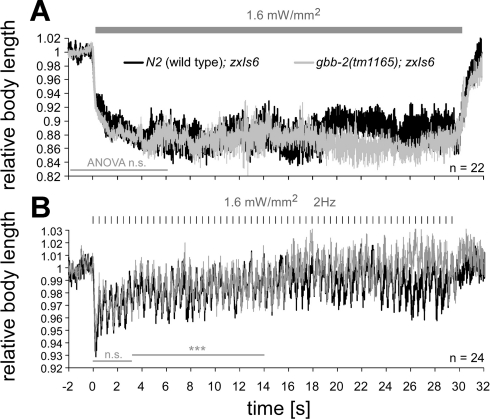
Because of the innervation pattern in the C. elegans nerve cord, cholinergic neurons stimulate GABAergic neurons (White et al. 1986) and apparently detect spillover GABA through the GBB-1/2 receptor as a feedback mechanism. Thus far we have optically manipulated GABA MNs to support this hypothesis. We next asked whether the GBB-1/2 receptor could further act to achieve plastic alterations in cholinergic MNs, e.g., when spillover GABA builds up in the nerve cords for prolonged periods of time. To this end, we used photoactivation of cholinergic neurons via transgene zxIs6[punc-17::ChR2(H134R)::YFP; lin-15+], which we have previously established and extensively characterized (Almedom et al. 2009; Liewald et al. 2008). Prolonged photoevoked ACh transmission, causing concomitant GABA transmission, could for example cause a progressive reduction of further ACh release in response to temporally intensifying GBB-1/2 receptor signaling. We thus measured body contraction in response to continuous, photoevoked ACh transmission in zxIs6 animals, both in the wild type and in gbb-2(tm1165) backgrounds. Contractions in the wild type remained essentially unaltered at ∼88% of the initial body length during a 30-s stimulus of 1.6 mW/mm2 (Fig. 5A), and basically identical contractions were observed in gbb-2(tm1165) mutants.
Fig. 5.
Photostimulated ACh release triggers body contractions that are slightly affected in gbb-2(tm1165) mutants and demonstrate synaptic rundown. A: ChR2, expressed in cholinergic MNs (transgene zxIs6; Liewald et al. 2008), was photostimulated for 30 s to evoke sustained ACh release and, consequently, body contractions (calculated from video frames, obtained at 15 frames/s, before, during, and after illumination with 1.6 mW/mm2 blue light). Contractions remained essentially constant throughout the illumination period (shaded bar) for wild-type and gbb-2(tm1165) mutant animals. B: to exaggerate potential plastic alterations in ACh MNs, we used a pulsed illumination protocol (10-ms stimuli, 2 Hz, shaded tick marks). Body contractions declined over the first 5 s of the stimulus train, indicating synaptic rundown. Contractions between 3 and 14 s into the stimulus train differed between wild-type and gbb-2 mutant animals. Values are means ± SE; n = no. of animals analyzed. Two-factorial ANOVAs were used to analyze statistically significant differences (***P < 0.001) for time periods indicated by shaded bars.
Constant photostimulation of cholinergic MNs did not induce a time-dependent alteration of body contractions. Thus no plastic changes were apparent, and ACh transmission remained sufficiently high to cause sustained full body contraction. As we and others previously showed, constant photostimulation of cholinergic MNs causes an initially large postsynaptic inward current (∼1,200 pA) that quickly declines to a steady-state current of roughly 80 pA (Almedom et al. 2009; Liewald et al. 2008; Liu et al. 2009). This is in large part due to desensitization of postsynaptic nicotinic ACh receptors and somewhat to depression of cholinergic MNs and partial inactivation of ChR2. Although currents in intact animals may differ, the small, light-induced steady-state currents may suffice to evoke prolonged and sustained muscle contractions, even if much less ACh than initially is released, e.g., by temporal summation. Thus a plastic alteration of cholinergic transmission based on GABAB receptor signaling may be masked at the behavioral level. We thus asked whether pulsed release of ACh could show time-dependent effects of GABAergic feedback on cholinergic MNs more pronouncedly, because temporal summation would be much less efficient. We presented 10-ms pulses of blue light over a period of 30 s, at 2 Hz, to zxIs6 wild type or gbb-2(tm1165) mutant animals. If GBB-1/2 receptors mediate plastic changes over time, contractions might show a depression in the wild type, and this depression should be abolished in the gbb-2 mutant. In the wild type, the contractions were reduced from ∼7% to ∼4% over the first 5 s of the stimulus train (93 vs. 96% body length; Fig. 5B), which could indeed reflect short-term synaptic depression under these stimulation conditions. Contractions in gbb-2(tm1165) mutants essentially showed the same decline over time, although as shown by analysis of variance (ANOVA), body length traces significantly differed 3–14 s into the stimulus train, with gbb-2 mutants contracting slightly more. Thus a strong stimulus train may possibly evoke some GBB-1/2-mediated plasticity.
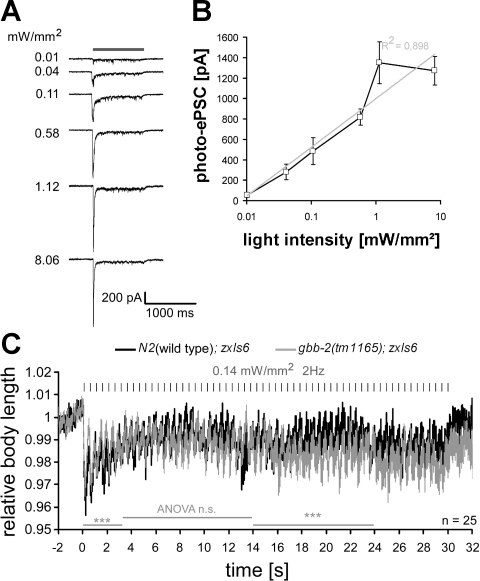
Thus far, we “hyper”-stimulated the cholinergic MNs (stimulus intensity 1.6 mW/mm2), and it appeared possible that the photoactivation was too strong and thus overrode any plastic changes. Because no voltage-gated sodium channels are found in C. elegans, cholinergic MNs likely fire no action potentials, and the amount of ACh released depends on membrane depolarization in a graded fashion (Bargmann 1998). As reported previously (Liu et al. 2009), we found that the size of photoevoked postsynaptic currents (photo-ePSCs) in muscle correlated with increasing light intensities and could be fitted with a single exponent (Fig. 6, A and B), and no all-or-none responses could be observed. To investigate potential plastic effects depending on GBB-1/2 function at low stimulus strength, we lowered the light intensity to approximately one-tenth strength. Photo-ePSCs using 0.11 mW/mm2 were reduced to ∼40% of the maximal photo-ePSCs, which could be reached at 1.12 mW/mm2 (Fig. 6, A and B). In behavioral assays, using 0.14 mW/mm2 caused contractions of the animals to only 96% (Fig. 6C; compared with 93% for 1.6 mW/mm2; Fig. 5B). Stimulus trains (2 Hz, 10 ms) at 0.14 mW/mm2 in wild-type and gbb-2(tm1165) mutant animals showed no major differences between genotypes, and although wild-type animals had significantly stronger contractions during the first 3 s of the train, a general reduction of the contractions (to 98%) was observed over the first 4–5 s in both genotypes, and significantly less pronounced contractions of the wild type were apparent during the second half of the train.
Fig. 6.
Graded responses of cholinergic MNs to increasing stimulus strength and different effects of low-intensity stimulus trains on ACh-evoked contractions in wild-type vs. gbb-2 mutant animals. A: photoevoked postsynaptic currents (photo-ePSCs) were measured in wild-type animals expressing ChR2 in cholinergic neurons (transgene zxIs6). Currents were recorded from voltage-clamped muscle cells (shown are representative single experiments) in response to a 1-s light pulse (470 nm, indicated by shaded bar) of the indicated light intensity. Peak inward currents were followed by a steady-state current that returned to baseline after the end of the stimulus. B: the peak currents were averaged (n = 6–7) and fitted with a single exponent. Values are means ± SE. C: wild-type or gbb-2(tm1165) animals with transgene zxIs6 were assayed as in Fig. 5B, with a light intensity of only 0.14 mW/mm2, and body contractions were quantified. Values are means ± SE; n = no. of animals analyzed. Two-factorial ANOVAs were used to analyze statistically significant differences (***P < 0.001) for time periods indicated by shaded bars.
Altered GABA levels in snf-11 GABA (reuptake) transporter mutants and concomitant photostimulation of GABA/ACh MNs unravels GBB-1/2 effects.
We observed slight effects of GABA, released in response to photoevoked ACh transmission and detected by GBB-1/2 receptors, inducing some possibly plastic changes in ACh neurons. However, these effects were subtle, perhaps because the amount of GABA released could have been too small. Thus we wanted to test whether increased levels of GABA in the synaptic cleft or increased acute release of GABA could enhance these minor effects.
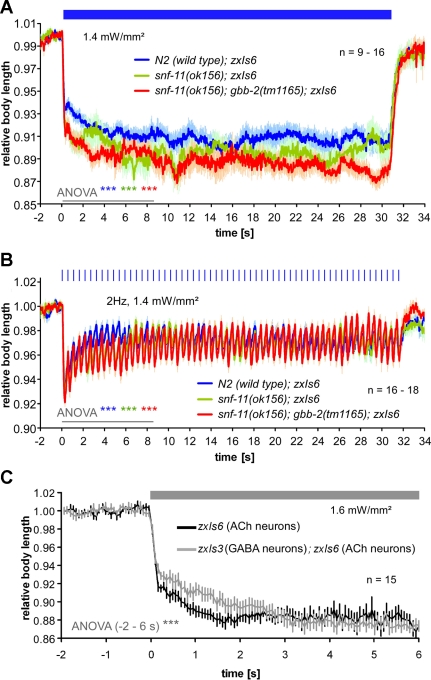
First, we tried to achieve higher GABA levels in the synaptic cleft at steady state. A high-affinity GABA transporter, SNF-11, has been described, which according to one study is expressed in muscles, as well as some neurons, excluding most of the inhibitory, GABAergic MNs (Mullen et al. 2006). This receptor is likely to act as a reuptake transporter, and not a transporter required in GABAergic MNs to provide GABA for release. Consequently, snf-11 mutants were shown to be resistant to aldicarb, since higher steady-state inhibition may be expected due to increased basal levels of GABA in the cleft, and this may even act through the GBB-1/2 receptor. However, in another study, SNF-11 expression was reported for the GABAergic MNs, and when the SNF-11 protein was knocked down by RNA interference, aldicarb hypersensitivity resulted, indicating reduced inhibition (Jiang et al. 2005). The findings of this report thus rather indicate that SNF-11 may be required for recycling of GABA in GABAergic neurons, which also express the GABA biosynthetic enzyme UNC-25 (glutamic acid decarboxlyase). To further investigate the two possibilities suggested by the two studies (Jiang et al. 2005; Mullen et al. 2006), we crossed the zxIs6 transgene into the snf-11(ok156) mutant background. We found that loss of SNF-11 caused significantly increased contractions in response to both continuous and pulsed photostimulation of cholinergic neurons (Fig. 7, A and B). This could indicate that steady-state levels of GABA in the synaptic cleft are not increased in snf-11 mutants but that SNF-11 rather functions to provide normal GABA levels in GABAergic MNs. Alternatively, if steady-state GABA levels were increased in snf-11 mutants, this may have long-term desensitized GABAA receptors, causing enhanced effects of photoevoked ACh release. ACh effects in snf-11(ok156) animals were further increased by additional deletion of gbb-2 (Fig. 7, A and B), in line with the hypothesis that GBB-1/2 receptors act in feedback inhibition of cholinergic MNs. The pulsed stimulation of cholinergic neurons in snf-11 or snf-11; gbb-2 mutants did not reveal any obviously abolished or enhanced plastic alterations in the contractions, although the differences to wild type were less pronounced toward the end of the 30-s stimulus train for both genotypes.
Fig. 7.
Increased synaptic GABA by elimination of the GABA (reuptake) transporter SNF-11 or by concomitantly photoevoked GABA release alters ACh-evoked body contractions. Body contractions (means ± SE) were evoked by 30-s continuous (indicated by blue bar in A) or by 2-Hz, 10-ms pulsed 1.4 mW/mm2 photostimulation (indicated by blue tick marks in B) of wild-type, snf-11(ok156), and snf-11(ok156); gbb-2(tm1165) animals carrying transgene zxIs6 (ACh). ANOVA for the first 8.5 s of the light stimulus showed significant differences between the wild type and the 2 mutant strains, as well as between mutants. C: wild-type animals expressing ChR2 in cholinergic neurons only (zxIs6) or in both cholinergic and GABAergic neurons (zxIs3; zxIs6) were exposed to a constant light stimulus (indicated by shaded bar), and body contractions were quantified. Values are means ± SE; n = no. of animals tested. ANOVA was performed from −2 to 6 s relative to the light stimulus. ***P < 0.001.
Because of the likely constantly elevated levels of GABA in the synaptic cleft of snf-11 mutants, compensatory mechanisms may have occurred, e.g., desensitization of GABAA (or GABAB) receptors. To acutely maximize synaptic GABA levels, concomitant with ACh release, we generated a strain expressing ChR2 in both cholinergic and GABAergic neurons (zxIs3; zxIs6 double transgenic animals). As expected, contractions evoked in these animals were significantly reduced compared with those in the zxIs6 animals, at least during the first 2.5 s of the stimulation (Fig. 7C). Since the initially strong GABA effects diminish over time (Fig. 4, D and E) (Liewald et al. 2008; Schultheis et al. 2011), this may explain that at later times both strains show similar contractions.
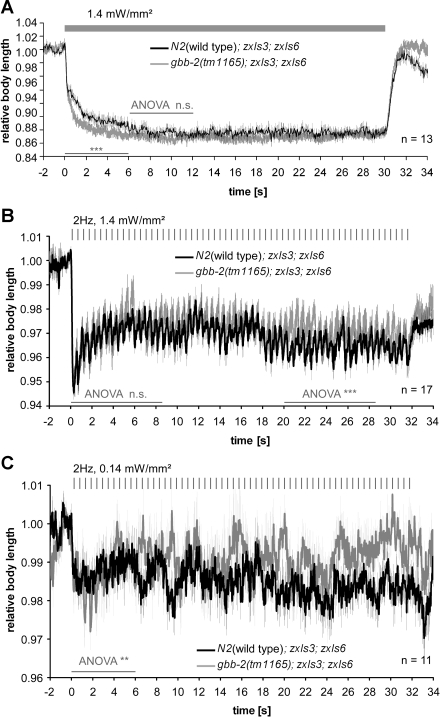
We next compared wild type with gbb-2(tm1165) mutant animals, both containing zxIs3 and zxIs6 transgenes. On continuous stimulation (Fig. 8A), gbb-2(tm1165) mutants contracted more strongly than the wild type for the initial 6 s of the stimulus, indicating that under conditions of enhanced GABA release, heterosynaptic inhibition via the GBB-1/2 receptor indeed has a modulating, time-dependent effect on ACh MNs. We also performed similar experiments with 2-Hz pulsed photostimulation, at both high (1.4 mW/mm2; Fig. 8B) and low stimulus intensity (0.14 mW/mm2; Fig. 8C). Whereas at the low stimulus intensity, gbb-2; zxIs3; zxIs6 animals showed significantly stronger contractions than wild-type zxIs3; zxIs6 animals during the first 6 s of the stimulus train, the effect was opposite for the remaining train. For the high stimulus intensity, differences were only observed late in the stimulus train, i.e., after 20 s, and wild-type animals contracted more strongly. In essence, there mainly appear to be acute modulating effects of heterosynaptic inhibition of ACh neurons via GBB-1/2 GABAB receptors, and if at all, only minor long-term plastic effects of GBB-1/2 in ACh MNs.
Fig. 8.
Concomitant GABA and ACh MN photostimulation shows initial inhibitory effect of GBB-1/2 GABAB receptors on ACh MNs and small but variable long-term effects. A: experiments were as described in Fig. 7C, and the genotypes used are indicated. ANOVA was performed for the indicated time periods (thin shaded bars); thick shaded bar indicates illumination period. B and C: animals of the same genotypes as in A were tested with 2-Hz, 10-ms photostimulus trains (tick marks) at 2 different stimulus intensities, 1.4 (B) and 0.14 mW/mm2 (C). Values are means ± SE; n = no. of animals tested. ANOVA was performed as indicated by thin shaded bars.
DISCUSSION
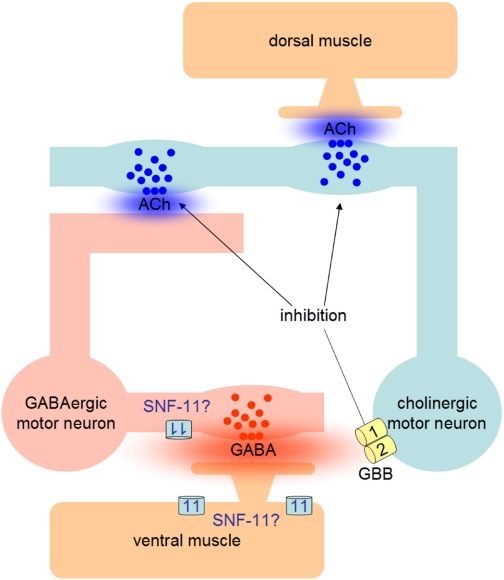
GABAB receptors, expressed by cholinergic MNs in C. elegans, mediate feedback inhibition via spillover GABA, released by GABAergic MNs that are stimulated by the cholinergic MNs. We asked whether this feedback might induce plastic changes at the cholinergic synapse, e.g., a progressive depression upon sustained activity. To investigate this, we used optogenetic methods, i.e., photostimulation of cholinergic neurons, to evoke behavioral changes (contractions) in vivo and analyzed whether these contractions dynamically changed during prolonged activity, dependent on GBB-1/2 receptors. Although we indeed found significant differences in the extent of the light-evoked, ACh-dependent contractions between gbb-2(tm1165) mutants and wild-type animals, these alterations were rather small, and their temporal occurrence was not consistent under different experimental conditions (e.g., strong vs. weak, continuous vs. pulsed stimuli). In one case, changing the stimulus protocol even reversed the effects from enhancing cholinergic function to reducing it. Although there may be complex interactions in the motor nervous system that could explain the variability of the observed effects, we cannot explain them satisfyingly and thus suggest that GBB-1/2 receptors mainly serve to provide a negative feedback to cholinergic MNs (model, Fig. 9) that has no major dynamic component. In agreement with this model, locomotion behavior during photostimulation of GABAergic neurons was more exaggerated (deeper body bends, increased speed) in gbb-2 animals, in line with a partial loss of inhibition, but these differences were roughly constant during the 120-s stimulus period. Our method is able to measure synaptic plasticity at the behavioral level, as we could previously show for mutants affecting synaptic vesicle recycling, e.g., the phospholipid phosphatase synaptojanin UNC-26: these animals showed a progressive reduction of the contractions over time (Liewald et al. 2008). Our method allows to quantitatively address the action of spillover transmitter in intact animals, rather than by electrophysiology in dissected preparations, where such transmitter effects would be abolished by dilution due to bath perfusion. We acknowledge, however, that photostimulation of motor neurons may evoke the co-release of transmitters other than GABA or ACh, since these cells also are likely to contain neuropeptides, whose (modulatory) action could contribute to some of the effects we observed.
Fig. 9.
Model of GBB-1/2 GABAB receptor function in feedback inhibition of cholinergic MNs at the Caenorhabditis elegans neuromuscular junction and putative site of action of the SNF-11 high-affinity GABA transporter.
GBB-1/2 receptors are responsible for some part of the GABA effects on body relaxation in optogenetic experiments, i.e., upon acute GABA release. We did not find any influence of the GBB-1/2 receptor on the effects of acutely photoevoked ACh release unless we photoevoked GABA release in addition to ACh release. These effects were somewhat dynamic and time dependent, because they were seen only during the initial 2.5-s of a constant stimulation of the two MN classes in gbb-2 (tm1165) mutants. Pulsed photostimulation of GABAergic and cholinergic neurons, however, initially showed more pronounced contractions of gbb-2 animals, whereas later during the stimulus train wild-type animals showed stronger contractions, and this depended on stimulus intensity. It is possible that even lower stimulus intensities may be required to uncover subtle plastic changes better; yet, given the minor extent of the behavioral effects we observed at the lower stimulus intensity we used, we were not confident that they could be accurately measured at even lower stimulus intensities. Instead, we explored whether continuously elevating GABA levels in the synaptic cleft could have effects similar to acute GABA signaling. When the SNF-11 high-affinity GABA transporter was absent, ACh-evoked contractions were stronger, and they were even further enhanced when the GBB-1/2 receptor was missing. This could be in line with a function of SNF-11 as a reuptake transporter or with a possible function of SNF-11 in GABAergic neurons, providing/recycling some of the GABA produced and released by these cells, as suggested by two conflicting previous reports (Jiang et al. 2005; Mullen et al. 2006). However, both continuously elevated and reduced levels of GABA in the cleft may affect compensatory mechanisms in GABA receptors, making it difficult to interpret our findings conclusively.
Although GBB-1/2 receptors are widely expressed in the nervous system (Dittman and Kaplan 2008), their influence on locomotion appears to be exerted in cholinergic MNs. Cell type-specific expression of a mutated version of the GBB-2 subunit (A484V; V572A) in cholinergic MNs at least partially rescued the gbb-2(tm1165) phenotypes. Although other GABA receptors are encoded in C. elegans [Ringstad et al. (2009) reported GABA-evoked currents when expressing LGC-35 and LGC-38 in Xenopus oocytes], no evidence of inhibition in the motor system could be detected in double mutants lacking both the ionotropic GABAA receptor UNC-49 and the GBB-1/2 receptor.
Using a fast optogenetic approach in vivo, we were able to show that inhibitory signaling via GBB-1/2 receptors likely occurs immediately, since it becomes apparent right upon stimulus onset, when gbb-2 mutants are compared with wild-type. This indicates that GABAB receptor signaling acts locally, e.g., to shape C. elegans locomotion, possibly to “smoothen” abrupt bending evoked by cholinergic transmission, and our locomotion analyses under GABA neuron photostimulation support this hypothesis. Because of its high affinity, the GBB-1/2 receptor can detect small amounts of free, spillover GABA, and no additional physical connections between GABAergic and cholinergic MNs are required for this function. The minor dynamic or plastic effects of GABAB receptor signaling on cholinergic neurons occur more slowly, after a few hundred milliseconds (up to seconds) on the level of our optogenetic behavioral analyses, and do not require extensive stimulus protocols over extended periods, as for example in the induction of long-term synaptic plasticity. These changes, however, may be of minor importance or will require more elaborate experimental approaches to be fully understood.
GRANTS
This work was funded by the Deutsche Forschungsgemeinschaft Grants GO1011/2-1 and SFB807-TP11 and by the Cluster of Excellence Frankfurt-Macromolecular Complexes.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank the Caenorhabditis elegans Genetics Center, which is supported by the National Institutes of Health-National Center for Research Resources, as well as the Japanese National Bioresource Project for the Experimental Animal “Nematode C. elegans” for providing strains.
Present address of M. Brauner: Center for Human Genetics, Heinrich-von-Stephan-Strasse 5, D-79100 Freiburg, Germany.
REFERENCES
- Almedom RB, Liewald JF, Hernando G, Schultheis C, Rayes D, Pan J, Schedletzky T, Hutter H, Bouzat C, Gottschalk A. An ER-resident membrane protein complex regulates nicotinic acetylcholine receptor subunit composition at the synapse. EMBO J 28: 2636–2649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J Neurosci 19: 5348–5359, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science 282: 2028–2033, 1998 [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev 84: 835–867, 2004 [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 77: 71–94, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature 349: 609–611, 1991 [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kaplan JM. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J Neurosci 28: 7104–7112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380: 258–262, 1996 [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380: 255–258, 1996 [DOI] [PubMed] [Google Scholar]
- Jiang G, Zhuang L, Miyauchi S, Miyake K, Fei YJ, Ganapathy V. A Na+/Cl−-coupled GABA transporter, GAT-1, from Caenorhabditis elegans: structural and functional features, specific expression in GABA-ergic neurons, and involvement in muscle function. J Biol Chem 280: 2065–2077, 2005 [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396: 674–679, 1998 [DOI] [PubMed] [Google Scholar]
- Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346, 1999 [DOI] [PubMed] [Google Scholar]
- Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A. Optogenetic analysis of synaptic function. Nat Methods 5: 895–902, 2008 [DOI] [PubMed] [Google Scholar]
- Liu Q, Hollopeter G, Jorgensen EM. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc Natl Acad Sci USA 106: 10823–10828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695, 1997 [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. Facilitation of the induction of long-term potentiation by GABAB receptors. Science 252: 1718–1720, 1991 [DOI] [PubMed] [Google Scholar]
- Mullen GP, Mathews EA, Saxena P, Fields SD, McManus JR, Moulder G, Barstead RJ, Quick MW, Rand JB. The Caenorhabditis elegans snf-11 gene encodes a sodium-dependent GABA transporter required for clearance of synaptic GABA. Mol Biol Cell 17: 3021–3030, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Brauner M, Liewald JF, Adeishvili N, Bamberg E, Gottschalk A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol 15: 2279–2284, 2005 [DOI] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2: 791–797, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Abe N, Horvitz HR. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science 325: 96–100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature 424: 775–778, 2003 [DOI] [PubMed] [Google Scholar]
- Schultheis C, Liewald JF, Bamberg E, Nagel G, Gottschalk A. Optogenetic long-term manipulation of behavior and animal development. PLoS ONE 6: e18766, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuske K, Beg AA, Jorgensen EM. The GABA nervous system in C. elegans. Trends Neurosci 27: 407–414, 2004 [DOI] [PubMed] [Google Scholar]
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, Seddik R, Tiao JY, Rajalu M, Trojanova J, Rohde V, Gassmann M, Schulte U, Fakler B, Bettler B. Native GABAB receptors are heteromultimers with a family of auxiliary subunits. Nature 465: 231–236, 2010 [DOI] [PubMed] [Google Scholar]
- Stirman JN, Crane MM, Husson SH, Wabnig S, Schultheis C, Gottschalk A, Lu H. Real-time multimodal optical control of neurons and muscles in freely behaving Caenorhabditis elegans. Nat Methods 8: 153–158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger S, Schultheis C, Liewald JF, Erbguth K, Nagel G, Gottschalk A. PACalpha—an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J Neurochem 116: 616–625, 2011 [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314: 1–340, 1986 [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396: 679–682, 1998 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang L, Brauner M, Liewald J, Kay K, Watzke N, Wood P, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature 446: 633–639, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.