Abstract
The suprachiasmatic nucleus (SCN) is the locus of a hypothalamic circadian clock that synchronizes physiological and behavioral responses to the daily light-dark cycle. The nucleus is composed of functionally and peptidergically diverse populations of cells for which distinct electrochemical properties are largely unstudied. SCN neurons containing gastrin-releasing peptide (GRP) receive direct retinal input via the retinohypothalamic tract. We targeted GRP neurons with a green fluorescent protein (GFP) marker for whole cell patch-clamping. In these neurons, we studied short (0.5–1.5 h)- and long-term (2–6 h) effects of a 1-h light pulse (LP) given 2 h after lights off [Zeitgeber time (ZT) 14:00–15:00] on membrane potential and spike firing. In brain slices taken from light-exposed animals, cells were depolarized, and spike firing rate increased between ZT 15:30 and 16:30. During a subsequent 4-h period beginning around ZT 17:00, GRP neurons from light-exposed animals were hyperpolarized by ∼15 mV. None of these effects was observed in GRP neurons from animals not exposed to light or in immediately adjacent non-GRP neurons whether or not exposed to light. Depolarization of GRP neurons was associated with a reduction in GABAA-dependent synaptic noise, whereas hyperpolarization was accompanied both by a loss of GABAA drive and suppression of a TTX-resistant leakage current carried primarily by Na. This suggests that, in the SCN, exposure to light may induce a short-term increase in GRP neuron excitability mediated by retinal neurotransmitters and neuropeptides, followed by long-term membrane hyperpolarization resulting from suppression of a leakage current, possibly resulting from genomic signals.
Keywords: gastrin-releasing peptide, sustained inward current, GABAA receptor, patch-clamp
the suprachiasmatic nucleus (SCN) of the mammalian hypothalamus is the locus of the principal circadian pacemaker in the body, which serves to coordinate rhythmicity in many physiological and behavioral responses (Klein et al. 1991). Light is the most salient external cue synchronizing the free-running endogenous oscillation of the SCN to the day-night cycle of the local environment (Meijer and Schwartz 2003), but the mechanisms by which light modulates the excitability and rhythmicity of SCN neurons are not fully understood. One difficulty is that the SCN is functionally, anatomically, and peptidergically heterogeneous (Silver and Schwartz 2005). Directly retinorecipient neurons, concentrated in a ventrolateral core region, transmit signals to the SCN shell region (Leak et al. 1999); for terminological clarification, see Morin and Allen (2006). Extracellular single-unit recordings, performed primarily in rat and hamster, show that ∼33% of SCN neurons are light-responsive and exhibit increased firing rate during a light pulse (LP) (Aggelopoulos and Meissl 2000; Cui and Dyball 1996; Groos and Mason 1980; Jiang et al. 1997; Kim and Dudek 1993; Meijer et al. 1986, 1998). In these prior investigations, however, recordings were made from cells of unknown phenotype and connectivity. Moreover, long-term changes in neuronal excitability following exposure of the animal to a phase resetting LP were not explored; these two topics are the main focus of the present study.
The availability of green fluorescent protein (GFP) reporters permits the identification of specific peptidergic cell types of the SCN. One such phenotype is the cell containing gastrin-releasing peptide (GRP+). In the mouse, these cells lack detectable rhythmicity in clock gene expression and are directly retinorecipient (Karatsoreos et al. 2004). Following a LP, 72% are activated, based on coexpression of GFP and c-Fos. Moreover, tract-tracing indicates that 77% are contacted by retinal fibers following injection of cholera toxin-β into the vitreous. Comparison of morphological characteristics, processes, and connections of individual, tracer-filled GRP+ and immediately adjacent cells lacking GRP (GRP−) has revealed specialized circuitry within the SCN. Specifically, GRP+ neurons form a dense network of local circuits within the core. They make appositions onto other GRP+ cells and exhibit dye coupling, suggestive of gap junctions. Dendrites and axons of GRP+ cells make appositions onto arginine vasopressin (AVP) neurons, whereas the adjacent non-GRP cells have a less extensive fiber network, largely confined to the region of GRP+ cells (Drouyer et al. 2010).
In the present study, we used transgenic mice expressing GFP in SCN cells containing GRP (Drouyer et al. 2010; Karatsoreos et al. 2004). Using the whole cell patch-clamp method, we monitored changes in membrane properties in a population of GFP-labeled (GFP+) neurons over a 10-h interval that followed exposure to a 1-h light pulse (LP+). These altered properties were compared with those of GFP+ neurons from animals that had not been exposed to light (LP−) as well as with responses of immediately adjacent, non-GFP cells (GFP−). In GFP+ neurons, prior exposure to light induced marked alterations of GABA-dependent synaptic input and in a cation-dependent leakage current, both lasting hours beyond the exposure to light. These data provide specifics of how light influences neuronal excitability in a defined subset of SCN neurons over many hours following exposure and offer a framework for examining how photic cues reset the phase of the SCN.
MATERIALS AND METHODS
Animals
Mouse pups, 14–21 days old, were used for the experiments. Breeding parents were homozygote transgenic mice bearing a jellyfish GFP reporter (calbindin-D28K-BAC::GFP transgenic mouse; a generous gift of Dr. N. Heintz, Rockefeller University, New York, NY). We have previously shown that 87% of GFP+ neurons contain calbindin in 7-day-old pups (Drouyer et al. 2010) and 90% of GFP+ cells contain GRP in the adult (Karatsoreos et al. 2004). Here, we have assumed, based on the uniformity of the data set, that all GFP-immunoreactive neurons contain GRP. Mice were maintained in a 12:12-h light-dark cycle with lights off at Zeitgeber time (ZT) 12:00, room temperature at 21 ± 1°C, and ad libitum access to food and water. A dim red light (<1 lx) was on continuously. All animals were cared for in accordance with the Columbia University Institutional Animal Care and Use Committee and Animal Welfare regulations, and all experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
Experimental Groups
Animals either received LP+ (1,000 lx) at ZT 14:00–15:00 or no LP and were euthanized as follows: to test for the effects of light, slices were taken from animals euthanized at ZT 15:00, and cells were studied electrophysiologically between ZT 15:30 and 21:00. [Note: although it is known that preparation of SCN slices at night can shift the phase of oscillators (Gillette 1986), GRP cells do not express detectable rhythmicity in clock genes or proteins (Karatsoreos et al. 2004).] To control for time between euthanasia and recording, other animals were euthanized at ZT 16:30, and recordings were obtained from ZT 17:00 to 21:00. To establish time of return to baseline, a third group of animals was euthanized either at ZT 16:30 or 20:00, and recordings were made between ZT 21:00 and 01:30. Although slices were studied over a period of hours, individual cells typically were examined for only a few minutes. We collected data within 3–10 min of establishing the whole cell configuration to minimize the effects of dialysis of cell cytoplasm by the pipette solution.
Slice Preparation
Mouse pups were euthanized by decapitation under a dim red light, and the optic nerves were cut immediately before turning on the room light. The brains were quickly excised from the skull and placed for ∼2 min in cold cutting solution (in mM): 110 choline chloride, 25 NaHCO3, 25 dextrose, 11.6 sodium ascorbate, 3.1 sodium pyruvate, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgSO4 (all from Sigma-Aldrich, St. Louis, MO) bubbled with 95% O2-5% CO2 (Tech Air, White Plains, NY) at 295 ± 5 mOsm/l. A tissue block containing the hypothalamus was dissected. Coronal slices (250 μm) were cut on a Vibratome (Technical Products International, St. Louis, MO) and placed in artificial cerebrospinal fluid [ACSF; in mM, 126 NaCl, 3 KCl, 26 NaHCO3, 1 NaH2PO4, 10 dextrose, 2.4 CaCl2, 1.3 MgSO4 (all from Sigma-Aldrich except NaCl from Fisher Scientific, Pittsburgh, PA), bubbled with 95% O2-5% CO2] at room temperature (22–25°C) at 295 ± 5 mOsm/l.
Electrophysiology
Slices were placed in a submerged recording chamber at room temperature on an upright microscope (BX50WI; Olympus, New Hyde Park, NY) and held in place by a slice anchor (Warner Instruments, Hamden, CT). Whole cell patch-clamp recordings were performed using pipettes with resistances of 4–10 MΩ, pulled from borosilicate glass (outer diameter 1.5 mm, inner diameter 0.86 mm; Warner Instruments) filled with an intracellular solution (in mM): 135 potassium gluconate, 5 KCl, 5 NaCl, 10 HEPES, 2.5 MgATP, 0.3 GTP (all from Sigma-Aldrich), 1% biocytin (ε-biotinoyl-l-lysine; Molecular Probes/Invitrogen, Carlsbad, CA), at 285 ± 5 mOsm/l. Slices were perfused at 1 ml/min with ACSF (same composition as above).
The pipette was aimed at GFP+ cells within the ventrolateral SCN (Fig. 1A), detected through the microscope fitted with a GFP filter (Fig. 1B; excitation: 470 ± 20 nm, emission: 525 ± 25 nm; Chroma Technology, Brattleboro, VT). To patch, the filter was removed, and cells were visualized under dim white light. A seal was formed in voltage-clamp (range 3.6–98 GΩ), and the cell membrane was broken by gentle suction. The amplifier (BVC-700A; Cornerstone/Dagan) was then switched to current-clamp, and whole cell recordings were made using Pulse version 8.63 (Heka Instruments, Bellmore, NY). Baseline activity was recorded for 3–5 min to establish resting membrane potential and firing pattern. A liquid junction potential of +8 mV for pipettes in contact with intracellular solution relative to ACSF was measured against an Ag/AgCl pellet connected by a salt bridge (Neher 1992). Membrane potential values reported in results were corrected accordingly. Input resistance and decay time constant were measured at −55 mV in response to 2- to 7-pA hyperpolarizing pulses of 200- or 300-ms duration. The spike firing pattern of the test cell was evaluated during 500-ms depolarizing pulses of 0- to 12-pA steps in 2-pA increments. Although LP+ neurons were in a hyperpolarized state between ZT 17:00 and 21:00, that group's input resistance and time constant were also measured at −55 mV. Moreover, to test whether the membrane hyperpolarization seen in this group was due to synaptic influences, in a few experiments we added 1 μM TTX (Sigma-Aldrich) to the ACSF bath (n = 5) and glutamatergic ionotropic receptor blockers AP5 (25 μM) and CNQX (10 μM; both from Sigma-Aldrich) to five other cells. These drugs had no effect on membrane potential. GABAergic input was studied with hyperpolarizing or depolarizing current pulses in 5-pA increments before and after exposure of LP− cells to the GABAA receptor blocker, bicuculline methiodide (10 μM; Sigma-Aldrich), and LP+ cells to GABA (100 μM; Sigma-Aldrich). To evaluate the reversal potential for the GABA-induced events, spike firing was blocked by adding QX-314, which blocks sodium-dependent spikes from the cytoplasmic side of the membrane (5 mM; Tocris Bioscience, Ellisville, MO), to the pipette solution.
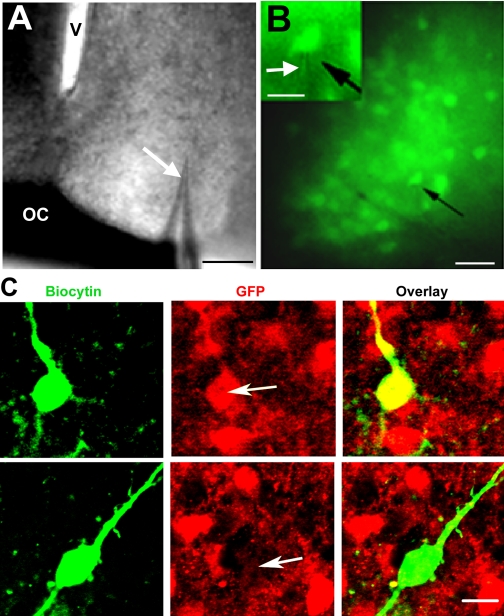
Fig. 1.
Photomicrographs of cell patching and identification of patched cells by immunocytochemistry. A: bright-field view of a brain slice containing the suprachiasmatic nucleus (SCN) with the recording pipette (white arrow) in position. Scale bar = 100 μm. V, 3rd ventricle; OC, optic chiasm. B: green fluorescent protein (GFP) cells seen during patch-clamping under fluorescent light with a GFP filter. The black arrow indicates the patched cell (scale bar = 30 μm). The inset shows the electrode (white arrow) patching a GFP-labeled (GFP+) cell (black arrow; scale bar = 20 μm). C: confocal images of 2 patched cells stained for biocytin (green) and GFP (red) and the overlay, confirming the peptidergic identity of the cell (white arrows indicate location of the filled cell). The cell in the 1st row is a GFP+ cell, and the cell in the 2nd row is a non-GFP cell (GFP−; z-axis = 2 μm; scale bar = 10 μm).
The contribution of a sustained inward current to the resting membrane potential of GRP neurons was evaluated in voltage-clamp mode using voltage steps or ramps. Initially, we used a modified ACSF designed to block voltage-dependent K and Ca currents (K-Ca blocking sol). The composition of external and pipette blocker solutions were altered as follows, in mM: external, 131 NaCl, 10 HEPES, 3 KCl, 10 glucose, 2 CaCl2, 2 MgCl2, 10 tetraethylammonium chloride, 10 CsCl, 1 4-aminopyridine, 0.3 CdCl, at 295 ± 5 mOsm/l; pipette solution, 130 cesium gluconate, 9 NaCl, 10 HEPES, 10 EGTA, 1 MgCl2, 3 K2-ATP, 1 NaGTP (Sigma-Aldrich), 1% biocytin, at 285 ± 5 mOsm/l. Subsequent experiments were done in normal ACSF, some substituting 140 mM choline chloride for an equivalent amount of NaCl. Other cells were tested in the absence or presence of flufenamic acid, an inhibitor of transient receptor potential (TRP) channels (100 μM; Sigma-Aldrich) in the bath. All the measurements were digitized, stored, and analyzed with Pulsefit version 8.63 (Heka Instruments).
Fast Fourier Transforms
GABA-dependent membrane potential fluctuations were analyzed using a fast Fourier transform (FFT) program in Microsoft Excel (Klingenberg 2005). For the purposes of comparing records from different cells or the same cell under different pharmacological conditions, we measured bicuculline-sensitive synaptic noise when the cell was stepped to −90 mV. Data were collected at the rate of 5,000 points/s.
Immunocytochemistry
After recording, slices were fixed in phosphate-buffered 4% paraformaldehyde for 48 h and then rinsed with 0.1 M PBS. Triple-label immunocytochemistry was performed on all slices as follows: to label for biocytin, slices were incubated overnight at 4°C with Cy2 streptavidin (1:200; Jackson ImmunoResearch, West Grove, PA) in 0.1 M PBS containing 0.3% Triton X-100 and then washed three times in PBS. To label GFP and AVP, slices were incubated in normal donkey serum (Jackson ImmunoResearch) in PBS for 1 h on a shaker at room temperature and then in rabbit polyclonal anti-GFP (1:10,000; Molecular Probes) and guinea pig polyclonal anti-AVP (1:5,000; Peninsula Laboratories, San Carlos, CA) antibodies in PBS containing 0.3% Triton X-100 for 48 h at 4°C. Slices were washed 3 × 10 min with PBS containing 0.1% Triton X-100. They were then incubated in secondary antibodies: Cy3 donkey anti-rabbit, Cy5 donkey anti-guinea pig, and Cy2 streptavidin (all from Jackson ImmunoResearch) for 2 h at room temperature. Finally, slices were washed 3 × 10 min with PBS, mounted onto glass slides, and dried overnight. Slides were dehydrated using alcohol and xylene baths and coverslipped with Krystalon (EMD Chemicals, Gibbstown, NJ).
Antibody Specificity
The anti-GFP rabbit polyclonal IgG was purified by ion exchange chromatography from an antiserum generated against GFP isolated from the jellyfish Aequorea victoria and affinity column-purified. Its specificity has been confirmed in rat neurons transfected with GFP-expressing vectors (Billig et al. 2000). The GFP antibody recognizes native GFP, recombinant GFP, and GFP fusion proteins by Western blot analysis and by immunoprecipitation. On Western blot using the native protein, the antibody detects a 27-kDa band corresponding to GFP (Molecular Probes).
The polyclonal AVP antibody was collected from guinea pigs immunized with a synthetic peptide as the immunogen. Radioimmunoassay shows that the cross-reaction with (Arg8)-vasopressin is 100%; [d(CH2)51,d-Ile2,Ile4,Arg8]-vasopressin, 0.06%; (Lys8)-vasopressin, 0.04%; (desamino-Cys1,d-Arg8)-vasopressin, 0.02%; PACAP38 (human, mouse, ovine, porcine, rat), Met-enkephalin, and oxytocin, 0% (Peninsula Laboratories Technical Service). Staining was eliminated by preadsorption of the diluted antiserum (1:5,000) with 2 μg/ml immunogenic protein.
Confocal Microscopy: Identification of the Recorded Cell
The slides were observed under a Zeiss Axiovert 200 MOT fluorescence microscope (Carl Zeiss, Thornwood, NY) with a Zeiss LSM 510 laser scanning confocal attachment. The sections were excited with an argon-krypton laser using the excitation wavelengths 488 nm for Cy2, 543 nm for Cy3, and 633 nm for Cy5. The Cy5 images were captured with blue pseudocolor. The images were collected as 2-μm multitract optical sections with sequential excitation by each laser to avoid cross talk between the three wavelengths. Each cell was examined (LSM 4.0.0.157; Zeiss) to determine whether it was singly or doubly labeled for biocytin and GFP or AVP.
RESULTS
Identification of Studied Cells
Electrodes were directed toward the region containing GFP+ neurons in the ventrolateral SCN (Fig. 1A). Although the electrode invariably was aimed at GFP+ cells (Fig. 1B), subsequent immunocytochemical staining indicated that some of the recordings were made from GFP− cells. All recorded cells were filled with biocytin and were identified either as GFP+ (double-labeled for biocytin and GFP) or GFP− (contain biocytin immunoreactivity but not GFP; Fig. 1C). No triple-labeled neuron was identified, indicating that GFP does not colocalize with AVP. If the recorded cell could not be recovered for immunocytochemical identification, its electrophysiological responses were excluded from the data set.
Light Induces Changes in Excitability of GFP+ but not GFP− Neurons
Short-term changes: ZT 15:30–17:00.
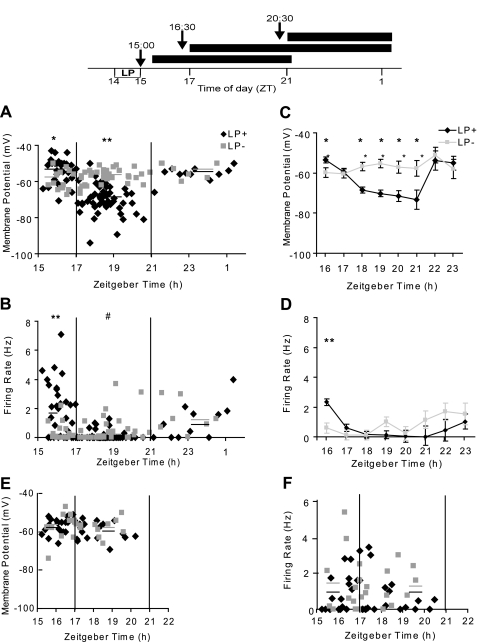
Our experimental protocols euthanizing animals at various times after the experimental or control manipulations (see materials and methods and schematic at top of Fig. 2) resulted in four functional categories of SCN neuron: light-exposed GFP neurons (GFP+LP+); GFP neurons not exposed to light (GFP+LP−); and the corresponding two groups of non-GFP neurons (GFP−LP+ and GFP−LP−). Electrophysiological data were not obtainable before ZT 15:30 because of the time required for slice preparation. During the 1st h of recording, we noted striking differences in resting membrane potential (Fig. 2A) and action potential firing rate (Fig. 2B) of GFP+ cells from mice exposed to a LP compared with no LP. Specifically, GFP+LP+ cells were depolarized, on average, by 6 mV (Fig. 2C) and had a significantly higher spike firing rate relative to the corresponding values for the GFP+LP− population (Fig. 2D). The raw data of Fig. 2, A and B, indicate that the differences between GFP+LP+ and GFP+LP− groups were greatest close to ZT 15:30 and gradually converged at later times, such that by ZT 17:00 the mean values for these two populations were not significantly different. In contrast to their GFP+ neighbors, GFP− neurons showed no significant differences between LP+ and LP− subtypes during this interval (Fig. 2, E and F).
Fig. 2.
Top: experimental design. Experimental animals housed in a light-dark cycle were exposed to a light pulse (LP+) from Zeitgeber time (ZT) 14:00 to 15:00. Control mice were treated identically but were not exposed to light (LP−). The arrows indicate the time of euthanasia and are coded to the horizontal bars, which indicate the period of recording for animals euthanized at each of 3 indicated times. Typically, an individual cell was followed for ∼3 min for measurements of membrane properties and an additional 5–7 min for effect of drug treatment. Middle: time-dependent changes in membrane properties of cells of light-exposed GFP neurons (GFP+LP+) and GFP neurons not exposed to light (GFP+LP−). GFP+ neurons from mice were exposed to a 1-h light pulse (LP+) or not (LP−) between ZT 14:00 and 15:00. Resting membrane potential (A) and spike firing rate (B) of individual GFP+LP+ and GFP+LP− neurons as a function of ZT are shown. Solid lines are the mean for each time group. *P < 0.05; **P < 0.01 for GFP+LP+ vs. GFP+LP− in same time group. #P < 0.01 for GFP+LP+ at ZT 17:00–21:00 vs. 15:30–17:00. C and D: statistical treatment of data in A and B, respectively. Error bars indicate SE. *P < 0.05, **P < 0.01, GFP+LP+ vs. GFP+LP−. Bottom: time-dependent changes in membrane properties of cells of non-GFP neurons GFP−LP+ and GFP−LP−. The figure shows resting membrane potential (E) and spike firing rate (F) of GFP− cells as a function of ZT. Solid lines are the mean for each time group. There is no statistically significant difference between data of GFP−LP+ and GFP−LP− neurons.
Long-term changes (ZT 17:00–21:00) and recovery (ZT 21:00–01:30) from exposure to light.
Beginning at about ZT 17:00, the GFP+LP+ population underwent a second change, consisting of a dramatic hyperpolarization of 10–15 mV, from near −55 mV to about −70 mV (Fig. 2, A and C; ∼ZT 17:00–21:00). This increase in transmembrane potential was associated with a further decline in spike firing rate (Fig. 2, B and D), resulting in the majority of GFP+LP+ neurons being silent between ZT 17:00 and 21:00. These alterations in excitability were accompanied by a significant increase in membrane resistance from 1.5 to 1.9 GΩ.
These changes were not a consequence of a deteriorating slice preparation, as evidenced by the following observations: 1) the GFP+LP− cell population showed no significant change in mean membrane potential over time between ZT 16:00 and 21:00 [1-way ANOVA: F(5–46) = 1.8, not significant (n.s.); Fig. 2, A and C]. The spike firing rate of GFP+LP− neurons varied over the same time interval but showed no significant trend [F(5–46) = 2.3, n.s.]; 2) we tested whether a longer interval between light exposure and animal euthanasia (cf. materials and methods and Fig. 2, top) would alter the results and found that it made no difference; 3) the population of GFP− cells, whether exposed to light or not, showed no changes in either mean membrane potential or spike firing rate at any time between ZT 15:30 and 21:00 (Fig. 2, E and F); and 4) finally, we note that although most GFP+LP+ cells were silent between ZT 17:00 and 21:00, at still later times (ZT 21:00–00:30) they subsequently recovered, as indicated by a resumption of spike firing and a depolarization of the membrane potential (Fig. 2, A and B). The light- and time-dependent changes in membrane properties of GFP+LP+ and GFP+LP− neurons are summarized in Table 1.
Table 1.
Membrane properties of GFP-labeled (GFP+) cells
| ZT 15:30–17:00 |
ZT 17:00–21:00 |
ZT 21:00–00:30 |
||||
|---|---|---|---|---|---|---|
| LP− | LP+ | LP− | LP+ | LP− | LP+ | |
| Resting potential, mV | −59.7 ± 1.6 | −53.2 ± 1.0* | −57.2 ± 0.7 | −68.4 ± 1.9† | −53.9 ± 1.6 | −55.7 ± 1.2 |
| n = 13 | n = 36 | n = 33 | n = 51 | n = 7 | n = 11 | |
| Firing rate, Hz | 0.49 ± 0.2 | 2.1 ± 0.3* | 0.49 ± 0.2 | 0.12 ± 0.04† | 1.48 ± 0.4 | 1.12 ± 0.4 |
| Input resistance, GΩ | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.9 ± 0.1* | 1.8 ± 0.2 | 1.7 ± 0.1 |
| n = 14 | n = 18 | n = 21 | n = 37 | n = 5 | n = 8 | |
| Decay time constant, ms | 27.7 ± 1.6 | 28.8 ± 2.3 | 29.0 ± 2.1 | 42.5 ± 2.7* | 28.3 ± 4.6 | 31.6 ± 2.7 |
Resting potential: *different from not been exposed to light (LP−) Zeitgeber time (ZT) 15:30–17:00 (P < 0.03); †different from LP− ZT 17:00–21:00 (P < 0.001), exposed to 1-h light pulse (LP+) ZT 15:30–17:00, LP− and LP+ ZT 21:00–00:30 (P < 0.001). Firing rate: *different from LP− ZT 15:30–17:00 (P = 0.01), LP− and LP+ ZT 17:00–21:00 (P = 0.001); †different from LP+ ZT 15:30–17:00 (P < 0.001), LP+ ZT 21:00–00:30 (P = 0.05), LP− ZT 21:00–00:30 (P = 0.02). Input resistance: *different from LP− ZT 17:00–21:00, LP+ ZT 15:00–17:00 (P < 0.05). Decay time constant: *different from LP− and LP+ ZT 15:30–17:00 (P = 0.003).
Mechanisms of Short-Term Changes ZT 15:30–17:00
Relation of firing rate to membrane potential.
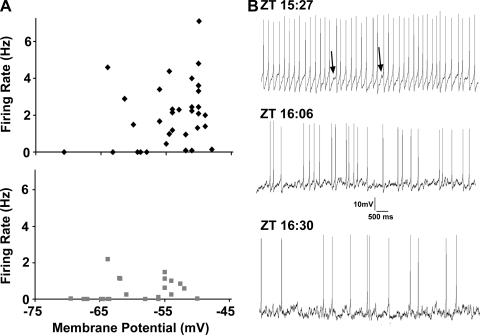
We used the data of Fig. 2, A and B, to plot spike firing rate against membrane potential for GFP+LP+ and GFP+LP− neurons at ZT 15:30–17:00 (Fig. 3A). The data show that for GFP+LP+ neurons, spike firing rates tend to rise steeply as their membrane potentials are depolarized positive to −60 mV. For GFP+LP− neurons, this relation is much less steep.
Fig. 3.
A: relation of membrane potential to spike firing rate for GFP+LP+ neurons (black diamonds; top; r = 0.7, P < 0.0001) and GFP+LP− neurons (gray squares; bottom; r = 0.3, P = 0.04). B: spike firing of GFP+LP+ neurons around the ZT 16:00 transition time. Traces are 10-s segments of spontaneous spike firing of 3 different GFP+LP+ neurons at the ZT indicated. At relatively early times, firing rates are both high and regular. Each spike is preceded by a smoothly depolarizing ramp, and the spike is followed by a large afterhyperpolarization, although small fluctuations in the depolarizing ramps are noted (arrows). These fluctuations in membrane potential become larger and more frequent over time as the firing rate decreases.
Relation of membrane noise to regularity of firing rate.
At all time points studied, GFP+LP− neurons exhibited irregular spike discharge patterns, and their membrane potentials showed rapid fluctuations, suggestive of a tonic synaptic input. In contrast, at times close to ZT 15:30, GFP+LP+ cells with mean firing rates >3 Hz were characterized by a regular discharge rate. Each spike was preceded by a smoothly depolarizing ramp, and the spike was followed by a large afterhyperpolarization characteristic for neurons showing pacemaker activity (Jackson et al. 2004). Even at the fastest firing rates observed, however, small depolarizing fluctuations in the ramps were noted, and these became larger and more frequent between ZT 15:30 and 17:00, resulting in a disruption of the depolarizing ramp and a reduction in spike frequency. Figure 3B illustrates these features of the spiking discharge pattern for three representative GFP+LP+ neurons. These data suggest that a tonic synaptic input interacts negatively with spike firing.
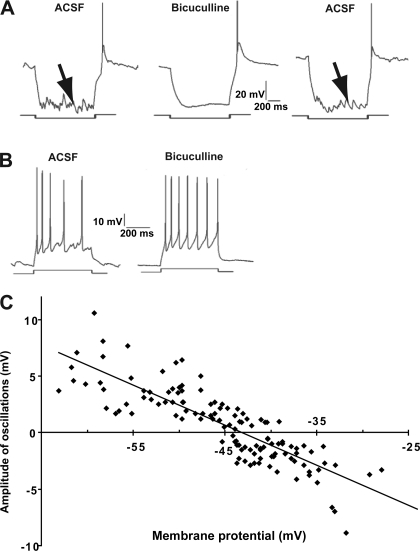
Given previous evidence that membrane fluctuations in SCN neurons are synaptic in origin and reflect, primarily, GABAA-mediated synaptic inputs (Itri et al. 2004; Jiang et al. 1997; Kim and Dudek 1992; Strecker et al. 1997), we examined GFP+ neurons for the presence of GABA-mediated postsynaptic potentials (PSPs). GFP+ neurons were subjected to a series of hyperpolarizing and depolarizing current steps, which displaced the membrane potential from about −100 mV to −30 mV. The membrane fluctuations became larger with increased membrane hyperpolarization. Furthermore, the membrane fluctuations (Fig. 4A, arrow on left) were blocked completely by exposure to the GABAA blocker, bicuculline (10 μM; Fig. 4A, middle), and this effect was reversible when the preparation was superfused with ACSF (Fig. 4A, arrow on right), identifying the membrane noise as GABAA-dependent synaptic input. Blockage of GABAA receptors with bicuculline also resulted in a restoration of pacemaker spiking activity, as evidenced by a depolarizing ramp preceding each spike that was free of PSPs (Fig. 4B, right) and with a more regular and higher frequency spiking pattern.
Fig. 4.
A: effects of bicuculline on GFP+LP− neurons tested with hyperpolarizing steps. Horizontal bars indicate the timing of a hyperpolarizing pulse to −90 mV. Different amounts of hyperpolarizing current were needed to reach −90 mV for individual cells; current injection did not significantly alter the cell input resistance (P = 0.7). In artificial cerebrospinal fluid (ACSF; left), the membrane shows synaptic fluctuations (arrow) that are blocked by 10 μM bicuculline (middle) and restored by a wash in ACSF (arrow, right). B: effects of bicuculline on GFP+LP− neurons tested with depolarizing steps. A depolarizing pulse (bottom bar) elicits irregular spike firing in ACSF (left), but an identical pulse elicits a more regular spike train in 10 μM bicuculline (right). C: reversal potential of GABA-dependent membrane oscillations. The amplitudes and polarity of bicuculline-sensitive membrane oscillations illustrated in A are plotted as a function of membrane potential for 10 GFP+ neurons. The solid line is the best-fit linear regression. It indicates a reversal potential near −43 mV.
In a group of 27 GFP+LP− neurons, we tested the effects of bicuculline on the spontaneous discharge rate. A fraction of the test population (n = 19) had no spontaneous firing, and for these neurons exposure to bicuculline did not elicit spike discharges. The remaining neurons (n = 8) had irregular firing rates ranging from 0.2 to 2 spikes/s. For each of these cells, bicuculline increased firing from 0.4 ± 0.1 to 1.1 ± 0.2 Hz [t(7) = 4.4, P = 0.003] without changing membrane potential [−45.6 ± 1.6 mV with ACSF to −45.6 ± 1.8 mV with bicuculline, t(7) = 0.0, n.s].
Reversal Potential of GABA-Dependent PSPs
Although PSP amplitude in GFP+ neurons increased with hyperpolarization (noted above), it was not possible to measure the reversal potential due to the interference of the spike mechanism. We therefore repeated these measurements in neurons in which the spike mechanism was blocked internally by adding QX-314 (5 mM) to the pipette solution. Test pulses to between −70 and −25 mV revealed a reversal potential for the PSPs of −43 mV (Fig. 4C). The measured reversal potential is positive relative to the Nernstian equilibrium potential for Cl−, which is estimated at −67.6 mV based on the Cl values for ACSF and pipette solution. We consider the possible reasons for this difference in discussion.
Fourier Analysis of GABAergic Input to GRP+ Neurons
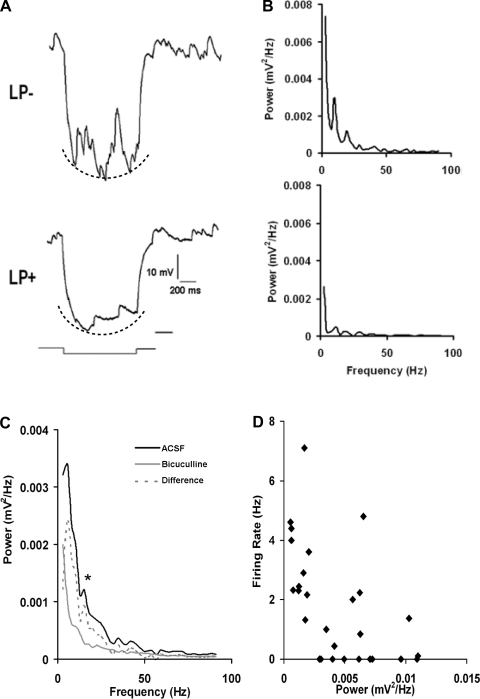
The occurrence of membrane noise (Figs. 3 and 4) indicated that GABAergic input varied markedly as a function of light exposure. To quantify the GABAergic input to GRP+ neurons, we used FFTs to measure the power and harmonic frequency of membrane fluctuations. The membrane noise evoked by a voltage step to −90 mV was the standard test permitting us to examine GABA-dependent synaptic input. We compared the responses of 28 GFP+LP− and 28 GFP+LP+ neurons in the period ZT 17:00–21:00 during which the membrane noise of LP+ neurons decreased markedly, whereas that of LP− neurons was robust [FFT for 0–20 mV: t(54) = 2.6, P = 0.01]. Figure 5A shows the responses of a LP− and a LP+ neuron. The corresponding FFTs for these 2 neuronal responses are shown in Fig. 5B. The dotted lines under the voltage responses of Fig. 5A, which reflect the curved envelope of the voltage responses, give rise to a steeply rising FFT component at frequencies <5 Hz; this component is not GABA-dependent. To isolate the GABA-sensitive FFT, we obtained spectra before and after application of bicuculline (Fig. 5C). The data shown in Fig. 5C are the averaged FFTs from 7 GFP+LP− neurons studied between ZT 17:00 and 21:00. The averaged difference spectrum illustrated shows a prominent low-frequency response near 5 Hz, a second peak at 14 Hz, and relatively minor components between 35 and 55 Hz. Based on these records, we used the integrated FFT power between 5 and 20 Hz as a relative index of GABAergic input.
Fig. 5.
Fast Fourier transforms (FFTs) of GABA-dependent membrane oscillations. A: representative responses of an GFP+LP− and an GFP+LP+ neuron to hyperpolarizing pulses to −90 mV, showing large and small membrane oscillations, respectively. Dotted lines indicate the curved shape of the voltage response. B: FFTs corresponding to the responses in A. The response component indicated by dotted line gives rise to a low-frequency response in the FFT. C: an averaged FFT was calculated for 7 GFP+LP− neurons in ACSF and after exposure to 10 μM bicuculline (FFT values at 5–20 mV: *P = 0.001). The difference FFT is the bicuculline-sensitive component. Note that it lacks the low-frequency response. D: each data point represents the integrated FFT power between 5 and 20 Hz for the bicuculline-sensitive component plotted against spike firing rate. Note that increased FFT power is associated with a lower firing rate.
The Fourier data permit us to relate the magnitude of GABAA-dependent synaptic activity to spike firing frequency. We previously noted that the fall in spike firing rate of GFP+LP+ neurons between ZT 15:30 and 17:00 was associated with an increase in membrane noise (Fig. 3B). We quantified the relationship between GABA-mediated noise and spike firing for the GFP+LP+ group between ZT 15:30 and 17:00 using the FFT power to represent the relative strength of GABA input (Fig. 5D). The results show that the strength of GABA input is negatively correlated with spike firing frequency. Thus, despite the reversal potential for the GABAergic input being at a relatively depolarized value (−43 mV), the effect of the GABA input on spike firing frequency of GFP+ neurons was invariably inhibitory, irrespective of light input and ZT.
Mechanism of Long-Term Changes in Excitability: Possible Sources of Increased Membrane Resistance and Hyperpolarization in LP+ Neurons
To gain further insight into what factors governed membrane excitability in LP+ and LP− neurons, we measured input resistance and looked at membrane responses to depolarizing and hyperpolarizing current pulses at different times in the night cycle between ZT 15:30 and ZT 00:30. In the period ZT 17:00–21:00, during which GFP+LP+ neurons showed a large hyperpolarization, the input resistance of this group rose markedly compared with ZT 15:00–17:00 [t(53) = 2.3, P = 0.03] and was higher compared with that of GFP+LP− cells at ZT 17:00–21:00 [t(56) = 2.2, P = 0.03]. As expected, we noted an associated increase in the membrane time constant of GFP+LP+ neurons between ZT 17:00 and 21:00 relative to ZT 15:00–17:00 [t(53) = 3.2, P = 0.002; LP+ vs. LP− at ZT 17:00–21:00: t(55) = 3.3, P = 0.001]. As summarized in Table 1, during the period ZT 17:00–21:00, GFP+LP+ neurons experienced a reduced conductance, presumably attributable to the loss or reduction of synaptic input and/or an intrinsic membrane current. On the other hand, there were only small and inconsistent differences in mean input resistance between GFP+LP+ and GFP+LP− groups either between ZT 15:30 and 17:00 or ZT 21:00 and 00:30.
Loss of GABA Input Does Not Result in Significant Membrane Hyperpolarization
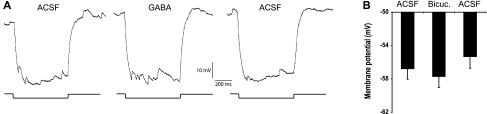
Synaptic noise in GFP+LP+ neurons was sharply reduced between ZT 17:00 and 21:00 (Fig. 5). However, an unresolved question was whether the marked reduction in GABA input reflected the loss of synaptic drive or rather a loss of sensitivity of the GFP+LP+ neurons to GABA. We tested this by exposing GFP+LP+ neurons to extrinsic GABA during ZT 17:00–21:00. Application of 5 mM GABA clamped the membrane potential at −43 mV, indicating that GFP+LP+ cells remain sensitive to GABA and confirming the PSP reversal potential measured previously (Fig. 4C). Application of a lower concentration of GABA (0.1 mM) reversibly increased membrane fluctuations measured during a hyperpolarizing pulse (Fig. 6A). We found that application of the GABAA antagonist, bicuculline, at a concentration sufficient to abolish GABAergic PSPs (10 μM), hyperpolarized GFP+LP− neurons by only 0.96 ± 1.4 mV (n = 9; Fig. 6B). This indicates that the 10–15 mV hyperpolarization observed during ZT 17:00–21:00 is not accounted for by the loss of GABAergic input to GRP neurons following a LP.
Fig. 6.
Effects of GABA on GRP+LP+ neurons between ZT 17:00 and 21:00. A: a GFP+LP+ neuron was subjected to a hyperpolarizing pulse to −90 mV in ACSF (left), 0.1 mM GABA (middle), and an ACSF wash (right). Membrane oscillations were weak in ACSF, more prominent in GABA, and again weak following an ACSF wash. The current injected to bring cells to −90 mV varied, reflecting GABA-induced decreases in membrane resistance [F(2–31) = 7.3, P = 0.003]. B: membrane potentials (mean ± 1 SE) are plotted for GFP+LP− cells in ACSF, treated with 10 μM bicuculline and in ACSF wash (n = 9; not significant, right).
Identification of a Steady Inward Membrane Current in GRP Neurons
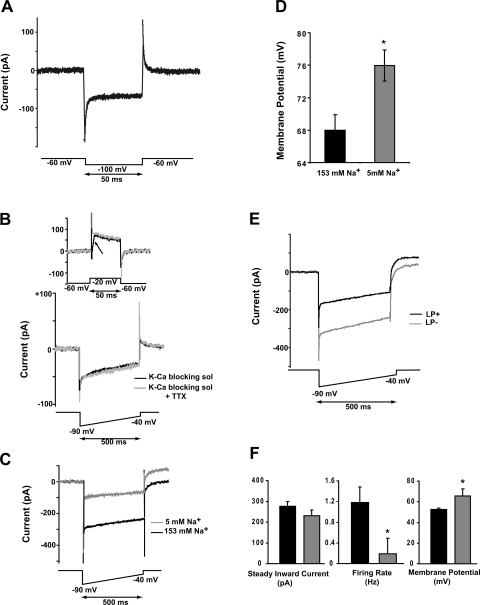
Given the above result, we sought another explanation for the large negative shift in membrane potential. Accordingly, we examined GFP+ neurons under voltage-clamp, using steps in 20-mV increments between −100 and +60 mV from a holding potential of −60 mV. The step to −100 mV revealed a noninactivating inward current that was prominent in GFP+ neurons (Fig. 7A). This current was investigated further by a combination of pharmacological and electrophysiological techniques. A persistent Na current (INaP) has been identified in numerous central nervous system (CNS) neurons, including SCN neurons of unidentified subtype (Kononenko and Dudek 2005; Kononenko et al. 2004). They showed that INaP is activated near −80 mV, reaches a peak near −60 mV, and is inhibited both by riluzole and TTX. We found, however, that the properties of the inward current in GFP+LP− neurons did not correspond to those of INaP. In initial experiments, we used a cocktail of blockers to reduce voltage-gated currents (Enomoto et al. 2006) and ramped the membrane potential from −90 to −40 mV over 2 s. This rate of change is too slow to activate the fast Na current that underlies the spike (Kononenko et al. 2004). The GFP+LP− current, however, showed no voltage-dependent threshold (not illustrated) and was not sensitive to TTX. TTX (1.0 μM) completely abolished the fast inward Na current (Fig. 7B, arrow) but had no effect on the inward current (Fig. 7B, bottom), demonstrating that it is a different current from that previously described (Konenko and Dudek 2005; Kononenko et al. 2004). Therefore, we refer to this current by the neutral term steady inward current (SIC).
Fig. 7.
Properties of a steady inward current in GFP+ neurons. A: in voltage-clamp, a hyperpolarizing step to −100 mV induces a noninactivating inward current. B: 1 μM TTX completely blocked the fast sodium current (arrow on top graph) but was without effect on the sustained inward current. Data for A and B were obtained in a blocking solution (K-Ca blocking sol; see materials and methods). C: the large inward current was reduced by ∼68% in a low-sodium medium (n = 9). D: the mean resting potential was hyperpolarized by ∼8 mV in the low-sodium medium (same cells as in C; *P = 0.0001). E: in normal ACSF, a 500-ms ramp from −90 to −40 mV elicited an inward current that was linear with voltage. This current was relatively large in LP− cells and smaller in LP+ neurons. Data illustrated in A–E were obtained between ZT 17:00 and 21:00. F: the steady inward current of GFP+LP+ and GFP+LP− cells was similar when tested at ZT 15:30–16:30, although firing rate and membrane potential were different (*P ≤ 0.003).
Given the foregoing results, all further experiments were conducted in normal ACSF. The SIC was largest at the most hyperpolarized potential tested (−90 mV) and decreased approximately linearly at more depolarized voltages. We attempted to measure its reversal potential by extending the ramp to more positive voltages. However, incompletely blocked and relatively large outward (presumed K) currents were activated at voltages positive to −20 mV, making a direct measurement of reversal potential infeasible. Linear extrapolation of the currents illustrated in Fig. 7C suggests a reversal potential between +30 and +40 mV. The Nernstian reversal potential for Na calculated from the internal and external media employed is +66 mV, suggesting that Na may be the primary but not exclusive current carrier. We examined this possibility further by ion substitution (n = 9). The ACSF medium containing 153 mM Na salts was substituted with a solution containing 140 mM choline chloride and 5 mM NaHCO3. In this sodium-poor medium, the SIC of GFP+LP− cells was reduced by 67.5% at −80 mV [t(8) = 6.8, P = 0.0001; Fig. 7C], and membrane potential hyperpolarized by 8 mV [t(8) = 6.9, P = 0.0001; Fig. 7D], confirming that Na is the primary current carrier.
The evidently important contribution of the SIC to the membrane potential of GFP+ neurons (Fig. 7D) suggested that its modulation might underlie the strong hyperpolarization of GFP+LP+ neurons we observed between ZT 17:00 and 21:00. As such, we compared the amplitudes of the SIC in GFP+LP+ and GFP+LP− subpopulations after ZT 17:00. The mean SICs evoked by a 500-ms ramp in ACSF are illustrated in Fig. 7E. Although the kinetics of the responses were identical, the mean SIC amplitude in GFP+LP+ neurons (n = 7) was ∼57% that of the GFP+LP− neurons (n = 4; Fig. 7E).
Zhou et al. (2008) described a leakage current carried by TRPC3 in GABAergic neurons and showed that it was blocked by flufenamic acid. For that reason, we tested the responses of 7 GFP+LP− neurons to 100 μM flufenamic acid but found that it did not reduce the SIC current even after 10-min exposure [SIC current at −80 mV: −190 ± 31 pA with ACSF and 243 ± 52 pA with flufenamic acid; t(12) = 2.2, P = 0.4].
Does the SIC Contribute to GFP+LP+ Neuronal Excitability Between ZT 15:30 and 16:30?
Whatever the identity of the channel underlying the SIC, a property of interest is its apparent modifiability. This possibility directed our attention to the interval between ZT 15:30 and 16:30 in which the cohort of GRP+LP+ neurons was more depolarized and had a faster spiking rate than its GRP+LP− counterpart. We asked whether these differences could be accounted for by a larger SIC in GRP+LP+ neurons, leading to increased depolarization and spike firing. Accordingly, we measured SIC amplitude, membrane potential, and spike firing rate in eight GFP+LP+ and nine GFP+LP− neurons between ZT 15:30 and 16:30. The results were that these neuronal subgroups had statistically indistinguishable values of SIC amplitude, but in contrast, GRP+LP+ neurons had more depolarized membrane potentials and higher spike firing rates than did GRP+LP− cells (Fig. 7F). Our data indicate that although the SIC contributes to setting the membrane potential 2.5–6.5 h following photic stimulation, other factors are instrumental in setting membrane potential and spike firing rate earlier on, between ZT 15:30 and 16:30.
DISCUSSION
Influence of Light on Circadian Rhythmicity
The present investigation demonstrates that photic stimulation has profound effects on the membrane properties of retinorecipient SCN neurons that express the peptide GRP but not on those of immediately adjacent cells that do not express this peptide. In the short-term (0.5–1.5 h after photic stimulation), light increased both spike firing and spike train regularity in GRP-expressing neurons (Fig. 2). These effects on spiking were accompanied by a decrease in GABAergic input. In the same neuronal class, long-term effects of light (2.5–6.5 h after photic stimulation) were a profound hyperpolarization and a loss of spiking (Fig. 2), effects associated with a steep reduction in a novel intrinsic current, the SIC, as well as with a loss of GABA-mediated input.
Within the SCN, only 20–33% of SCN cells are light-responsive (Meijer and Schwartz 2003). Most light-responsive cells of the SCN depolarize and show increased firing rate during a LP (Groos and Mason 1980; Meijer and Schwartz 2003). However, long-term effects of a LP on these cells have not been examined previously, even though it is well-known that the effects on circadian rhythmicity of a LP administered at night greatly outlast the stimulus (Meijer et al. 2010).
It is well-established that the SCN clock is synchronized to local solar time by retinal innervation (Morin and Allen 2006). LPs that reset the clock induce calcium-dependent signaling cascades, leading to time-gated changes in gene expression. For example, blocking Per1 induction by light in vivo and in vitro attenuates phase delays of behavioral and electrical rhythms (Akiyama et al. 1999). One model of the mechanisms mediating oscillator resetting posits that rhythmic- and light-regulated Per expression occurs in the same cell, and that in early night, photic resetting entails a temporary reversal of the spontaneously occurring decline in SCN expression of Per1 and Per2 (Shigeyoshi et al. 1997). We favor a second model, based on work showing that GRP neurons lack detectable rhythms of the clock genes and proteins PER1 and PER2 (Karatsoreos et al. 2004). Moreover, an anatomic study of SCN GRP neurons (Drouyer et al. 2010) indicates that they contact vasopressin-containing neurons, which have high amplitude rhythmic expression of clock genes (Dardente et al. 2002; Kuhlman et al. 2003).
This view of serial processing of photic information, from retinohypothalamic tract (RHT) to directly retinorecipient neurons to neurons that do not receive direct RHT input is supported by studies of SCN circuitry, demonstrating that cells of the SCN core send projections to cells of the shell, whereas projections in the reverse direction are very sparse (Leak et al. 1999). Furthermore, nighttime administration of GRP produces a phase delay in SCN shell neurons, associated with an induction of mPer1 and mPer2 RNA, whereas these responses are absent in mice deficient in the GRP receptor (Aida et al. 2002). A more complete analysis of the mechanism of light-induced phase shifts will require understanding the nature and time course of photic stimulation on membrane properties and firing rate of other phenotypically identified SCN cells, as has been done for GRP-expressing neurons in the present study.
Possible Sources of Error
Many investigators have noted that the whole cell patch-clamp method dialyzes the cytoplasm of the studied cell, resulting in altered transmembrane currents. This is unlikely to be a large source of error in the present study as dialysis occurs slowly, providing a window of 10–20 min during which the composition of the cytoplasm is relatively undisturbed, and this is the time frame within which we made measurements. That said, we recognize that the concentrations of cytoplasmic constituents will have been modified by our recording technique to an undetermined degree.
We can also exclude the possibility that our data are a result of a preparation artifact. Specifically, the GFP−LP+ cells acted as in-slice controls, and their responses differed significantly from those seen in GFP+LP+ cells in both the short- and long-term. In addition, these differences were not observed in GFP+LP− cells.
GABA actions on GRP neurons.
GABAA-mediated synaptic responses are ubiquitous throughout the CNS, including the SCN (De Jeu and Pennartz 2002; Jiang et al. 1997). The effects of GABA input on postsynaptic targets, however, are quite variable (Choi et al. 2008; De Jeu and Pennartz 2002; Gribkoff et al. 2003; Wagner et al. 1997). Although GABA can act either to depolarize or hyperpolarize the postsynaptic neuron, the direction of polarization does not predict whether GABA action is excitatory or inhibitory (reviewed in Farrant and Kaila 2007). Whatever the sign of GABA-induced polarization, opening of GABAA-gated channels always results in shunting inhibition, which is inhibitory to spike production. This inhibitory effect can be offset by a very depolarized reversal potential dependent on transporter activity (up to −20 mV), resulting in a net GABA-mediated excitation (Marty and Llano 2005). A second potential mechanism for bicuculline action is through inhibition of a small conductance Ca-activated K channel (SKCa). Bicuculline increased spontaneous firing in some SCN cells and reduced the spike afterhyperpolarization, which is dependent in part on SKCa (Teshima et al. 2003). On the other hand, Cloues and Sather (2003) found that neither bicuculline nor picrotoxin modified SKCa in cluster I SCN neurons. Whatever the resolution to this question, a putative effect of bicuculline on the afterhyperpolarization will act in parallel with its effects on membrane potential and the regularity of spike firing demonstrated in the present report.
In the present study, the reversal potential for GABA-dependent PSPs is −43 mV, quite positive to the calculated value for Cl equilibrium potential (ECl) of −67.6 mV. GABA-gated channels flux HCO3− in addition to Cl−; although the pipette solution lacked HCO3−, entry of CO2 from carbogen bubbling of ACSF undoubtedly generated some [HCO3−]i. Nevertheless, based on a [Cl−]i of 10 mM (cf. materials and methods), HCO3− flux through GABA-gated channels would not be expected to depolarize EGABA by >5 mV (Fig. 2 of Farrant and Kaila 2007), much less than the 24.6 mV spread between EGABA and estimated ECl. It therefore appears probable that GRP neurons express an active Cl− transporter, similar to what has been reported in other SCN neurons (Choi et al. 2008).
In GRP+ neurons, the actions of GABA were invariably inhibitory, as evidenced by a reduction in spike firing frequency and increased irregularity of the spike firing rhythm. The strength of the GABA signal was negatively correlated with spike firing frequency (Fig. 5D). Addition of the GABAA blocker, bicuculline, to the bathing medium resulted in increased frequency and regularity of spike firing (Fig. 4B). Thus our data are consistent with the finding that bicuculline was mostly excitatory to neuronal firing in ventral SCN but primarily inhibitory in dorsal SCN (Albus et al. 2005).
Time Course of Effects of Light on GRP Neurons
GABA-mediated PSPs were reduced in light-exposed neurons both in the 1.5 h following exposure to light (when a gradual recovery of GABAergic input was noted) and beginning ∼2.5 h after light exposure and lasting for several hours thereafter. It seems probable that the mechanisms of PSP suppression differ between these two periods.
Light alters the spike firing rate of SCN neurons, associated with alterations in input resistance and membrane potential (reviewed in Meijer and Schwartz 2003). In part, these changes are dependent on glutamatergic and GABAergic synaptic inputs. The short-term increase in firing rate following a LP is consistent with synaptic mechanisms that modify electrophysiological properties of GRP neurons. Changes that begin hours after the light stimulus has been extinguished, however, are inconsistent with ionotropic synaptic events and are more likely attributable to metabotropic pathways and gene activation, as has been shown to occur during long-term potentiation in hippocampal cells (Anwyl 2009).
In GRP neurons, we found that the SIC was very strongly suppressed beginning 2.5 h after the animal was exposed to light for 1 h. GRP cells from animals not exposed to light showed no suppression of the SIC. The long delay between light and current suppression strongly suggests that gene activation is an intermediary step leading to the synthesis of a protein capable of inhibiting the SIC. It is suggestive that, in hippocampus and ventral tegmental areas, the peptides substance P and neurotensin activate a channel complex consisting of NALCN and UNC-80 (Lu et al. 2009); both of these peptides are found in SCN (Abrahamson and Moore 2001; Moore and Speh 1993).
SIC in GRP Neurons
We identified a noninactivating inward current in GRP neurons with the following properties: 1) increased by hyperpolarizing steps but with no evident threshold voltage; 2) linear decrease in magnitude with depolarization between the test values of −110 and −40 mV; 3) resistance to TTX; and 4) Na is the primary current carrier. The SIC evidently contributes importantly to the resting conductance of GRP neurons. In light-exposed GRP neurons, suppression of SIC was associated with a 10- to 15-mV hyperpolarization and an increase of input resistance from 1.5 to 1.9 GΩ, although an undetermined fraction of this resistance increase is attributable to loss of GABA input.
Prior work identified a slowly inactivating Na current, INaP, in SCN neurons (Kononenko and Dudek 2005; Kononenko et al. 2004), but the characteristics of INaP differ markedly from those of the SIC, in that INaP is sensitive to TTX, has a threshold near −80 mV, and displays a nonlinear current-voltage function. The SIC we describe behaves as a TTX-resistant, nonspecific cation channel. To our knowledge, ours is the first description of a cation leakage current in any SCN neuron, but leakage currents with very similar properties have been described both within and outside of the CNS (Snutch and Monteil 2007). For example, certain TRP channels, e.g., TRPC3 and TRPM2, are nonspecific cation channels. However, both of these TRP channels are blocked by flufenamic acid (Hill et al. 2004; Zhou et al. 2008), whereas the SIC is insensitive to this agent at the same concentration. A nonselective cation channel, NALCN, which is a crucial component of respiratory rhythm circuitry, has been described in Lu et al. (2007). NALCN is voltage-insensitive and resistant to both TTX and Cs. Evaluation of the possibility that the SIC represents flux through a NALCN channel awaits the development of specific blockers.
Overview: Circuitry and Function of GRP Cells
How photic cues from the environment regulate the autoregulatory molecular feedback loop underlying rhythmicity within SCN neurons is a critical issue in circadian rhythm studies. Given that the monosynaptic projection from the RHT reaches a peptidergically diverse population of neurons within the nucleus (Morin and Allen 2006), a full understanding of photic resetting will require delineation of the response properties of these diverse cell types. The post-LP hyperpolarization of GRP cells described here contributes to understanding the sequence of changes associated with phase resetting. A LP at night induces c-Fos, Per1, and Per2 mRNA and protein in directly retinorecipient cells concentrated in the SCN core (Hastings et al. 1996; Shigeyoshi et al. 1997; Silver et al. 1996). Expression of these genes then spreads to the rest of the nucleus. Behavioral phase shifts occur only when light-induced expression of Per1 and Per2 mRNA and protein extends from the SCN core to the shell (Yan and Silver 2002, 2004). The time course of changes in gene activation and protein expression occurs over 2 h after a phase-delaying LP (Best et al. 1999; Yan and Silver 2004). SCN GRP cells project to the rest of the nucleus where they contact oscillators including AVP-containing cells (Dardente et al. 2004; Drouyer et al. 2010; Van der Zee et al. 2005). Based on this evidence, we hypothesize that the retinorecipient core cells inhibit the activity of oscillator cells in the shell. Accordingly, the light-induced delayed hyperpolarization of GRP cells would be associated with disinhibition of shell oscillator cells leading to resetting of circadian phase.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant NS-37919 (R. Silver) and the Richard H. Chartrand Foundation (P. Witkovsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. S. Siegelbaum (Columbia University) and F. E. Dudek (University of Utah) for helpful suggestions and advice and Margaret Robotham and Tina Tong (Barnard College) for help with immunocytochemistry and analyses.
REFERENCES
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916: 172–191, 2001 [DOI] [PubMed] [Google Scholar]
- Aggelopoulos NC, Meissl H. Responses of neurones of the rat suprachiasmatic nucleus to retinal illumination under photopic and scotopic conditions. J Physiol 523: 211–222, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol 61: 26–34, 2002 [DOI] [PubMed] [Google Scholar]
- Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19: 1115–1121, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15: 886–893, 2005 [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology 56: 735–740, 2009 [DOI] [PubMed] [Google Scholar]
- Best JD, Maywood ES, Smith KL, Hastings MH. Rapid resetting of the mammalian circadian clock. J Neurosci 19: 828–835, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci 20: 7446–7454, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim do Y, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci 28: 5450–5459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci 23: 1593–1604, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LN, Dyball RE. Synaptic input from the retina to the suprachiasmatic nucleus changes with the light-dark cycle in the Syrian hamster. J Physiol 497: 483–493, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardente H, Klosen P, Caldelas I, Pevet P, Masson-Pevet M. Phenotype of Per1- and Per2-expressing neurons in the suprachiasmatic nucleus of a diurnal rodent (Arvicanthis ansorgei): comparison with a nocturnal species, the rat. Cell Tissue Res 310: 85–92, 2002 [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res Mol Brain Res 124: 143–151, 2004 [DOI] [PubMed] [Google Scholar]
- De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J Neurophysiol 87: 834–844, 2002 [DOI] [PubMed] [Google Scholar]
- Drouyer E, LeSauter J, Hernandez AL, Silver R. Specializations of gastrin-releasing peptide cells of the mouse suprachiasmatic nucleus. J Comp Neurol 518: 1249–1263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Wu N, Chandler SH. Participation of sodium currents in burst generation and control of membrane excitability in mesencephalic trigeminal neurons. J Neurosci 26: 3412–3422, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res 160: 59–87, 2007 [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res 379: 176–181, 1986 [DOI] [PubMed] [Google Scholar]
- Gribkoff VK, Pieschl RL, Dudek FE. GABA receptor-mediated inhibition of neuronal activity in rat SCN in vitro: pharmacology and influence of circadian phase. J Neurophysiol 90: 1438–1448, 2003 [DOI] [PubMed] [Google Scholar]
- Groos GA, Mason R. The visual properties of rat and cat suprachiasmatic neurones. J Comp Physiol A 135: 349–356, 1980 [Google Scholar]
- Hastings MH, Best JD, Ebling FJ, Maywood ES, McNulty S, Schurov I, Selvage D, Sloper P, Smith KL. Entrainment of the circadian clock. Prog Brain Res 111: 147–174, 1996 [DOI] [PubMed] [Google Scholar]
- Hill K, Benham CD, McNulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 47: 450–460, 2004 [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol 92: 311–319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci 24: 7985–7998, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Yang Y, Liu ZP, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J Physiol 499: 141–159, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci 24: 68–75, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: inhibitory synaptic mechanisms. J Physiol 458: 247–260, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Membrane properties of rat suprachiasmatic nucleus neurons receiving optic nerve input. J Physiol 464: 229–243, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. (editors). Suprachiasmatic Nucleus. The Mind's Clock. New York: Oxford Univ. Press, 1991, p. 467 [Google Scholar]
- Klingenberg L. Frequency Domain Using Excel (Online) School of Engineering, San Francisco State Univ., San Francisco, CA: http://userwww.sfsu.edu/∼larryk/Common%20Files/Excel.FFT.pdf [15 Apr. 2010] [Google Scholar]
- Kononenko NI, Dudek FE. Noise of the slowly inactivating Na current in suprachiasmatic nucleus neurons. Neuroreport 16: 981–985, 2005 [DOI] [PubMed] [Google Scholar]
- Kononenko NI, Shao LR, Dudek FE. Riluzole-sensitive slowly inactivating sodium current in rat suprachiasmatic nucleus neurons. J Neurophysiol 91: 710–718, 2004 [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci 23: 1441–1450, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Card JP, Moore RY. Suprachiasmatic pacemaker organization analyzed by viral transynaptic transport. Brain Res 819: 23–32, 1999 [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Liu J, Xia J, Ren D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129: 371–383, 2007 [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 457: 741–744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A, Llano I. Excitatory effects of GABA in established brain networks. Trends Neurosci 28: 284–289, 2005 [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and the hamster. Brain Res 382: 109–118, 1986 [DOI] [PubMed] [Google Scholar]
- Meijer JH, Michel S, Vanderleest HT, Rohling JH. Daily and seasonal adaptation of the circadian clock requires plasticity of the SCN neuronal network. Eur J Neurosci 32: 2143–2151, 2010 [DOI] [PubMed] [Google Scholar]
- Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms 18: 235–249, 2003 [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. J Neurosci 18: 9078–9087, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett 150: 112–116, 1993 [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev 51: 1–60, 2006 [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992 [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91: 1043–1053, 1997 [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport 7: 1224–1228, 1996 [DOI] [PubMed] [Google Scholar]
- Silver R, Schwartz WJ. The suprachiasmatic nucleus is a functionally heterogeneous timekeeping organ. Methods Enzymol 393: 451–465, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch TP, Monteil A. The sodium “leak” has finally been plugged. Neuron 54: 505–507, 2007 [DOI] [PubMed] [Google Scholar]
- Strecker GJ, Wuarin JP, Dudek FE. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J Neurophysiol 78: 2217–2220, 1997 [DOI] [PubMed] [Google Scholar]
- Teshima K, Kim SH, Allen CN. Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience 120: 65–73, 2003 [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Roman V, Ten Brinke O, Meerlo P. TGFalpha and AVP in the mouse suprachiasmatic nucleus: anatomical relationship and daily profiles. Brain Res 1054: 159–166, 2005 [DOI] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature 387: 598–603, 1997 [DOI] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci 16: 1531–1540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci 19: 1105–1109, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Matta SG, Zhou FM. Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci 28: 473–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]