Abstract
Neurotoxic effects of oxaliplatin chemotherapy, including proprioceptive impairments, are debilitating and dose limiting. Here, we sought to determine whether oxaliplatin interrupts normal proprioceptive feedback by impairing sensory transduction of muscle length and force by neurons that are not damaged by dying-back neuropathy. Oxaliplatin was administered over 4 wk to rats in doses that produced systemic changes, e.g., decreased platelets and stunted weight gain, but no significant abnormality in the terminal ends of primary muscle spindle sensory neurons. The absence of neuropathy enabled the determination of whether oxaliplatin caused functional deficits in sensory encoding without the confounding issue of axon death. Rats were anesthetized, and action potentials encoding muscle stretch and contraction were recorded intra-axonally from dorsal roots. In striking contrast with normal proprioceptors, those from oxaliplatin-treated rats typically failed to sustain firing during static muscle stretch. The ability of spindle afferents to sustain and centrally conduct trains of action potentials in response to rapidly repeated transient stimuli, i.e., vibration, demonstrated functional competence of the parent axons. These data provide the first evidence that oxaliplatin causes persistent and selective deficits in sensory transduction that are not due to axon degeneration. Our findings raise the possibility that even those axons that do not degenerate after oxaliplatin treatment may have functional deficits that worsen outcome.
Keywords: chemotherapy, neuropathy, muscle spindle, electrophysiology, afferent
peripheral neuropathy is the most common dose-limiting factor of oxaliplatin chemotherapy (for reviews, see Argyriou et al. 2008; Krishnan et al. 2005; Pasetto et al. 2006). Acute neurotoxicity, predominantly sensory in nature, is seen in nearly all patients and is characterized by cold intolerance, dysthesias, and, less commonly, laryngeal spasms (Wilson et al. 2002). With continued treatment, a cumulative sensory neuropathy can develop similar to that seen in patients treated with other platinum compounds. The onset of the neuropathy is gradual, and the severity worsens with an increase in cumulative dose. Chronic neuropathy is thought to primarily affect large-diameter sensory fibers (for evidence and earlier citations, see Jamieson et al. 2005), which include muscle afferents that provide the central nervous system with proprioceptive information. Consistent with this notion, patients often lose deep tendon reflexes, mediated by muscle spindle afferents, and develop proprioceptive loss seen as problems with coordinating movement, e.g., loss of dexterity and sensory ataxia.
Sensory contributions to behavior and experience depend on conduction and central transmission of signals that are encoded by specialized sensory receptors. Behavior can be affected, therefore, by interrupting the flow of sensory information at any point in peripheral receptor transduction, axon conduction, and/or central synaptic transmission. With oxaliplatin treatment, axon degeneration, i.e., neuropathy, interrupts axonal conduction in sensory nerves, as suggested by the reduction of compound sensory nerve action potential (SNAP) amplitudes and by the sensory loss that often occurs in a “stocking and glove” distribution affecting the distal limbs and progressing proximally (Argyriou et al. 2008). There are also changes in axon excitability that can disturb normal conduction of sensory signals (Krishnan et al. 2005; Lehky et al. 2004). These effects of oxaliplatin undoubtedly contribute in altering sensory feedback, although the relative contribution of impaired conduction to sensory loss is undetermined. Substantial numbers of sensory axons maintain conduction as indicated by the partial presence of SNAPs, but their ability to properly encode sensory stimuli in the periphery is untested and, if modified, could contribute significantly to the sensory losses experienced by some patients in the advance of neuropathy (Cascinu et al. 2002).
The goal of this study was to determine whether functional deficits in sensory encoding exist after oxaliplatin administration. To avoid the confound of recording from axons that might be in the process of degenerating, we found a dose of oxaliplatin that did not cause neurodegeneration in rats and recorded from individual sensory axons in vivo during muscle stretch to characterize sensory encoding of proprioceptive information. We demonstrate abnormal sensory transduction that persists long after removal from treatment, even in the absence of peripheral degeneration.
METHODS
All procedures involving animals were approved by the Wright State University Laboratory Animal Care and Use Committee. Data were collected from 20 adult female Wistar rats (240–300 g). All animals were assigned to either the vehicle control (VC) group (n = 7) or to the oxaliplatin-treated (OX) group (n = 13), housed individually in barrier-protected cages, and given food and water ad libitum. Animals were monitored daily for general signs of distress, and body weights and food intake were recorded twice per week. None of the animals exhibited signs of discomfort reaching preset levels for removal from the study; all were killed by an intraperitoneal injection of euthasol (150 mg/kg) after terminal experiments.
Oxaliplatin (Sigma-Aldrich, St. Louis, MO) dissolved in 5% dextrose (4 mg/ml) was administered through intraperitoneal injections weekly for 4 wk. Doses of 9–10 mg/kg were used to reach cumulative doses of 36–40 mg/kg. Five animals were used solely for immunohistochemical analysis (see below). VC rats were administered 5% dextrose using similar volumes (adjusted for weight) and dosing schedules.
At 1-wk intervals preceding terminal experiments, the 15 remaining rats not used for immunohistochemical analysis were anesthetized (inhalation of isoflurane in 100% O2) for purposes of recording SNAPs evoked electrically in the tail exactly as described by Novak et al. (2009). SNAPs were stored on a computer and analyzed for amplitude, latency, and duration. Before anesthesia was discontinued, blood samples were taken from the lateral tail vein of OX rats for the measurement of complete blood counts.
Terminal experiments were performed on all rats 3–5 wk after the final treatment with oxaliplatin or vehicle alone. Anesthesia was induced and maintained by isoflurane inhalation (1–3% in 100% O2). Either the response properties of muscle sensory nerves or the neural morphology of muscle spindle receptors were studied as detailed in our earlier studies (Haftel et al. 2004, 2005). Briefly, with rats secured in a rigid recording frame, triceps surae muscles in the left hindlimb were dissected and attached through their common Achilles tendon to a servomotor, which measured force and controlled muscle length. Triceps surae nerves were placed in continuity on bipolar stimulating electrodes, and other hindlimb nerves were crushed, including common peroneal and posterior tibial nerves and nerves supplying the hamstring muscles. From dorsal roots L4 and L5 exposed by laminectomy and positioned on a recording platform, individual sensory axons were penetrated with sharp glass micropipettes (25–35 MΩ, 2M K-acetate) to record their action potentials produced in response to various stimuli.
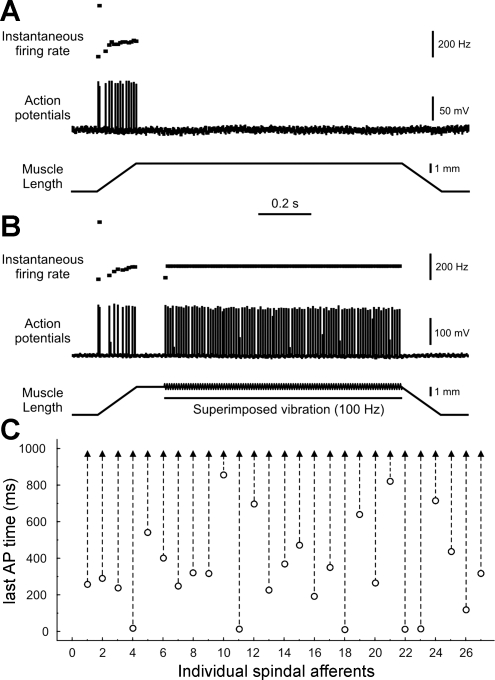
Sensory axons were randomly sampled in dorsal roots and selected for further study when they responded with orthodromic action potentials having a conduction delay of <3 ms upon electrical stimulation of the triceps surae nerves. Sensory axons were classified as either muscle-spindle or tendon-organ afferents, respectively, depending on whether they paused or accelerated firing during the rising phase of force in isometric twitch contractions of the triceps surae muscles. Further classification and characterization of mechanotransduction by these neurons were obtained from their responses to muscle stretch in the forms of ramp-hold-release (20 mm/s ramp and release, 3 mm), triangular stretch-release, and vibration (50–250 Hz, 80 μm). Muscle spindle afferents were classified as group IA when they 1) produced high-frequency bursts of firing at the onset of ramp or triangular stretch (4 mm/s, 3 mm) and 2) fired in response to each cycle of vibrations at frequencies of ≥100 Hz (e.g., see Fig. 3) (Matthews 1972). Those muscle spindle afferents exhibiting neither property 1 nor 2 were designated group II, whereas those displaying mixed responses were unclassified and were not considered further. In the VC group, 52 of 153 (34%) afferents were unclassifiable versus 25/116 (22%) in the OX group. All stretches began at resting length (Lr) and were also performed at Lr + 1 mm (Lr + 1), Lr + 2 mm (Lr + 2), and Lr − 1 mm (Lr − 1). Records of intra-axonal action potentials and muscle length force were collected, digitized (20 kHz), and stored on a computer for later analysis using Spike2 software.
Fig. 3.
Chronic oxaliplatin selectively affects sensory transduction. A and B: records from group IA afferent in an OX rat showed that failure to fire during the hold phase of muscle stretch (A) was overcome by superimposing vibration (not to scale; B). C: time of occurrence of the last AP during the hold phase of stretch alone (○) extended to the end of the hold phase when vibration was superimposed (▴) for 27 of 27 spindle afferents tested.
Several parameters were measured from afferent firing responses during ramp-hold-release stretch. The first action potential in response to ramp stretch specified the thresholds associated with muscle length and force traces. The slope of best-fit linear regressions on instantaneous firing rate versus muscle length represented the change in firing rate with muscle length. Peak firing rate was measured at the peak of ramp stretch, and dynamic index was taken as the difference in the firing rate between the peak and 0.5 s into the hold phase of stretch. The time of occurrence of the last spike during the hold phase was noted. Finally, history-dependent afferent firing was characterized by the reduced dynamic response (see Haftel et al. 2004), which was measured as the difference in the number of spikes fired in the first and third in a series of three successive triangular stretches, excluding the initial burst.
From five OX rats, the left medial gastrocnemius (one of the triceps surae muscles) was excised to examine the sensory nerve supply of muscle spindles for signs of neuropathy (Haftel et al. 2005). Briefly, fixed and frozen muscles were cut in 100-μm sections for immunohistochemical labeling of muscle spindle axons using rabbit polyclonal antibody against protein gene product 9.5 (1:500, MorphoSys, Munich, Germany). Visualization of axons was achieved using fluorescein-conjugated secondary antibodies (1:100, Jackson ImmunoResearch, West Grove, PA). z-Axis stacks of images were obtained using a Fluoview FV 1000 confocal microscope and a ×60 objective (Olympus optical). Illustrated images are flat plane in focus projections obtained from z-series images using Fluoview software.
RESULTS
Oxaliplatin toxicity without neuropathy.
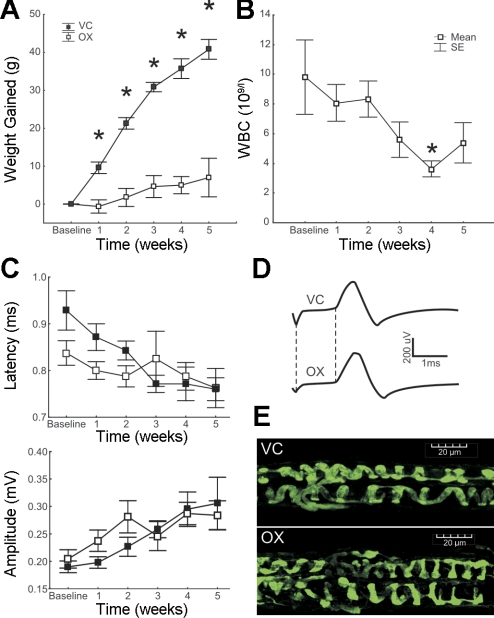
Rats maintained a healthy appearance and behavior throughout treatment, but there were clear systemic effects of oxaliplatin. Mean body weight increased over time in VC rats but remained relatively unchanged in OX rats (Fig. 1A). Some OX rats (4 of 13 rats) developed abdominal bloating with a purulent fluid found in the abdominal cavity at necropsy, as reported in a previous study using similar intraperitoneal doses (Jamieson et al. 2005). Because results from these four rats appeared indistinguishable from others, the data were pooled. No animals died during the course of the experiment.
Fig. 1.
Chronic oxaliplatin toxicity without sensory neuropathy. A–C: plots of rat body weight (A), white blood cell count (WBC; B), and sensory nerve action potential (SNAP) properties (C), all versus time (in wk) from the beginning of oxaliplatin treatment (baseline data taken immediately before treatment) until the time that the first rats were taken for terminal experiments. *P < 0.05 by ANOVA. D: records of SNAPs from individual rats at week 5 representing the similarity between the vehicle control (VC) and oxaliplatin-treated (OX) groups. The difference between the vertical dashed lines measures the latency from stimulus to SNAP onset. E: annulospiral endings were indistinguishable in OX and VC rats.
A tendency toward decreased white blood cell count was observed in OX rats (Fig. 1B), consistent with observations reported for humans (de Gramont et al. 2000) treated with oxaliplatin. The decrease achieved significance beginning week 4, just 1 wk after the oxaliplatin dosage was completed, and by week 5 began to recover.
We found no evidence of appreciable neuropathy in nerve conduction experiments, similar to patients that have been treated with OX but have no reduction in SNAP amplitude. Figure 1C shows that SNAP amplitude and latency (clinical measures of axon degeneration and demyelination, respectively) were not significantly different between VC and OX rats at any time point (P > 0.1). Tendencies toward larger amplitudes and shorter latencies with time in both groups may reflect maturation of peripheral nerves.
Morphological inspection of the distal-most extent of sensory nerves yielded little evidence of degeneration or structural disruption. Figure 1E shows that annulospiral nerve endings formed by group IA sensory axons appeared structurally normal in OX rats. In the medial gastrocnemius muscle of two untreated control animals, we observed 20 and 21 intact muscle spindles, each exhibiting neural innervation and annulospiral endings. The same normal morphology was observed in muscles from 5 OX rats, in which normal numbers of muscle spindles were found (19, 20, 21, 21, and 21 muscle spindles), and the great majority displayed normally appearing annulospiral endings. In rare cases, annulospiral endings were absent (3/102) or diminished in number (9/102 <5 annulospiral rings/spindle). These results demonstrate that even the most distal portion of axons encoding stretch was intact after the dose of oxaliplatin used. Thus, there was no distal neuropathy in these rats that could contribute to deficits in sensory transduction.
Oxaliplatin modifies sensory transduction.
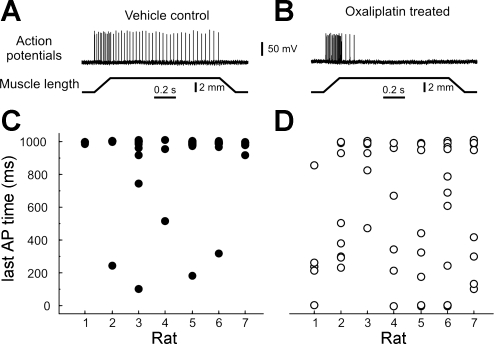
Muscle proprioceptors in OX rats exhibited a conspicuous failure to sustain firing during static muscle stretch. Figure 2 shows this failure, i.e., rapid adaptation, for one spindle afferent from an OX rat (B) compared with the sustained, slowly adapting firing from a VC rat (A). Spindle afferents are normally characterized as slowly adapting sensory neurons (Matthews 1972), as were 93% (50/54) of spindle afferents, which fired continuously through most of the hold phase of muscle stretch (≥0.9 s) for VC rats, in contrast with only 56% (34/61) that sustained firing in OX rats (Fig. 2, C and D). On average, the last action potential occurred ∼300 ms earlier than normal for all spindle and tendon-organ afferents (see Table 1).
Fig. 2.
Chronic oxaliplatin modified sensory transduction. A and B: intra-axonal records of action potentials (APs) recorded from group IA muscle-spindle afferents in response to ramp-hold-release muscle stretch from VC (A) and OX rats (B). C and D: time of occurrence of the last AP during a 1-s muscle stretch-hold phase for all spindle afferents sampled per rat [n = 54 afferents in VC rats (C) and n = 61 in OX rats (D)].
Table 1.
Sensory encoding by muscle proprioceptors
| Muscle Spindle Group IA |
Muscle Spindle Group II |
Tendon Organ Group IB |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC group |
OX group |
VC group |
OX group |
VC group |
OX group |
|||||||
| Means ± SD | n | Means ± SD | n | Means ± SD | n | Means ± SD | n | Means ± SD | n | Means ± SD | n | |
| Axonal conduction delay, ms | 1.6 ± 0.8 | 37 | 1.7 ± 0.6 | 52 | 1.8 ± 0.3 | 17 | 2.0 ± 0.6 | 9 | 2.4 ± 2.3 | 47 | 1.8 ± 2.8 | 30 |
| Ramp | ||||||||||||
| Length threshold, mm | 0.2 ± 0.1 | 37 | 0.3 ± 0.4 | 52 | 0.7 ± 0.5 | 17 | 1.2 ± 1.0 | 9 | ||||
| Force threshold, g | 29 ± 22 | 47 | 62 ± 29* | 30 | ||||||||
| Initial burst, pulses/s | 344 ± 102 | 12 | 373 ± 101 | 27 | ||||||||
| Slope, pulses · s · mm−1 | 32 ± 14 | 37 | 38 ± 14 | 50 | 36 ± 24 | 16 | 27 ± 3 | 5 | 20 ± 10 | 34 | 33 ± 10* | 10 |
| Peak firing rate, pulses/s | 167 ± 52 | 37 | 149 ± 47 | 52 | 86 ± 24 | 17 | 74 ± 27 | 7 | 74 ± 31 | 43 | 71 ± 19 | 20 |
| Hold | ||||||||||||
| Average firing rate, pulses/s | 57 ± 30 | 37 | 34 ± 21* | 52 | 39 ± 17 | 17 | 27 ± 25 | 9 | 37 ± 17 | 47 | 21 ± 18* | 30 |
| Last spike time, ms | 920 ± 217 | 37 | 631 ± 375* | 52 | 913 ± 228 | 17 | 615 ± 436 | 9 | 866 ± 325 | 47 | 536 ± 492 | 30 |
| Slope, pulses · s · s−1 | −32 ± 22 | 35 | −43 ± 25 | 45 | −17 ± 5 | 17 | −23 ± 6 | 6 | −13 ± 6 | 41 | −21 ± 13 | 16 |
| Miscellaneous | ||||||||||||
| Dynamic Index | 115 ± 39 | 34 | 113 ± 43 | 31 | 50 ± 22 | 17 | 46 ± 13 | 7 | 36 ± 23 | 41 | 37 ± 17 | 16 |
| Reduced dynamic response, spike number | 14 ± 7 | 36 | 10 ± 6 | 52 | 8 ± 5 | 17 | 6 ± 3 | 6 | 7 ± 7 | 46 | 3 ± 4 | 18 |
n, No. of afferents pooled within groups. VC group, vehicle control group; OX group, oxaliplatin-treated group. Nested ANOVA and Tukey's honestly significant difference post hoc tests were used to test for the significance of group differences (
P < 0.05).
Rapid adaptation of spindle afferent firing recorded in the dorsal roots might have been explained by impaired function of either excitability of the parent axon, which has been reported to change with oxaliplatin treatment (Park et al. 2009) or mechanotransduction by sensory receptors. Possible problems with action potential production were ruled out by demonstrating that axons fired continuously and at normal rates when muscle vibration was superimposed on the hold phase of stretch (Fig. 3). Of the 27 muscle spindle afferents with rapidly adapting responses, all 27 were capable of resuming initiation and conduction of action potentials when vibration was added to the muscle stretch (Fig. 3B). Thus, all spindle afferents maintained the capacity to generate and conduct action potentials in OX rats, but many lost the ability to transduce selected features of mechanical stimuli, namely, static muscle stretch.
Apart from selective impairment of sustained firing, the sensory code generated by proprioceptors in OX rats was indistinguishable from normal. Spindle afferent encoding of the dynamic features of muscle stretch was similar in VC and OX rats (Table 1). The firing rate increased with muscle length during the ramp portion of muscle stretch (e.g., Figs. 2B and 3A), with a relationship (slope pulses·s·mm−1) that was unchanged by oxaliplatin. The absolute firing rates achieved at the peak of the ramp were not significantly different, although the nominally lower firing rate for group IA afferents (18 pulses/s) in OX rats was noteworthy since it could contribute to reduced deep tendon reflexes. Several other response properties associated with the ramp portion of stretch were unchanged. Included were the length threshold for firing and the high frequency burst of firing (initial burst) observed in spindle group IA afferents, which was quantified here for cases when it occurred at Lr. The tendency for firing to decrease progressively in the ramp phases of successive triangular stretches [reduced dynamic response (Haftel et al. 2004)] did not differ significantly in OX and VC rats.
Sensory axon hyperexcitability has been observed in acute oxaliplatin toxicity experienced by patients. We tested for the expression of hyperexcitability in chronic OX rats and found no abnormality. No spontaneous firing was observed, nor was the variance in action potential intervals during ramp stretch greater in OX rats than in VC rats. Thus, the functional deficit reported here is distinct from that observed in acute oxaliplatin toxicity.
The data sample included tendon organ afferents, which respond most readily to active muscle contraction but also respond to stretch of a passive muscle. As for group IA spindle afferents, tendon-organ afferents were less responsive (lower average firing rate) during the hold phase of muscle stretch in OX rats compared with VC rats (Table 1). In contrast with spindle afferents, however, the responses of tendon-organ afferents during the ramp phase of stretch (Table 1) were modified in OX rats.
DISCUSSION
The results presented here provide the first evidence that oxaliplatin treatment in rats causes persistent deficits in sensory transduction, the process whereby sensory neurons transduce features of physical stimuli, e.g., duration of muscle stretch, into trains of action potentials. The deficits cannot be accounted for by axon degeneration, at least for group IA afferents, for which there was no apparent evidence for degeneration of annulospiral endings at the oxaliplatin dose used. Studies in patients will be necessary to determine whether persistent deficits in sensory transduction contribute to sensory loss after treatment with oxaliplatin.
The most common etiology of sensory deficits in neuropathy is axon degeneration. In oxaliplatin neuropathy, however, some patients have sensory deficits in the absence of electrophysiological evidence for degeneration, e.g., no detectable abnormality in SNAPs (Cascinu et al. 2002). One theoretical concern when one does not find evidence of axon degeneration in the setting of sensory deficits is that axons were not examined distally enough. We examined the distal-most extent of sensory terminal axon morphology by examining annulospiral endings. No signs of significant degeneration were observed for group IA axons in direct visualization of muscle spindle annulospiral endings. In addition, oxaliplatin treatment had no detectable effect on several response properties of muscle proprioceptors, which exhibited their normal brief conduction delay and were classifiable by various measures of sensory modality as normal spindle and tendon-organ afferents. If the terminal axons had disconnected from their receptors, then we would have observed changes in some, although not all (Johnson and Munson 1991) response properties. Thus, we ruled out axon degeneration as a contributor to deficits observed during muscle stretch in the rats studied. Despite the absence of axon degeneration and the retention of some normal properties, muscle proprioceptors exhibited distinct signs of abnormality in selected functions. Muscle proprioceptors were in some, but not all, respects less sensitive to muscle stretch and were notably less able to sustain firing throughout the stimulus as they are normally. These abnormalities would alter muscle length and force feedback from spindle and tendon-organ afferents, respectively, potentially leading to dysfunction at multiple sites in the sensory motor system.
Oxaliplatin neuropathy is already known to include functional effects distinct from degeneration. Functional deficits (dysesthesias and paresthesias) occur within hours of oxaliplatin administration, presumably reflecting the direct action of oxaliplatin on ion channels in sensory axons (Gamelin et al. 2002; Grolleau et al. 2001; Wilson et al. 2002). The best-known acute cellular effects of oxaliplatin on neurons are its actions on voltage-gated Na+ channels. Oxaliplatin modifications of Na+ current include a slowing of inactivation kinetics (Adelsberger et al. 2000; Wu et al. 2009), reduction in current amplitude (Benoit et al. 2006; Grolleau et al. 2001; Wu et al. 2009), and hyperpolarized shift in the voltage dependence of inactivation (Benoit et al. 2006). Effects on Na+ channels have been suggested to alter axon excitability in patients treated with oxaliplatin (Kiernan and Krishnan 2006; Webster et al. 2005). We considered the possibility that these direct effects of oxaliplatin might have produced results presented here because oxaliplatin can take many weeks to clear the blood (Levi et al. 2000). However, the sensory deficits we found are not consistent with acute effects of oxaliplatin on axon excitability. Sensory axons expressed normal excitability, giving no evidence of spontaneous firing or firing that was more variable than normal in response to muscle stretch. If anything, the cessation of firing during stretch suggests reduced axon excitability. This cannot be the case, however, since vibration superimposed on stretch completely restored normal action potential generation and propagation. Thus, sensory axons appeared to have normal excitability as they were as competent as normal in propagating action potentials from peripheral receptors to dorsal roots entering the spinal cord.
The failure of muscle proprioceptors to maintain firing during static muscle stretch provides insights into the underlying mechanism. The receptor potential produced in muscle spindle proprioceptors during sustained stretch is produced by persistent inward Na+ current (Simon et al. 2010). The ability of vibration to restore sensory transduction demonstrates that transient potentials underlying sensory transduction function normally. Cessation of firing during static stretch may be caused, therefore, by an effect of oxaliplatin on a mechanically gated Na+ channel involved in generating persistent inward current. Reduction of persistent Na+ current underlying the receptor potential in proprioceptors would reduce their sensitivity and lead to negative symptoms, including impaired proprioceptive function.
Earlier studies demonstrated two mechanisms contributing to sensory deficits in oxaliplatin neuropathy. One is an acute effect on sensory axon excitability causing dysaesthesias, and the other is axon degeneration that leads to chronic sensory deficits. Our results from studies in rats suggest another possible mechanism by which altered sensory transduction could impair sensory feedback. Proprioceptive deficits occurring with oxaliplatin therapy might be intensified if sensory encoding is truncated in those axons that do not degenerate. Further study will be necessary to determine whether the deficits in sensory transduction identified in rats occur in patients treated with oxaliplatin.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant P01-NS-057228.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors are grateful to Lori Goss for technical assistance.
REFERENCES
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na+ channel kinetics on rat sensory neurons. Eur J Pharmacol 406: 25–32, 2000 [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev 34: 368, 2008 [DOI] [PubMed] [Google Scholar]
- Benoit E, Brienza S, Dubois JM. Oxaliplatin, an anticancer agent that affects both Na and K channels in frog peripheral myelinated axons. Gen Physiol Biophys 25: 263–276, 2006 [PubMed] [Google Scholar]
- Cascinu S, Catalano V, Cordella L, Labianca R, Giordani P, Baldelli AM, Beretta GD, Ubiali E, Catalano G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 20: 3478–3483, 2002 [DOI] [PubMed] [Google Scholar]
- de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18: 2938–2947, 2000 [DOI] [PubMed] [Google Scholar]
- Gamelin E, Gamelin L, Bossi L, Quasthoff S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol 29: 21–33, 2002 [DOI] [PubMed] [Google Scholar]
- Grolleau F, Gamelin L, Boisdron-Celle M, Lapied B, Pelhate M, Gamelin E. A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channels. J Neurophysiol 85: 2293–2297, 2001 [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol 91: 2164–2171, 2004 [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Wang QB, Prather JF, Pinter MJ, Cope TC. Central suppression of regenerated proprioceptive afferents. J Neurosci 25: 4733–4742, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson SM, Liu J, Connor B, McKeage MJ. Oxaliplatin causes selective atrophy of a subpopulation of dorsal root ganglion neurons without inducing cell loss. Cancer Chemother Pharmacol 56: 391–399, 2005 [DOI] [PubMed] [Google Scholar]
- Johnson RD, Munson JB. Regenerating sprouts of axotomized cat muscle afferents express characteristic firing patterns to mechanical stimulation. J Neurophysiol 66: 2155–2158, 1991 [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Krishnan AV. The pathophysiology of oxaliplatin-induced neurotoxicity. Curr Med Chem 13: 2901–2907, 2006 [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle Nerve 32: 51–60, 2005 [DOI] [PubMed] [Google Scholar]
- Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve 29: 387, 2004 [DOI] [PubMed] [Google Scholar]
- Levi F, Metzger G, Massari C, Milano G. Oxaliplatin: pharmacokinetics and chronopharmacological aspects. Clin Pharmacokinet 38: 1–21, 2000 [DOI] [PubMed] [Google Scholar]
- Matthews PBC. The Structure of the Receptors. In: Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold, 1972, p. 1–59 [Google Scholar]
- Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J Clin Invest 119: 1150–1158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Oxaliplatin-induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain 132: 2712–2723, 2009 [DOI] [PubMed] [Google Scholar]
- Pasetto LM, D'Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol 59: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol 588: 171–185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. Br J Pharmacol 146: 1027–1039, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH, Lehky T, Thomas RR, Quinn MG, Floeter MK, Grem JL. Acute oxaliplatin-induced peripheral nerve hyperexcitability. J Clin Oncol 20: 1767–1774, 2002 [DOI] [PubMed] [Google Scholar]
- Wu SN, Chen BS, Wu YH, Peng H, Chen LT. The mechanism of the actions of oxaliplatin on ion currents and action potentials in differentiated NG108-15 neuronal cells. Neurotoxicology 30: 677–685, 2009 [DOI] [PubMed] [Google Scholar]