Abstract
Deaf humans implanted with a cochlear prosthesis depend largely on temporal cues for speech recognition because spectral information processing is severely impaired. Training with a cochlear prosthesis is typically required before speech perception shows improvement, suggesting that relevant experience modifies temporal processing in the central auditory system. We tested this hypothesis in neonatally deafened cats by comparing temporal processing in the primary auditory cortex (AI) of cats that received only chronic passive intracochlear electric stimulation (ICES) with cats that were also trained with ICES to detect temporally challenging trains of electric pulses. After months of chronic passive stimulation and several weeks of detection training in behaviorally trained cats, multineuronal AI responses evoked by temporally modulated ICES were recorded in anesthetized animals. The stimulus repetition rates that produced the maximum number of phase-locked spikes (best repetition rate) and 50% cutoff rate were significantly higher in behaviorally trained cats than the corresponding rates in cats that received only chronic passive ICES. Behavioral training restored neuronal temporal following ability to levels comparable with those recorded in naïve prior normal-hearing adult deafened animals. Importantly, best repetitition rates and cutoff rates were highest for neuronal clusters activated by the electrode configuration used in behavioral training. These results suggest that neuroplasticity in the AI is induced by behavioral training and perceptual learning in animals deprived of ordinary auditory experience during development and indicate that behavioral training can ameliorate or restore temporal processing in the AI of profoundly deaf animals.
Keywords: auditory deprivation, deafness, neuroplasticity, perceptual learning, primary auditory cortex, signal detection training
in ordinary listening conditions, humans with normal hearing rely on the spectral content and temporal features of the speech signal for speech recognition and comprehension (Ahisssar et al. 2001; Rosen 1992; Shannon et al. 1995). If spectral information is degraded while the temporal envelope pattern of the speech signal is preserved, the temporal information alone is often sufficient for speech recognition (Drullman 1995; Shannon et al. 1995).
Moreover, temporal processing of speech is an essential feature of neural coding in the auditory cortex for speech comprehension to occur. Evoked potential responses to low-frequency temporal modulation are observed at the cortical level, and phase locking to the temporal envelope of a sentence is correlated with comprehension of the sentence (Ahissar et al. 2001; Joris et al. 2004). These findings indicate that neural responses in the human auditory cortex represent slow temporal events within the range of modulation frequencies (∼2–30 Hz) that correspond to the temporal envelopes present in speech signals. Lesions of the auditory cortex may interfere with timing tasks including the detection of low-frequency temporal modulations that are prominent in the modulation spectrum of speech signals (Joris et al. 2004), although a recent study in rats reported that ablation of the auditory cortex failed to produce significant deficits in the detection of signals presented at a modulation rate of 10 Hz (Cooke et al. 2007).
Speech recognition can be very challenging in profoundly deaf humans implanted with a cochlear prosthesis. Auditory frequency selectivity is severely degraded by deafness, and performance on speech comprehension must rely primarily on temporal factors (Rosen 1992; Shannon et al. 1995). However, cochlear implant users often show improvement in speech recognition over time, suggesting that relevant experience is an important factor for enhanced performance in electric hearing. Clinical reports and psychophysical studies have documented that perceptual training can improve speech recognition and production in deaf children (Busby and Clark 1999; Busby et al. 1993; Svirsky et al. 2004), and even prelingual deaf individuals implanted as adults can demonstrate some gradual improvements in speech recognition (Busby et al. 1991). Improved performance in electric hearing suggests that temporal processing mechanisms in the central auditory system can be modified or remodeled by relevant auditory experience.
The primary auditory cortex (AI) expresses neuronal plasticity, and many studies have shown that this cortical field can be functionally modified and reorganized by behavioral training in normal-hearing animals. Most studies have reported modifications in the spectral features of receptive fields (for reviews, see Edeline 1998; Recanzone et al. 1993; Scheich 1991; Weinberger and Bakin 1998). However, several studies in normal-hearing adult animals have shown that auditory detection and discrimination learning based on temporal cues significantly improved temporal processing or temporal precision in the auditory cortex (Bao et al. 2004; Beitel et al. 2003; Engineer et al. 2008; Leon et al. 2008; Schnupp et al. 2006). Temporal processing deficits induced in the infant rat auditory cortex by exposure to modulated noise have been reversed in developmentally impaired animals by auditory discrimination training later in life (Zhou and Merzenich 2009).
We (Beitel et al. 2000a, 2000b; Vollmer et al. 2001) have previously shown that neonatally deafened cats deprived of normal auditory experience during development learn to detect and discriminate temporally modulated intracochlear electric stimulation (ICES). Importantly, this research established that behavioral detection thresholds as well as minimum neural response thresholds in the inferior colliculus and cortical field AI were virtually identical in the profoundly deaf cat. In the present study, we compared temporal processing in AI neurons recorded from four groups of profoundly deaf young adult cats to assess the role of behavioral training on cortical temporal processing capacity. Cats in three groups were neonatally deafened. Prior normal-hearing cats in the fourth group served as controls and were adults when they were deafened. All of the animals received chronic passive stimulation during the study, and two groups of neonatally deafened cats were also behaviorally trained with temporally modulated ICES. Our main objective was to compare the effects of experience with behaviorally relevant versus chronic passive ICES on temporal processing in AI neurons when the auditory stimulation occurs before the central auditory system has fully matured. In this study, behaviorally relevant ICES signifies that the electric signals are associated with psychophysically documented evidence of perceptual learning and behavioral signal detection in the trained animals.
METHODS
Animal group identifications and the histories of deafness, exposure to ICES, and behavioral training in the 10 cats used in the present study are shown in Table 1. Many of the procedures have been described in detail in previous reports (Beitel et al. 2000a; Leake et al. 2008; Moore et al. 2002; Raggio and Schreiner 1999; Schreiner and Raggio 1996; Vollmer et al. 2001). All procedures followed National Institutes of Health and University of California (San Francisco, CA) Institutional Animal Care and Use Committee guidelines for the care and use of laboratory animals.
Table 1.
Deafness, chronic ICES, and behavioral training histories
| Deafness |
Chronic Stimulation |
Behavioral Stimulation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Duration, wk | Type | Electrode | Age begun, wk | Total chronic ICES, h | Age begun, wk | Duration, wk | Number of sessions | Total behavioral ICES, min | Behavioral Training | |
| Untrained | |||||||||||
| A300/30 group | |||||||||||
| C158 | Adult | 24 | 300/30 | 1,2 | Adult | 466 | N/A | N/A | N/A | N/A | |
| C87 | Adult | 33 | 300/30 | 1,2 | Adult | 542 | N/A | N/A | N/A | N/A | |
| J300/30 group | |||||||||||
| K92 | Neonate | 32 | 300/30 | 1,2 | 8 | 295 | N/A | N/A | N/A | N/A | |
| K89 | Neonate | 37 | 300/30 | 1,2 | 10 | 414 | N/A | N/A | N/A | N/A | |
| K190 | Neonate | 34 | 300/30 | 1,2 | 8 | 476 | N/A | N/A | N/A | N/A | |
| Trained | |||||||||||
| JT300/30 group | |||||||||||
| K99 | Neonate | 45 | 300/30 | 1,2 | 8 | 200 | 19.3 | 25.0 | 118 | 82 | Detection threshold (300/30); successive discrimination |
| K91 | Neonate | 38 | 300/30 | 1,2 | 7 | 310 | 19.8 | 5.7 | 28 | 19 | Detection threshold (300/30) |
| K102 | Neonate | 46 | 300/30 | 1,2 | 8 | 400 | 14.7 | 31.0 | 136 | 121 | Detection threshold (300/30); detection threshold (20, 300 pulses/s); detection threshold (300/8) |
| JT30 group | |||||||||||
| K80 | Neonate | 35 | 30 | Mono | 7 | 356 | 23.1 | 5.6 | 27 | 18 | Detection threshold (30 pulses/s); detection threshold (100 Hz) |
| K82 | Neonate | 36 | 30 | Mono | 7 | 375 | 24.4 | 5.2 | 22 | 14 | Detection threshold (30 pulses/s); detection threshold (100 Hz) |
Group identification was as follows: adult (A), juvenile (J), and behaviorally trained (T). Sinusoidal amplitude modulation was 300/30 [300 pulses/s modulated (100%) at 30 Hz]. Electrodes were apical scala tympani bipolar (1,2) or intracochlear monopolar (Mono). Behavioral training of each cat occurred in the order shown. ICES, intracochlear electric stimulation; N/A, not applicable.
Deafening
Two young adult cats with normal hearing (A300/30 group) were deafened by the coadministration of kanamycin (300 mg/kg sq) and ethacrynic acid (1 mg/ml iv to effect), and eight kittens (J300/30, JT300/30, and JT30 groups) were deafened by systemic injections of neomycin sulfate (60 mg/kg im/sid) for 16–21 days starting the day after birth. Profound hearing loss (>110 dB sound pressure level) was confirmed by the absence of auditory brain stem responses to clicks (0.2 ms/phase, 20 pulses/s). The JT300/30 group had the longest (mean: 43 wk) and the A300/30 group the shortest (mean: 28.5 wk) duration of deafness at the time of the acute terminal electrophysiological experiment (Table 1). Postmortem histological analysis confirmed the absence of auditory hair cells in the cochleae of neonatally deafened animals; spiral ganglion cell density (percent normal) ranged from 35.3% to 53.7% (mean ± SD: 45.2 ± 6.1%). As judged by an absence of reflex pinna or head movements to environmental sounds, none of the neonatally deafened or prior normal-hearing animals in this study demonstrated residual acoustic hearing.
Feline Cochlear Prostheses and Implantation
Cochlear electrodes were fabricated from Teflon-coated platinum-iridium wires embedded in a silicone rubber carrier (Rebscher et al. 2001). Intracochlear electrode arrays implanted in animals in the A300/30, J300/30, and JT300/30 groups had four bipolar contacts (∼250-μm diameter) arranged as two offset radial pairs (1-mm radial separation). Apical pair (1,2) was located ∼9–11 mm from the round window; basal pair (3,4) was located ∼3–4 mm closer to the round window. In the JT30 group, the implant was an intracochlear monopolar electrode (∼400-μm diameter) located 2–3 mm from the round window with a ground lead located beneath the temporal muscle.
In two adult and eight weaned juvenile cats, prostheses were implanted into the left scala tympani. Before the surgical procedure, the animal was sedated [ketamine (22–33 mg/kg im) and acepromazine maleate (0.1 mg/kg im)], and anesthesia was induced by pentobarbital sodium (10–20 mg/kg) delivered via an intravenous catheter. Surgery was conducted in a University of California-San Francisco-approved recovery surgical suite under sterile conditions. An areflexic level of anesthesia was maintained during surgery by an intravenous infusion of pentobarbital sodium in Ringer solution, and vital parameters (temperature, heart rate, and respiration) were monitored and maintained within normal physiological limits. A prophylactic antibiotic and buprenorphine HCl (0.005 mg/kg) for analgesia were administered, and animals were monitored continuously during recovery.
Chronic Passive Stimulation
A regimen of continuous ICES (∼3–5 h/day, 5 days/wk) was applied to each cat, usually beginning within 1 wk after implantation surgery, to provide at least a rudimentary engagement of the auditory system during maturational processes in the developmental period (Leake et al. 2008). Chronic electric stimuli were charge-balanced biphasic rectangular current pulses (0.2 ms/phase) applied at 2 dB above the electric evoked auditory brain stem response (EABR) threshold. The electric stimuli were computer generated (LabView, National Instruments) and delivered through an audio attenuator (HP 350B) to an optically isolated constant-current stimulator. ICES was applied to the cochlear electrodes via a percutaneous cable located behind a cat's left pinna.
In the A300/30, J300/30, and JT300/30 groups, pulses were delivered at 300 pulses/s, amplitude modulated at 30 Hz (300/30; modulation depth: 100%) to apical cochlear electrode pair (1,2). In the JT30 group, 30 pulses/s were delivered to the intracochlear monopolar electrode. Based on an estimate from a previous study (Beitel et al. 2000a), at 2 dB above the EABR threshold, the chronic passive ICES was ∼8.5 ± 2.1 (mean ± SD) dB higher than AI minimum neural thresholds.
The total hours of chronic passive ICES are shown for the 10 cats in Table 1. In the behaviorally trained cats (JT300/30 and JT30 groups), the mean total hours (328.2 ± 78.9) and mean hours/day (3.7 ± 0.33) were not statistically different (P > 0.05) than the mean total hours (438.6 ± 92.3) and mean hours/day (3.9 ± 0.20) in the untrained cats (A300/30 and J300/30 groups). In the JT300/30 group, chronic passive stimulation was suspended during the period of behavioral training. EABR thresholds and electrode impedances were measured at regular intervals after implantation to assess the stability and reliability of the cochlear implants. In all cats, chronic passive stimulation was applied for months before the animals were studied electrophysiologically.
Electrophysiological Procedures
Animal preparation.
The eight animals deafened as kittens were sexually mature young adults (age: 32–46 wk) at the time of the acute electrophysiological recording experiments. The areflexic anesthetic procedures described above for implantation of the feline prosthesis were also implemented for the recording experiments. The cat's head was immobilized by a rod attached to the cranium, and, after a craniotomy contralateral to the cochlear implant, the right auditory cortex was exposed by removing the overlying dura. The cortex was protected with silicone oil, and an image of the cortex was stored in a computer. The surface locations of electrode penetrations into the auditory cortex were displayed on the cortical image to produce topographic maps (Raggio and Schreiner 1999).
ICES.
Electric charge-balanced biphasic rectangular current pulses (0.2 ms/phase) generated by a computer (TMS32010) at a sampling rate of 60 kHz were delivered through an audio attenuator to an optically isolated constant-current stimulator. Before each recording session, this system was calibrated to a common reference level [0 dB = 1 μA peak to peak (μApp)]. Every increase in stimulus intensity by a factor of 10 was equivalent to an increase of 20 dB (e.g., 1 μApp = 0 dB, 10 μApp = 20 dB, 100 μApp = 40 dB, and 1,000 μApp = 60 dB).
In the trained JT30 group, the intracochlear monopolar electrode used for chronic passive stimulation and behavioral training was also used to evoke cortical responses in the physiological experiments. In the untrained J300/30 group, the electrode configuration used for chronic passive stimulation [apical pair (1,2)] was used also for ICES in the physiological experiments. In the trained JT300/30 group and untrained A300/30 group, the electrode configuration used in the physiological experiments was either apical pair (1,2) used to deliver chronic passive and behaviorally relevant ICES, basal pair (3,4), a longitudinal configuration consisting of one contact from each radial pair, or a monopolar configuration.
Cortical recording.
Neuronal activity was recorded differentially using two impedance-matched tungsten microelectrodes (0.8–1.2 MΩ). The active electrode, oriented orthogonally to the surface of the cortex, was lowered with a hydraulic micropositioner (Kopf) to intracortical depths of 600–1,200 μm (cortical layers IIIb and IV) at locations distributed across the AI at spatial intervals of ∼0.5 mm. When responses from a multineuronal cluster (unit) were isolated, the response threshold to a single electric pulse delivered at the minimal intensity required to elicit a spiking response was determined using audiovisual criteria. For each unit, the ICES intensity was then increased sufficiently to produce reliable, discriminable responses evoked by trains of biphasic current pulses at increasing pulse rates (2 to ≤48 pulses/s, 500-ms sweep duration, 2 to ≤12 dB above the unit's minimal response threshold, 15 or 20 repetitions, 1,000-ms intervals between repetitions). Responses were bandpass filtered, amplified, and monitored on an oscilloscope and an audio monitor. Spiking activity was isolated from background noise and stimulus artifacts using a window discriminator (BAK-DIS-1). Beginning at the pulse train onset in each repetition, the numbers of spikes and arrival time of each spike were recorded and stored in a computer. During the experiment, neuronal responses at all pulse rates were displayed automatically in poststimulus time histograms (PSTHs), dot raster plots, or period histograms derived from 1-period folding of the corresponding PSTHs.
Neuronal Data Analysis
Data were analyzed offline using customized software (Matlab, The Math Works) to obtain the number of phase-locked spikes [phase-locked spikes = total spikes × vector strength (Eggermont 1991)] evoked by different pulse rates for all studied units in the four groups of cats. Except at the lowest pulse rate (2 pulses/s), the response to the first pulse in each repetition was excluded from the analyses to avoid stimulus onset effects, and at all pulse rates the first 8 ms of the response after an electric pulse were excluded to eliminate contamination of the recording by artifacts produced by the electric stimulus pulses. Note that cortical neuronal response latency was usually >8 ms. Given the shorter latencies of thalamocortical axons, this procedure could also reduce or minimize a potential contribution of spiking activity from thalamocortical axons to the discriminated response (Schreiner and Urbas 1988).
For every cortical unit included in this report (n = 307), we plotted the number of phase-locked spikes versus the stimulus pulse repetition rate to obtain a repetition rate transfer function (RRTF) (Schreiner and Raggio 1996). From the RRTF, two phase-locked parameters were used to estimate neuronal temporal processing in a cortical unit: the best repetition rate (BRR) is the pulse rate that evoked the largest number of phase-locked spikes, and the cutoff rate is the higher pulse rate at which the number of phase-locked spikes was just <50% of the number of spikes at the BRR. For all units, the vector strength values included to calculate the two phase-locked parameters were significant (P = 0.001, Rayleigh statistic). The cutoff rate is approximately equivalent to a −6 dB criterion for frequency following (Schreiner and Raggio 1996).
In addition, response latency variables were measured to evaluate temporal processing and temporal precision. To obtain an estimate of unit minimum spike latency, a single stimulus pulse delivered at the lowest stimulus pulse rate was used to evoke a response (15 or 20 repetitions), and the mean minimum latency and variability (jitter) were calculated (Phillips et al. 1989; Phillips and Hall 1990; Ter-Mikaelian et al. 2007). Due to a computer malfunction, minimum latency data for the JT30 group and for one cat in the J300/30 group were lost before we could analyze the latency data in these animals.
Finally, to investigate the occurrence of response suppression duration and response rebound after a single stimulus pulse (15 or 20 repetitions), the modal peak latency of the onset response and the modal peak latency of the rebound response were measured. If a rebound response occurred, an estimate of the duration of response suppression was calculated (rebound latency − onset latency = suppression duration). Minimal response latency, peak latency, suppression durations, and rebound results are shown in Table 2 and are described at the end of the results.
Table 2.
Total units, percentages of bandpass, low-pass, and high-pass units, and latency, rebound, and suppression data
| Latency Data |
Rebound and Suppression Data |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Total units, n | Percent Bandpass Units | Percent Low-Pass Units | Percent High-Pass Units | Peak onset latency, n | Median peak onset latency, ms | Median first spike latency, ms | Units, n | Rebound, n | Percent rebound units | Median suppression duration, ms |
| A300/30 | 45 | 73.3 | 8.9 | 17.8 | 45 | 10.0 | 8.7† | 44 | 26 | 59.1 | 115 |
| J300/30 | 74 | 56.8 | 33.8 | 4.0 | 60 | 11.0 | 10.7 | 74 | 42 | 56.8 | 125 |
| JT300/30 | 135 | 77.8 | 8.9 | 5.9 | 124 | 11.0* | 9.8 | 125 | 63 | 50.4 | 140‡ |
| JT30 | 53 | 92.4 | 1.9 | 5.7 | 48 | 9.6 | 53 | 36 | 67.9 | 134 | |
| Total, n | 307 | 229 | 42 | 22 | 277 | 296 | 167 | ||||
n, Number of multineuronal clusters (units).
P < 0.05, JT300/30 group vs. JT30 group;
P < 0.01, A300/30 group vs. J300/30 and JT300/30 groups;
P < 0.05, JT300/30 group vs. A300/30 group.
Statistical Analysis
Nonparametric statistics used in data analysis included χ2, Spearman rank order correlation, and Mann-Whitney rank sum tests, Kruskal-Wallis one-way ANOVA on ranks, and Dunn pairwise multiple-comparison tests. Rank order statistics are shown in box plots that illustrate the median and four percentiles (10th, 25th, 75th, and 90th) of the ranked data for each group included in the analysis (see Figs. 6 and 7). Statistical values are reported as means ± SD or as medians + Q, the quartile deviation.
Fig. 6.
Comparisons of BRRs and cutoff rates among the four groups of cats for stimulation with all bipolar (offset radial or longitudinal) and monopolar electrode configurations. A: number of units versus BRR (in pulses/s). The number of units in each group is shown. The distributions for the four groups are shown in black and blue for the untrained A300/30 and J300/30 groups and in red and green for the behaviorally trained JT300/30 and JT30 groups. Bin width: 2 pulses/s. B: cumulative probability functions (integrated data) versus BRR in the four groups of cats. Intersections of the functions with the dashed horizontal line illustrate that 90% of units in a group are at the corresponding BRR on the abscissa. C: comparison of BRRs for the four groups. *P < 0.05; **P < 0.01. D: number of units versus cutoff rate (in pulses/s). The number of units in each group is shown. Bin width: 4 pulses/s. E: cumulative probability functions (integrated data) versus cutoff rate in the four groups of cats. Intersections of the functions with the dashed horizontal line illustrate that 90% of units in a group are at the corresponding cutoff rate on the abscissa. F: comparison of cutoff rates for the four groups. **P < 0.01. In A and D, the numbers in parentheses indicate the total number of units analyzed in each group. In C and F, boxes indicate the 25th and 75th percentiles, horizontal lines indicate the medians, and whiskers indicate the 10th and 90th percentiles.
Fig. 7.
Effects of stimulating electrode configuration on BRRs and cutoff rates. The electrode configuration used in the electrophysiological recording experiments was either apical pair (1,2) used prior in chronic passive stimulation (A300/30, J300/30, and JT300/30 groups) and behavioral training (JT300/30 group) or other electrode configurations used only in the electrophysiological experiments. A and B: BRRs (A) and cutoff rates (B) in the A300/30 and JT300/30 groups. In the A300/30 group, the BRR and cutoff rate for chronic passive apical pair (1,2) versus other configurations were not different. In the JT300/30 group, the BRR and cutoff rate for behavior apical pair (1,2) versus other configurations were significantly different. C and D: BRRs (C) and cutoff rates (D) for ICES with apical electrode pair (1,2) in the A300/30, J300/30, and JT300/30 groups. In the JT300/30 group, the BRR and cutoff rate were significantly higher for stimulation with behavioral electrode pair (1,2) than BRRs and cutoff rates in the A300/30 and J300/30 groups, which were passively stimulated with apical pair (1,2). Open symbols indicate values above the 90th percentile and below the 10th percentile. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant.
Psychophysical Procedures and Data Analysis
In five deaf juvenile cats, behavioral training was initiated at an average age of 20.3 ± 3.8 wk (Table 1). Four of the cats (cats K80, K82, K99, and K102) were trained on two or more behavioral tasks for a combined training period ranging from 5.2 to 31 wk; cat K91 was trained on a single task for 5.7 wk. The total behaviorally relevant ICES delivered during training ranged from 14 to 121 min in the trained cats. Behavioral sessions were monitored on a closed-video system.
This report describes the procedures and results associated with the initial behavioral tasks used to train the naïve cats in the JT30 group (30 pulses/s task) and the JT300/30 group (300 pulses/s modulated at 30-Hz task). Cats were trained to detect suprathreshold ICES, perceptual learning was documented, and, finally, stable mean end of training thresholds were determined for each cat.
Training was based on a conditioned avoidance paradigm combined with a preferred food incentive (Fig. 1A). This method rather than behavior motivated by food or fluid restriction was selected because the immature juvenile cats required food and water ad libitum to sustain normal growth and development during the study. The cat was placed in a wire cage located inside an acoustic chamber (Industrial Acoustics) and trained to lick a metal spoon located at one end of the wire cage to obtain a preferred food reward (meat puree). Contact with the spoon was monitored by computer (sampling rate: 50 Hz), and the puree was delivered at a constant rate on safe trials (70–80% of trials) from a motor-driven syringe pump during periods when the cat was licking the spoon. On safe trials (2-s duration), electric signals were never delivered to the cochlea. On warning trials (20–30% of trials), a warning signal [conditioned stimulus (CS)] was presented, and the cat was required to interrupt licking within 2 s from the CS onset to avoid a mild electrocutaneous shock [unconditioned stimulus (US)] applied to the spoon. The US was a 140- to 210-ms-duration sinusoidal pulse train (60 Hz) adjusted for each cat to the minimum current intensity (0.5–1.0 mA) required to maintain avoidance behavior. The US was perceived as “annoying,” “itchy,” or “irritating” rather than “painful” when applied to an investigator's lips. A light located above the spoon was turned on and off with the US to provide visual feedback that it was safe to resume licking the spoon.
Fig. 1.
Conditioned avoidance paradigm and early behavioral performance associated with training to detect suprathreshold intracochlear electric stimulation (ICES). A: the safe trial illustrates a false alarm when licking is interrupted during the timed trial. ITI, intertrial interval. The warning trial illustrates a “hit” when licking is stopped during the timed trial and a “miss” when licking continues. Variable-duration shock and light (140–210 ms) are not drawn to scale. RT, reaction time. B: performance illustrates improved detection of suprathreshold conditioned stimuli (CSs) as a function of early training sessions in five deaf cats (cats K80, K82, K91, K99, and K102). P(hit) represents the total avoidance responses/total warning trials. The proportion of avoidance responses was above 50% within four training sessions in all of the animals. Numbers in parentheses are the suprathreshold CS intensities (in dB) used to assess performance.
Charge-balanced biphasic rectangular pulses (0.2 ms/phase) were used as the CS in this study. In the JT300/30 group, the CS was a sinusoidal amplitude modulated pulse train [300 pulses/s, 30-Hz modulation, modulation depth: 100%, 1 s-duration, apical electrode pair (1,2)]; in the JT30 group, the CS was an unmodulated pulse train (30 pulses/s, 2-s duration, intracochlear monopolar electrode). The stimuli were computer generated (LabView, National Instruments) and delivered through an audio attenuator (HP 350B) to an optically isolated constant-current stimulator. The output from the stimulator was delivered to the implanted feline prosthesis through a percutaneous cable. Before each behavioral session, this system was calibrated to a common reference level (0 dB = 1 μApp).
Behavioral training began with the CS presented over a range of suprathreshold intensities that bracketed the cat's EABR threshold. Within one or two training sessions, the cat typically began to avoid the US by breaking contact with the spoon during the CS. A suprathreshold signal intensity was then identified for each cat (e.g., 39 dB for cat K82), and the animal's conditioned avoidance performance at that signal intensity was tracked over several sessions (Fig. 1B). To document perceptual learning, the psychophysical method of constant stimuli or the descending method of limits was used to estimate thresholds. The CS was presented during a session within a range of intensities (5–10 dB) estimated to include near-threshold stimulus intensities. The minimum difference between successive CS presentations was a 1-dB step. During training, the intensities of the CS were gradually reduced as performance improved.
Although the detection of suprathreshold ICES improved from session to session (Fig. 1B), threshold training sessions run to document perceptual learning were particularly challenging for the deaf animals. In daily threshold training sessions in each cat, at least one session did not include a documented threshold. Only successive sessions that included documented thresholds were used in the analysis of perceptual learning. For example, as shown for cat K82 (Fig. 2A), detection thresholds (50% correct avoidance responses) were estimated from psychometric functions in four successive threshold sessions. These detection thresholds were plotted against training sessions to obtain the perceptual learning function (connected symbols), as shown for cat K82 in Fig. 2B. Perceptual learning functions are also shown for the other trained cats in Fig. 2B.
Fig. 2.
Perceptual learning functions and final detection thresholds. A: psychometric functions from four successive threshold sessions document perceptual learning in cat K82. Each threshold (50% avoidance) was estimated by interpolation (arrow) from the intersection of a psychometric function with the dashed horizontal line. The four thresholds are plotted for cat K82 in B (connected symbols) to illustrate perceptual learning. Perceptual learning functions are also shown in B for successive thresholds estimated from psychometric functions in the other animals. The symbols located above “F” in B represent the mean final stable detection thresholds shown in C. These thresholds are based on estimates from three or four threshold sessions at the end of training for cats in the JT300/30 group (hatched bars) trained with an apical bipolar electrode pair (1,2) and for cats in the JT30 group (shaded bars) trained with an intracochlear monopolar electrode. Error bars are SDs (in dB).
To estimate final stable thresholds, the following response measures were obtained by pooling data from three or four threshold sessions run at the end of training when the SDs of thresholds in each cat were within 1 dB (Fig. 2C). First, the false alarm (FA) rate was calculated as P(FA) = total FA/total safe trials, where P is probability. A FA occurred whenever a cat was not in contact with the spoon during the final 200 ms of a safe trial. Second, the hit rate at each level of the CS was calculated as P(hit) = total avoidance responses at each level of the CS/total warning trials at each level of the CS. A hit occurred whenever a cat was not in contact with the spoon during the final 200 ms of a warning trial, i.e., whenever a cat successfully avoided the US. Finally, a correction based on P(FA) was calculated to obtain the probability for detection at each level of the CS (Green and Swets 1961), as follows: P(detection) = P(hit) − P(FA)/1 − P(FA).
RESULTS
Improved Conditioned Avoidance, Perceptual Learning, and Stable Detection Thresholds
In early training sessions with suprathreshold ICES, performance over the course of training improved in detecting the CS and avoiding the aversive US (Fig. 1B). As shown for cat K82 in Fig. 2A, perceptual learning was documented as a decrease in thresholds estimated from psychometric functions for successive sessions in which a threshold was obtained. Psychometric estimates of threshold were obtained for successive threshold sessions in each of the trained cats.
Figure 2B shows perceptual learning functions for the five deaf cats. During the learning phase of auditory detection and discrimination tasks, sessions with and without thresholds typically occur over the course of training deaf cats. Although performance improved with training, in this study a total of 42 training sessions was run to obtain 20 perceptual learning thresholds, as shown in Fig. 2B. A clear trend is apparent in the results: a significant decrease in thresholds occurred over successive threshold sessions. The mean difference in detection thresholds on the first and last threshold sessions shown in Fig. 2B was 2.8 ± 1.3 dB [mean ± SD, paired t-test = 4.92, 4 degrees of freedom (df), P = 0.008].
The symbols located above “F” (abscissa) in Fig. 2B represent the final stable mean detection thresholds shown in Fig. 2C. At the end of training, only 21 sessions were required to obtain 18 threshold sessions (3 or 4 threshold sessions/cat) to document final stable thresholds. These thresholds are essentially identical to the lowest perceptual learning thresholds shown in Fig. 2B (P > 0.6).
In summary, the behavioral results show that 1) in previously naïve deaf juvenile cats, conditioned avoidance performance improved when the animals were trained with suprathreshold ICES; 2) perceptual learning was expressed by a significant reduction in detection thresholds over successive threshold training sessions; and 3) in the final stage of training, detection thresholds were stable over three or four sessions in the five deaf animals.
The range of final detection thresholds shown in Fig. 2C is 33.8–43.4 dB. Interindividual differences in thresholds reflect variability in the position of the stimulating electrodes relative to the surviving spiral ganglion neurons in the cochlea as well as differences in the spread of current and current shunting in monopolar and bipolar electric stimulation of the cochlea (Beitel et al. 2000a; Rebscher et al. 2001). The FA rates were below 9.0% in every deaf cat (mean ± SD: 7.4 ± 1.5%). The FA rates were similar to FA rates reported in normal-hearing cats trained in a conditioned avoidance paradigm (Heffner and Heffner 1985), indicating that performance was under stimulus control.
In the signal detection tasks described in this report (CS: 300/30 or 30 pulses/s), the mean total duration of behaviorally relevant ICES per session was <1 min (44.4 ± 13 s), and differences in ICES duration per session among the trained cats were not significant (ANOVA, P > 0.05). In a behavioral training session, ∼70% of the CSs were delivered at threshold or suprathreshold intensities, indicating that the central auditory system was stimulated by behaviorally relevant trains of electric pulses on most warning trials.
Characteristics of Neuronal Responses to ICES
Response thresholds and ICES suprathreshold intensities.
The mean neuronal response thresholds in the four groups of cats were 52.0 ± 3.4 dB in the A300/30 group, 41.4 ± 2.6 dB in the J300/30 group, 43.3 ± 4.4 dB in the JT300/30 group, and 48.4 ± 4.3 dB in the JT30 group. The range of mean thresholds across the four groups of cats was ∼11 dB, and this range was similar to the ranges of behavioral and cortical thresholds reported in deaf cats for pulsatile ICES (Beitel et al. 2000a). Differences in response thresholds within and between groups of cats represent variability in the position of the stimulating electrode configurations in the cochlea, differences in the spread and shunting of current in monopolar and bipolar electric stimulation of the cochlea (Rebscher et al. 2001), and variation in the specific region of the auditory cortex activated by ICES (Raggio and Schreiner 1999).
In the four groups of cats, the mean suprathreshold stimulus intensities used to activate cortical units for analysis of temporal processing were 5.1 ± 1.9 dB in the A300/30 group, 6.9 ± 3.1 dB in the J300/30 group, 7.0 ± 3.2 dB in the JT300/30 group, and 8.2 ± 2.2 dB in the JT30 group. Juvenile group differences in mean suprathreshold stimulus intensities were ≤1.3 dB, and group differences in stimulus intensities had no apparent effect on the quality of recordings in any of the groups.
Neuronal responses to ICES pulse trains.
Our main goal in the electrophysiological portion of this study was to compare temporal processing capacity in the AI in deaf young adult cats that had been behaviorally trained versus deaf young adult cats that had not received behavioral training. Two phase-locked response parameters, the BRR and the cutoff rate, were evaluated in cortical units.
Figures 3–5 show the sequence of analyses used to evaluate neuronal temporal following at different ICES pulse rates in two representative units, one from a behaviorally trained animal (JT300/30 group) and one from a naïve animal (J300/30 group). In Fig. 3, unit responses are displayed in PSTHs (A) or dot raster plots (B); Fig. 4, A and B, shows period histograms derived from the data in Fig. 3. An examination of the results shown in Fig. 3, A and B, clearly reveals that robust onset responses, followed by rebound responses, occurred at lower pulse rates (2–6 pulses/s), and this is likewise apparent in Fig. 4, A and B, top. At pulse rates of ≥14 pulses/s, the response to even numbered pulses was diminished (Fig. 3A, 14–18 pulses/s), the second pulse evoked no spikes (Fig. 3A, 20–30 pulses/s; Fig. 3B, 16–20 pulses/s), and, although spiking activity occurred to the third and subsequent pulses (Fig. 3A, 20–30 pulses/s), response adaptation tended to increase with increasing pulse rate. As shown in Fig. 4, A and B, as the pulse rate increased (>6 pulses/s), the proportion of asynchronous spikes decreased in both units, and the vector strength and number of phase-locked spikes increased to maxima at the BRR. Thus, in cortical units, ICES can produce complex behavior that includes stimulus-evoked responses, suppression and rebound responses, response adaptation, and changes in the ratio of asynchronous to synchronous spikes. (Response suppression and rebound results are shown in Table 2 and are described at the end of the results.)
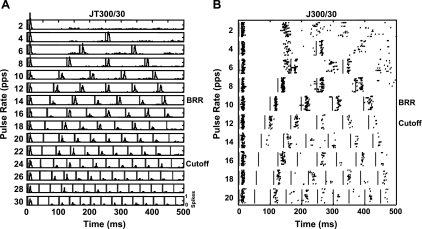
Fig. 3.
A and B: neuronal responses from a unit recorded in a behaviorally trained cat (JT300/30 group; A) and from a unit recorded in an untrained cat (J300/30 group; B). A: poststimulus time histograms (PSTHs). Pulse rate: 2–30 pulses/s (pps); bin width: 1 ms; 20 repetitions. The scale is relative to the maximum response (number of spikes/repetition/bin). Vertical lines mark the onset of stimulus pulse artifacts. The unit was spontaneously active. B: raster plots with each dot representing a spike. Pulse rate: 2–20 pulses/s; bin width: 1 ms; 20 repetitions. There are no stimulus artifacts; vertical lines mark the onset of stimulus pulses. The unit was not spontaneously active. Best repetition rates (BRRs) and cutoff rates are shown for both units.
Fig. 4.
A and B: period histograms for the units shown in Fig. 3. Spikes per bin for 20 repetitions is plotted versus one period. At all pulse rates, the first 8 ms of the response after an electric pulse was excluded to eliminate contamination by electric stimulus artifacts, and at pulse rates of >2 pulses/s, responses to the first pulse in each repetition were excluded to avoid stimulus onset effects. At 2 pulses/s, the onset and rebound responses are shown. The pulse rate, vector strength, and number of phase-locked spikes are shown in A and B. All vector strength values were significant (Rayleigh statistic, P = 0.001).
Fig. 5.
A and B: repetition rate transfer functions (RRTFs) for bandpass units recorded in a behaviorally trained cat (JT300/30 group; A) and an untrained cat (J300/30 group; B). Phase-locked spikes are plotted against the pulse rate. BRRs and cutoff rates are shown in the plots. A bandpass RRTF was identified when the low and high cutoffs (lower dashed line) intersected the RRTF at <50% the number of phase-locked spikes at the BRR. C: functions showing the mean number of phase-locked spikes per second versus pulse rate are shown in black and blue for the untrained A300/30 and J300/30 groups and in red and green for the behaviorally trained JT300/30 and JT30 groups. Error bars are SEs. Inset: proportions of units that contributed to the phase-locked spike rates. As pulse rate increased, the proportions of contributing units declined.
Figure 5, A and B, shows RRTFs for the units shown in Figs. 3 and 4. Both units had bandpass profiles identified by low and high cutoff rates that were <50% of the number of phase-locked spikes at the BRR. In our sample of 307 units, 95.4% had bandpass, low-pass, or high-pass RRTFs. High-pass and low-pass RRTFs are essentially either increasing or decreasing monotonic response functions, respectively. Based on the <50% criterion, the majority of units in each of the four groups of cats had bandpass RRTFs (Table 2). This result is consistent with investigations that have reported a majority of AI units with bandpass profiles in synchronized response to amplitude-modulated tones (Schreiner and Urbas 1988), acoustic click trains (Eggermont 1991, 1992), and ICES in deaf cats (Schreiner and Raggio 1996). Although a χ2 analysis found no difference in the proportions of bandpass units in the four groups of cats (P > 0.05), in the J300/30 group, the percent bandpass RRTFs was relatively low, and compared with other groups, the percent low-pass RRFTs was much higher (Table 2). However, when the criterion used for low cutoff rate was set at <75% of the number of phase-locked spikes at the BRR, 74.3% of units in the J300/30 group had bandpass profiles.
Figure 5C shows mean phase-locked spikes per second in the four groups of cats over the range of ICES pulse rates (3–21 pulses/s) that produced the most dynamic spike rate responses. In all groups of cats, peak phase-locked spike rates occurred at pulse rates between 9 and 12 pulses/s; spike rates were highest in the JT30 group, lowest in the J300/30 group, and intermediate in the A300/30 and JT300/30 groups. At pulse rates between 14 and 21 pulses/s, mean phase-locked spike rates were higher in the behaviorally trained groups of cats than in untrained animals.
As shown in the inset in Fig. 5C, the proportions of units contributing to the phase-locked spike rate functions declined as the pulse rate increased. A steep decline occurred after 12 pulses/s in the J300/30 group, and at 20 pulses/s fewer than half the units in this group contributed to the phase-locked spike rate. In the A300/30, JT300/30, and JT30 groups, the steep decline occurred after 14, 18, and 21 pulses/s, respectively, and at 36 pulses/s, <25% of units in any group contributed to the phase-locked spike rate. The relatively robust spike rate function at 3–21 pulses/s in the JT30 group may reflect the cats' unique training and chronic passive stimulation history with salient fast rise time signals identical to those used in the electrophysiological experiments (biphasic pulses, 0.2 ms/phase).
Effects of Behavioral Training and Chronic Passive Stimulation
The effects of behavioral training and chronic passive stimulation on temporal processing in the four groups of animals are based on responses of small multineuronal clusters or units located in the right AI. In neonatally deafened cats, chronic passive stimulation was initiated at an age (median: 8 wk or 55 days) that was close to the age (∼60 days) when the BRR has reached adult values but earlier than the age (∼150 days) when the cutoff rate has matured (Eggermont 1996). In the A300/30, J300/30, and JT30 groups, chronic passive stimulation was applied until the end of the study; in the JT300/30 group, chronic passive stimulation ended when behavioral training began. Behavioral training was initiated in juvenile cats (median age: 19.8 wk or 138 days) and concluded when the cats were mature young adults.
TEMPORAL PROCESSING: BRR AND CUTOFF RATE.
In contrast to chronic passive stimulation alone, behavioral training restored a normal range of temporal processing capacity. In Fig. 6, comparisons of BRRs (left column) and cutoff rates (right column) are shown for the four groups of cats. The histograms in Fig. 6, A and D, show the number of units versus BRR and cutoff rate, respectively. In each group, the number of BRRs is larger than the number of cutoff rates because recording in several units (n = 8) was not continued to pulse rates high enough to determine a cutoff rate, and, by our definition, high-pass units (n = 22) could not have a cutoff rate.
In the behaviorally trained JT300/30 group, the modal BRR was 12 pulses/s, and the modal BRR in the other groups was 10 pulses/s (Fig. 6A). The modal cutoff rate was 14 pulses/s in the untrained J300/30 group (Fig. 6D); in the A300/30 group and in the behaviorally trained JT300/30 and JT30 groups, the cutoff rate mode was at 18 pulses/s (Fig. 6D). In the untrained J300/30 group, the distribution of units toward higher pulse rates was reduced compared with the distribution of units toward higher pulse rates in the other groups (Fig. 6, A and D). This result is clearly evident in Fig. 6, B and E, which show the corresponding cumulative probabilities for the four groups of animals. Intersections of the functions with the dashed horizontal lines indicate that 90% of units in a group of cats are at the corresponding BRR (Fig. 6B) or cutoff rate (Fig. 6E) on the abscissas. In the untrained J300/30 group, the BRR and cutoff rate intersections were at 11 pulses/s (Fig. 6B) and 18 pulses/s (Fig. 6E), whereas in the other groups, the BRR and cutoff rate intersections occurred at ≥14 pulses/s (Fig. 6B) and ≥22 pulses/s (Fig. 6E). In the untrained J300/30 group, <3% of the units had BRRs of >12 pulses/s, and <6% of the units had cutoff rates of >20 pulses/s.
Statistical comparisons of BRRs and cutoff rates in the four groups of cats are shown in Fig. 6, C and F, in box plots (Kruskal-Wallis one-way ANOVAs on ranks: 3 df, P < 0.001; Dunn pairwise multiple-comparison tests: *P < 0.05 and **P < 0.01). Ranked BRRs and cutoff rates in the untrained JT300/30 and JT30 groups were significantly higher than the corresponding values in the untrained J300/30 group, and BRRs and cutoff rates in prior normal-hearing adult cats (A300/30 group) were likewise higher than BRRs and cutoff rates in the untrained J300/30 group. However, BRRs and cutoff rates were equivalent among the A300/30, JT300/30, and JT30 groups.
The results indicate that behavioral training initiated in deaf juvenile cats enhanced temporal processing in the AI to levels comparable with those observed in young adult cats with histories of prior normal hearing. Conversely, temporal following rates were lowest in the J300/30 group relative to the other groups of deaf cats. Compared with behavioral training with ICES, chronic passive electric stimulation alone was unattended and meaningless and had no beneficial or ameliorative effect on temporal following in the AI.
With respect to the total duration of behavioral training (cats K99 and K102: median 28 wk vs. cats K80, K82, and K91: median 5.6 wk, cf. methods and Table 1), there was no effect of training duration on either BRRs or cutoff rates (Mann-Whitney rank-sum tests, P > 0.05), and there was no effect of the interval between the completion of detection training and the electrophysiological experiments on BRRs or cutoff rates (range: 1–45 days, Spearman rank order correlation, P > 0.05).
ELECTRODE CONFIGURATION.
Given the restored temporal processing capacity associated with behavioral training, we compared BRRs and cutoff rates obtained with acute physiological stimulation by the electrode configuration used in behavioral training [apical pair (1,2)] with BRRs and cutoff rates obtained with acute stimulation of the other [e.g., basal pair (3,4)] electrode configurations in two groups: JT300/30 and A300/30. The mean response thresholds for apical pair (1,2) were 51.5 ± 4.2 dB in the A300/30 group and 41.5 ± 2.6 dB in the JT300/30 group; for other electrode configurations, the mean response thresholds were 52.4 ± 2.8 dB in the A300/30 group and 45.5 ± 3.8 dB in the JT300/30 group.
We hypothesized 1) that the effects on temporal processing of stimulation with apical pair (1, 2) used in training would be larger than the effects of stimulation with the other electrode configurations not used in training and 2) that the stimulating electrode configuration would have no effect on temporal processing in the untrained A300/30 group. Comparisons between electrode configurations could not be made in the JT30 and J300/30 groups because only one electrode configuration was used in the electrophysiological experiments in these cats (cf. methods).
The results of the analysis for the JT300/30 and A300/30 groups are shown in Fig. 7A for BRRs and in Fig. 7B for cutoff rates. Statistical comparisons are summarized in the box plots and indicate that in the JT300/30 group, the median BRR for the apical (1,2) electrode configuration [13.92 + 2.0 (Q) pulses/s] was significantly higher (Mann-Whitney rank-sum test, P < 0.001) than the median BRR for the pool of other electrode configurations [9.96 + 2.2 (Q) pulses/s]. In the JT300/30 group, the difference in median cutoff rate for the apical [22.0 + 5.2 (Q) pulses/s] and other [16.0 + 4.0 (Q) pulses/s] electrode configurations was also significant (Mann-Whitney rank-sum test, P < 0.001). In contrast, the electrode configuration [apical pair (1,2) vs. other configurations] had no effect on either BRRs (Fig. 7A) or cutoff rates (Fig. 7B) in the A300/30 group (P > 0.05).
The results shown in Fig. 7, A and B, indicate that when cortical neurons were repeatedly activated by ICES delivered from the apical electrode configuration used in behavioral training, the stimulus had a disproportionately large effect on temporal processing at the stimulated cortical sites in the JT300/30 group. The median BRR and median cutoff rate were 39.8% and 37.5% higher, respectively, for stimulation by apical pair (1,2) compared with other electrode configurations. If the effects are expressed as the percent difference in the duration of the interpulse interval at median BRR and median cutoff rate, the interpulse intervals were 28.6% and 27.4% shorter, respectively, for activation by the electrode configuration used in training compared with other electrode configurations. In the A300/30 group, chronic passive stimulation was delivered by apical pair (1,2). However, neuronal responses evoked by ICES delivered from the apical electrode configuration were similar to neuronal responses evoked by stimulation delivered from other electrode configurations that were not used to deliver chronic passive stimulation. These results suggest that chronic passive stimulation has no effect on cortical temporal processing.
Statistical comparisons for acute physiological stimulation with apical pair (1,2) on BRRs and cutoff rates in the A300/30, J300/30, and JT300/30 groups are shown in Fig. 7, C and D. The A300/30 and J300/30 groups received only chronic passive stimulation with apical pair (1,2); the JT300/30 group received chronic passive stimulation and behavioral training with apical pair (1,2). The median BRR and cutoff rate were significantly higher in the trained JT300/30 group than those in the untrained groups (Kruskal-Wallis one-way ANOVAs on ranks: 2 df, P < 0.001; Dunn pairwise multiple-comparison tests: P < 0.05 and P < 0.01, respectively). This result is important because it eliminates any possible confounding with electrode configuration and shows that in the three groups of cats that received chronic passive stimulation with apical electrode pair (1,2), BRRs and cutoff rates increased only when stimulation of the apical electrode configuration was relevant for conditioned avoidance learning in the JT300/30 group. In the untrained animals in the A300/30 and J300/30 groups, chronic passive stimulation with apical electrode pair (1,2) had no apparent qualitative or quantitative effects on cortical temporal processing.
Temporal Precision: Minimum Latency and Jitter
We also analyzed the first spike timing (minimum latency) and latency variability of AI units recorded in the A300/30, J300/30, and JT300/30 groups. As noted in the methods, minimum latency data were not available for cats in the JT30 group and for one cat in the J300/30 group. The mean and SD of minimum latencies were determined across repetitions for each unit. The SD (jitter) of minimum latency and the median mean minimum latency were compared across the three groups of animals. Median mean minimum latency was significantly shorter in the A300/30 group [8.74 + 0.5 (Q) ms] than in the JT300/30 [9.79 + 1.3 (Q) ms] and J300/30 [10.68 + 1.0 (Q) ms] groups (Kruskal-Wallis one-way ANOVA on ranks: 2 df, P < 0.001; Dunn pairwise multiple-comparison test: P < 0.01). However, analysis of median jitter in the A300/30 (0.81 ms), JT300/30 (0.85 ms), and J300/30 (1.14 ms) groups found no differences in precision among the three groups of cats (P > 0.05).
Influence of Response Suppression and Rebound on Neuronal Responses to ICES
A common feature of the responses of AI neurons to acoustic clicks in anesthetized cats is the occurrence of response suppression and response rebound or oscillation after a click, and these events can influence neuronal responses to subsequent clicks (Eggermont 1991, 1992). Depending on the click rate, the duration of suppression, and the size of the rebound response, the response to subsequent clicks may be augmented or reduced. These effects are particularly apparent at low click rates (<10 pulses/s) and can affect periodic indices of temporal processing that are measured by the number of phase-locked spikes or by the vector strength of the response.
We observed similar effects on unit responses to ICES. Representative examples of suppression and rebound responses are shown in Fig. 3, A and B, top (PSTH and dot raster plots) and in Fig. 4, A and B (period histograms). After the onset response to an individual pulse (2 pulses/s, 500-ms sweep duration), suppression of spontaneous activity and a rebound response occurred (Figs. 3A, top, and 4A), and the rebound response in one unit was strongly oscillated (Figs. 3B and 4B).
To analyze suppression and rebound responses, we focused on responses to an individual pulse to allow the investigation of a postpulse period without spiking activity and rebound events from subsequent pulses. We conducted several statistical analyses to assess the effects of suppression and rebound on neuronal responses. The results are shown in Table 2. The peak (modal) latencies of the onset responses in the four groups of cats were significantly different (Kruskal-Wallis one-way ANOVA on ranks, 3 df, P < 0.001), although the only significant comparison was between the two behaviorally trained groups (Dunn pairwise multiple-comparison test, P < 0.05). The duration of the suppression interval was estimated by the difference between the peak (modal) latencies of the rebound and onset responses (Kruskal-Wallis one-way ANOVA on ranks, 3 df, P = 0.016). The only significant comparison was between the adult A300/30 and JT300/30 groups (Dunn pairwise multiple-comparison test, P < 0.05), i.e., no differences were found among the juvenile cats. A χ2 analysis of the proportions of rebound units in the four groups was not significant (P > 0.05), indicating that the distribution of expected and observed frequencies of occurrence across the four groups was not different from a random distribution.
Finally, we compared BRRs in units with rebound versus units without rebound for each of the four groups of cats (Mann-Whitney rank-sum tests). The only significant difference was obtained for the A300/30 group, the prior normal-hearing adult cats (with rebound, median BRR = 12 pulses/s; without rebound, median BRR = 10 pulses/s, t = 315.5, P = 0.033). Application of a Bonferroni correction for multiple comparisons indicated that rebound responses had no effect on BRRs in the four groups of cats (Bonferroni required P = α/4 = 0.05/4 = 0.0125 vs. observed P = 0.033).
The absence of a pattern of results showing differences between groups of cats indicates that the effects of suppression and rebound events on the response of AI neurons were essentially the same in the four groups of animals.
DISCUSSION
In this study, temporal processing in AI neurons was investigated in profoundly deaf young adult cats that had different hearing histories. In cats deafened before the onset of hearing, chronic passive stimulation with a feline cochlear prosthesis for hundreds of hours over a period of several months had no apparent effect on cortical temporal processing. This kind of stimulation did not produce behavioral responses or motor reactions and appeared to be unattended or completely ignored by the animals. The ability of neurons to respond to higher stimulus repetition rates was significantly impaired in these cats compared with neurons recorded in adult deafened cats with a prior history of normal hearing. In contrast, cortical neurons recorded in neonatally deafened cats that received behavioral training were capable of reliable temporal following responses at stimulus repetition rates equivalent to or higher than the BRRs and cutoff rates recorded in prior normal-hearing animals. Delivering <1 min of meaningful ICES to an attentive cat during each training session resulted in a significant increase in neuronal temporal following in the AI. In animals deprived of all auditory experience until they were juveniles, enhancement of temporal processing was dependent on training with behaviorally relevant ICES.
Perceptual Learning and Behavioral Training Enhance Cortical Temporal Processing
The deaf cats in this study were necessarily vigilant during a behavioral task that demanded detection, avoidance of an aversive CS, and disciplined, intermittent responses to obtain a preferred food. As we have shown, several training sessions were obligatory to consolidate successful learning. The brain mechanisms involved in normal or deaf auditory perceptual learning are poorly understood and are essentially conjectural. We assume that temporal neuroplasticity in the AI occurs in response to a challenging behavioral context that engenders modification of cortical sensory processing mechanisms.
Behavioral training in this study was initiated in juvenile cats before the AI was fully mature, and cortical temporal plasticity was expressed, although the behavioral task did not require discrimination or identification based on temporal features of the ICES. Other investigators have shown that AI temporal processing is enhanced by enrichment of the auditory environment in adult animals (Engineer et al. 2004). Likewise, in adult animals, merely pairing acoustic stimuli with electric activation of the basal forebrain modified minimum cortical response latency and maximum cortical following rate, and these effects were induced selectively by the schedule and statistical properties of the auditory stimuli paired with basal forebrain stimulation (Kilgard et al. 2001). These studies show that discrimination or identification of specific temporal cues is not required for temporal remodeling of the auditory cortex.
In the present study, the temporally modulated CS was delivered at threshold or suprathreshold amplitudes on ∼70% of warning trials at a modulation rate (30 Hz or 30 pulses/s) designed to challenge the slower temporal following rates typically observed in the cortical field AI of anesthetized naïve cats (Eggermont 1991, 1992; Joris et al. 2004; Schreiner and Urbas 1988; Schreiner and Raggio 1996). Temporally modulated cortical spiking responses were associated on warning trials with salient cues: ICES, tongue shock if the cat failed to interrupt licking, or interruption of access to a preferred food if the animal avoided the shock. Behavioral training afforded conditions for enhanced representation and encoding of the temporally modulated signals in the cortical field AI. The combination of a challenging CS, temporally modulated cortical responses, and subsequent reinforcement provided an appropriate context for perceptual associative learning and cortical plasticity to occur.
Importantly, neither the number of behavioral training sessions nor the proximity of training to the acute electrophysiological experiment at the end of the study (range: 1–45 days) appear to have influenced the physiological results described above. For example, in the JT300/30 group, the BRR and cutoff rate in the animal with the shortest duration of behavioral training (5.7 wk) were slightly higher than the corresponding values obtained in an animal that was trained for a longer duration (25 wk). In the cat with the longest interval between training and the acute physiological experiment (45 days), the BRR and cutoff rate were essentially identical to the corresponding values in two animals with much shorter intervals (2 and 3 days). It is unlikely that potential correlations between behavior and physiology in individual animals were obscured by a variation in either the number of behavioral training sessions or the proximity of training to the electrophysiological experiments.
Our results are consistent with reports that auditory behavioral training with either positive reward or negative reinforcement can induce cortical neuroplasticity that is retained for weeks or months after training (Bakin et al. 1996; Bakin and Weinberger 1990; Weinberger and Bakin 1998; Zhou and Merzenich 2009). Recent evidence has shown that functional reorganization of synaptic networks in the motor and sensory cortex in mammals occurs dynamically over days or weeks during learning and may persist also for even longer periods of time after training is concluded (Yang at al. 2009; Yotsumoto et al. 2008; but see Molina-Luna et al. 2008).
Temporal Processing in the AI
Studies in the AI of anesthetized cats have reported average BRRs of ∼6–14 Hz for synchronization to temporally modulated acoustic or electric signals (Eggermont 1991, 1992; Joris et al. 2004; Schreiner and Urbas 1988; Schreiner and Raggio 1996), and the average BRRs for the four groups of cats in the present study fall within that range. Behavioral training with temporally modulated ICES markedly influenced temporal following ability in cortical neurons. Neurons were enabled to respond at shorter interpulse intervals, the proportion of phase-locked spikes increased at higher stimulus repetition rates, and both BRRs and cutoff rates were significantly enhanced in the behaviorally trained animals.
Our results are concordant with previously reported results in normal-hearing adult animals showing that auditory discrimination training based on temporal cues significantly improved temporal processing in the AI (Bao et al. 2004; Beitel et al. 2003; Engineer et al. 2008; Leon et al. 2008; Schnupp et al. 2006). In our study, neuronal temporal processing was improved in developmentally impaired deaf cats if the animals were behaviorally trained as juveniles or young adults to detect temporally modulated ICES.
Most noteworthy, however, are the neuroplasticity results obtained with different electrode configurations. Each electrode configuration was located within the cochlea close to a surviving portion of auditory nerve fibers and spiral ganglion cells that in the normal cochlea would be excited by acoustic stimulation of sensory hair cells with a preferred range of best frequencies (Moore et al. 2002; Vollmer et al. 1999, 2007). Specifically, radial electrode pair (3,4) was located toward the basal higher-frequency region of the cochlea, whereas radial electrode pair (1,2) was located toward the apical lower-frequency region. When ICES was applied to an electrode configuration, neurons in a sector of the inferior colliculus (Snyder et al. 1990) and a limited area in the AI (Fallon et al. 2009; Raggio and Schreiner 1999) would be preferentially stimulated, that is, the residual cochleotopic spatial representation in the central auditory system of the deaf cats would influence the spatial pattern of neuronal activation in the auditory cortex.
Cats in the untrained A300/30 and J300/30 groups and the trained JT300/30 group received chronic passive stimulation with apical electrode pair (1,2) for hundreds of hours at suprathreshold stimulus levels that would activate a broad array of central auditory neurons. We predicted that stimulation with behaviorally relevant ICES in a conditioned avoidance paradigm would have its largest effect on AI neurons that were activated most consistently by electric stimulation applied to the apical electrode configuration [electrode pair (1,2)] used in training.
The physiological results confirmed our prediction. In the JT300/30 group, BRRs and cutoff rates were significantly higher for apical pair (1,2) used in behavioral training compared with other electrode configurations used in the electrophysiological experiments. As represented by the interpulse interval at median BRR and median cutoff rate in the JT300/30 group, the cycle-by-cycle timing for apical pair (1,2) was shortened by >25% compared with other electrode configurations. However, electrode configuration had no effect on temporal following in the A300/30 group, indicating that chronic passive stimulation without behavioral training did not differentially influence temporal processing at different cortical locations. Furthermore, for apical pair (1,2), BRRs and cutoff rates were significantly higher in the trained JT300/30 group than in the untrained A300/30 and J300/30 groups. Thus, the results indicate that stimulation with apical pair (1,2) enhanced temporal processing only if that electrode pair was used in behavioral training. Similarly, temporal plasticity specific to a 7-kHz frequency tone has been reported in rats that were exposed to the temporally modulated tone during early development (Kim and Bao 2009).
Cortical temporal reorganization was robust in the present study. Consistent with several previous studies in anesthetized animals (Bao et al. 2004; Beitel et al. 2003; Engineer et al. 2008; Leon et al. 2008; Schnupp et al. 2006; Zhou and Merzenich 2009), modification of temporal processing in the AI has been documented in anesthetized animals. Anesthesia affects cortical networks involved in attention, planning, and execution of responses, and it may influence cortical temporal tuning, but it does not prevent the expression of behavior-driven neuroplasticity in the AI.
However, not all of the temporal processing parameters evaluated in this study were modified by behavioral training or perceptual learning. There were no differences between trained and untrained cats in the peak (modal) latencies of the onset response to a single electric pulse, and minimum first spike latencies were shorter in untrained adult cats with prior normal hearing compared with juvenile trained and untrained cats. Variability (jitter) in response latency did not vary among groups. A similar training-induced increase in AI neuronal temporal following rate without a change in response latencies has been reported in normal-hearing adult rats (Bao et al. 2004). One model of cortical activity has demonstrated that input synchrony contributed by peripheral mechanisms determines response latency and jitter in the auditory cortex (Eggermont 2002). However, learning-induced changes in cortical field AI first spike latencies are difficult to interpret because increased latencies (Recanzone et al. 1993; Leon et al. 2008), decreased latencies (Brown et al. 2004), and no effect on latencies (Bao et al. 2004) have been reported in hearing animals.
Mechanisms and Models for Cortical Temporal Processing
Mauk and Buonomano (2004) have argued that the basic understanding of neural temporal processing is rather immature. The many “potential” mechanisms in the literature on temporal processing suggest, at least, creative guesswork. Nevertheless, some mechanistic aspects of temporal processing have been reasonably well documented. Schreiner and Raggio (1996) have reviewed evidence indicating that the onset, duration, and interaction of inhibitory and excitatory synaptic dynamics in the AI affect cycle-by-cycle timing and contribute to temporal repetition rate coding in hearing and deaf animals. Recent evidence from in vivo whole cell recordings has suggested that synaptic inhibition and synaptic depression are involved in postexcitatory hyperpolarization (suppression) and that the interaction of synaptic excitation and inhibition determines the timing of spiking activity in AI neurons (Tan et al. 2004; Wehr and Zador 2003, 2005). Importantly, Tan and colleagues (2004) reported durations of synaptic inhibition (100–200 ms) that are essentially the same as the durations of suppression in AI neurons obtained with extracellular recordings in deaf cats (present study) and hearing cats (Eggermont 1991, 1992).
Several models have been proposed that in principle are compatible with learning-induced temporal reorganization of the AI. One model suggests a temporal filtering process based on synaptic mechanisms that produces the short recovery time constants required for neuronal following of temporally modulated environmental sounds (Eggermont 2002), another model includes a frequency response parameter, modified by synaptic dynamics, that is responsible for the enhanced, behaviorally driven rate tuning observed experimentally in the AI (Saeb et al. 2007), and a third model emphasizes temporal interactions between excitation and inhibition mechanisms and short-term plasticity at the thalamocortical synapse (Sakai et al. 2009). Two influential models have been developed that emphasize the contributions of the cholinergic basal forebrain system (Weinberger and Bakin 1998) and the corticofugal system (Suga and Ma 2003) to learning-induced modification of spectral receptive field properties in AI neurons. Given that electric stimulation of cholinergic nucleus basalis modulates temporal processing in the AI (Kilgard and Merzenich 1998), these models can presumably be developed to include cortical temporal reorganization.
Limitations of the Present Work
Two important issues not addressed in this study can be rectified in future research. First, we focused on neuroplasticity in the AI, but contributions to the cortical effects from subcortical auditory structures, especially the magnocellular medial geniculate body (Weinberger and Bakin 1998) and perhaps the inferior colliculus (Gao and Suga 2000), cannot be excluded. Collicular neuronal latencies are shorter, and following frequencies are higher in deaf cats that received chronic ICES compared with deaf cats that did not receive ICES (Snyder et al. 1995; Vollmer et al. 1999, 2005). However, we have recently reported that behaviorally relevant ICES significantly enhanced cortical temporal processing in deaf juvenile and long-deaf cats, but in the same deaf animals, we found no evidence that behavioral training modified temporal processing in the inferior colliculus (Vollmer and Beitel 2010). Thus, even unattended, meaningless ICES might enhance temporal processing in the inferior colliculus, and these effects are apparently not driven or modulated by behavioral training or perceptual learning.
The second issue concerns our choice of ICES temporal parameters. We selected electric signals (simple unmodulated or sinusoidal amplitude-modulated pulse trains) based on experimental criteria. Although our research is compatible with clinical and psychophysical evidence that experience and training with behaviorally relevant stimulation is beneficial for speech discrimination performance in cochlear prosthesis users (Busby and Clark 1999; Busby et al. 1991, 1993; Fu and Galvin 2008; Svirsky et al. 2004), the stimuli currently used in human cochlear implant systems provide a more complex spatial-temporal array of signals from multiple channels (Kirby and Middlebrooks 2010; Zeng et al. 2008). An important goal in future work is the investigation of signal parameters widely used in human cochlear implants to determine their effects on neuronal processing in the central auditory system.
Finally, it seems worthwhile to note that our experimental design did not include a neonatally deafened control group that received ICES only during the electrophysiological portion of the study. This kind of control group, if compared with a passively stimulated group, could test whether passive ICES degrades or reduces the BRR and cutoff rate. However, compared with unstimulated animals, chronic passive ICES in neonatally deafened cats maintains temporal processing in the auditory cortex in long-deaf cats (Vollmer et al. 2009), and we are not aware of any evidence showing that passive ICES degrades temporal processing in the auditory cortex.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants N01-DC-3-1006 and HHS-N-263-2007-00054-C (Primary Investigator: P. A. Leake) and R01-DC-02260 (Primary Investigator: C. E. Schreiner). Other support was provided by The Coleman Memorial Fund, The Saul and Ida Epstein Fund, Hearing Research Incorporated and by DGFVo 640/1-1 from the German Research Association (M. Vollmer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Patricia Leake for cochlear implantation surgeries and preparation of cochlear specimens for histology, Gary Hradek for sectioning and analysis of cochlear spiral ganglion data, Stephen Rebscher for the design and fabrication of feline cochlear prostheses, Russell Snyder for computer programming, Charlotte Moore for participation in electrophysiological recording, Elizabeth Dwan for laboratory animal care and participation in behavioral training, Marshall Fong for engineering support, and Patricia Leake and Brian Malone for important comments on the manuscript.
REFERENCES
- Ahissar E, Nagarajan S, Ahissar M, Protopapas A, Mahncke H, Merzenich MM. Speech comprehension is correlated with temporal response patterns recorded from auditory cortex. Proc Natl Acad Sci USA 98: 13367–13372, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, South DA, Weinberger NM. Induction of receptive field plasticity in the auditory cortex of the guinea pig during instrumental avoidance conditioning. Behav Neurosci 110: 905–913, 1996 [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res 536: 271–286, 1990 [DOI] [PubMed] [Google Scholar]
- Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci 7: 974–980, 2004 [DOI] [PubMed] [Google Scholar]
- Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci USA 100: 11070–11075, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel RE, Snyder RL, Schreiner CE, Raggio MW, Leake PA. Electrical cochlear stimulation in the deaf cat: comparisons between psychophysical thresholds and central auditory thresholds. J Neurophysiol 83: 2145–2162, 2000a [DOI] [PubMed] [Google Scholar]
- Beitel RE, Vollmer M, Snyder RL, Schreiner CE, Leake PA. Behavioral and neurophysiological thresholds for electrical cochlear stimulation in the deaf cat. Audiol Neurootol 5: 31–38, 2000b [DOI] [PubMed] [Google Scholar]
- Brown M, Irvine DR, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: Association with changes in primary auditory cortex. Cereb Cortex 14: 952–965, 2004 [DOI] [PubMed] [Google Scholar]
- Busby PA, Clark GM. Gap detection by early-deafened cochlear-implant subjects. J Acoust Soc Am 105: 1841–1852, 1999 [DOI] [PubMed] [Google Scholar]
- Busby PA, Roberts SA, Tong YC, Clark GM. Results of speech perception and speech production training for three prelingually deaf patients using a multiple-electrode cochlear implant. Br J Audiol 25: 291–302, 1991 [DOI] [PubMed] [Google Scholar]
- Busby PA, Tong YC, Clark GM. Electrode position, repetition rate, and speech perception by early- and late-deafened cochlear implant patients. J Acoust Soc Am 93: 1058–1067, 1993 [DOI] [PubMed] [Google Scholar]
- Cooke JE, Zhang H, Kelly JB. Detection of sinusoidal amplitude modulated sounds: deficits after bilateral lesions of auditory cortex in the rat. Hear Res 231: 90–99, 2007 [DOI] [PubMed] [Google Scholar]
- Drullman R. Temporal envelope and fine structure cues for speech intelligibility. J Acoust Soc Am 97: 585–592, 1995 [DOI] [PubMed] [Google Scholar]
- Edeline JM. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: a critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Prog Neurobiol 57: 165–224, 1998 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Rate and synchronous measures of periodicity coding in cat primary auditory cortex. Hear Res 56: 153–167, 1991 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Stimulus induced and spontaneous rhythmic firing of single units in cat primary auditory cortex. Hear Res 61: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Differential maturation rates for response parameters in cat primary auditory cortex. Aud Neurosci 2: 309–327, 1996 [Google Scholar]
- Eggermont JJ. Temporal modulation transfer functions in cat primary auditory cortex: separating stimulus effects from neural mechanisms. J Neurophysiol 87: 305–321, 2002 [DOI] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Chen YTH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP. Cortical activity patterns predict speech discrimination ability. Nat Neurosci 11: 603–608, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol 92: 73–82, 2004 [DOI] [PubMed] [Google Scholar]
- Fallon JB, Irvine DR, Shepherd RK. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats. J Comp Neurol 512: 101–114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin J., 3rd Maximizing cochlear implant patients' performance with advanced speech training procedures. Hear Res 242: 198–208, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci USA 97: 8081–8086, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Heffner RS, Heffner HE. Hearing range of the domestic cat. Hear Res 19: 85–88, 1985 [DOI] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev 84: 541–577, 2004 [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci 1: 727–731, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol 86: 326–338, 2001 [DOI] [PubMed] [Google Scholar]
- Kim H, Bao S. Selective increase in representations of sounds repeated at an ethological rate. J Neurosci 29: 5163–5169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AE, Middlebrooks JC. Auditory temporal acuity probed with cochlear implant stimulation and cortical recording. J Neurophysiol 103: 531–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hradek GT, Hetherington AM. Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hear Res 242: 86–99, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon MI, Poytress BS, Weinberger NM. Avoidance learning facilitates temporal processing in the primary auditory cortex. Neurobiol Learn Mem 90: 347–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci 27: 307–340, 2004 [DOI] [PubMed] [Google Scholar]
- Molina-Luna K, Hertler B, Buitrago MM, Luft AR. Motor learning transiently changes cortical somatotopy. Neuroimage 40: 1748–1754, 2008 [DOI] [PubMed] [Google Scholar]
- Moore CM, Vollmer M, Leake PA, Snyder RL, Rebscher SJ. The effects of chronic intracochlear electrical stimulation on inferior colliculus spatial representation in adult deafened cats. Hear Res 164: 82–96, 2002 [DOI] [PubMed] [Google Scholar]
- Phillips DP, Hall SE, Hollett JL. Repetition rate and signal level effects on neuronal responses to brief tone pulses in cat auditory cortex. J Acoust Soc Am 85: 2437–2549, 1989 [DOI] [PubMed] [Google Scholar]
- Phillips DP, Hall SE. Response timing constraints on the cortical representation of sound time structure. J Acoust Soc Am 88: 1403–1411, 1990 [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III. Activation patterns in short- and long-term deafness. J Neurophysiol 82: 3506–3526, 1999 [DOI] [PubMed] [Google Scholar]
- Rebscher SJ, Snyder RL, Leake PA. The effect of electrode configuration and duration of deafness on threshold and selectivity of responses to intracochlear electrical stimulation. J Acoust Soc Am 109: 2035–2048, 2001 [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13: 87–103, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci 336: 367–373, 1992 [DOI] [PubMed] [Google Scholar]
- Saeb S, Gharibzadeh S, Towhidkhah F, Farajidavar A. Modeling the primary auditory cortex using dynamic synapses: can synaptic plasticity explain the temporal tuning? J Theor Biol 248: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- Sakai M, Chimoto S, Qin L, Sato Y. Neural mechanisms of interstimulus interval-dependent responses in the primary auditory cortex of awake cats. BMC Neurosci 10: 10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheich H. Auditory cortex: comparative aspects of maps and plasticity. Curr Opin Neurobiol 1: 236–247, 1991 [DOI] [PubMed] [Google Scholar]
- Schnupp JWH, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci 26: 4785–4795, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Raggio MW. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation: II. Repetition rate coding. J Neurophysiol 75: 1283–1300, 1996 [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Urbas JV. Representation of amplitude modulation in the auditory cortex of the cat. II. Comparison between cortical fields. Hear Res 32: 49–64, 1988 [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science 270: 303–304, 1995 [DOI] [PubMed] [Google Scholar]
- Snyder RL, Leake PA, Rebscher SJ, Beitel RE. Temporal resolution of neurons in cat inferior colliculus to intracochlear electrical stimulation: Effects of neonatal deafening and chronic stimulation. J Neurophysiol 73: 449–467, 1995 [DOI] [PubMed] [Google Scholar]
- Snyder RL, Rebscher SJ, Cao K, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I: expansion of central representation. Hear Res 50: 7–34, 1990 [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4: 783–794, 2003 [DOI] [PubMed] [Google Scholar]
- Svirsky MA, Teoh SW, Neuberger H. Development of language and speech perception in congenitally, profound deaf children as a function of age at cochlear implantation. Audiol Neurootol 9: 224–233, 2004 [DOI] [PubMed] [Google Scholar]
- Tan AYY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol 92: 630–643, 2004 [DOI] [PubMed] [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory mibbrain and cortex in the awake mongolian gerbil. J Neurosci 27: 6091–6102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer M, Beitel RE. Behaviorally relevant auditory experience improves temporal processing in primary auditory cortex (AI) but not in inferior colliculus (ICC) in deaf cats (Abstract). Assoc Res Otolaryngol 33: 120, 2010 [Google Scholar]
- Vollmer M, Beitel RE, Raggio MW, Schreiner CE. Auditory experience affects temporal processing in the long-deafened cat primary auditory cortex (Abstract). Conf Implant Aud Prostheses: 209, 2009 [Google Scholar]
- Vollmer M, Beitel RE, Snyder RL. Auditory detection and discrimination in deaf cats: psychophysical and neural thresholds for intracochlear electrical signals. J Neurophysiol 86: 2330–2343, 2001 [DOI] [PubMed] [Google Scholar]
- Vollmer M, Beitel RE, Snyder RL, Leake PA. Spatial selectivity to intracochlear electrical stimulation in the inferior colliculus is degraded after long-term deafness in cats. J Neurophysiol 98: 2588–2603, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer M, Leake PA, Beitel RE, Rebscher SJ, Snyder RL. Degradation of temporal resolution in the auditory midbrain after prolonged deafness is reversed by electrical stimulation of the cochlea. J Neurophysiol 93: 3339–3355, 2005 [DOI] [PubMed] [Google Scholar]
- Vollmer M, Snyder RL, Leake PA, Beitel RE, Moore CM, Rebscher SJ. Temporal properties of chronic cochlear electrical stimulation determine temporal resolution of neurons in cat inferior colliculus. J Neurophysiol 82: 2883–2902, 1999 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426: 442–446, 2003 [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron 47: 437–445, 2005 [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Bakin JS. Learning-induced physiological memory in adult primary auditory cortex: receptive field plasticity, model, and mechanisms. Audiol Neurootol 3: 145–167, 1998 [DOI] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature 462: 920–925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57: 827–833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Rebscher S, Harrison W, Sun X, Feng H. Cochlear implants: system design, integration, and evaluation. IEEE Rev Biomed Eng 1: 115–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Merzenich MM. Developmentally degraded cortical temporal processing restored by training. Nat Neurosci 12: 26–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]