Abstract
Cognitive flexibility depends on the integrity of the prefrontal cortex (PFC). We showed previously that impaired decision making in pain results from amygdala-driven inhibition of medial PFC neurons, but the underlying mechanisms remain to be determined. Using whole cell patch clamp in rat brain slices and a cognitive behavioral task, we tested the hypothesis that group I metabotropic glutamate receptors (mGluRs) activate feed-forward inhibition to decrease excitability and output function of PFC pyramidal cells, thus impairing decision making. Polysynaptic inhibitory postsynaptic currents (IPSCs) and monosynaptic excitatory postsynaptic currents (EPSCs) were evoked in layer V pyramidal cells by stimulating presumed amygdala afferents. An mGluR1/5 agonist [(S)-3,5-dihydroxyphenylglycine, DHPG] increased synaptic inhibition more strongly than excitatory transmission. The facilitatory effects were blocked by an mGluR1 [(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid, LY367385], but not mGluR5, antagonist, 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine. IPSCs were blocked by bicuculline and decreased by 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX). Facilitation of synaptic inhibition by DHPG was glutamate driven because it was blocked by NBQX. DHPG increased frequency but not amplitude of spontaneous IPSCs; consistent with action potential-dependent synaptic inhibition, tetrodotoxin (TTX) prevented the facilitatory effects. DHPG decreased synaptically evoked spikes (E-S coupling) and depolarization-induced spiking [frequency-current (f-I) relationship]. This effect was indirect, resulting from glutamate-driven synaptic inhibition, because it persisted when a G protein blocker was included in the pipette but was blocked by GABAA receptor antagonists and NBQX. In contrast, DHPG increased E-S coupling and f-I relationships in mPFC interneurons through a presynaptic action, further supporting the concept of feed-forward inhibition. DHPG also impaired the ability of the animals to switch strategies in a decision-making task; bicuculline restored normal decision making, whereas a GABAA receptor agonist (muscimol) mimicked the decision-making deficit. The results show that mGluR1 activates feed-forward inhibition of PFC pyramidal cells to impair cognitive functions.
Keywords: metabotropic glutamate receptor, cognition, pain, synaptic plasticity
cognitive inflexibility refers to the inability to adapt cognitive processing and behavioral responses to changing conditions (see Stalnaker et al. 2009). Dysfunction of the prefrontal cortex (PFC) has been identified as a key neurobiological correlate of cognitive inflexibility that is associated with neuropsychiatric disorders such as drug addiction, obsessive-compulsive disorder, and schizophrenia (Bowie and Harvey 2006; Clarke et al. 2004; Goto et al. 2010; Gu et al. 2008; Stalnaker et al. 2009). Perseveration of high-risk decision making in a gambling task was also shown in pain patients (Apkarian et al. 2004a) and in animal pain models (Galhardo and Pais-Vieira 2005; Ji et al. 2010). Pain-related functional and structural abnormalities were observed in the PFC (Apkarian et al. 2009; Ji et al. 2010; Metz et al. 2009).
We showed recently that pain-related hyperactivity in the amygdala inhibits activity of medial PFC pyramidal cells and impairs decision making (Ji et al. 2010). The underlying pharmacological and synaptic cortical mechanisms remain to be determined. Here we tested the hypothesis that group I metabotropic glutamate receptors (mGluRs) activate feed-forward inhibition of medial PFC pyramidal cells, resulting in decreased output function and decision-making deficits. Disynaptic feed-forward inhibition is found in various brain areas and typically involves activation of inhibitory interneurons and their target cells by the same excitatory input (Doyle and Andresen 2001; Ferrante et al. 2009; Silberberg and Markram 2007). In support of our hypothesis, anatomical evidence showed that glutamatergic afferents from the amygdala target GABAergic interneurons that synapse on pyramidal cells in layer V of the medial PFC, indicating that amygdalocortical projections can engage feed-forward inhibitory mechanisms (Bacon et al. 1996; Gabbott et al. 2006).
Our hypothesis further holds that group I mGluRs activate feed-forward inhibition of PFC pyramidal cells. Group I mGluRs can modulate excitatory and inhibitory synaptic transmission, are involved in synaptic plasticity in various brain areas, and have become important therapeutic targets for neurological and psychiatric disorders that involve cortical dysfunction (Lesage and Steckler 2010; Niswender and Conn 2010; Olive 2010; Pinheiro and Mulle 2008). Both mGluR1 and mGluR5 subtypes are expressed in the PFC at pre- and postsynaptic sites (Cauli et al. 2000; Muly et al. 2003). However, surprisingly little is known about their synaptic function in the medial PFC. Activation of mGluR5 by systemic application of a positive allosteric modulator increased activity of presumed pyramidal cells in normal animals but reversed hyperexcitability of pyramidal cells, inhibition of interneurons, and behavioral inflexibility in a model of N-methyl-d-aspartate (NMDA) receptor hypofunction (Homayoun and Moghaddam 2010). A systemically administered mGluR5 antagonist [2-methyl-6-(phenylethynyl)-pyridine, MPEP] inhibited pyramidal cell activity (Homayoun and Moghaddam 2010).
At the synaptic level, an mGluR1/5 agonist [(S)-3,5-dihydroxyphenylglycine, DHPG] increased the frequency of spontaneous excitatory postsynaptic currents (EPSCs) but had no effect in the presence of tetrodotoxin (TTX) (Marek and Zhang 2008), indicating network activity. MPEP inhibited the facilitatory effect of DHPG. DHPG also had mixed “membrane” effects, which were interpreted as the result of indirect synaptic mechanisms. This is in agreement with older studies that showed, in unspecified brain areas of frontal cortical slices, a broad-spectrum mGluR agonist (1-aminocyclopentane-1,3-dicarboxylic acid, 1S,3R-ACPD) increased frequency of spontaneous but not miniature inhibitory postsynaptic currents (IPSCs) onto presumed GABAergic interneurons (Zhou and Hablitz 1997) and onto pyramidal cells (a selective mGluR1/5 agonist, DHPG, was also tested in this study; Chu and Hablitz 1998), suggesting action-potential-dependent synaptic inhibition. 1S,3R-ACPD also inhibited evoked excitatory and inhibitory synaptic potentials in layer II/III frontal cortical neurons, suggesting a rather general inhibition of synaptic transmission, but this effect was not blocked by a broad-spectrum mGluR antagonist (α-methyl-4-carboxyphenylglycine, MCPG) (Burke and Hablitz 1994). 1S,3R-ACPD increased neuronal excitability of layer II/III frontal cortical neurons through a G protein-dependent postsynaptic process that did not involve PKA or PKC and was not blocked by MCPG (Burke and Hablitz 1996). The contribution of individual mGluR subtypes was not determined in these studies. Consistent with these mixed effects of group I mGluRs in neocortical areas, 1S,3R-ACPD increased GABA release (Segovia and Mora 2005) and DHPG increased glutamate release in the medial PFC, which was blocked by an mGluR1 antagonist [(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid, LY367385] (Melendez et al. 2005).
These findings suggest that group I mGluRs can regulate inhibitory and excitatory synaptic transmission in frontal cortical areas possibly through different receptor subtypes and mechanisms. Whereas evidence from studies in other brain areas, such as Purkinje cells of the cerebellum, suggests that mGluR1 can activate feed-forward inhibition (Karakossian and Otis 2004), this has not been demonstrated in medial PFC neurons. The significance of this hypothesis is that mGluR-driven feed-forward inhibition could explain the pain-related deactivation of pyramidal cells observed in our previous study (Ji et al. 2010). On the other hand, exogenous activation of feed-forward inhibition might be a useful strategy to restore normal cortical activity in disorders that are associated with impaired or loss of inhibition such as schizophrenia (Beasley et al. 2002; Goto et al. 2010; Lewis and Gonzalez-Burgos 2008; Wang et al. 2008).
METHODS
Adult male Sprague-Dawley rats (150–220 g) were housed in a temperature-controlled room and maintained on a 12-h:12-h day/night cycle. Water and food were available without restriction. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch and conform to the guidelines of the International Association for the Study of Pain (IASP) and of the National Institutes of Health.
Electrophysiology in Brain Slices
Slice preparation.
Brain slices containing the medial PFC were obtained from normal naïve rats (120–200 g) as previously described (Fu and Neugebauer 2008; Ji et al. 2010; Orozco-Cabal et al. 2006). Coronal brain slices (300–500 μm) were cut at 3.0–3.2 rostral to bregma. At this level, slices contain both the prelimbic cortex (recording site) and infralimbic cortex [stimulation of basolateral amygdala (BLA) afferents passing through]. A single brain slice was transferred to the recording chamber and submerged in artificial cerebrospinal fluid (ACSF) (31 ± 1°C), which superfused the slice at ∼2 ml/min. ACSF contained (in mM): 117 NaCl, 4.7 KCl, 1.2 NaH2PO4, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, and 11 glucose. The ACSF was oxygenated and equilibrated to pH 7.4 with a mixture of 95% O2-5% CO2. Only one or two brain slices per animal were used, one neuron was recorded in each slice, and a fresh slice was used for each new experimental protocol. Numbers in the text refer to the number of neurons tested for each parameter.
Patch-clamp recording.
Whole cell patch-clamp recordings were obtained from visually identified layer V pyramidal cells and interneurons in the medial PFC (∼700 μm lateral to the interhemispheric fissure) using differential interference contrast and infrared videomicroscopy as described previously (Ji et al. 2010). Recording pipettes (3–5 MΩ tip resistance) made from borosilicate glass were filled with intracellular solution containing (in mM): 122 K-gluconate, 5 NaCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, and 0.4 Na3-GTP; pH was adjusted to 7.2–7.3 with KOH and osmolarity to 280 mOsm/kg with sucrose. Data acquisition and analysis of voltage and current signals were done using a dual four-pole Bessel filter (Warner Instruments), low-noise Digidata 1322 interface (Axon Instruments), Axoclamp-2B amplifier (Axon Instruments), Pentium PC, and pClamp9 software (Axon Instruments). Headstage voltage was monitored continuously on an oscilloscope to ensure precise performance of the amplifier. Neurons were voltage clamped at −70 mV or 0 mV for the study of excitatory and inhibitory transmission, respectively. The calculated equilibrium potential for chloride in this system was −68.99 mV (Nernst equation, pClamp9 software). High-seal (GΩ) and low-series (<20 MΩ) resistances were checked throughout the experiment (using pClamp9 membrane test function) to ensure high-quality recordings.
Synaptic transmission.
Using concentric bipolar stimulating electrodes (Kopf Instruments), EPSCs, IPSCs, excitatory postsynaptic potentials, and action potentials (E-S coupling) were evoked in pyramidal cells by focal stimulation (150 μs square-wave pulses) of presumed BLA afferents in the infralimbic cortex as described previously (Ji et al. 2010). The stimulating electrode was placed in layer IV (500 μm from the medial surface of the slice) of the infralimbic cortex, where BLA afferents were identified by the fluorescent signal originating from anterogradely labeled fibers following stereotaxic injections of a fluorescent tracer (1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate, DiI; Molecular Probes; dissolved in N,N-dimethylformamide dimethyl acetal, Sigma-Aldrich; 2 μl at 40 nl/min) into the BLA (3.3 caudal to bregma, 5.0 lateral to midline, depth 9.0) 10–12 days before brain tissue was taken. The distance between stimulation and recording sites was 600–800 μm.
For paired-pulse ratio (PPR) analysis, two orthodromic synaptic stimuli of equal intensity were applied at varying intervals. PPR was used to determine presynaptic modulations of IPSCs in the PFC. Two orthodromic synaptic stimuli of equal intensity were applied at varying intervals, and the resulting EPSCs were recorded. Peak amplitudes of the initial EPSC (EPSC1) and the second EPSC (EPSC2) were measured as the difference between the current level before the stimulus artifact and the peak of the EPSC. PPR was calculated as the ratio of EPSC2 over EPSC1. Any alterations in PPR, a measure of short-term synaptic plasticity, suggest a presynaptic site of action (Fu and Neugebauer 2008; McKernan and Shinnick-Gallagher 1997).
Spontaneous and miniature (in TTX 1 μM) EPSCs and IPSCs (s/mEPSCs and s/mIPSCs) were recorded at −70 mV and 0 mV, respectively, as described previously (Fu and Neugebauer 2008; Ji et al. 2010). A fixed length of traces (5 min) was analyzed for frequency and amplitude distributions using MiniAnalysis program 5.3 (Synaptosoft). The root mean square of the background noise was computed for each set of data. Detection threshold for an event was set to three to four times the root means square value. Peaks were detected automatically, but each detected event was then visually inspected to prevent the inclusion of false data.
Drug application.
Drugs were applied by gravity-driven superfusion of the brain slice in the ACSF (∼2 ml/min). Solution flow into the recording chamber (1 ml volume) was controlled with a three-way stopcock.
Behavioral Tests
Gambling task.
Decision making was measured in a behavioral task (Ji et al. 2010; Pais-Vieira et al. 2007; 2009) using a custom-designed computerized behavioral system (Coulbourn Instruments). The system included a behavioral arena (octagon) with two levers on each side for automated delivery of chocolate-coated food pellets (Research Diets). The levers were separated by a Plexiglas divider so that the animal needed to choose between the two sides/levers. A runway connected to the octagon by an automated guillotine door housed the animal briefly (10 s) between each trial. The rat was given 20 s to choose between two levers to recover a food reward in 90 consecutive trials. Following the nongambling training phase in which each of two levers provided one food pellet in nine of ten visits, animals were tested on decision making in a single session of 90 consecutive trials in which one lever was altered to return three pellets in three of ten trials (high-risk choice), whereas the other lever continued to deliver one pellet in nine of ten trials (low-risk choice). The side of the high-risk lever remained the same throughout the experiment but changed randomly between different animals to avoid any lateralization bias.
Main outcome measure was the preference index defined as the proportion of choices between the levers during the gambling task. Preference index was calculated for each group of 10 consecutive trials using the formula: (low-risk lever choices) − (high-risk lever choices)/number of completed trials. A negative preference index reflects high-risk decision making.
Drug application by microdialysis in awake behaving animals.
As described in detail previously (Ji et al. 2010; Neugebauer et al. 2007), a guide cannula was implanted stereotaxically the day before behavioral measurements, using a stereotaxic apparatus (David Kopf Instruments). The animal was anesthetized with pentobarbital sodium (Nembutal, 50 mg/kg ip), and a small unilateral craniotomy was performed at the sutura frontoparietalis level. The guide cannula was implanted on the dorsal margin of the prelimbic PFC, using the following coordinates (Paxinos and Watson 1998) in mm: 3.2 rostral to bregma, 0.7 lateral to midline, depth 3.0. The cannula was fixed to the skull with dental acrylic (Plastics One). Antibiotic ointment was applied to the exposed tissue to prevent infection. On the day of the experiment, a microdialysis probe (CMA/11, CMA/Microdialysis; membrane diameter: 250 μm; membrane length: 1 mm; 8 kDa cut-off) was inserted through the guide cannula so that the probe protruded by 1 mm. The probe was connected to a Harvard infusion pump and perfused with ACSF (oxygenated and equilibrated to pH = 7.4).
Before each drug application, ACSF was pumped through the fiber for at least 1 h to establish equilibrium in the tissue. Drugs were dissolved in ACSF on the day of the experiment at a concentration 100-fold that was predicted to be needed on the basis of our in vitro data (Fu and Neugebauer 2008; Ji et al. 2010; Neugebauer et al. 2003; Pernia-Andrade et al. 2009) because of the concentration gradient across the dialysis membrane. The appropriateness of the factor 100 for microdialysis concentrations of mGluR-related drugs was validated in our previous in vivo studies that used microdialysis to administer these agents into the brain (Ji and Neugebauer 2010; Li and Neugebauer 2004). Drugs were administered into the prelimbic PFC by microdialysis at a rate of 5 μl/min. Numbers in the text refer to drug concentrations in the microdialysis fiber.
Drugs
The following selective compounds were used: DHPG (mGluR1/5 agonist); LY367385 (mGluR1 antagonist); 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP, mGluR5 antagonist); DL-2-amino-5-phosphonopentanoic acid (AP5; NMDA receptor antagonist); 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX; non-NMDA receptor antagonist); [R-(R*,S*)]-6-(5,6,7,8-tetrahydro-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)furo[3,4-e]-1,3-benzodioxol-8(6H)-one (bicuculline) and picrotoxin (GABAA receptor antagonists); 5-aminomethyl-3-hydroxyisoxazole (muscimol; GABAA receptor agonist); (2S)-3-[[(1S)-1-(3,4-Dichlorophenyl) ethyl]amino-2-hydroxypropyl] (phenylmethyl)phosphinic acid hydrochloride (CGP55845; GABAB receptor antagonist). These were purchased from Tocris Cookson. Guanosine 5-O-(2-thiodiphosphate) (GDP-β-S, nonhydrolysable GDP analog, G-protein inhibitor) was purchased from Sigma. Stock solutions of DHPG, muscimol, and GDP-β-S were prepared with water. LY367385 was dissolved in NaOH (3%). MTEP, NBQX, bicuculline, picrotoxin, and CGP55845 were dissolved in DMSO (10%). Drugs were dissolved in ACSF to their final concentrations (see text) on the day of the experiment. The dilution factor was 1:10,000 for NaOH and DMSO. ACSF served as vehicle control in all experiments. Selectivity and target concentrations have been established in the literature (Fu and Neugebauer 2008; Ji et al. 2010; Lea and Faden 2006; Lesage 2004; Niswender and Conn 2010; Pernia-Andrade et al. 2009).
Histology
At the end of a behavioral experiment, the animal was euthanized, and the brain was removed and submerged in 10% formalin. Tissues were stored in 20% sucrose before they were frozen sectioned at 50 μm. Sections were stained with Neutral Red, mounted on gel-coated slides, and coverslipped. Positions of the microdialysis fibers were identified under the microscope and plotted on standard diagrams adapted from Paxinos and Watson (1998). The boundaries of the different amygdala nuclei were easily identified under the microscope (Fu and Neugebauer 2008; Ji et al. 2010; Ji and Neugebauer 2010).
Statistical Analysis
Statistical significance was accepted at the level P < 0.05. GraphPad Prism 3.0 software (Graph-Pad Software) was used for all statistical analyses. For multiple comparisons, one-way ANOVA or two-way ANOVA was used with appropriate posttests as indicated in the text and figure legends (Tukey's multiple comparison tests to compare all pairs of data; Bonferroni posttests to compare selected pairs of data; Dunnett's posttests to compare all sets of data to a control value). Student's t-test (paired or unpaired where appropriate) was used to compare two sets of data that have Gaussian distribution and similar variances. Statistical analysis was performed on the raw data (firing rate measured as spikes per second). All averaged values are given as the means ± SE. Statistical significance was accepted at the level P < 0.05.
RESULTS
Synaptic effects of group I mGluR activation were studied in layer V pyramidal cells in the medial PFC (prelimbic cortex), using whole cell patch-clamp recordings in a brain slice preparation as described before (Ji et al. 2010; Orozco-Cabal et al. 2006). Specifically, we tested the hypothesis that group I mGluRs activate feed-forward inhibition of pyramidal cells and increase inhibitory synaptic transmission more strongly than excitatory transmission, thus decreasing pyramidal cell output and impairing cognitive behavior such as decision making.
Monosynaptic EPSCs and Polysynaptic IPSCs in Medial PFC Pyramidal Cells
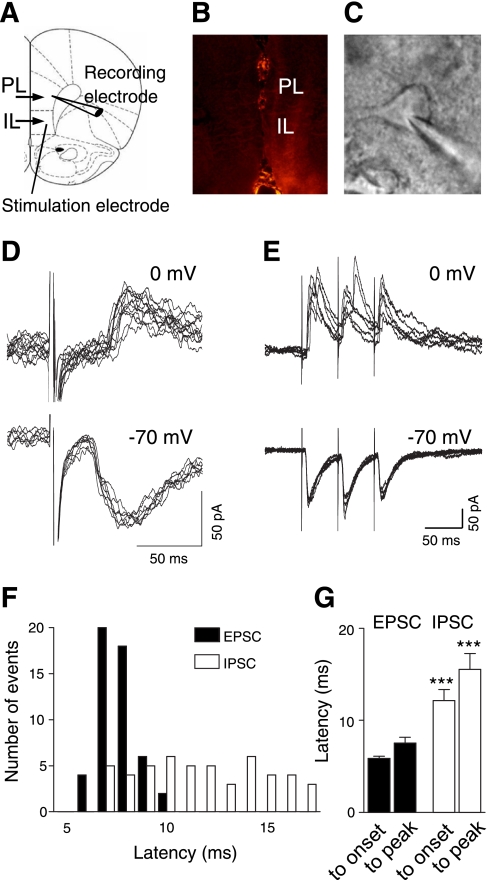
EPSCs and IPSCs were evoked in visually identified prelimbic layer V pyramidal cells (Fig. 1, A and C) by stimulation of presumed afferents from the BLA (see methods) that traverse the infralimbic cortex as described previously (Ji et al. 2010; Orozco-Cabal et al. 2006). The stimulation electrode was positioned on the track of anterogradely labeled fibers from the BLA (Fig. 1, A and B). Low stimulus intensities near threshold evoked monosynaptic EPSCs of constant latencies (Fig. 1D) that followed high-frequency stimulation reliably (Fig. 1E). In contrast, latencies and magnitude of polysynaptic IPSCs showed larger variability (Fig. 1, D and E). Average latencies of IPSCs were significantly longer than those of EPSCs (n = 6 neurons, paired t-test, P < 0.001; Fig. 1G; individual example is shown in Fig. 1F). EPSCs were recorded at a holding potential of −70 mV near the equilibrium potential for chloride (calculated as −68.99 mV, Nernst equation, pClamp9 software); IPSCs were recorded at 0 mV.
Fig. 1.
Polysynaptic inhibitory and monosynaptic excitatory transmission in the medial prefrontal cortex (PFC). A: stimulation and recording sites in a coronal brain slice containing the medial PFC, consisting of infralimbic (IL) and prelimbic (PL) cortical areas. Level is 3.2 mm anterior to bregma. B: course of afferent fibers in the medial PFC labeled anterogradely by fluorescent dye injection into the basolateral amygdala (BLA). Brain slices obtained 10–12 days after BLA injection show fluorescent labeling of fiber bundles that divide to reach layers II and V of IL and PL (Ji et al. 2010). No cell bodies are stained in layer V (arguing against retrograde labeling). C: individual pyramidal cell visually identified with infrared differential interference contrast videomicroscopy. D: whole cell patch-clamp recordings of excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs, respectively) evoked by stimulating presumed BLA afferents with minimal stimulus intensities near threshold. Individual traces recorded at −70 mV (downward deflections, EPSCs) or at 0 mV (upward deflections, IPSCs). E: monosynaptic EPSCs, but not polysynaptic IPSCs, follow high-frequency stimulation (20 Hz) reliably with constant latency. F: distribution of latencies measured from the stimulus artifact to the onset of EPSCs and IPSCs in an individual pyramidal cell. G: average latencies of IPSCs and EPSCs (n = 6 neurons). Latencies were measured from stimulus artifact to onset of synaptic current and from stimulus artifact to peak amplitude. Bar histograms show means ± SE. Latencies of IPSCs were significantly longer. ***P < 0.05 (paired t-test).
Activation of mGluR1, But Not mGluR5, Increases Inhibitory Transmission More Strongly Than Excitatory Transmission
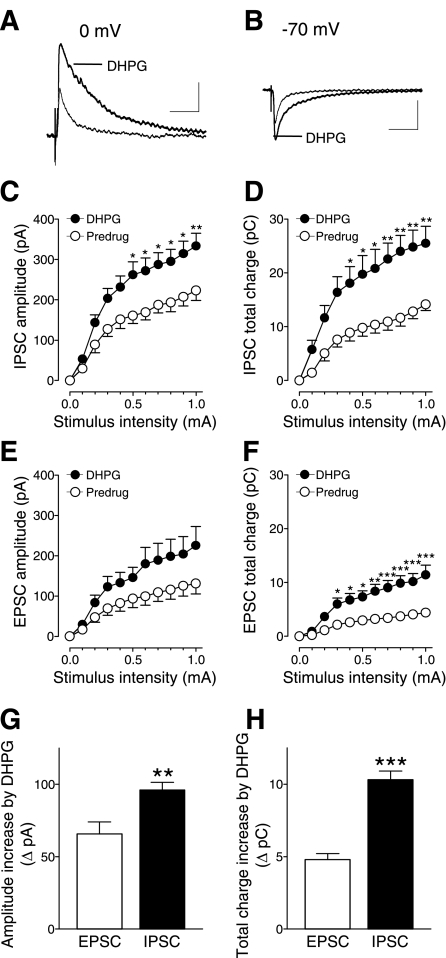
Input-output functions of IPSCs (Fig. 2, C and D) and EPSCs (Fig. 2, E and F) were obtained by measuring peak amplitude (Fig. 2, C and E) and total charge transfer (Fig. 2, D and F). Activation of mGluR1/5 with DHPG (1 μM) increased input-output functions of inhibitory and excitatory synaptic transmission significantly (Fig. 2, C and F; IPSC amplitude, n = 15 neurons, 2-way ANOVA, F1,308 = 63.24, P < 0.0001; IPSC total charge, n = 15, 2-way ANOVA, F1,308 = 87.20, P < 0.0001; EPSC amplitude, n = 9, 2-way ANOVA, F1,176 = 24.28, P < 0.0001; EPSC total charge, n = 9, 2-way ANOVA, F1,308 = 108.67, P < 0.0001). The facilitatory effect of DHPG on inhibitory transmission was significantly greater than on excitatory transmission (Fig. 2, G and H). This was evident from the comparison of DHPG-induced changes of amplitude and total charge transfer (area under the curve; P < 0.01–0.001, unpaired t-test).
Fig. 2.
(S)-3,5-dihydroxyphenylglycine (DHPG) increases inhibitory synaptic transmission more strongly than excitatory transmission. Whole cell voltage-clamp recordings of visually identified prelimbic layer V pyramidal cells as in Fig. 1. A: DHPG (1 μM) increased evoked IPSCs (recorded at 0 mV). B: DHPG also increased evoked EPSCs (recorded at −70 mV). A and B: individual traces are averages of 8–10 PSCs. Scale bars = 100 pA, 100 ms. C and D: DHPG (1 μM) significantly increased input-output functions of IPSC peak amplitude (n = 15 neurons, 2-way ANOVA, F1,308 = 63.24, P < 0.0001, C) and area under the curve (2-way ANOVA, F1,308 = 87.20, P < 0.0001, D). E and F: DHPG (1 μM) also significantly increased input-output functions of EPSC peak amplitude (n = 9 neurons, 2-way ANOVA, F1,176 = 24.28, P < 0.0001, E) and area under the curve (2-way ANOVA, F1,308 = 108.67, P < 0.0001, F). C–F: *P < 0.05, **P < 0.01, ***P < 0.001 (Bonferroni posttests). G and H: difference between DHPG and predrug values of amplitude (G) and total charge (H) was significantly greater for IPSCs compared with EPSCs (P < 0.01–0.001, unpaired t-test). Stimulation intensity was 700 μA. C–H: symbols and bar histograms show means ± SE.
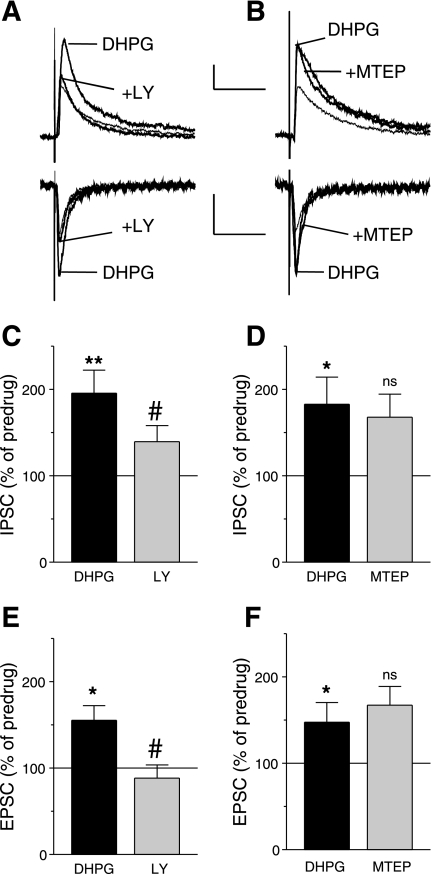
We next determined the receptor subtype involved in the facilitatory effect of DHPG. A selective mGluR1 antagonist (LY367385, 10 μM) inhibited the DHPG-induced increase of inhibitory (n = 5 neurons, Fig. 3C) and excitatory (n = 5, Fig. 3E) transmission. Figure 3A shows an individual example. In contrast, a selective mGluR5 antagonist (MTEP, 10 μM) did not block the facilitatory effect of DHPG on inhibitory (n = 13 neurons, Fig. 3D) and excitatory (n = 7, Fig. 3F) transmission (see individual example in Fig. 3B). The data suggest that mGluR1 activation increases inhibitory transmission more strongly than excitatory transmission.
Fig. 3.
(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385, LY), but not 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP), antagonizes the effects of DHPG on inhibitory and excitatory transmission. Whole cell voltage-clamp recordings of visually identified prelimbic layer V pyramidal cells. A: LY367385 (10 μM) inhibited the increase of IPSCs (recorded at 0 mV) and EPSCs (recorded at −70 mV) induced by DHPG (1 μM). B: MTEP (10 μM) had no effect on the DHPG-induced increase of IPSCs and EPSCs. A and B: individual traces are averages of 8–10 PSCs. Scale bars = 100 pA, 100 ms. C and D: effects of DHPG (1 μM) alone and in the presence of LY367385 (10 μM, n = 5) or MTEP (10 μM, n = 13) on IPSC total charge normalized to predrug control values (set to 100%). E and F: effects of DHPG (1 μM) alone and in the presence of LY367385 (10 μM, n = 5) or MTEP (10 μM, n = 7) on EPSC total charge normalized to predrug control values (set to 100%). Bar histograms show means ± SE. *P < 0.05, **P < 0.01 compared with predrug; #P < 0.05, compared with DHPG; ns, not significant, compared with DHPG (Tukey's multiple-comparison tests).
Increased Synaptic Inhibition by mGluR1 is GABAA-R Mediated, Action Potential Driven, and Requires Non-NMDA-R
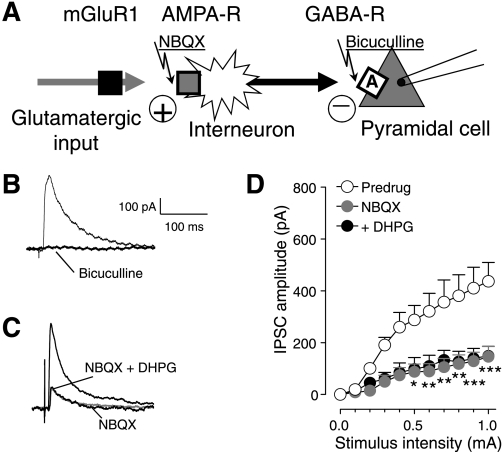
We next tested the hypothesis that mGluR1 activates feed-forward inhibition of medial PFC pyramidal cells (Fig. 4A). IPSCs were blocked by a GABAA receptor antagonist (bicuculline, 10 μM; see individual example in Fig. 4B). GABAergic IPSCs were also significantly inhibited by a non-NMDA (3-hydroxy-5-methylisoxazole propionic acid, AMPA) receptor antagonist (NBQX, 10 μM, n = 5 neurons, 2-way ANOVA, F1,88 = 80.14, P < 0.0001; Fig. 4D; see individual example in Fig. 4C). In the presence of NBQX, DHPG had no significant effect on inhibitory synaptic transmission (n = 5, 2-way ANOVA, F1,88 = 0.95, P > 0.05; Fig. 4D; see individual example in Fig. 4C). A general reduction of excitability by the blockade of postsynaptic AMPA receptors on pyramidal cells cannot account for the block of DHPG effects because inhibitory effects of NBQX on IPSCs were recorded at the reversal potential for AMPA receptors (0 mV). The results rather indicate that DHPG-induced synaptic inhibition requires the activation of non-NMDA (AMPA) receptors through glutamate release onto GABAergic interneurons (see Fig. 4A).
Fig. 4.
2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) blocks the facilitatory effect of DHPG on inhibitory synaptic transmission. Whole cell voltage-clamp recordings of visually identified prelimbic layer V pyramidal cells. A: diagram illustrates our hypothesis that DHPG (through metabotropic glutamate receptor 1, mGluR1) activates feed-forward inhibition of medial PFC cells through a mechanism that involves non-N-methyl-d-aspartate (NMDA) (3-hydroxy-5-methylisoxazole propionic acid, AMPA) and GABAA receptors. Squares indicate receptors. B: individual traces (averages of 8–10 IPSCs recorded at 0 mV) show that IPSCs were blocked by a GABAA receptor antagonist (bicuculline, 10 μM). C: individual traces (averages of 8–10 IPSCs) show that IPSCs were inhibited by a non-NMDA (AMPA) receptor antagonist (NBQX, 10 μM). DHPG (1 μM) coapplied with NBQX had no effect. D: NBQX inhibited GABAergic IPSCs significantly (n = 5, 2-way ANOVA, F1,88 = 80.14, P < 0.0001) and blocked the effect of DHPG (n = 5, 2-way ANOVA, F1,88 = 0.95, P > 0.05, compared with NBQX alone). Symbols show means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
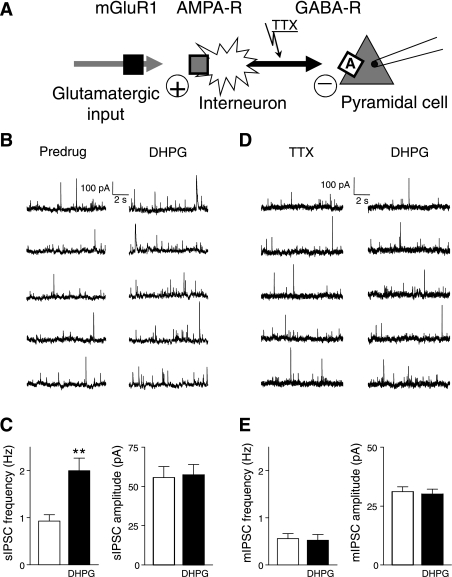
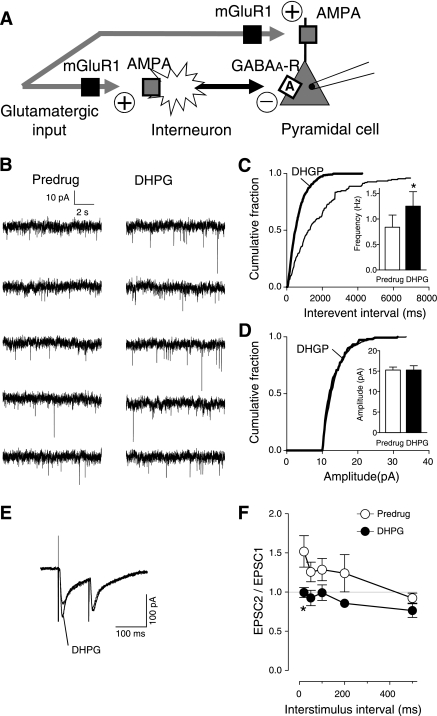
Consistent with the activation of GABAergic interneurons by DHPG, the analysis of spontaneous and miniature IPSCs showed that DHPG increased synaptic inhibition through the action potential-dependent release of GABA, arguing against the axon terminal of GABAergic interneurons as the site of action of DHPG (Fig. 5A). DHPG (1 μM) increased frequency but not amplitude of spontaneous IPSCs (n = 10 neurons, paired t-test, P < 0.01; Fig. 5C; individual example is shown in Fig. 5B). Analysis of mIPSCs showed that TTX (1 μM; to block action potential-dependent synaptic activity) prevented the facilitatory effect of DHPG (n = 5 neurons; Fig. 5E; see individual example in Fig. 5D), suggesting that mGluR1-mediated synaptic inhibition requires action potential-driven GABA release.
Fig. 5.
DHPG-induced synaptic inhibition is action potential dependent. Whole cell voltage-clamp recordings of visually identified prelimbic layer V pyramidal cells. A: diagram illustrates the hypothesis that DHPG activates feed-forward inhibition through an action potential-dependent mechanism rather than a site of action on the GABAergic terminal. B: individual traces show spontaneous IPSCs before (predrug) and during DHPG application (1 μM). C: DHPG (1 μM) increased frequency but not amplitude of spontaneous IPSCs (sIPSC) (n = 10 neurons, paired t-test, P < 0.01). Bar histograms show averaged values before (white bars) and during DHPG application. D: individual traces show miniature IPSCs (mIPSC) in tetrodotoxin (TTX) (1 μM) before (predrug) and during application of DHPG (1 μM). E: in the presence of TTX (1 μM) DHPG had no effect (n = 5 neurons). Bar histograms show averaged values before (white bars) and during application of DHPG. **P < 0.01.
Synaptic Inhibition by mGluR1 Decreases Output of PFC Pyramidal Cells
The consequence of mGluR1-mediated synaptic inhibition on pyramidal output was studied on synaptically evoked (Fig. 6) and depolarization-induced (Fig. 7) spikes. The hypothesis was that mGluR1-driven feed-forward inhibition would decrease pyramidal output through an action potential-dependent GABAergic mechanism (Fig. 6A).
Fig. 6.
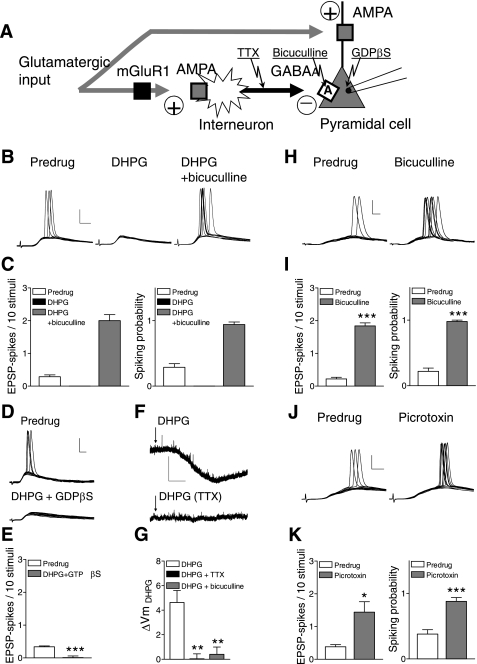
DHPG-induced synaptic inhibition inhibits pyramidal output (synaptically evoked spiking). Whole cell current-clamp recordings were made of visually identified prelimbic layer V pyramidal cells. A: diagram illustrates our hypothesis that feed-forward inhibition decreases pyramidal output through an action potential-dependent GABAergic mechanism. B: individual traces (10 each) show excitatory postsynaptic potentials (EPSPs) and spikes evoked with near-threshold stimulus intensity from a holding potential of −60 mV before (predrug) and during DHPG (1 μM) and in the presence of DHPG together with bicuculline (10 μM). C, left: bar histograms show average number of evoked spikes per synaptic stimulation in 10 successive trials; right: probability of synaptically evoked spikes were calculated as follows: (number of trials with evoked spikes)/(number of trials). DHPG (1 μM) (n = 5 neurons); DHPG together with bicuculline (10 μM), n = 5 neurons. D and E: inhibitory effects of DHPG persist when guanosine 5-O-(2-thiodiphosphate) (GDP-β-S) (1 mM) is included in the patch pipette (n = 5 neurons). F and G: membrane hyperpolarization by DHPG is blocked with TTX (1 μM; n = 5 neurons) and bicuculline (10 μM, n = 7). Mean resting membrane potential, 65 ± 1 mV. H and I: bicuculline (10 μM, n = 5) increases the number of synaptically evoked spikes (left) and spiking probability (right); same display as in C. J and K: picrotoxin (30 μM, n = 5) also increases synaptically evoked spiking (see C and I). Scale bars in B, D, H, and J = 25 mV, 5 ms; scale bars in F = 5 mV, 60 s. Bar histograms show means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 (E, I, and K, paired t-test; G, ANOVA with Bonferroni posttests, compared with DHPG).
Fig. 7.
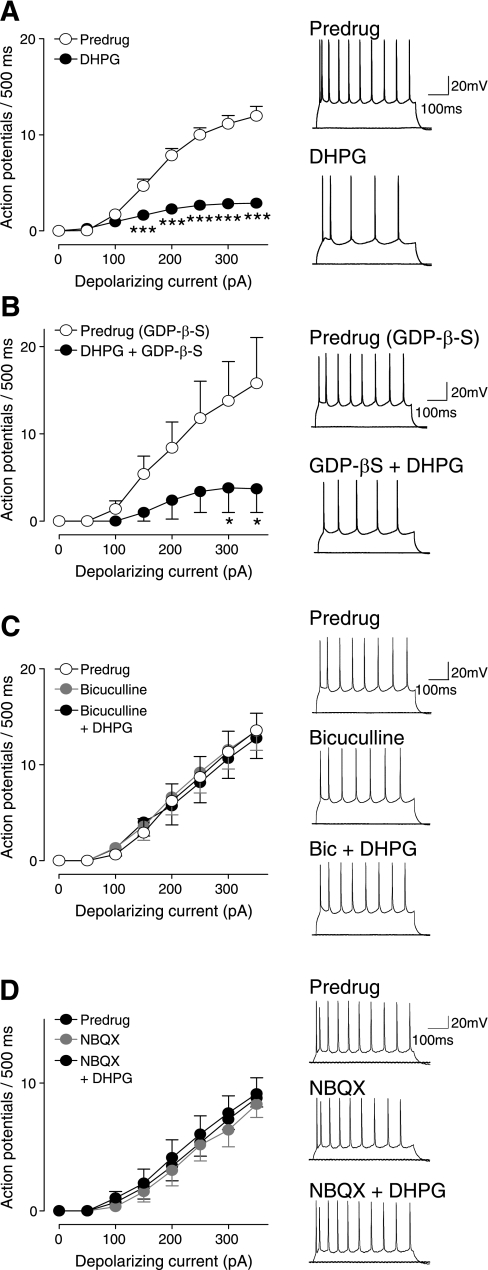
DHPG-induced synaptic inhibition inhibits pyramidal output (depolarization-induced spiking). Whole cell current-clamp recordings of visually identified prelimbic layer V pyramidal cells. Action potentials were generated by direct intracellular current injections (500 ms) of increasing magnitude (in 50-pA steps) from a holding potential of −60 mV. Right: original voltage traces showing action potentials evoked in individual cells by current injections of 0 pA and 300 pA. Left: graphs showing input-output functions (f-I relationships) averaged for each sample of neurons. A: DHPG (1 μM) decreased the input-output function significantly (n = 28 neurons, 2-way ANOVA, F1,432 = 257.01, P < 0.0001). ***P < 0.001 (Bonferroni posttests). B: when GDP-β-S (1 mM) was included in the patch pipette (predrug), DHPG still had inhibitory effects (n = 5 neurons, 2-way ANOVA, F1,64 = 16.84, P < 0.001). *P < 0.05 (Bonferroni posttests). C: bicuculline (10 μM) itself did not affect neuronal excitability but blocked the inhibitory effect of DHPG (n = 7 neurons, 2-way ANOVA, F1,96 = 0.28, P > 0.05). D: NBQX (10 μM) also had no effect on action potential firing but blocked the effect of DHPG (n = 6, 2-way ANOVA, F1,80 = 0.57, P > 0.05). Symbols show means ± SE.
DHPG inhibits synaptically evoked spiking.
DHPG (1 μM, n = 5 neurons) blocked synaptically evoked spikes (E-S coupling) in medial PFC pyramidal cells recorded in current clamp (Fig. 6, B and C). Coapplication of bicuculline (10 μM, n = 5 neurons) restored spike firing, which is consistent with DHPG-activated synaptic inhibition of pyramidal cell output. Synaptically evoked spiking was measured as the average number of evoked spikes per synaptic stimulation and as probability of evoked spikes. The inhibitory effects of DHPG persisted when GDP-β-S (1 mM), a nonhydrolysable GDP analog, was included in the patch pipette to inhibit G protein-mediated intracellular effects (n = 5 neurons; Fig. 6, D and E). At this concentration GDP-β-S (1 mM) effectively blocked the effect of DHPG on amygdala neurons in our previous study (Li et al. 2011). Consistent with an indirect action potential-dependent action, DHPG-evoked membrane hyperpolarization was blocked with TTX (1 μM, n = 5 neurons; Fig. 6, F and G). Hyperpolarization by DHPG was not observed in the presence of a GABAA receptor antagonist (bicuculline, 10 μM, n = 7 neurons; Fig. 6G). Blockade of GABAA receptors with bicuculline (10 μM, n = 5; Fig. 6, H and I) or picrotoxin (30 μM, n = 5; Fig. 6, J and K) increased synaptically evoked spiking, suggesting that synaptic stimulation activates a GABAergic mechanism that controls pyramidal output.
DHPG decreases depolarization-induced spiking.
DHPG (1 μM) decreased the frequency-current (f-I) relationship significantly (n = 28 neurons, 2-way ANOVA, F1,432 = 257.01, P < 0.0001; Fig. 7A). Action potentials were generated by direct intracellular current injections (500 ms) of increasing magnitude (in 50 pA steps). A depolarizing current of 24.44 ± 1.69 pA was injected to repolarize the membrane potential to predrug levels (∼ −65 mV). The inhibitory effects of DHPG on excitability were associated with a significantly decreased input resistance (from 172 ± 6.8 MΩ to 148 ± 6.7 MΩ; P < 0.01, paired t-test), which did not depend on DHPG-induced membrane hyperpolarization (see Fig. 6F) because it was measured when the membrane potential was held constant. The inhibitory effect of DHPG also persisted when GDP-β-S (1 mM) was included in the patch pipette to inhibit G protein-mediated effects (n = 5 neurons, 2-way ANOVA, F1,64 = 16.84, P < 0.001; Fig. 7B). However, DHPG had no significant effect on excitability in the presence of bicuculline (n = 7 neurons, 2-way ANOVA, F1,96 = 0.28, P > 0.05; Fig. 7C) or NBQX (n = 6, 2-way ANOVA, F1,80 = 0.57, P > 0.05; Fig. 7D). DHPG also did not affect input resistance when coapplied with bicuculline (control, 170 ± 8.3 MΩ; DHPG with bicuculline, 171 ± 7.5 MΩ; P > 0.05, paired t-test). The results suggest that the decrease of pyramidal output (f-I function) by DHPG was not attributable to a direct membrane effect of DHPG but resulted from the activation of non-NMDA and GABAA receptors, consistent with the proposed model of mGluR1-activated feed-forward inhibition, involving GABA release from interneurons and GABAergic effects on pyramidal cells.
Presynaptic mGluR1/5 Activation Increases Output of PFC Interneurons
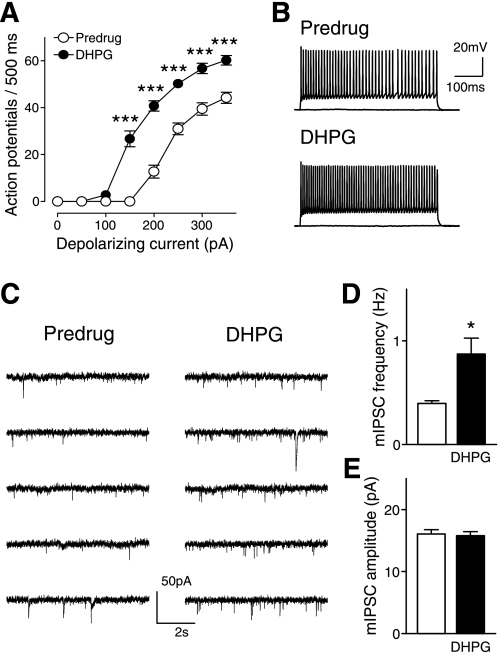
Amygdala (BLA) inputs target local inhibitory interneurons, including fast-spiking parvalbumin-positive cells, in layer V of the medial PFC, which form synapses with pyramidal cells (Gabbott et al. 2006). Our hypothesis (Figs. 4A, 5A, and 6A) holds that DHPG increases output of GABAergic interneurons through a presynaptic mechanism that results in increased glutamatergic activation of these interneurons, thus increasing synaptic inhibition of pyramidal cells. We recorded directly from fast-spiking interneurons that were visually identified as nonpyramidal cells and showed nonaccommodating spike-firing properties and strong fast afterhyperpolarization (Markram et al. 2004; Zhou and Hablitz 1996) (see example in Fig. 8A). DHPG (1 μM) increased the frequency-current (f-I) relationship of PFC interneurons significantly (n = 5 neurons, 2-way ANOVA, F1,64 = 210.94, P < 0.0001; Fig. 8, A and B). DHPG also increased frequency, but not amplitude, of mEPSCs in mPFC interneurons (n = 5, paired t-test, P < 0.05; Fig. 8, C–E), consistent with a presynaptic site of action (see diagrams in Figs. 4A and 5A).
Fig. 8.
DHPG increases output of interneurons through a presynaptic mechanism. Whole cell current-clamp recordings were made of fast-spiking nonaccommodating interneurons with fast afterhyperpolarization (Markram et al. 2004; Zhou and Hablitz 1996) that were visually identified as nonpyramidal cells in layer V of the prelimbic cortex. A: DHPG (1 μM) increased the frequency-current (f-I) function of PFC interneurons significantly (n = 5 neurons, 2-way ANOVA, F1,64 = 210.94, P < 0.0001). Input-output functions were averaged for each sample of neurons (mean ± SE). B: action potentials evoked in an individual interneuron by current injections of 0 pA and 300 pA. Action potentials were generated by direct intracellular current injections (500 ms) of increasing magnitude (in 50-pA steps) from a holding potential of −65 mV. C: individual traces show miniature EPSCs in TTX (1 μM) before (predrug) and during application of DHPG (1 μM). D and E: DHPG decreased frequency (n = 5, paired t-test, P < 0.05) but not amplitude of mEPSCs in mPFC interneurons, consistent with a presynaptic site of action. Bar histograms show means ± SE. ***P < 0.001, *P < 0.05.
Facilitation of Excitatory Synaptic Transmission onto Pyramidal Cells by mGluR1 is Presynaptic
The results so far show that DHPG activates PFC interneurons through a presynaptic mechanism to inhibit output of PFC pyramidal cells. Thus feed-forward inhibition controls synaptically evoked spiking and overrides facilitatory effects of DHPG on excitatory transmission onto pyramidal cells (see Fig. 2, E and F). We hypothesized that the facilitatory effect on pyramidal cells involved a presynaptic mechanism (Fig. 9A) like the facilitatory effect on interneurons (Fig. 8, C–E). The analysis of mEPSCs in the presence of TTX (1 μM) showed that DHPG (1 μM) increased frequency (Fig. 9C), but not amplitude (Fig. 9D), of mEPSCs (see original traces in Fig. 9B) in pyramidal cells significantly (cumulative frequency distribution, P < 0.001, Kolmogorov-Smirnov test; mean frequency, P < 0.05, paired t-test, n = 5 neurons). PPR analysis (see methods) also indicated a presynaptic modulation of evoked EPSCs in PFC pyramidal cells. Two orthodromic synaptic stimuli of equal intensity were applied at varying intervals, and the resulting EPSCs were recorded (Fig. 9E). PPR was calculated as the ratio of the initial EPSC (EPSC1) and the second EPSC (EPSC2; see Fig. 9F). Any alterations in PPR suggest a presynaptic site of action (Fu and Neugebauer 2008). DHPG (1 μM) decreased PPR significantly (n = 5 neurons, 2-way ANOVA, F1,40 = 16.77, P < 0.001; Fig. 9F), which is consistent with a presynaptic site of action. Thus mGluR1 increases excitatory input to pyramidal cells and also to interneurons to activate feed-forward inhibition. The net effect is suppression of pyramidal output.
Fig. 9.
DHPG acts presynaptically to increase excitatory transmission onto pyramidal cells. Whole cell voltage-clamp recordings of visually identified prelimbic layer V pyramidal cells. A: diagram illustrates our hypothesis that mGluR1 acts presynaptically to regulate glutamatergic transmission onto interneurons as well as pyramidal cells. B: individual traces show mEPSCs (in TTX, 1 μM) recorded before and during DHPG (1 μM). C and D: cumulative distribution analysis (see methods) of mEPSC frequency (C) and amplitude (D). Bar histograms show mean frequency and amplitude averaged for the sample of neurons (n = 5) before (predrug) and during DHPG. DHPG (1 μM) increased frequency but not amplitude of mEPSCs significantly (cumulative frequency distribution, P < 0.001, Kolmogorov-Smirnov test; mean frequency, P < 0.05, paired t-test, n = 5 neurons). E: individual traces (averages of 3–4 EPSCs) show that DHPG (1 μM) decreased paired-pulse facilitation of evoked EPSCs. F: paired-pulse ratio of peak amplitudes of 2 consecutive EPSCs (EPSC2/EPSC1) was measured at different interstimulus intervals under control conditions (predrug) and in the presence of DHPG (1 μM). The overall effect of DHPG was significant (n = 5 neurons, 2-way ANOVA, F1,40 = 16.77, P < 0.001). Bonferroni posttests showed a significant difference for the 20 ms interstimulus interval. *P < 0.05.
Activation of Group I mGluRs in the Medial PFC Impairs Decision Making Through GABAergic Inhibition
We next we tested the hypothesis that group I mGluR-induced inhibition of pyramidal cells in the medial PFC results in cognitive deficits that were previously described in a pain model, using a computerized behavioral task in which rats decide between high-risk and low-risk strategies (Ji et al. 2010). This rodent “gambling task” is based on serial choices between food rewards of different value and probability (Pais-Vieira et al. 2007; 2009). In 90 consecutive trials, a rat entered the test arena to press one of two levers associated with a food reward before the animal was returned to the entry chamber. Following the nongambling training phase in which each of two levers provided one food pellet in nine of ten visits, the animal was tested on decision making in a single session of 90 consecutive trials in which one lever was altered to return three pellets in three of ten visits (high-risk choice), whereas the other lever continued to deliver one pellet in nine of ten visits (low-risk choice; see methods).
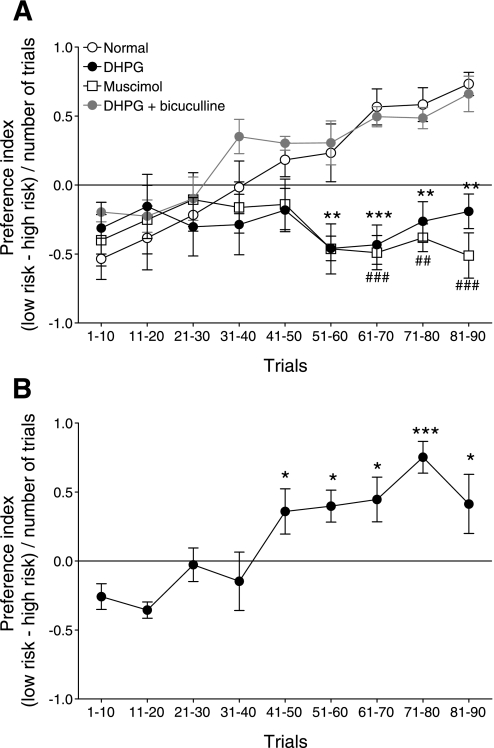
As in our previous study (Ji et al. 2010), rats initially preferred the high-risk lever, which is reflected in a negative preference index (see methods and Fig. 10). Normal animals (n = 6) developed, after about 40 trials, a preference for the low-risk lever that provided smaller but more reliable rewards (Fig. 10A). In contrast, administration of DHPG (100 μM, concentration in microdialysis fiber) into the medial PFC by microdialysis (see methods) caused these animals (n = 5) to perseverate in preferring the high-risk lever associated with larger but infrequent rewards (Fig. 10A). Preference index of DHPG-treated animals was significantly different from that of normal rats after about 50 trials (2-way ANOVA, F1,81 = 28.16, P < 0.0001). Coapplication of bicuculline (1 mM) with DHPG to block GABAA-receptors in the medial PFC restored normal decision making (n = 5 rats; Fig. 10A). Accordingly, their preference index was not significantly different from that of normal rats (2-way ANOVA, F1,81 = 2.38, P > 0.05).
Fig. 10.
DHPG impairs decision making through a GABAergic mechanism in the PFC. Animals chose between low-risk (1 food pellet in 9 of 10 visits) and high-risk (3 pellets in 3 of 10 visits) levers in 90 consecutive trials (see methods). Symbols (mean ± SE) show the preference index [(low-risk − high-risk choices)/number of trials] averaged for 10 consecutive trials of animals in 3 experimental groups. A: normal animals (n = 6) switched from high- to low-risk choices. Rats in which DHPG (100 μM) was administered into the medial PFC by microdialysis (see methods) failed to switch strategies (n = 5). Their preference index was significantly different from that of normal rats (2-way ANOVA, F1,81 = 28.16, P < 0.0001). Coapplication of bicuculline (1 mM) with DHPG restored normal decision making (n = 5). Preference index was not significantly different from that of normal rats (2-way ANOVA, F1,81 = 2.38, P > 0.05). Administration of a GABAA receptor agonist (muscimol, 0.5 mM, n = 5) (Nattie and Li 2008) into the medial PFC mimicked the effect of DHPG and resulted in a preference index that was significantly different from that of normal animals (F1,81 = 32.31, P < 0.0001 compared with normal). **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with normal (Bonferroni posttests). B: placement control for drug diffusion. Administration of DHPG into the anterior cingulate cortex dorsal to the prelimbic PFC (n = 5) did not impair the ability to switch strategies, which is reflected in the significant change of the preference index compared with the initial preference index value (F = 6.06, P < 0.0001 repeated-measures ANOVA). *P < 0.05, ***P < 0.001 compared with initial preference index (Dunnett's posttests).
The data suggest that activation of mGluR1/5 impairs decision-making abilities through a GABAergic mechanism. Accordingly, administration of a GABAA receptor agonist (muscimol, 0.5 mM, n = 5) (Nattie and Li 2008) into the medial PFC also prevented the animals from developing a preference for the low-risk reward (Fig. 10A; F1,81 = 32.31, P < 0.0001 compared with normal). As a placement control for drug diffusion, administration of DHPG into the anterior cingulate cortex dorsal to the prelimbic PFC did not impair the ability to switch strategies and develop a preference for the low-risk lever (Fig. 10B; n = 5, F = 6.06, P < 0.0001 repeated-measures ANOVA, compared with initial preference index).
Impaired decision making observed with DHPG or muscimol was not due to motor or attention deficits because reaching each lever involved the same movements and choices were not cue based in this task (Pais-Vieira et al. 2007; 2009). The results suggest that group I mGluRs impair decision making through the activation of GABAA receptors in the medial PFC.
DISCUSSION
This study tested the hypothesis that group I mGluR-activated feed-forward inhibition decreases PFC pyramidal cell output, thus impairing cognitive behavior. Our hypothesis was based on anatomical evidence that in the medial PFC glutamatergic afferents from extracortical areas such as the amygdala target not only pyramidal cells but also nearby inhibitory GABAergic interneurons synapsing on pyramidal cells in layer V (Bacon et al. 1996; Gabbott et al. 2006; Kita and Kitai 1990). Functional evidence linked synaptic inhibition of pyramidal cells to impaired decision making in a pain model (Ji et al. 2010).
Our data clearly show that DHPG-activating mGluR1 1) increases output of medial PFC interneurons (f-I relationship) through a presynaptic action (mEPSC analysis), thus increasing synaptic inhibition of medial PFC pyramidal cells (increased IPSCs) to decrease their output (E-S coupling and f-I relationship) through a mechanism that does not require G proteins in the pyramidal cell (not blocked with GDP-β-S), 2) is action potential dependent (lost in TTX), and 3) requires non-NMDA receptors (blocked by NBQX). The results are consistent with a model in which mGluR1 activation increases glutamate-driven GABAA-receptor-mediated feed-forward inhibition of medial PFC pyramidal cells to decrease their output (spike generation).
A potential shortcoming of this study is that the mechanism by which synaptic inhibition overrides synaptic excitation remains to be determined. Our data show that DHPG facilitated glutamatergic transmission onto interneurons to produce feed-forward inhibition of pyramidal cells, but DHPG also acted presynaptically to increase excitatory transmission onto pyramidal cells. Mechanisms that favor synaptic inhibition over excitation may involve intracellular processes such as shunting and the modulation of the generation and timing of action potentials, hence control pyramidal output by GABAergic cortical interneurons (Cruikshank et al. 2007; Markram et al. 2004). Cortical interneurons show greater sensitivity than pyramidal cells to glutamatergic input attributable to different cellular properties or synaptic targeting and timing (Cruikshank et al. 2007; Markram et al. 2004; Povysheva et al. 2006).
mGluR1-driven feed-forward inhibition impairs cognitive performance measured as the inability to switch strategies in a decision-making task. Cognitive inflexibility resulting from the loss or impairment of PFC function is well documented for a number of neuropsychiatric disorders (Bowie and Harvey 2006; Clarke et al. 2004; Goto et al. 2010; Gu et al. 2008; Stalnaker et al. 2009). Recent evidence also implicates PFC areas in pain-related cognitive deficits such as impaired emotion-based decision making (Apkarian et al. 2004b; Ji et al. 2010; Pais-Vieira et al. 2009). Structural and functional changes of layer II/III pyramidal cells in the medial PFC were observed in a model of neuropathic pain (Metz et al. 2009).
Our previous study showed increased synaptic inhibition of layer V pyramidal cells as the consequence of hyperactivity of amygdala neurons in a model of arthritis pain (Ji et al. 2010). Functional interactions between amygdala and PFC play an important role in emotional learning and behavior (Herry et al. 2008; Holland and Gallagher 2004; Laviolette and Grace 2006; McGaugh 2004; Roozendaal et al. 2009; Stalnaker et al. 2007a). Both PFC (Bechara et al. 1999; Kouneiher et al. 2009; Pais-Vieira et al. 2007; Stalnaker et al. 2007a; Vertes 2006) and amygdala (Bechara et al. 1999; 2003; Seymour and Dolan 2008; Stalnaker et al. 2007b) contribute to emotion-based decision making. Glutamatergic projections from the basolateral amygdala form monosynaptic connections with parvalbumin-positive local-circuit interneurons in the medial PFC (Bacon et al. 1996; Gabbott et al. 2006), which are known to control pyramidal cell output (Markram et al. 2004). Therefore, enhanced amygdala output would increase synaptic inhibition of medial PFC neurons. Accordingly, increased inhibitory synaptic transmission in the medial PFC, decreased activity of pyramidal cells, and impaired decision making were observed in our previous study in a model of arthritis pain (Ji et al. 2010).
In contrast, disruption of GABAergic inhibitory systems in the PFC is one consistent finding in schizophrenia pathology (Beasley et al. 2002; Lewis and Gonzalez-Burgos 2008), a disorder that is characterized by cognitive deficits such as behavioral inflexibility (Bowie and Harvey 2006; Goto et al. 2010). A specific deficit of parvalbumin-interneurons in the PFC was described in the PCP model of schizophrenia (Wang et al. 2008) and was found in postmortem studies of schizophrenic brains (Lewis et al. 2005). Loss of GABAergic function is believed to result in the disinhibition of pyramidal cells, impaired regulation of pyramidal cell networks and pyramidal output, and cognitive deficits (Homayoun and Moghaddam 2007; Lewis and Gonzalez-Burgos 2008; Lisman et al. 2008). Cognitive deficits resulting from enhanced (pain model) or impaired (schizophrenia) synaptic inhibition of PFC pyramidal cells are consistent with the finding that abnormally low or high action potential firing rates of PFC pyramidal cells correlate with impaired cognitive performance (Chang et al. 2005). Therefore, maintenance of an optimum firing rate and pyramidal output is required for normal cortical function and cognitive performance.
Here we analyzed mechanisms and consequences of feed-forward inhibition in the medial PFC. Our data show that mGluR1, but not mGluR5, can increase inhibition through the activation of non-NMDA receptors that regulate GABAA-receptor-mediated inhibitory transmission onto pyramidal cells. Furthermore, mGluR1-activated synaptic inhibition decreases synaptically evoked spikes (E-S coupling) and depolarization-induced action potential firing (f-I relationship) in pyramidal cells. Activation of group I mGluRs in the medial PFC of freely behaving animals impairs decision making through a mechanism that involves GABAergic inhibition because it is blocked by a GABAA receptor antagonist and mimicked by a GABAA receptor agonist.
The following observations support these conclusions. DHPG facilitated polysynaptic inhibitory transmission onto pyramidal cells. The polysynaptic nature of the GABAergic IPSCs is supported by the pharmacological profile (blockade by NBQX and bicuculline) and synaptic characteristics (high jitter, longer latencies, inability to follow high-frequency stimulation reliably). In this serial two-step pathway, NBQX blocked an initial unobserved EPSC that was responsible for evoking the bicuculline-sensitive GABAergic IPSC (for discussion see Doyle and Andresen 2001). The facilitatory effect of DHPG was blocked by LY367385 but not MTEP, indicating the involvement of mGluR1. Both antagonists are subtype-selective at the concentrations used in this study (Lea and Faden 2006; Lesage 2004; Niswender and Conn 2010), and their differential effects further argue in favor of subtype selectivity. DHPG-activated synaptic inhibition was blocked by NBQX and TTX, suggesting that DHPG did not act directly on the GABAergic terminal but through an action potential-dependent mechanism that involved the activation non-NMDA (AMPA) receptors. Recordings of mPFC interneurons showed that DHPG increased spike firing through a presynaptic mechanism. In other words, mGluR1 activation triggered release of glutamate onto GABAergic interneurons, thus causing them to fire action potentials and release GABA onto mPFC pyramidal cells to inhibit their output.
The underlying mechanisms of DHPG-driven feed-forward inhibition of pyramidal cell function remain to be determined. DHPG produced GABAergic (bicuculline-sensitive) membrane hyperpolarization and decreased input resistance and inhibition of excitability and output (E-S coupling) of pyramidal cells. The DHPG-induced increase in spontaneous IPSCs is consistent with the contribution of GABA release onto pyramidal cells. Excitability changes are likely due to the decreased input resistance, which is a well-documented effect of GABAA receptor activation, but they are at least in part independent of the DHPG-induced hyperpolarization because they persist even when the membrane potential is kept constant (repolarized to control levels). Thus the data suggest that excitability changes are not attributable to hyperpolarization-activated secondary ionic membrane effects.
Glutamate-driven disynaptic feed-forward inhibition has been shown in several brain areas (Doyle and Andresen 2001; Ferrante et al. 2009; Ling and Benardo 1995; Silberberg and Markram 2007). Importantly, mGluR1 has been implicated in feed-forward inhibition in the cerebellum, where mGluR1 activation increased the spontaneous firing of stellate-basket cells and also the frequency of spontaneous inhibitory postsynaptic currents in Purkinje cells (Karakossian and Otis 2004). Activation of group I mGluRs in the medial PFC increased the release of GABA (Segovia and Mora 2005) and glutamate (Melendez et al. 2005), and the latter effect was blocked by an mGluR1 antagonist (LY367385). On the other hand, mGluR5 mediated the increase of spontaneous EPSCs but not miniature EPSCs by DHPG in PFC pyramidal cells, suggesting network effects (Marek and Zhang 2008). Similar to the results of our study, DHPG increased the frequency of spontaneous but not miniature IPSCs onto pyramidal cells in not further specified regions of prefrontal brain slices, but the contribution of mGluR1 and mGluR5 was not determined (Chu and Hablitz 1998). To the best of our knowledge, the present study is the first to show that mGluR1, but not mGluR5, activates feed-forward inhibition compared with synaptic excitation of pyramidal cells in the medial PFC.
The consequence of DHPG-activated feed-forward inhibition is decreased output of pyramidal cells, which correlated with decision-making deficits in a behavioral task. The DHPG-induced cognitive deficit mimicked that observed in pain models (Ji et al. 2010; Pais-Vieira et al. 2009), suggesting that blocking mGluR1 may have beneficial effects in these conditions. The link between electrophysiology and behavior in this study rests on pharmacological data. As a note of caution, several issues need to be considered when interpreting the electrophysiological results as showing a neural mechanism of impaired decision making. Simultaneous activation of all mGluRs in the slice with the exogenously applied mGluR1/5 agonist and synaptic activation of all inputs from the BLA to the mPFC would not likely occur under physiological conditions. Temporal and spatial patterns and magnitude of mGluR1 activation by the endogenous release of ligands in decision-making behavior could be quite different from the situation in the reduced slice preparation.
Interestingly, positive allosteric modulators of mGluR1 (Lesage and Steckler 2010) or mGluR5 (Niswender and Conn 2010) have been proposed as novel therapeutic approaches in the treatment of cognitive symptoms of schizophrenia. Importantly, whereas cognitive inflexibility associated with schizophrenia resembles the decision-making deficits in pain, the underlying neural mechanisms are different. The well-documented loss of GABAergic interneurons in schizophrenia (Beasley et al. 2002; Goto et al. 2010; Lewis and Gonzalez-Burgos 2008; Wang et al. 2008) is in stark contrast to the increased GABAergic tone in the medial PFC in a pain model (Ji et al. 2010). The data of the present study would suggest that activating mGluR1 to increase synaptic inhibition could be a useful strategy in disorders that are accompanied by impaired GABAergic function.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-38261 and NS-11255.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol 87: 81–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain 108: 129–136, 2004a [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 24: 10410–10415, 2004b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res 720: 211–219, 1996 [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 52: 708–715, 2002 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann NY Acad Sci 985: 356–369, 2003 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 19: 5473–5481, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat 2: 531–536, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Hablitz JJ. Presynaptic depression of synaptic transmission mediated by activation of metabotropic glutamate receptors in rat neocortex. J Neurosci 14: 5120–5130, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JP, Hablitz JJ. G-protein activation by metabotropic glutamate receptors reduces spike frequency adaptation in neocortical neurons. Neuroscience 75: 123–131, 1996 [DOI] [PubMed] [Google Scholar]
- Cauli B, Porter JT, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci USA 97: 6144–6149, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex 15: 409–418, 2005 [DOI] [PubMed] [Google Scholar]
- Chu Z, Hablitz JJ. Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex. J Neurophysiol 80: 621–627, 1998 [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science 304: 878–880, 2004 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10: 462–468, 2007 [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001 [DOI] [PubMed] [Google Scholar]
- Ferrante M, Migliore M, Ascoli GA. Feed-forward inhibition as a buffer of the neuronal input-output relation. Proc Natl Acad Sci USA 106: 18004–18009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28: 3861–3876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139: 1039–1048, 2006 [DOI] [PubMed] [Google Scholar]
- Galhardo V, Pais-Vieira M. Decision-making cognitive deficits in a rat model of chronic pain. Soc Neurosci Abstr 50.3, 2005 [Google Scholar]
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry 67: 199–207, 2010 [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain 131: 155–164, 2008 [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature 454: 600–606, 2008 [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol 14: 148–155, 2004 [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27: 11496–11500, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol 639: 33–39, 2010 [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Reactive oxygen species are involved in group I mGluR-mediated facilitation of nociceptive processing in amygdala neurons. J Neurophysiol 104: 218–229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30: 5451–5464, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakossian MH, Otis TS. Excitation of cerebellar interneurons by group I metabotropic glutamate receptors. J Neurophysiol 92: 1558–1565, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 298: 40–49, 1990 [DOI] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci 12: 939–945, 2009 [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci 26: 6458–6468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev 12: 149–166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage A, Steckler T. Metabotropic glutamate mGlu1 receptor stimulation and blockade: therapeutic opportunities in psychiatric illness. Eur J Pharmacol 639: 2–16, 2010 [DOI] [PubMed] [Google Scholar]
- Lesage ASJ. Role of group I metabotropic glutamate receptors mGlu1 and mGlu5 in nociceptive signalling. Curr Neuropharm 2: 363–393, 2004 [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology 33: 141–165, 2008 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324, 2005 [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol 91: 13–24, 2004 [DOI] [PubMed] [Google Scholar]
- Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci 31: 1114–1127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS. Recruitment of GABAA inhibition in rat neocortex is limited and not NMDA dependent. J Neurophysiol 74: 2329–2335, 1995 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci 31: 234–242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Zhang C. Activation of metabotropic glutamate 5 (mGlu5) receptors induces spontaneous excitatory synaptic currents in layer V pyramidal cells of the rat prefrontal cortex. Neurosci Lett 442: 239–243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28, 2004 [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390: 607–611, 1997 [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cysteine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther 314: 139–147, 2005 [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci USA 106: 2423–2428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol 467: 521–535, 2003 [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis into the caudal aspect of the Nucleus tractus solitarii of conscious rats inhibits chemoreception. Respir Physiol Neurobiol 164: 394–400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain 3: 8–20, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23: 52–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol 639: 47–58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Pollandt S, Liu J, Vergara L, Shinnick-Gallagher P, Gallagher JP. A novel rat medial prefrontal cortical slice preparation to investigate synaptic transmission from amygdala to layer V prelimbic pyramidal neurons. J Neurosci Meth 151: 148–158, 2006 [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience 145: 225–231, 2007 [DOI] [PubMed] [Google Scholar]
- Pais-Vieira M, Mendes-Pinto MM, Lima D, Galhardo V. Cognitive impairment of prefrontal-dependent decision-making in rats after the onset of chronic pain. Neuroscience 161: 671–679, 2009 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain In Stereotaxic Coordinates. New York: Academic Press, 1998 [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325: 760–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci 9: 423–436, 2008 [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Gonzalez-Burgos G, Zaitsev AV, Kroner S, Barrionuevo G, Lewis DA, Krimer LS. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex 16: 541–552, 2006 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433, 2009 [DOI] [PubMed] [Google Scholar]
- Segovia G, Mora F. Effects of the metabotropic glutamate receptor agonist, ACPD, on the extracellular concentrations of GABA and acetylcholine in the prefrontal cortex of the rat during the normal process of aging. Brain Res Bull 65: 11–16, 2005 [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron 58: 662–671, 2008 [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by martinotti cells. Neuron 53: 735–746, 2007 [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron 54: 51–58, 2007a [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Roesch MR, Franz TM, Calu DJ, Singh T, Schoenbaum G. Cocaine-induced decision-making deficits are mediated by miscoding in basolateral amygdala. Nat Neurosci 10: 949–951, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology 56, Suppl 1: 63–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142: 1–20, 2006 [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology 33: 2442–2455, 2008 [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Layer I neurons of rat neocortex. I. Action potential and repetitive firing properties. J Neurophysiol 76: 651–667, 1996 [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Metabotropic glutamate receptor enhancement of spontaneous IPSCs in neocortical interneurons. J Neurophysiol 78: 2287–2295, 1997 [DOI] [PubMed] [Google Scholar]