Abstract
Ketogenic diets are very low in carbohydrates and can reduce epileptic seizures significantly. This dietary therapy is particularly effective in pediatric and drug-resistant epilepsy. Hypothesized anticonvulsant mechanisms of ketogenic diets focus on increased inhibition and/or decreased excitability/excitation. Either of these consequences might not only reduce seizures, but also could affect normal brain function and synaptic plasticity. Here, we characterized effects of a ketogenic diet on hippocampal long-term potentiation, a widely studied form of synaptic plasticity. Adult male rats were placed on a control or ketogenic diet for 3 wk before recording. To maintain the most physiological conditions possible, we assessed synaptic transmission and plasticity using chronic in vivo recordings in freely behaving animals. Rats underwent stereotaxic surgery to chronically implant a recording electrode in the hippocampal dentate gyrus and a stimulating electrode in the perforant path; they recovered for 1 wk. After habituation and stable baseline recording, 5-Hz theta-burst stimulation was delivered to induce long-term potentiation. All animals showed successful plasticity, demonstrating that potentiation was not blocked by the ketogenic diet. Compared with rats fed a control diet, rats fed a ketogenic diet demonstrated significantly diminished long-term potentiation. This decreased potentiation lasted for at least 48 h. Reduced potentiation in ketogenic diet-fed rats is consistent with a general increase in neuronal inhibition (or decrease in excitability) and decreased seizure susceptibility. A better understanding of the effects of ketogenic diets on synaptic plasticity and learning is important, as diet-based therapy is often prescribed to children with epilepsy.
Keywords: hippocampus, epilepsy, metabolic therapy, paired-pulse, perforant path, plasticity, theta-burst
a ketogenic diet (KD) is a high-fat, low-carbohydrate diet used most often to treat epilepsy in young children unresponsive to anticonvulsant medications. The low availability of carbohydrates (and thus limited glucose) creates a state of ketosis in which ketone bodies become the primary source of energy for the nervous system. Introduced in the 1920s, a KD reduces seizure occurrence significantly in a majority of epileptic children and eliminates seizures in a sizable minority (Kossoff and Rho 2009; Neal et al. 2009), often with fewer cognitive side effects than anticonvulsant drugs. These benefits are seen across sex and seizure etiology but can reverse quickly with the reintroduction of dietary carbohydrates. A KD is also effective in reducing seizures in adults (Klein et al. 2010; Sirven et al. 1999), and there is growing interest in and evidence for the application of a KD for clinical benefits in other conditions, such as pain (Ruskin et al. 2009) and brain injury (Appelberg et al. 2009; Hu et al. 2009).
Despite the clinical efficacy of the KD in reducing seizures, researchers and clinicians remain uncertain as to the precise mechanism by which it raises seizure threshold to control epilepsy. Proposed mechanisms include (but are not limited to) increases in inhibitory neuromodulators, activated potassium channels, enhanced inhibitory neurotransmission, or diminished excitatory neurotransmission (Hartman et al. 2007; Juge et al. 2010; Kawamura et al. 2010; Ma et al. 2007; Masino et al. 2011; Masino and Geiger 2008; Nylen et al. 2009). Because it is effective in drug-resistant epilepsy, the diet is likely to activate mechanisms other than those targeted by any one type of antiepileptic drug.
The anticonvulsant effects of a KD might also affect normal synaptic functions. Thus far, some electrophysiological evidence has been reported for such effects in hippocampus and neocortex (Bough et al. 2003; Cantello et al. 2007), although not all published work using KD or diet-related manipulations are consistent (Stafstrom 2004; Thio et al. 2010). Changes in synaptic function might, in turn, lead to diverse cognitive and behavioral side effects such as changes in learning and memory. Some studies have found unchanged cognitive function (Appelberg et al. 2009; Silva et al. 2005; Thio et al. 2010; Todorova et al. 2000); other rodent studies have found impaired spatial learning in water maze paradigms (Su et al. 2000; Zhao et al. 2004).
To date, no research has characterized the impact of a KD on synaptic transmission in vivo without the use of anesthetics, which necessarily alter central inhibition and/or excitability (Cain et al. 1992; MacIver et al. 1989). To achieve the most physiologically relevant conditions possible, the current study used chronic electrophysiological recordings in freely behaving rats to evaluate the effects of a KD on transmission in the perforant pathway-hippocampal dentate gyrus (DG) synapse, a well-established model of rodent synaptic plasticity in vivo (Blaise and Bronzino 2003; Blaise et al. 2008). After feeding a KD to adult rats, we assessed both short-term plasticity via recording of paired-pulse responses and long-term plasticity via induction of long-term potentiation (LTP) using theta-burst stimulation (TBS). Results indicate long-term synaptic plasticity was reliably induced in all animals. KD significantly reduced the magnitude of TBS-induced potentiation at time points up to 48 h after induction. However, we found no significant effects of KD on baseline synaptic parameters or paired-pulse ratios.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats were assigned diets starting at an age range of 51–73 days. Each cage of animals was randomly assigned to be fed ad libitum either a KD [F3666; Bio-Serv, Frenchtown, NJ; with a >6:1 ratio of fat-to-(carbohydrate + protein)] or a regular control diet (CD; Purina 5001, W.F. Fisher, Somerville, NJ). Animals were maintained on diets for 14 days before surgery. We previously showed that this KD fed to adult male rats ad libitum had no significant effect on body weight over a 3-wk period but did elevate plasma ketone bodies significantly (Ruskin et al. 2009), and we replicated this effect in a separate group of animals (data not shown). All surgical and experimental protocols were approved by the Trinity College Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic implantation surgery.
Details of our stereotaxic surgical procedures have been described in detail elsewhere (Blaise and Bronzino 2003). Briefly, anesthetized (50 mg/kg ip sodium pentobarbital) male rats were chronically implanted with a concentric bipolar stimulating electrode positioned in the angular bundle to activate the medial perforant path and with a monopolar single-strand flat-point tungsten recording electrode positioned in the ipsilateral DG. Ground and reference electrodes consisting of stainless steel machine screws were positioned contralaterally on the cortical surface of the parietal cortex. Dorsal-ventral positioning of the recording and stimulating electrodes was optimized by maximizing the amplitude of the evoked field potential on a digital oscilloscope (Nicolet Instruments, Madison, WI). The electrode wires were then led to a contact pin headstage assembly, which was fixed to the skull with fast-drying dental acrylic (Lang Dental Manufacturing, Wheeling, IL). Throughout surgery, body temperature was maintained at 37°C with heat lamps, and animals were allowed a 7-day recovery before testing. During recovery, animals were housed individually and maintained on their respective diets.
Electrophysiology.
On the day of recording, rats were placed in a sound-attenuating recording chamber and attached to the recording apparatus via low-noise cabling through a counterweighted commutator assembly, which allowed free movement of the animal about the chamber. Animals were allowed 1 h to habituate to the recording environment, and recordings were acquired only when animals were in a vigilance state of quiet waking (characterized behaviorally by animals posturally relaxed with eyes open and electrographically by desynchronized activity in the DG with occasional low-amplitude spindles and delta waves) to minimize evoked response variability (Blaise and Bronzino 2003; Hargreaves et al. 1990). Details of our stimulation parameters and data acquisition/analysis systems are available elsewhere (Blaise and Bronzino 2003; Blaise et al. 2008).
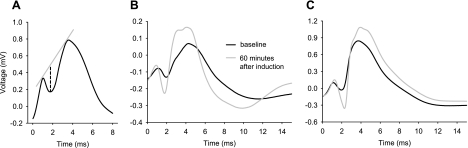
Figure 1A illustrates the methodology for measuring the amplitude of the population spike (PS). After verifying an appropriate evoked response from the chronic implant, an input/output curve was constructed for each animal by averaging 10 PSs induced by single-pulse current stimuli at current intensities from 200 to 1,500 μA. From this input/output curve, the stimulation intensity required to evoke 50% of the maximum recorded PS amplitude was determined for each individual animal; this current intensity was then used for both paired-pulse analysis and LTP induction. With these parameters, the excitatory postsynaptic potential is contaminated by the PS; thus we limited our analysis to PS amplitude. Paired-pulse ratios were also determined for a varying range of interstimulus intervals (ranging from 20 to 500 ms) to assess local circuit short-term plasticity. Following paired-pulse analysis, a pretetanus baseline of evoked responses to single-pulse stimuli was established. To induce LTP, a train consisting of a 100-pulse, 5 Hz TBS (10 bursts of 10 pulses; 400 Hz within-burst frequency) was delivered to the perforant path at the same 50% stimulus intensity. All animals were monitored closely for signs of seizure during and immediately after tetanus using behavioral and electrographic criteria; this never occurred. Five evoked DG PSs were recorded and averaged for each minute for the 1st 15 min. The average of 5 responses within 1 min was again recorded at 30, 60, 120, and 180 min, 24 h, and 48 h after tetanization. The mean percentage change in PS amplitude relative to baseline was determined at each time point for each animal in each treatment group. Data from 5 out of 19 animals were removed from analysis due to input/output curves that were nonmonotonic or did not reach a clear maximal asymptote or due to signal loss during recording. Significance was determined by analysis of variance with Newman-Keuls post hoc comparisons. Data are shown as means ± standard error.
Fig. 1.
Representative examples of field postsynaptic potentials and superimposed population spikes (PSs) in the dentate gyrus (DG), recorded during quiet waking. A: illustration of PS measurement. The gray line tangents the beginning and ending of the PS; the dashed line rising from the spike nadir to the tangent line is the measured amplitude. B: traces from baseline and 60 min after theta-burst stimulation (TBS) to induce long-term potentiation (LTP) in a rat fed the control diet (CD). Baseline stimulation was calibrated to produce 50% of maximal PS amplitude, and, using this same stimulation intensity, robust potentiation was produced by TBS. All traces are averages of 5 single traces. C: as in B, except from a rat fed the ketogenic diet (KD). TBS produces significant potentiation but of lesser magnitude on average (Fig. 3) than in CD-fed animals.
RESULTS
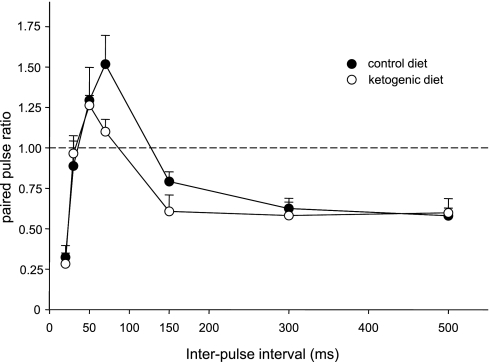
We recorded input/output curves, paired-pulse ratios, and synaptic plasticity in 14 animals (CD: n = 7; KD: n = 7). Treatment with a KD did not affect the input/output relationship significantly (data not shown; diet: F = 2.8, not significant; diet-stimulation current interaction: F = 0.3, not significant). Paired-pulse analysis demonstrated the characteristic depression-facilitation-depression pattern with increasing interpulse intervals (from 20 to 500 ms) at the perforant path-DG synapse (Bekenstein and Lothman 1991; Blaise and Bronzino 2000) regardless of diet (Fig. 2). Although there was a trend for the paired-pulse ratios to be reduced by KD treatment at the 70-ms (facilitation) and 150-ms (late depression) intervals, and a trend toward a reduced latency to the maximal paired-pulse ratio (50 vs. 70 ms), these effects were not significant (Fig. 2).
Fig. 2.
Paired-pulse analysis in the DG of CD- vs. KD-fed awake adult rats. The paired-pulse ratio was calculated across a 20- to 500-ms interpulse interval. All rats demonstrated the expected paired-pulse facilitation (50- to 70-ms interstimulus interval) and early- and late-phase depression as published previously. Feeding with a KD had no significant effect on this pattern or on the amplitude of the paired-pulse ratio at any interstimulus interval. Diet, F = 2.1, not significant (P = 0.15); current × diet, F = 1.0, not significant; n = 7 for each group.
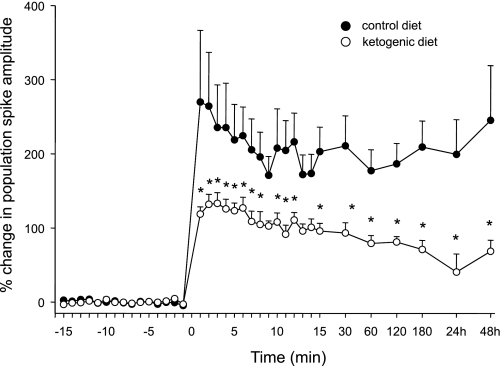
TBS tetanization successfully potentiated PS amplitude in all animals (representative PSs at baseline vs. 1 h post-TBS shown in CD- and KD-fed rats; Fig. 1, B and C, respectively). Synaptic potentiation was significantly less in KD-treated rats at nearly all time points (Fig. 3). At the later time points qualifying as LTP, KD treatment reduced PS potentiation consistently, including the longest time points recorded (24 and 48 h; Fig. 3). No animals from either treatment group showed behavioral or electrographic seizures during tetanization, and observed behavior throughout the recording process did not differ between CD- and KD-fed animals.
Fig. 3.
Synaptic potentiation induced by TBS of the perforant path-DG pathway of CD- vs. KD-fed awake adult rats. KD-fed rats show a significantly smaller degree of short-term potentiation (defined as <30 min after TBS, delivered at time 0) and a significantly smaller degree of LTP (defined as >30 min after TBS) than CD-fed rats. This increased potentiation persisted in CD- vs. KD-fed rats for at least 48 h. Diet, F = 7.1, P < 0.05; time × diet, F = 3.0, P < 0.001. *P < 0.05, compared with CD; n = 7 for each group.
DISCUSSION
Feeding with a KD decreased synaptic potentiation but had no significant effects on baseline synaptic transmission or paired-pulse ratios at the perforant path-DG synapse in freely behaving rats. TBS-induced potentiation, including stable and significant LTP, was induced successfully in all animals in all diet groups, underscoring that the KD reduced the magnitude of but did not block synaptic plasticity. Decreased potentiation in KD-fed rats is consistent with increased seizure threshold and thus reduced seizure susceptibility: these two measures are modified in parallel by a number of treatments and conditions (Casasola et al. 2004; Johnston 1996; Lopez de Armentia et al. 2007; Mazarati et al. 2000). Here, we used normal, nonepileptic animals; to our knowledge, the relationship between a KD and synaptic plasticity in in vivo animal models of epilepsy remains unknown.
Our findings contrast somewhat with those of a recent paper using the identical KD. Thio et al. (2010) reported no significant effect of 2- to 3-wk KD feeding on LTP in the DG in vivo recorded out to ∼90 min postinduction. This difference might arise from the use of halothane-anesthetized rats by Thio et al. (2010) and of awake, freely behaving rats in our study: a putative moderate inhibition and/or moderately reduced excitability of the KD impacting LTP might be overwhelmed by anesthetic actions. Interestingly, a parallel finding appears in the literature for anticonvulsant drugs, which inhibit LTP induction in awake animals but have no effect on LTP in anesthetized animals (Kubota et al. 1994; Stringer 2000; Xiong and Stringer 1997). This pattern suggests that anesthetized subjects may be of limited value in electrophysiological studies of the effects of KD or other ketogenic treatments on synaptic plasticity. Other methodological differences between Thio et al. (2010) and our study include subject age [Thio et al. (2010) used juvenile rats: postnatal day (PD) > 21, we used adult rats: PD > 73], acute vs. chronic recording, and type of electrical stimulation: the theta-burst-patterned stimulation used presently might be more physiologically relevant than the sustained high-frequency tetanus used by Thio et al. (2010) and thus more sensitive to moderate experimental treatments (Jedlicka et al. 2009; Morozov et al. 2003; Stäubli et al. 1999).
Nevertheless, both we and Thio et al. (2010) found no significant effect of KD feeding on paired-pulse depression or facilitation in the DG. A lack of effect was also reported in an in vitro study of another hippocampal region, CA1 (Stafstrom et al. 1999). In contrast, Bough et al. (2003) found that a KD enhanced early-phase paired-pulse depression in urethane-anesthetized rats. This effect might be attributable to caloric restriction, as only a restricted KD was tested and the same effect occurred in animals on a restricted standard pellet diet. A similar explanation might underlie shifted input/output relationships in Bough et al. (2003), whereas such an effect was not found in CA1 (Stafstrom et al. 1999) or DG (present study) with ad libitum KD treatment; more research is needed to resolve these issues.
Although a major effect of any KD is to increase circulating ketones, ketones might not be directly responsible for the present effect. Ketones applied in vitro do not appear to affect inhibitory synaptic transmission in the hippocampus (Thio et al. 2000) and actually prevent oxidative stress-induced impairment of hippocampal LTP (Maalouf and Rho 2008); there are conflicting data on ketones affecting hippocampal excitatory synaptic transmission (Juge et al. 2010; Thio et al. 2000). KD-induced reduction of LTP thus might be secondary to ketonemia (or mild hypoglycemia). Because all diets were provided ad libitum, and CD- and KD-fed groups did not differ in weight, these findings are unlikely to be due to caloric restriction. At this time, we cannot completely rule out that altered intake of vitamins and minerals may have influenced the results; the high caloric density of the KD leads animals to eat less (by weight) (Al-Khalifa et al. 2009).
Animal studies report a variety of cognitive outcomes with KD treatment in normal animals of varying ages and in multiple models of epilepsy, ranging from impairment (Su et al. 2000; Zhao et al. 2004) to no negative effect (Appelberg et al. 2009; Silva et al. 2005; Thio et al. 2010; Todorova et al. 2000). Clinical studies of pediatric epileptic patients on the KD have reported improvements in general cognitive/behavioral measures such as alertness, attention, and social functioning (Kinsman et al. 1992; Pulsifer et al. 2001; Svedova et al. 2010). Any negative cognitive effects of the KD in pediatric patients, however, might be overpowered by positive effects due to seizure control or reduction or elimination of antiepileptic drugs. Whereas explicit effects of a KD on cognition in nonepileptic children have not been examined, some studies have been performed in nonepileptic adults. One study found a transient, moderate impairment in 1 cognitive task (out of 3), present at 1 wk of diet treatment but not at later time points (Wing et al. 1995). Two studies examining chronic KD treatments reported improved processing speed and working memory lasting up to 1 yr (Brinkworth et al. 2009; Halyburton et al. 2007). Thus negative cognitive effects of KD feeding might be minimal and/or transient, at least in adults.
It is important to recognize that our diet formulation was stricter than that typically applied clinically; even so, all animals did display significant synaptic plasticity. Furthermore, there are no established guidelines and limited scientific discussions regarding “how much” plasticity is best, and mechanisms underlying synaptic plasticity are thought to overlap those associated with epileptogenesis (Gall and Lynch 2004; Golarai and Sutula 1996). It is clear that more in vivo electrophysiological characterization of KD effects needs to be performed in unanesthetized animals with clinically relevant diet formulations. This work should include juvenile animals, epilepsy models, and parallel cognitive/behavioral analyses. In particular, additional work in juveniles will help characterize the effects of a KD during development and maximize the clinical relevance to the mainly pediatric target of current KD treatments.
GRANTS
This study was supported by Grants NS-065446, NS-065957, and NS-066392 from the National Institute of Neurological Disorders and Stroke, Grant IOS-0843585 from the National Science Foundation, and Trinity College.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Urey Chow and Kaitlin E. Haines for technical assistance and Jenny Nord for animal care.
Present address of J. L. Koranda: Committee on Neurobiology, University of Chicago, Chicago, IL 60637.
REFERENCES
- Al-Khalifa A, Mathew TC, Al-Zaid NS, Mathew E, Dashti HM. Therapeutic role of low-carbohydrate ketogenic diet in diabetes. Nutrition 25: 1177–1185, 2009 [DOI] [PubMed] [Google Scholar]
- Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma 26: 497–506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. A comparison of the ontogeny of excitatory and inhibitory neurotransmission in the CA1 region and dentate gyrus of the rat hippocampal formation. Dev Brain Res 63: 237–243, 1991 [DOI] [PubMed] [Google Scholar]
- Blaise JH, Bronzino JD. Effects of stimulus frequency and age on bidirectional synaptic plasticity in the dentate gyrus of freely moving rats. Exp Neurol 182: 497–506, 2003 [DOI] [PubMed] [Google Scholar]
- Blaise JH, Bronzino JD. Modulation of paired-pulse responses in the dentate gyrus: effects of normal maturation and vigilance state. Ann Biomed Eng 28: 128–134, 2000 [DOI] [PubMed] [Google Scholar]
- Blaise JH, Koranda JL, Chow U, Haines KE, Dorward EC. Neonatal isolation stress alters bidirectional long-term synaptic plasticity in amygdalo-hippocampal synapses in freely behaving adult rats. Brain Res 1193: 25–33, 2008 [DOI] [PubMed] [Google Scholar]
- Bough KJ, Schwartzkroin PA, Rho JM. Caloric restriction and ketogenic diet diminish neuronal excitability in rat dentate gyrus in vivo. Epilepsia 44: 752–760, 2003 [DOI] [PubMed] [Google Scholar]
- Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Intern Med 169: 1873–1880, 2009 [DOI] [PubMed] [Google Scholar]
- Cain DP, Boon F, Hargreaves EL. Evidence for different neurochemical contributions to long-term potentiation and to kindling and kindling-induced potentiation: role of NMDA and urethane-sensitive mechanisms. Exp Neurol 116: 330–338, 1992 [DOI] [PubMed] [Google Scholar]
- Cantello R, Varrasi C, Tarletti R, Cecchin M, D'Andrea F, Veggiotti P, Bellomo G, Monaco F. Ketogenic diet: electrophysiological effects on the normal human cortex. Epilepsia 48: 1756–1763, 2007 [DOI] [PubMed] [Google Scholar]
- Casasola C, Montiel T, Calixto E, Brailowsky S. Hyperexcitability induced by GABA withdrawal facilitates hippocampal long-term potentiation. Neuroscience 126: 163–171, 2004 [DOI] [PubMed] [Google Scholar]
- Gall CM, Lynch G. Integrins, synaptic plasticity and epileptogenesis. Adv Exp Med Biol 548: 12–33, 2004 [DOI] [PubMed] [Google Scholar]
- Golarai G, Sutula TP. Functional alterations in the dentate gyrus after induction of long-term potentiation, kindling, and mossy fiber sprouting. J Neurophysiol 75: 343–353, 1996 [DOI] [PubMed] [Google Scholar]
- Halyburton AK, Brinkworth GD, Wilson CJ, Noakes M, Buckley JD, Keogh JB, Clifton PM. Low- and high-carbohydrate weight-loss diets have similar effects on mood but not cognitive performance. Am J Clin Nutr 86: 580–587, 2007 [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Cain DP, Vanderwolf CH. Learning and behavioral long-term potentiation: importance of controlling for motor activity. J Neurosci 10: 1472–1478, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol 36: 281–292, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZG, Wang HD, Qiao L, Yan W, Tan QF, Yin HX. The protective effect of the ketogenic diet on traumatic brain injury-induced cell death in juvenile rats. Brain Inj 23: 459–465, 2009 [DOI] [PubMed] [Google Scholar]
- Jedlicka P, Schwarzacher SW, Winkels R, Kienzler F, Frotscher M, Bramham CR, Schultz C, Orth CB, Deller T. Impairment of in vivo theta-burst long-term potentiation and network excitability in the dentate gyrus of synaptopodin-deficient mice lacking the spine apparatus and the cisternal organelle. Hippocampus 19: 130–140, 2009 [DOI] [PubMed] [Google Scholar]
- Johnston MV. Developmental aspects of epileptogenesis. Epilepsia 37, Suppl 1: S2–S9, 1996 [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron 68: 99–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors and KATP channels. J Neurosci 30: 3886–3895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman SL, Vining EP, Quaskey SA, Mellits D, Freeman JM. Efficacy of the ketogenic diet for intractable seizure disorders: review of 58 cases. Epilepsia 33: 1132–1136, 1992 [DOI] [PubMed] [Google Scholar]
- Klein P, Janousek J, Barber A, Weissberger R. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav 19: 575–579, 2010 [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics 6: 406–414, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Jibiki I, Fukushima T, Kurokawa K, Yamaguchi N. Carbamazepine-induced blockade of induction of long-term potentiation in the perforant path-dentate gyrus pathway in chronically prepared rabbits. Neurosci Lett 170: 171–174, 1994 [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Jancic D, Olivares R, Alarcon JM, Kandel ER, Barco A. cAMP response element-binding protein-mediated gene expression increases the intrinsic excitability of CA1 pyramidal neurons. J Neurosci 27: 13909–13918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci 27: 3618–3625, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM. Oxidative impairment of hippocampal long-term potentiation involves activation of protein phosphatase 2A and is prevented by ketone bodies. J Neurosci Res 86: 3322–3330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIver MB, Tauck DL, Kendig JJ. General anaesthetic modification of synaptic facilitation and long-term potentiation in hippocampus. Br J Anaesth 62: 301–310, 1989 [DOI] [PubMed] [Google Scholar]
- Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci 31: 273–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci 20: 6276–6281, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin DQ, Winder DG, Adams JP, Sweatt JD, Kandel ER. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron 39: 309–325, 2003 [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 50: 1109–1117, 2009 [DOI] [PubMed] [Google Scholar]
- Nylen K, Likhodii S, Burnham WC. The ketogenic diet: proposed mechanisms of action. Neurotherapeutics 6: 402–405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsifer MB, Gordon JM, Brandt J, Vining EP, Freeman JM. Effects of ketogenic diet on development and behavior: preliminary report of a prospective case study. Dev Med Child Neurol 43: 301–306, 2001 [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Kawamura M, Jr, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One 4: e8349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Rocha J, Pires CS, Ribeiro LC, Brolese G, Leite MC, Almeida LM, Tramontina F, Ziegler DR, Gonçalves CA. Transitory gliosis in the CA3 hippocampal region in rats fed on a ketogenic diet. Nutr Neurosci 8: 259–264, 2005 [DOI] [PubMed] [Google Scholar]
- Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O'Dwyer J, Sperling MR. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia 40: 1721–1726, 1999 [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Dietary approaches to epilepsy treatment: old and new options on the menu. Epilepsy Curr 4: 215–222, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Wang C, Jensen FE. Electrophysiological observations in hippocampal slices from rats treated with the ketogenic diet. Dev Neurosci 21: 393–399, 1999 [DOI] [PubMed] [Google Scholar]
- Stäubli U, Scafidi J, Chun D. GABAB receptor antagonism: facilitatory effects on memory parallel those on LTP induced by TBS but not HFS. J Neurosci 19: 4609–4615, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JL. A comparison of topiramate and acetazolamide on seizure duration and paired-pulse inhibition in the dentate gyrus of the rat. Epilepsy Res 40: 147–153, 2000 [DOI] [PubMed] [Google Scholar]
- Su SW, Sogawa MRCY, Silveira DC, Holmes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Dev Brain Res 125: 131–138, 2000 [DOI] [PubMed] [Google Scholar]
- Svedova J, Masino SA, DiMario FJ., Jr Retrospective analysis of the effects of the ketogenic diet on behavior and temperament of children with epilepsy. Soc Neurosci Abstr 561.6, San Diego, CA, 2010 [Google Scholar]
- Thio LL, Rensing N, Maloney S, Wozniak DF, Xiong C, Yamada KA. A ketogenic diet does not impair rat behavior or long-term potentiation. Epilepsia 51: 1619–1623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology 54: 325–331, 2000 [DOI] [PubMed] [Google Scholar]
- Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia 41: 933–940, 2000 [DOI] [PubMed] [Google Scholar]
- Wing RR, Vazquez JA, Ryan CM. Cognitive effects of ketogenic weight-reducing diets. Int J Obes Relat Metab Disord 19: 811–816, 1995 [PubMed] [Google Scholar]
- Xiong ZQ, Stringer JL. Effects of felbamate, gabapentin and lamotrigine on seizure parameters and excitability in the rat hippocampus. Epilepsy Res 27: 187–194, 1997 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res 55: 498–506, 2004 [DOI] [PubMed] [Google Scholar]