Abstract
Background: This prospective investigation assessed success rates of a pain management program for patients with and without DSM-III-R Axis I and II psychiatric disorders.
Method: Subjects included 40 consecutive patients with chronic pain who were referred to a physical therapy–oriented, “standard” pain management program. Serial ratings of pain levels were measured via a visual analogue scale (VAS) at baseline, weekly throughout a 12-week program, and during a follow-up interval 1 month after completion of the program. Weekly reports of hours of gainful employment were recorded. VAS scores and number of hours worked per week were combined into a measure of pain improvement. This dependent variable was used to compare groups of patients across psychiatric disorders diagnosed via the Diagnostic Interview Schedule (DIS). Percentages of patients in each diagnostic group who met minimal criteria for improvement were computed and compared. A chi-square analysis was conducted on success rates between patients with and without any Axis I disorder, any Axis II disorder, and any substance abuse/dependence disorder.
Results: Overall, 70% of patients (N = 28) were found to have a DIS psychiatric disorder. There were differences in improvement between patients with and without Axis I disorders and between those with and without Axis II disorders. The presence of a diagnosis was associated with significantly lower improvement rates (p < .05).
Conclusion: Patients with chronic pain enrolled in this clinic had a high prevalence of comorbid psychiatric disorders, and these comorbid patients were less likely to improve with standard chronic pain treatment. In a population of patients seeking treatment for chronic pain, these results suggest a need for detection and diagnosis of psychiatric disorders and further research on the efficacy of psychiatric treatment interventions in chronic pain management.
Increasing evidence from an array of sources suggests that psychiatric factors play an important role in chronic pain. Reich et al.1 reported that 98% of chronic pain patients reviewed by a University Pain Board had an Axis I disorder, and 37% had an Axis II disorder. A more naturalistic study of psychiatric and chronic pain comorbidity was conducted by Atkinson et al.2 In this study, the authors evaluated 100 consecutive admissions to a Veterans Affairs Medical Center Low Back Pain Clinic. They compared lifetime and current rates of depressive, anxiety, and alcohol abuse disorders between the chronic back pain patients and controls. Lifetime and 6-month rates of major depression were 32% and 22%, respectively (vs. 16% and 6% for controls). More importantly, it was found that after onset of pain, patients had a relative risk of 9.0 for developing major depression. The lifetime alcohol abuse/dependence rate for chronic pain patients was 65%, compared with 39% for controls. The rates for generalized anxiety, panic, and obsessive-compulsive disorders were also comparatively high for pain patients, but failed to reach significance in a chi-square analysis.
Several other studies have demonstrated that psychiatric illness is strongly associated with chronic pain. Most of these investigations have found a relationship between chronic pain and depressive spectrum symptoms.3–5 Furthermore, the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),6 demonstrates an increased awareness of pain and its comorbidity patterns. The DSM-IV includes the categories of pain disorder with and without psychological factors. It also allows for the diagnosis of comorbid pain and psychiatric conditions via the categories of anxiety or depressive disorder due to a “general medical” condition (which can include various pain-producing conditions).
Despite the frequent comorbidity of chronic pain disorders and psychiatric illness, we are unaware of any prior studies that have investigated the impact of the latter on recovery from pain among those participating in pain management programs. The primary purpose of this study was to evaluate the outcomes of a pain management program in the presence or absence of various psychiatric disorders. The frequency of Axis I and II disorders in this chronic pain patient population was also investigated.
METHOD
Subjects
Forty consecutive patients with chronic pain were enrolled in a multidisciplinary, non–Veterans Affairs, private practice pain management center for evaluation and treatment. These patients were referred via physician or insurance company. In all cases, the reason for referral was chronic pain. Patient ages ranged between 30 and 65 years, with a mean age of 46 years. Pain syndromes included neck, back, and limb pain. Documented etiologies included discogenic pain, facet arthrosis, reflex sympathetic dystrophy, arthritic conditions, myofascial pain, and fibromyalgia. The average duration of the patients' pain syndromes was 34 months for localized/regional pain and 49 months for fibromyalgia. Forty-five percent of subjects were male and 55% were female. The average education level was 11 years.
Pain Management Program
All patients entered into a 12-week program that included the following treatment modalities as appropriate to the condition: trigger point injections, anti-inflammatory medications, low-dose tricyclic antidepressants (defined as less than 100 mg of imipramine per day), physical therapy focusing on stretching and range-of-motion exercises (2–5 days per week as tolerated), aerobic physical exercise (3–4 days per week), and electromyographic biofeedback with relaxation training (1–2 times per week). The center program personnel included a consulting psychiatrist, 3 clinical psychologists, 4 physical therapists, and occupational therapists. This type of program is similar to that of other multidisciplinary pain management programs in the United States.7,8
Psychiatric Diagnoses
Prior to starting formal treatment for their chronic pain, all patients received a structured interview using the DSM-III-R Diagnostic Interview Schedule for current Axis I and Axis II disorders.9 Of note, DSM-III-R and DSM-IV diagnoses of substance abuse and dependence are not based on specific type or quantity of substance used. A psychiatrist or a Ph.D.-level clinical psychologist conducted this structured interview. A different professional, either a psychiatrist or psychologist, verified each diagnosis via a second, semistructured interview. At the time of the initial evaluation, the interviewer was blind to the patients' specific diagnosis and specific pain complaints. Also at the time of the initial evaluation, each patient was administered the Computer-Assisted version of the Personality Diagnostic Questionnaire-Revised (PDQR-C).10–12 This is a well-validated screening device for the detection of the major personality disorders in Axis II of DSM-III-R. Those patients who received PDQR-C scores on personality disorder factors suggesting the presence of such disorders were referred to one of the staff psychologists or the psychiatrist for a full, structured Axis II diagnostic interview via the Axis II version of the Diagnostic Interview Schedule. Patients either received no Axis II diagnosis, received a formal Axis II diagnosis, or, if they exhibited Axis II criteria on the PDQR-C but were found to be without clinically significant impairment, were placed into the group of patients with Axis II “traits.” Axis I and Axis II data were known only to staff psychologists or the psychiatrist and were not recorded in the chart that was made available to physical and occupational therapy staff. All patients read and signed a detailed consent form describing the clinical regimen of the clinics and the possible use of data from clinic assessments in clinical research studies that might be published.
Outcome Measures and Improvement Ratings
Two outcome measures were used for the purposes of this study: (1) the number of hours of work per week in the month prior to admission to the pain management program and the number of hours of work per week in the follow-up month after the program and (2) weekly ratings of average daily pain. Each week, including the week the program was started (thus serving as a baseline), every patient completed a visual analogue scale (VAS) consisting of a 100-mm line anchored with the labels “no pain” and “worst pain I've ever experienced” on the left and right extremes, respectively. This method of evaluating pain has been validated previously.13,14 The VAS was also chosen because it is easily administered, is well accepted by patients, and is frequently used in other clinically based chronic pain treatment programs.
For the purposes of this study, patients were rated as “improved” if they (1) exhibited an increase in work hours per week from either 0 (at baseline) to 20+ hours per week after the program or from 15 to 20 hours at baseline to at least 35 hours per week after the program and (2) exhibited at least a 50% decrease in average weekly VAS pain ratings from baseline to the 1-month follow-up after participation in the program.
Data Analysis
For this study, patients were grouped into diagnostic categories and then cross-compared in terms of whether or not they were improved clinically as defined above. Data were examined in terms of the percentage of patients with a specific Axis I or II disorder and the percentage of patients who were improved in these categories. Extensive statistical analyses of improvement rates for all diagnostic categories were precluded by the large number of categories and relatively small cell sizes. To compare number of patients improved for Axis I and II categories, the McNemar chi-square test for 3 different 2 × 2 contingency tables was performed. The tables included (1) number improved and not improved for all patients and those with any Axis I disorder, (2) number improved and not improved for all patients and those with any Axis II disorder, and (3) number improved and not improved for all patients and those with any substance-related disorder.
RESULTS
The McNemar chi-square value for the differential improvement rates for all patients versus those with any Axis I disorder was 4.65 (p < .05). For the differential rates for all patients versus those with any Axis II disorder, the chi-square value was 9.48 (p < .01). The McNemar chi-square value for the rates of improvement for all patients versus those with any substance-related disorder was 12.96 (p < .001).
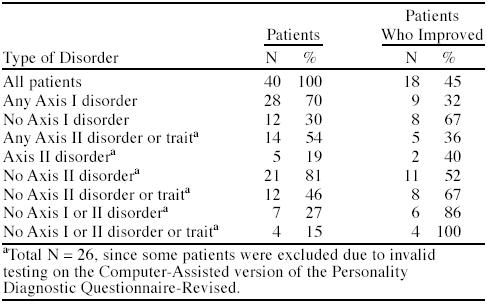
The proportions of chronic pain patients with Axis I and Axis II disorders and their improvement rates are shown in Table 1. As can be seen, 70% of patients had some Axis I disorder, 19% had a formal Axis II disorder, and 54% had either an Axis II disorder or Axis II traits. Overall, 45% of patients were improved with participation in a standard pain management program. For those without an Axis I or Axis II disorder, 86% improved. However, for those with any Axis I disorder, only 32% improved, and for those with an Axis II disorder, only 40% improved.
Table 1.
Percentages of Chronic Pain Patients With Psychiatric Disorders and Percentages Improved With Treatment
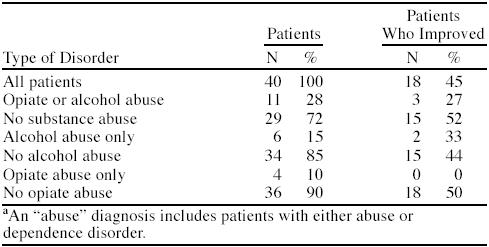
The percentages of patients with substance-related diagnoses are shown in Table 2. Associated rates of improvement are also shown in Table 2. For patients without a substance abuse diagnosis, 52% were improved at follow-up. For those patients with a diagnosis of opiate or alcohol abuse, only 27% were improved at follow-up. About 15% of patients had alcohol abuse only, of whom 33% improved. Of the 10% that abused opiates only, none improved (although the sample size was only 4 for this diagnostic subgroup).
Table 2.
Percentages of Patients With Substance Abuse and Percentages Improved With Treatment Among Chronic Pain Patientsa
The percentages of patients with specific Axis I disorders and their respective improvement rates are shown in Table 3. Those without an Axis I disorder had an improvement rate of 67%. Patients with generalized anxiety disorder had the highest rate of improvement (60%) among those with Axis I disorders. Panic disorder, major depression, dysthymia, and posttraumatic stress disorder patients exhibited improvement rates of only 25%, 31%, 0%, and 0%, respectively. However, the number of patients in each of these specific diagnostic categories was small.
Table 3.
Percentages of Patients With Axis I Disordersa and Percentages Improved With Treatment Among Chronic Pain Patients
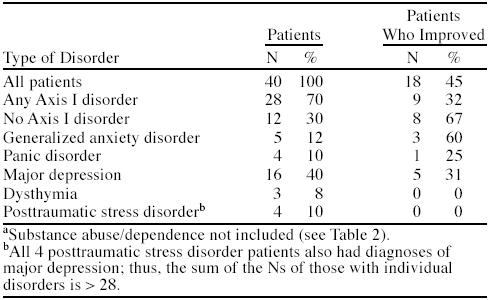
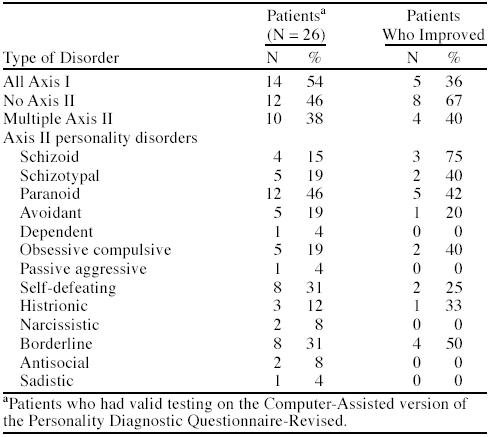
Table 4 shows the proportion of patients with specific personality disorders and their improvement rates. Improvement occurred in 40% of patients with at least 1 Axis II diagnosis compared with 67% of patients without an Axis II diagnosis. The percentage of improved patients with specific Axis II diagnoses was low. However, improvement rates for paranoid, self-defeating, and borderline personality disorders were higher at 42%, 25%, and 50%, respectively.
Table 4.
Percentages of Patients With Axis II Disorders and Percentages Improved With Treatment Among Chronic Pain Patients
DISCUSSION
This study found high rates of comorbid psychiatric disorders in patients with chronic pain and that these comorbid patients were less likely to improve with standard chronic pain treatment. The results of this study support prior evidence that psychiatric comorbidity is high in patients with chronic pain. The reported prevalence rates of 70% for comorbid Axis I diagnoses and 54% for comorbid Axis II disorders or traits resemble those previously published by Reich et al.,1 who reported frequencies of 98% for Axis I and 37% for Axis II disorders. Similar to other studies, major depression was the primary Axis I diagnosis. The 40% occurrence rate in this study was even higher than the 22% 6-month rate found by Atkinson et al.2
The 15% alcohol abuse frequency in this study was high compared with the current (as opposed to lifetime) frequency of about 2% to 8% in the general population.15 The opiate abuse frequency of 10% was also higher than the general population,15 as might be expected in patients who are frequently exposed to opiate treatment for their chronic pain. Importantly, substance-related disorders reduced the success rate of chronic pain treatment from 45% to 27%.
Taken together, these data indicate that comorbid psychiatric disorders are common in chronic pain patients and that the presence of these disorders significantly reduces the efficacy of a standard chronic pain treatment program. These findings suggest that psychiatric comorbid disorders need to be screened for and diagnosed. Results of this study do not indicate whether treatment of these disorders will impact treatment outcomes. However, until results from outcome studies are clear, these patients should be offered aggressive psychiatric treatment. The impact of early recognition and treatment of comorbid psychiatric disorders in the chronic pain management setting is, therefore, an important area that needs to be investigated further, since the importance of psychiatric care may be overlooked in many chronic pain programs.
Drug name: imipramine (Tofranil and others).
Footnotes
This report presents the findings and conclusions of the authors. It does not necessarily represent the views of The Department of Veterans Affairs.
All authors were employed by The Department of Veterans Affairs, Salem Veterans Affairs Medical Center, Salem, Va., when this work was completed. Time used to analyze data was supported by The Department of Veterans Affairs, Salem Veterans Affairs Medical Center, Salem, Va.
Dr. Hubbard has received book royalties and has given past expert testimony. Drs. Workman and Felker report no financial affiliation or other relationship relevant to the subject matter in this article.
REFERENCES
- Reich J, Tupin J, Abramowitz S. Psychiatric diagnosis of chronic pain patients. Am J Psychiatry. 1983;140:1495–1498. doi: 10.1176/ajp.140.11.1495. [DOI] [PubMed] [Google Scholar]
- Atkinson J, Slater M, Patterson T, et al. Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain. 1991;45:111–121. doi: 10.1016/0304-3959(91)90175-W. [DOI] [PubMed] [Google Scholar]
- Van Houdenhove B, Verstraeten D, Onghena P. Chronic idiopathic pain, mianserin and “masked” depression. Psychother Psychosom. 1992;58:46–53. doi: 10.1159/000288609. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Samborsky W, Kellner M, et al. Tender points, depressive and functional symptoms: comparison between fibromyalgia and major depression. Clin Rheumatol. 1997;16:76–79. doi: 10.1007/BF02238767. [DOI] [PubMed] [Google Scholar]
- Wadell G, Main C, Morris E, et al. Chronic low back pain, psychologic distress, and illness behavior. Spine. 1984;9:209–213. doi: 10.1097/00007632-198403000-00013. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association. 1994. [Google Scholar]
- Cohen M. The pain center: centerpiece of comprehensive medicine? In: Cohen M, Campbell J, eds. Pain Treatment Centers at a Crossroads: A Practical and Conceptual Reappraisal. Prog Pain Res Manage. 1996 7:123–141. [Google Scholar]
- Deardorff W, Rubin H, Scott D. Comprehensive multidisciplinary treatment of chronic pain: a follow-up study of treated and non-treated groups. Pain. 1991;45:35–43. doi: 10.1016/0304-3959(91)90162-Q. [DOI] [PubMed] [Google Scholar]
- Robins L, Helzer JE, Cottler L, and et al. NIMH Diagnostic Interview Schedule. Version III-R (DIS-III-R). St. Louis, Mo: Washington University School of Medicine. 1989. [Google Scholar]
- Hyler S. Computer Assisted version of the Personality Diagnostic Questionnaire Revised (PDQR-C). New York, NY: NiJo Software. 1993. [Google Scholar]
- Hyler S, Reider R. Personality Diagnostic Questionnaire-Revised (PDQ-R). New York, NY: New York State Psychiatric Institute. 1984. [Google Scholar]
- Reich J. Update on instruments to measure DSM-III and DSM-III-R personality disorders. J Nerv Ment Dis. 1989;177:366–370. doi: 10.1097/00005053-198906000-00008. [DOI] [PubMed] [Google Scholar]
- Scott J, Huskisson E. Graphic representation of pain. Pain. 1976;2:175–184. [PubMed] [Google Scholar]
- Huskisson E, Jones J, Scott P. Application of visual analogue scales to the measurement of functional capacity. Rheumatol Rehabil. 1976;15:185–187. doi: 10.1093/rheumatology/15.3.185. [DOI] [PubMed] [Google Scholar]
- Covington E, Kotz M. Chronic pain and addictive disorder: psychological interventions. In: Miller N, ed. Principles of Addiction Medicine. Washington, DC: American Society of Addiction Medicine. 1994 1–11. [Google Scholar]


