Abstract
Diabetes is the most prevalent metabolic disorder in the United States, and between 50% and 70% of diabetic patients suffer from diabetes-induced neuropathy. Yet our current knowledge of the functional changes in sensory nerves and their distal terminals caused by diabetes is limited. Here, we set out to investigate the functional and morphological consequences of diabetes on specific subtypes of cutaneous sensory nerves in mice. Diabetes was induced in C57Bl/6 mice by a single intraperitoneal injection of streptozotocin. After 6–8 wk, mice were characterized for behavioral sensitivity to mechanical and heat stimuli followed by analysis of sensory function using teased nerve fiber recordings and histological assessment of nerve fiber morphology. Diabetes produced severe functional impairment of C-fibers and rapidly adapting Aβ-fibers, leading to behavioral hyposensitivity to both mechanical and heat stimuli. Electron microscopy images showed that diabetic nerves have axoplasm with more concentrated organelles and frequent axon-myelin separations compared with control nerves. These changes were restricted to the distal nerve segments nearing their innervation territory. Furthermore, the relative proportion of Aβ-fibers was reduced in diabetic skin-nerve preparations compared with nondiabetic control mice. These data identify significant deficits in sensory nerve terminal function that are associated with distal fiber loss, morphological damage, and behavioral hyposensitivity in diabetic C57Bl/6 mice. These findings suggest that diabetes damages sensory nerves, leading to functional deficits in sensory signaling that underlie the loss of tactile acuity and pain sensation associated with insensate diabetic neuropathy.
Keywords: sensory nerve, C-fiber, skin-nerve, electron microscopy, mechanotransduction
diabetes is the most prevalent metabolic disorder in the United States and is projected to increase threefold by the year 2050 (Boyle et al. 2010). Approximately 50–70% of diabetic patients exhibit diabetic neuropathy (DN), which first emerges in the hands and feet as distal nerve fibers degenerate in a “glove and stocking pattern” (Harati 2007). Patients may experience painful burning and tingling sensations, but all gradually lose normal touch and temperature sensation, leading to accidental personal injuries and impaired quality of life. Despite the prevalence and severe sequelae of DN, the biological mechanisms underlying this devastating disorder remain elusive (Zochodne 1996; Feldman et al. 1999). Hyperglycemia has been identified as the fundamental metabolic disturbance; however, the relationship between early metabolic events, vascular damage, and structural and functional changes in nerves is unclear. Although controlling blood glucose and glycated hemoglobin (HbA1C) levels with insulin remains the most effective method to prevent DN, this approach fails for many patients as it only reduces the incidence of DN by 34% over a 9-yr period (DCCT Research Group 1993). Also, insulin treatment does little for the 7% of patients who already exhibit neuropathy when diagnosed with diabetes (Pirart 1977). Tricyclic antidepressants, duloxetine, gabapentin, aldose-reductase inhibitors, α-lipoic acid, and antioxidants have demonstrated mixed efficacy in treating DN, and practitioners cannot predict the medication(s) that will aid a given patient (Fedele and Giugliano 1997; Ziegler 2009). The combination of both painful symptoms and sensory loss caused by DN further complicates treatment.
From a morphological standpoint, chronic diabetes leads to the degeneration and regeneration of peripheral axons (Greene et al. 1999). Complicating this process, diabetes attenuates the expression of genes that are important in axon regeneration such as neurofilament, Tα1-tubulin, and growth-associated protein-43 mRNA (Mohiuddin et al. 1995; Maeda et al. 1996; Mohiuddin and Tomlinson 1997). Collectively, it is postulated that chronic diabetes damages the distal ends of sensory axons and suppresses axon regeneration, ultimately leading to chronic denervation of cutaneous tissues. Multiple studies using quantitative immunohistochemical assessments of cutaneous innervation have established that human diabetics experiencing decreased tactile sensation concomitantly have significantly reduced dermal and epidermal innervation (McCarthy et al. 1995; Kennedy et al. 1996; Shun et al. 2004; Lauria et al. 2005).
The molecular, anatomic, and behavioral changes associated with diabetes have been studied in numerous rodent models, including streptozotocin (STZ)-treated animals, animals on a high-fat diet, leptin-deficient animals, leptin receptor-deficient animals, insulinopenic animals, hyperinsulinemic animals, and others. These different diabetic models have produced either hyper- or hyposensitivity to heat and mechanical stimuli (for a review, see Obrosova 2009). In some models, the behavioral phenotype appears to depend on the time course of the study. For instance, STZ-treated rats develop thermal and mechanical hyperalgesia after 2–8 wk (Corteix et al. 1993; Calcutt et al. 2004; Li et al. 2005; Talbot et al. 2009) but become hypoalgesic after longer periods of diabetes (Pertovaara et al. 2001; Calcutt et al. 2004; Obrosova et al. 2008). The development of hyper- or hyposensitivity may also be species dependent, as STZ-treated mice develop hyposensitivity to heat and mechanical stimuli (Christianson 2003; Christianson et al. 2007; Drel et al. 2007; Vareniuk et al. 2008).
Here, we use electrophysiological approaches along with histological and behavioral assessments to determine the consequences of diabetes on sensory nerve fiber function in STZ-treated C57Bl/6 mice. Our results demonstrate that diabetes caused morphological changes in the distal saphenous nerve and decreased the proportion of functional Aβ-fibers that innervate diabetic skin. Furthermore, diabetes severely impaired the function of cutaneous C-fibers and rapidly adapting Aβ-fibers. These changes led to the development of behavioral mechanical hypoalgesia in diabetic mice, which contrasts sharply from the hypersensitivity and pain behavior observed in STZ-treated rats. The neuropathic changes in diabetic mice closely resemble the neuropathic changes in human DN, and these results suggest that impairment of sensory nerve function may play an important role in human insensate neuropathy.
MATERIALS AND METHODS
Experimentally induced diabetes.
Six-week-old male C57BL/6 mice (Charles River) were given a single intraperitoneal injection of 180 mg/kg STZ (Sigma) dissolved in 0.4 ml sodium citrate buffer to pH 4.5 (Wang et al. 1993). This dose was sufficient to induce diabetes in 26 of the 33 mice injected, and no mice died as a result of the injection. Nondiabetic control mice were injected with vehicle (0.4 ml sodium citrate buffer). After injection, mice were monitored for symptoms of diabetes, including polydipsia, polyuria, and weight loss. Mice were weighed before STZ injection and weekly thereafter to monitor weight loss. Blood glucose levels were monitored every 2 wk and at death using the Accu Chek Active glucometer (Roche). STZ-injected mice with blood glucose levels of >350 mg/dl (normal blood glucose < 150 mg/dl) were included in the diabetic groups. Diabetic and nondiabetic control (vehicle-injected) mice were killed after 6–8 wk for histology or electrophysiology experiments. Every attempt was made to blind the experimenter to the treatment group of the mice throughout the behavioral, electrophysiological, and morphological experiments. However, for behavioral assays, true blinding was often not possible due to the effects of diabetes on the appearance and body habits of the mice. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin and followed the guidelines established by the National Institutes of Health.
Behavioral testing.
For mechanical stimuli, mice were allowed to acclimate for at least 30 min in a Plexiglass cage on a wire mesh floor before testing. Von Frey monofilaments (0.04, 0.22, 0.27, 0.66, 1.63, 4.0, 6.8, 11.7, 14.6, 20.1, 35.6, 53.9, and 84.8 mN, Smith and Nephew, Germantown, WI) were applied to the plantar surface of each hindpaw. The mechanical stimulus that produced a 50% paw withdrawal threshold was determined using the up-down method (Chaplan et al. 1994). Heat hypersensitivity was assessed using a radiant heat paw withdrawal test (Hargreaves et al. 1988). Mice were allowed to acclimate for at least 30 min in a Plexiglass cage on a glass surface. Radiant noxious heat was applied to the plantar surface of the hindpaw and the latency to paw withdrawal was measured. Each hindpaw was tested four times, and the average paw withdrawal latency was recorded for each mouse. Light intensity was adjusted so that baseline paw withdrawal latencies averaged between 8 and 10 s.
Skin-saphenous nerve preparation.
The saphenous nerve and associated skin from the dorsal hindpaw were dissected free, placed corium side up in an organ bath, and superfused with buffer as previously described (Reeh 1986; Koltzenburg et al. 1997). Thin filaments were teased from the nerve until extracellular recordings were obtained from single fibers. Receptive fields were identified with a mechanical probe. Fibers conducting >10 m/s were classified as myelinated (Aβ) axons, fibers conducting 1.2–10 m/s were classified as thinly myelinated (Aδ) axons, and fibers conducting <1.2 m/s were classified as unmyelinated C-fibers (Koltzenburg et al. 1997).
The mechanical threshold for each fiber was determined by calibrated von Frey monofilaments (0.04–84.8 mN, Smith and Nephew). The mechanical stimulus response properties of each fiber were determined by applying constant force (5–200 mN, 10-s stimulus, 2-min interval between stimuli) via a feedback-controlled, computer-driven mechanical probe (Kwan et al. 2009). Aβ-fibers that fired throughout a sustained force were classified as slowly adapting. Aβ-fibers that fired only at the onset and/or offset of a sustained force were classified as rapidly adapting. Both slowly and rapidly adapting Aβ-fibers had characteristic small receptive fields (<2 mm). Similarly, Aδ-fibers that adapted rapidly to mechanical stimuli were classified as down hair (D-hair) fibers, and Aδ-fibers that adapted slowly were classified as A-mechanoreceptor (AM) fibers. As previously reported, D-hair fibers had large receptive fields (4–8 mm) and were sensitive to very low mechanical forces [<1.0 mN (Stucky et al. 1998)]. For AM and C-fibers, after mechanical stimuli, thermal and chemical stimuli were applied as follows. Receptive fields were superfused with heated buffer, increasing the temperature from 32 to 52°C over 10 s. Subsequently, a 2-min baseline was recorded followed by the application of 1 μM capsaicin for 2 min (Lennertz et al. 2010). The heat response threshold and number of action potentials elicited by heat or capsaicin were quantified. All stimulus traces were collected via a Powerlab/4sp multichannel recorder and analyzed via Chart software (AD Instruments).
Microscopy.
Samples for microscopy consisted of four STZ-treated mice and four nondiabetic control mice. Eight weeks after injection with STZ, animals were killed under anesthesia. The saphenous nerve (proximal nerve samples) and skin from the dorsal hindpaw (distal nerve samples) were exposed and incubated in primary fixative in situ (4% glutaraldehyde-1× PBS, pH 7.4, 5 min). Samples were dissected, immersion fixed at 4°C for 1.5 days, postfixed with 1% osmium tetroxide + 1.5% potassium ferricyanide-PBS for 2 h, dehydrated in ethanol and propylene oxide, and embedded in epon araldite resin, which was then polymerized at 60°C overnight. Semithin sections (0.5 μm) were cut using a RMC Powertome XL, poststained with toluidine blue, and examined using a Nikon Eclipse light microscope. Light micrographs of entire nerve bundles were analyzed for morphology and axon counts. In distal sections, this included subdermal nerve bundles surrounded by a perineurial sheath. Ultrathin sections for electron microscopy were cut on a Reichert Ultracut E, poststained with uranyl acetate and lead citrate, and examined on a JEOL 100CX transmission electron microscope. At least three high-power fields were selected at random from the proximal and distal nerve segments of each animal. Axon area, axon perimeter, and myelin thickness were measured using ImageJ 1.43 software. Separations between the axoplasm and myelin sheath were counted as “axon-myelin separations” when the area of the separation exceeded 6% of the total cross-sectional area of the axon.
Statistical analyses.
For normally distributed data, means ± SD were calculated, and comparisons were made between two groups using Student's t-test or across multiple measurements between two groups using two-way ANOVA followed by a Bonferroni post hoc test (*). For non-normally distributed data, medians ± interquartile ranges were calculated, and comparisons were made between two groups using a Wilcoxon rank-sum test, whereas comparisons between greater than or equal to three groups were made using a Kruskal-Wallace test followed by a post hoc Dunn's multiple-comparison test among selected groups (†). Analyses between two proportions were made using a Fisher's exact test, and between greater than or equal to three proportions were made using a χ2-test followed by the standardized residual method to compare the contributions of individual proportions (‡). Comparisons between sample locations were made using a Mantel-Haenszel test of homogeneity (§).
RESULTS
Diabetic C57Bl/6 mice develop mechanical and thermal hypoalgesia.
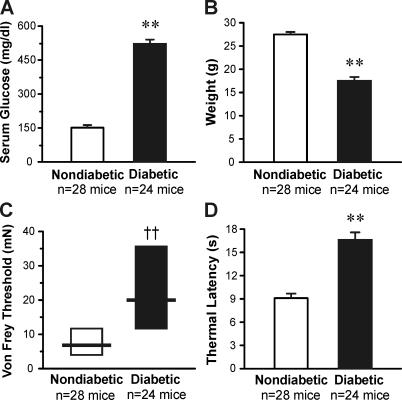
Eight weeks after STZ injection, the serum glucose of diabetic mice was elevated from 151 ± 7 mg/dl in control mice to an average of 521 ± 16 mg/dl (P < .01; Fig. 1A). Whereas nondiabetic control mice weighed an average of 28 ± 1 g, diabetic mice weighed only 17 ± 1 g on average (P < .01; Fig. 1B). Behavioral mechanical threshold and thermal response latency were also measured at this time. Diabetic mice displayed markedly increased mechanical thresholds compared with nondiabetic mice (median of 20 vs. 6.8 mN, respectively, P < .01; Fig. 1C). Diabetic mice also displayed significantly longer withdraw latencies to a noxious heat stimulus compared with nondiabetic animals (17 ± 1 vs. 9 ± 1 s, respectively, P < .01; Fig. 1D). Thus, C57Bl/6 diabetic mice exhibit hypoalgesia to both mechanical and heat stimuli.
Fig. 1.
Streptozotocin (STZ)-treated mice develop diabetes, weight loss, and sensory hypoalgesia over the course of 8 wk. A: average blood glucose level of diabetic (STZ-injected) and nondiabetic (vehicle-injected) mice. B: average weight of diabetic and nondiabetic mice. C: behavioral mechanical response threshold (von Frey filament applied to the plantar hindpaw). D: behavioral thermal response latency (stimulus applied to the plantar hindpaw). **P < 0.01 by Student's t-test; ††P < 0.01 by Wilcoxon rank-sum test.
Nerves from diabetic mice have nearly normal morphology in semithin sections.
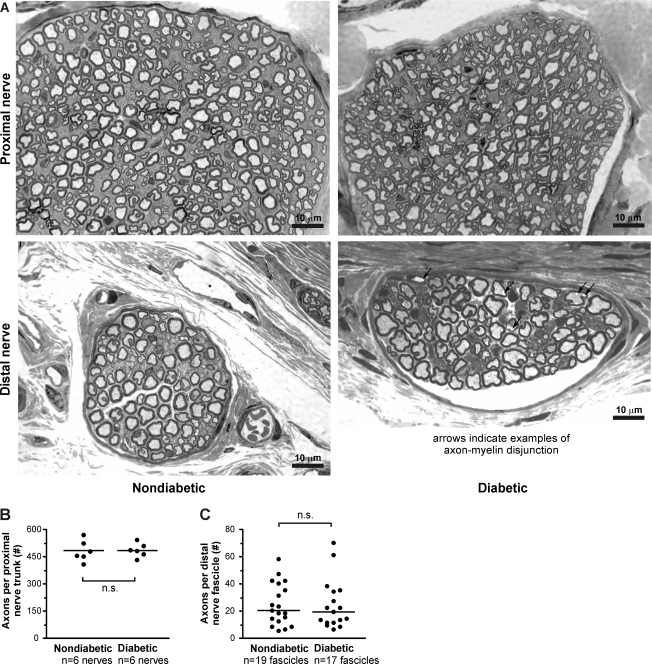
Next, we determined whether diabetes induces nerve fiber loss, demyelination, or changes in axon morphology using light microscopy. Samples were taken from the saphenous nerve trunk above the knee joint (proximal nerve) and at the level of the hindpaw from small fascicles coursing beneath the dermis in the hindpaw (distal nerve). Proximal nerve semithin sections from nondiabetic mice displayed normal morphology. Myelinated nerve fibers appeared mostly round and without pronounced compression, as can be caused by the hypertonic fixative solutions used to fix peripheral nerves (Fig. 2A, top left). Excluding fibers near an internode that naturally appeared crenellated, myelinated nerve fibers appeared slightly less round in proximal nerve semithin sections from diabetic mice. While this appearance could have been caused by high blood glucose levels in diabetic mice, we cannot rule out the possibility that diabetic tissue responded differently than nondiabetic tissue to our fixative solution. Otherwise, proximal nerve segments from diabetic mice exhibited normal myelination and morphology (Fig. 2A, top right). Furthermore, counts of myelinated axons in the proximal nerve trunk did not reveal any nerve fiber loss from diabetic saphenous nerves (Fig. 2B).
Fig. 2.
Diabetic nerves appear mostly similar to nondiabetic nerves at low magnification. A: plastic-embedded 0.5-μm sections of saphenous nerve stained with toluidine blue and imaged by oil immersion light microscopy (×800 magnification). Top, proximal nerve segments from nondiabetic (left) and diabetic mice (right) taken from above the knee; bottom, distal, subdermal nerve segments from nondiabetic (left) and diabetic mice (right) taken from the dorsal hindpaw. Myelinated axons from diabetic mice appeared marginally less circular than axons from nondiabetic mice. Note the separations between the axon and myelin sheath in the distal diabetic nerve (highlighted by arrows). B: myelinated axon counts from nondiabetic and diabetic proximal nerve segments (P > 0.05 by Wilcoxon rank-sum test). C: myelinated axon counts from distal nerve segments (P > 0.05 by Wilcoxon rank-sum test).
Similarly, distal nerve semithin sections from both nondiabetic and diabetic mice exhibited mostly normal structure (Fig. 2A, bottom left and bottom right). In particular, distal nerve sections from diabetic animals did not exhibit demyelination, axonal degeneration, or atrophy. Counts of myelinated axons did not reveal a loss of nerve fibers from distal nerve fascicles in diabetic mice (Fig. 2C). However, unmyelinated axons from diabetic mice consistently appeared more intensely stained by toludine blue. Also, separations between the axon and myelin sheath were noticeable in many myelinated nerve fibers in diabetic nerve fascicles (Fig. 2A, bottom right, highlighted by arrows).
Electron micrographs reveal altered morphology in distal diabetic nerves.
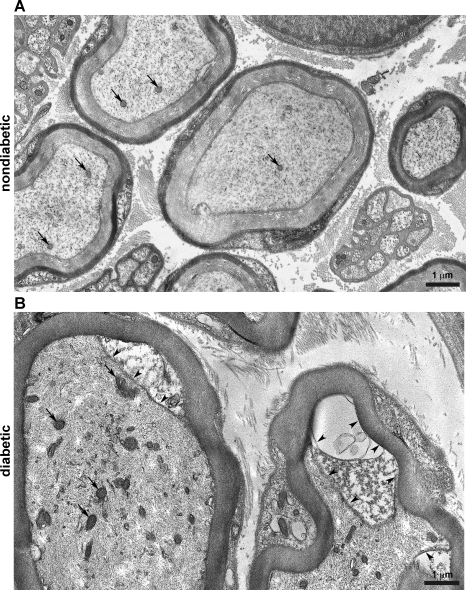
Changes in the morphology of diabetic nerves became increasingly noticeable in higher-magnification electron micrographs of distal nerve segments. Nondiabetic axons exhibited a fine, lightly stained filament network in the axoplasm with some more darkly stained microtubules and few mitochondria (Fig. 3A, arrows). In comparison, the axoplasm of diabetic axons appeared quite dense in neurofilaments and contained numerous mitochondria (Fig. 3B, arrows). The left axon shown in Fig. 3B displayed a small amount of Schwann cell cytoplasm at the axon-myelin junction (arrowheads). The right axon shown in Fig. 3B displayed increasing separation between the axoplasm and myelin, with a larger amount of Schwann cell cytoplasm and a partially extracted area. In many axons, this space became progressively larger near the internode (see Supplemental Material, Supplemental Fig. 2).1 It was noted that the density of axoplasm components was greater near the internode in both nondiabetic and diabetic axons, but nonetheless the density of the axoplasm appeared more dense in diabetic axons than in nondiabetic axons. Although the axoplasm appeared to be more dense in diabetic axons, there was no difference in the cross-sectional area of myelinated nondiabetic axons compared to diabetic axons (Supplemental Fig. 1A).
Fig. 3.
Diabetic nerves exhibit morphological changes at high magnification. A: high-magnification image of nondiabetic axons in a distal nerve segment stained with uranyl acetate and lead citrate (magnification: ×8,710). The central myelinated axon within the size range of a sensory Aβ-fiber (6–12 μm) exhibited a single mitochondrion, a lightly stained neurofilament network, and some more intensely stained microtubules. Smaller myelinated axons within the size range of sensory Aδ-fibers (1–5 μm) exhibited few mitochondria and a similar filament network. B: high-magnification image of diabetic axons in a distal nerve segment (magnification: ×9,260). Both axons fell within the size range of sensory Aβ-fibers. Both axons exhibited an increased number of mitochondria (arrows) and a dense neurofilament network compared with nondiabetic axons. The top right of the left axon demonstrated a widened interface between the Schwann cell and the axoplasm (arrowheads). The right axon demonstrated a similar area that was partly extracted (arrowheads).
Unmyelinated axons from diabetic mice stained more intensely than unmyelinated axons from nondiabetic mice (Fig. 4A, arrowheads). This made individual components of the axoplasm in unmyelinated axons more difficult to discern in diabetic mice. This effect was not due to a decrease in the size of unmyelinated axons. Rather, unmyelinated axons were slightly larger in proximal nerve segments from diabetic mice (P < 0.001; Supplemental Fig. 1B). However, as the size distribution of unmyelinated axons overlaps almost completely between nondiabetic and diabetic samples, it is not clear that this represents a meaningful change in the caliber of unmyelinated axons.
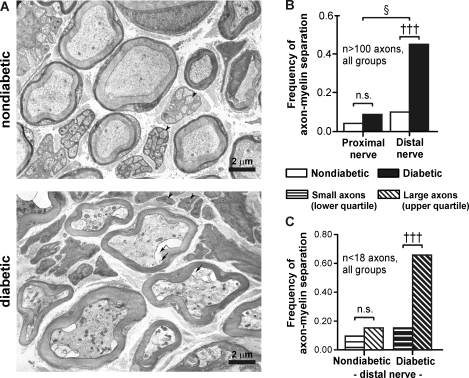
Fig. 4.
Axon-myelin separations are prevalent in distal axons from diabetic mice. A: electron micrographs of myelinated and unmyelinated axons in distal nerve segments from nondiabetic (top) and diabetic mice (bottom) stained with uranyl acetate and lead citrate (×3,430 magnification). Myelinated axons exhibited axon-myelin separations and numerous intracellular organelles compared with nondiabetic axons. Arrows highlight lipid-like material within axon-myelin separations. Arrowheads highlight some unmyelinated axons, which appeared more electron dense in diabetic nerves. B: frequency of axon-myelin separation in proximal versus distal nerve segments in nondiabetic and diabetic mice. C: frequency of axon-myelin separation in small-diameter axons (lower quartile) versus large-diameter axons (upper quartile). Quartiles were defined from the size distribution of all myelinated axons in nondiabetic or diabetic samples. †††P < 0.001 by a Kruskal-Wallis test followed by a post hoc Dunns multiple-comparison test between selected groups; §P < 0.05 by a Mantel-Haenszel test of homogeneity.
Separations between the axoplasm and myelin sheath were prevalent in distal diabetic nerves, occurring in 47% of myelinated axons, and contained varying amounts of material in the developing space (Fig. 4A, bottom, arrows). In comparison, separations between the axon and myelin sheath were smaller in size and were present in only 10% of distal nondiabetic nerve axons (P < 0.001; Fig. 4B). Axon-myelin separations were also infrequent in the proximal segments of nondiabetic and diabetic nerves (5% and 8%, respectively). Thus, axon-myelin separations were restricted to distal segments of diabetic nerves (P < 0.05; Fig. 4B).
In addition, we examined distal myelinated axons according to their size distribution (in μm2). Since Aβ-fiber axons are generally larger than Aδ-fiber axons, we reasoned that the upper quartile of the distribution would contain mostly Aβ-fiber axons and the lower quartile would contain mostly Aδ-fiber axons. In diabetic mice, the frequency of axon-myelin separations was strikingly high (66%) among axons in the upper quartile compared with axons in the lower quartile of the size distribution (17%, P < 0.001; Fig. 4C, right). In contrast, the frequency of these changes did not change between the upper and lower quartiles in nondiabetic mice (16% and 10%, respectively; Fig. 4C, left). These data suggest that Aβ-fiber axons exhibit axon-myelin separations more frequently than Aδ-fiber axons in diabetic mice. These morphological findings support electrophysiological data (see below) indicating that myelinated Aβ-fibers are particularly affected during diabetes in C57Bl/6 mice (Fig. 5).
Fig. 5.
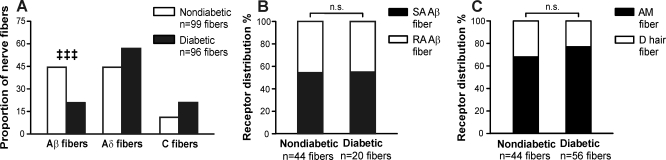
Diabetic mice preferentially lose functional Aβ-fiber innervation. A: proportion of functional Aβ-, Aδ-, and C-fibers identified in diabetic and nondiabetic skin-nerve preparations. B: proportion of functional Aβ-fibers that were rapidly adapting (RA) versus slowly adapting (SA) in diabetic and nondiabetic skin-nerve preparations. C: proportion of functional Aδ fibers that were rapidly adapting (D hair) versus slowly adapting (AM) in diabetic and nondiabetic skin-nerve preparations. ‡‡‡P < 0.001 by a χ2-test followed by analysis of standardized residuals.
Diabetic mice lose functional Aβ-fiber innervation.
To examine functional cutaneous innervation, diabetic and nondiabetic mice were killed for ex vivo teased fiber recordings from the hindpaw skin 1–2 days after the behavioral measurements were made. Mechanical search stimuli were used to identify isolated receptive fields of nerve fibers. Each fiber encountered was characterized according to its conduction velocity and mechanical adaptation. Aβ-fibers (>10 m/s) accounted for 44% of all nerve fibers classified from nondiabetic skin but only 21% of nerve fibers classified from diabetic skin (P < .001; Fig. 5A). A reciprocal trend was observed in the prevalence of Aδ-fibers and C-fibers, as would be the expected result of a relative loss of Aβ-fibers (1.2–10 and <1.2 m/s, respectively). No changes were observed in the ratio of rapidly adapting versus slowly adapting Aβ-fibers in diabetic versus nondiabetic preparations (Fig. 5B). Similarly, no significant changes were observed in the ratio of rapidly adapting Aδ (D-hair)-fibers versus slowly adapting Aδ (AM)-fibers (Fig. 5C). The loss in Aβ-fibers is consistent with the observed increase in behavioral mechanical thresholds among diabetic mice, as slowly adapting Aβ-fibers and rapidly adapting Aβ-fibers mediate light touch sensation.
C-fiber mechanical responses are markedly impaired in diabetic mice.
Although we observed few examples of overt axon degeneration, the majority of axons in the diabetic nerve fascicles exhibited separations from the myelin sheath and a density of axoplasm components that may indicate compromised axon function. Therefore, we tested the mechanical threshold and suprathreshold responsiveness of individual nerve fibers. Mechanical thresholds were assessed by applying calibrated von Frey filaments to the cutaneous receptive field of nerve fibers in the skin-nerve preparation. There was a nonsignificant trend toward increased mechanical thresholds in C-fibers from diabetic mice (P = 0.08; Table 1). However, no significant differences were detected in mechanical thresholds among the subtypes of nerve fibers in diabetic and nondiabetic mice (Table 1).
Table 1.
Sensory nerve fiber mechanical thresholds are unaffected in diabetic mice
| Control (n = 99) |
Diabetic (n = 96) |
|||||
|---|---|---|---|---|---|---|
| Median | Interquartile range | n | Median | Interquartile range | n | |
| Slowly adapting Aβ | 0.7 | 0.7–1.6 | 24 | 0.7 | 0.7–1.1 | 11 |
| Rapidly adapting Aβ | 0.7 | 0.3–0.7 | 20 | 0.2 | 0.04–0.3 | 9 |
| Aδ A-mechanoreceptor | 1.6 | 1.6–4.0 | 30 | 4.0 | 1.6–6.8 | 43 |
| Aδ down hair | 0.1 | 0.04–0.2 | 14 | 0.2 | 0.04–0.2 | 13 |
| C-fiber | 4.0 | 1.6–4.0 | 11 | 6.8 | 2.8–9.3 | 20 |
Values are von Frey thresholds (in mN); n, number of nerve fibers. Median von Frey thresholds and interquartile ranges for nerve fiber subtypes in diabetic and nondiabetic mice are shown. Subtypes were compared using Wilcoxon rank-sum tests.
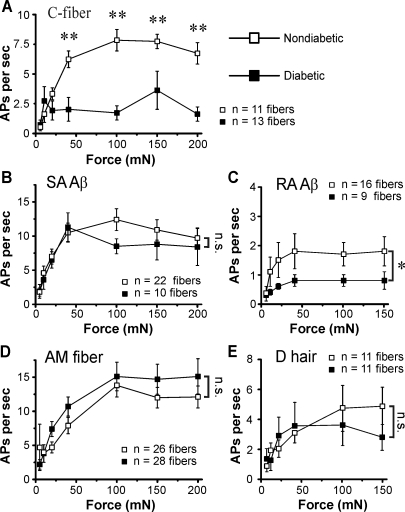
Subsequently, we assessed the mechanical responsiveness of nerve fibers to suprathreshold force. Increasing mechanical forces between 5 and 200 mN were applied to individual receptive fields, and the number of action potentials elicited at each force was compared between diabetic and nondiabetic mice. Diabetes had the greatest effect on unmyelinated C-fibers. C-fibers from diabetic mice exhibited tepid action potential firing to mechanical stimuli at forces of >20 mN (P < 0.01; Fig. 6A). Normally, C-fibers faithfully encode the intensity of mechanical stimuli by firing action potentials in direct relation to the stimulus intensity (Stucky et al. 1999). However, C-fibers from diabetic animals failed to encode the intensity of the stimulus as they responded weakly to mechanical stimuli, especially at high-intensity forces. Interestingly, other subtypes of neurons with slowly adapting response properties were essentially normal in diabetic mice. For example, AM nociceptors and slowly adapting Aβ-fibers were essentially normal in their responsiveness to mechanical stimuli (Fig. 6, B and D). Rapidly adapting Aβ-fibers from diabetic mice also responded significantly less to mechanical force than fibers from nondiabetic mice (P < 0.05; Fig. 6C). In fact, forces above 5 mN elicited only half as many action potentials in diabetic nerve fibers. In contrast, the mechanical response properties of rapidly adapting D-hair afferents were unaffected in diabetic animals (Fig. 6E). Thus, in addition to distal cutaneous denervation, these functional changes in mechanical responsiveness in the sensory terminal of mechanoreceptors would further contribute to the hypoalgesia observed in diabetic C57Bl/6 mice.
Fig. 6.
Diabetes impairs sensory nerve fiber function. A–E: force stimulus response curves in C-fibers (5–200 mN, 10 s each, 1-min interstimulus interval; A) and SA Aβ-fiber responses (B), RA Aβ-fiber responses (C), AM fiber responses (D), and D-hair fiber responses (E) to increasing mechanical stimuli in diabetic versus nondiabetic mice. AP, action potential. *P < 0.05 and **P < 0.01 by two-way ANOVA followed by a post hoc Bonferroni t-test.
C-fibers exhibit an increased threshold to heat stimuli in diabetic mice.
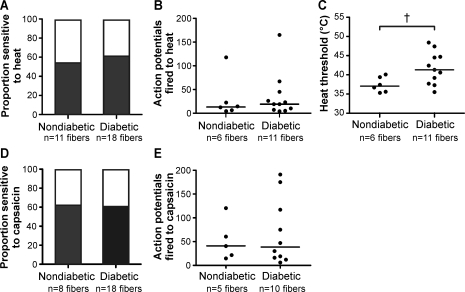
As diabetic mice exhibit significantly delayed behavioral responses to heat, we also examined the response of C-fibers to heat stimuli. Characterized C-fibers were superfused with heated buffer, warming the receptive field from 32 to 52°C over 10 s. We found no difference in the proportion of C-fibers that responded to the heat ramp (54% of nondiabetic C-fibers vs. 61% of diabetic C-fibers, P = 0.39; Fig. 7A). Also, the heat stimulus evoked similar numbers of action potentials from heat-sensitive C-fibers in nondiabetic and diabetic mice (mean of 35 vs. 29 action potentials, respectively, P = 0.55; Fig. 7B). However, C-fibers from diabetic mice exhibited significantly elevated heat response thresholds compared with nondiabetic mice (median 41.3 vs. 37.1°C, respectively, P < 0.05; Fig. 7C). Some AM fibers are sensitive to heat stimuli and contribute to behavioral heat responses. However, we did not record from enough heat-sensitive AM fibers to compare heat responses between diabetic and nondiabetic animals (n = 2 for nondiabetic mice and 3 for diabetic mice). Thus, diabetes increases the threshold at which C-fibers respond to heat stimuli. However, the proportion of heat-sensitive C-fibers and the magnitude of responses to heat stimuli were unaffected.
Fig. 7.
Diabetes increases heat response thresholds in C-fibers. A: proportion of C-fibers sensitive to heat. B: numbers of APs elicited by heat ramp (32 to 52°C over 10 s). C: heat response threshold. D: proportion of C-fibers sensitive to 1 μM capsaicin. E: numbers of APs elicited by 1 μM capsaicin. †P < 0.05 by a Wilcoxon rank-sum test.
After the heat stimuli, C-fibers were exposed to the transient receptor potential (TRP)V1 channel agonist capsaicin. The TRPV1 channel is sensitive to temperatures above 43°C and is important for normal behavioral responses to heat stimuli. As with heat responses, similar proportions of C-fibers responded to capsaicin in nondiabetic and diabetic animals (62% vs. 61%, respectively; Fig. 7D). Furthermore, the number of action potentials elicited by capsaicin was similar between nondiabetic and diabetic animals (median of 41 vs. 39 action potentials, respectively; Fig. 7E). These data suggest that neither TRPV1 expression or function are considerably altered in diabetic mice.
Nerve fiber conduction velocity is maintained in diabetic mice.
The in vitro skin-nerve preparation allowed the assessment of conduction velocities in individual sensory nerve axons. Here, the conduction velocities of subtypes of sensory nerve fibers in diabetic and nondiabetic animals were quantified. No significant changes in conduction velocity were evident among the various subtypes of sensory nerve fibers in diabetic animals (Table 2). It is plausible to suggest that no differences were observed because conduction velocity itself was one of the parameters used to classify nerve fibers and thus slow-conducting fibers may have been misclassified as a different fiber subtype. While this is possible, we observed no examples of oddly characterized fibers. For instance, we observed no “D-hairs with abnormally small receptive fields” that could be slow-conducting rapidly adapting Aβ-fibers. Of note, an overall decrease in conduction velocity would have been reported in diabetic mice if sensory nerve fibers had not been classified into subtypes. This resulted from the loss of functional Aβ-fibers in diabetic skin-nerve preparations.
Table 2.
Sensory nerve fiber conduction velocity is unaffected in diabetic mice
| Control (n = 99) |
Diabetic (n = 96) |
|||
|---|---|---|---|---|
| Average | n | Average | n | |
| Slowly adapting Aβ | 16.5 ± 1.1 | 24 | 16.4 ± 1.6 | 11 |
| Rapidly adapting Aβ | 17.1 ± 1.1 | 20 | 16.4 ± 1.5 | 9 |
| Aδ A-mechanoreceptor | 5.3 ± 0.3 | 30 | 4.6 ± 0.4 | 43 |
| Aδ down hair | 5.5 ± 0.8 | 14 | 6.6 ± 0.5 | 13 |
| C-fiber | 0.6 ± 0.0 | 11 | 0.6 ± 0.0 | 20 |
Values are conduction velocities (in m/s); n, number of nerve fibers. Average conduction velocities for nerve fiber subtypes in diabetic and nondiabetic mice are shown. Subtypes were compared using Student's t-tests.
DISCUSSION
The goal of this study was to identify the effects of diabetes on peripheral nerves of diabetic C57Bl/6 mice in the setting of insensate neuropathy. Behavioral, histological, and electrophysiological approaches revealed marked morphological and functional changes in peripheral nerve fibers 6–8 wk after the induction of diabetes. Notably, the mechanical responses of C-fibers and rapidly adapting Aβ light touch mechanoreceptors were markedly impaired in diabetic mice.
Our electrophysiological results provide novel insights into the function of sensory nerve fibers in mice with diabetic neuropathy. Unmyelinated C-fibers from diabetic mice responded poorly to mechanical stimuli across a range of forces (40–200 mN) and failed to increase their response to more intense stimuli. Furthermore, heat response thresholds were significantly increased in C-fibers from diabetic mice. These results differ from teased fiber recordings in STZ-treated rats, in which C-fibers exhibit decreased mechanical thresholds and increased responsiveness to suprathreshold stimuli, reflecting sensitization of nociceptors in the rat. Of note, these rat recordings were performed at a time point when the animals exhibited behavioral hypersensitivity to mechanical stimuli (Chen and Levine 2001; Suzuki et al. 2002).
C-fibers normally encode a variety of noxious and innocuous stimuli, encode the stimulus intensity, and play an important role in avoiding tissue injury. C-fibers in diabetic mice no longer fulfill this role, at least for mechanical stimuli, as noxious forces elicited similar numbers of action potentials as innocuous forces. Although C-fibers in diabetic animals respond to heat with similar numbers of action potentials, the increase in heat response threshold coincides with a clear behavioral hyposensitivity to heat. Interestingly, not all nociceptor-type populations were affected by diabetes, as myelinated AM fibers exhibited normal mechanical responsiveness in diabetic animals. Nonetheless, C-fibers account for 60–75% of all axons in peripheral cutaneous nerves, and many C-fibers summate on second-order spinal cord neurons. Therefore, the deficit in C-fiber function could greatly affect the detection of acute noxious and innocuous force (Griffin et al. 2001).
Diabetes also reduced the mechanical firing of rapidly adapting Aβ-fibers. Rapidly adapting Aβ-fibers are light touch mechanoreceptors that innervate guard hair follicles in hairy skin and Meissner corpuscles in glabrous skin. The exquisite sensitivity of rapidly adapting Aβ-fibers to dynamic stimuli greatly enhances our perception of object texture and edges. Thus, deficits in rapidly adapting Aβ function could lead to a considerable loss of normal tactile acuity in diabetic animals.
Significant changes to the structure of distal myelinated nerve fibers occurred in diabetic mice. Myelinated axons contained numerous organelles, possibly reflecting impaired transport along nerve fibers or an increase in the number of mitochondira within the axoplasm. Also, myelinated axons were frequently separated from their myelin sheath. These separations did not appear to result from axonal atrophy, as axons from diabetic mice were not significantly smaller than axons from nondiabetic mice (Supplemental Fig. 1). Several scorpion venom toxins have been shown to induce periaxonal edema, presumably due to an osmotic imbalance due to Na+ channel dysfunction (Love et al. 1986). Furthermore, high plasma galactose concentrations produce a hyperosmolar perineurial environment, increase the amount of glycogen present in Schwann cells, and lead to axon-myelin separation in rat studies (Powell and Myers 1983; Myers and Powell 1984). Thus, axon-myelin separations in diabetic mouse axons may reflect an osmotic imbalance due to hyperglycemia, accumulation of glycogen in the Schwann cell, or, secondarily, membrane channel dysfunction in diabetic mice. Separations between the axon and myelin sheath could also reflect axoglial disjunction in the paranodal region of diabetic nerve fibers, as previously described in diabetic rats (Sima et al. 1986). Importantly, changes in plasma osmolarity due to elevated glucose concentration have been demonstrated to directly affect sensory nerve fiber function in diabetic rats (Suzuki et al. 2002).
Morphological analyses highlighted two important features of neuropathy in STZ-treated mice. First, axon-myelin separations were restricted to distal nerve segments nearing their innervation territory in the skin of diabetic mice. This pattern is consistent with the pattern of peripheral neuropathy observed in human diabetic patients, where the distal aspects of long peripheral nerve fibers are affected first and most severely. Second, the axon-myelin separations were significantly more common among large-caliber axons in diabetic mice. This finding suggests that Aβ-fiber axons are most susceptible to damage after 6–8 wk of diabetes and supports electrophysiological data that demonstrate a preferential loss of functional Aβ-fiber innervation in the skin.
Morphological changes coincided with a shift in the relative abundance of functional cutaneous nerve fibers. A lesser proportion of heavily myelinated Aβ-fibers was encountered in diabetic skin-nerve preparations than in control preparations. However, there was no change in the proportion of rapidly adapting versus slowly adapting subtypes among either Aβ- or Aδ-fibers. Thus, diabetes equally affected both rapidly adapting Aβ-fibers, which predominately innervate hair follicles (in hairy skin) or Meissner corpuscles (in glabrous skin), and slowly adapting Aβ fibers, which terminate either on Merkel cells (SAI) or as free nerve endings (SAII) at the dermal-epidermal border (Lumpkin and Caterina 2007; Maricich et al. 2009). Previous studies have reported losses of both myelinated and unmyelinated nerve fibers in diabetic skin.
Although conduction abnormalities have been described in human diabetics and in animal models of diabetes, we did not observe significant changes to the conduction velocity of diabetic nerve fibers in mice. Human and rat studies have reported nerve conduction slowing after several months to several years of diabetes and in the context of severe nerve fiber pathology (Sima et al. 1982). In contrast, we observed only modest nerve fiber pathology after a relatively short (6–8 wk) duration of diabetes. In particular, there was no evidence of demyelination in diabetic mice. Thus, the absence of conduction velocity abnormalities may reflect the absence of segmental demyelination and remyelination in diabetic mice during the initial 8 wk of diabetes. A longer time course of diabetes may be necessary for diabetic mice to develop pronounced nerve fiber pathology and to observe a change in nerve fiber conduction velocity.
In summary, diabetes impaired the function of sensory nerve fibers and reduced the abundance of functional Aβ-fibers in diabetic skin, resulting in behavioral hyposensitivity to mechanical and heat stimuli. Histological analyses revealed changes that were restricted to distal segments of diabetic nerve fibers. Presumably, these represent early pathological changes associated with murine diabetes. Thus, diabetic mice develop a peripheral, insensate neuropathy that shares physiological and anatomical similarities with insensate DN in humans. Importantly, our findings suggest that the sensory losses experienced by human diabetic patients may be due to impaired nerve fiber function as well as sensory denervation.
GRANTS
This work was supported by the Juvenile Diabetes Research Foundation and National Institute of Neurological Disorders and Stroke Grants RO1-NS-43314 (to D. E. Wright) and NS040538 and NS070711 (to C. L. Stucky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Janelle Williams for help with the experimental procedures.
Footnotes
Supplemental Material for this article is available at the Journal of Neurophysiology website.
REFERENCES
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8: 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA, Freshwater JD, Mizisin AP. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia 47: 718–724, 2004 [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63, 1994 [DOI] [PubMed] [Google Scholar]
- Chen X, Levine JD. Hyper-responsivity in a subset of C-fiber nociceptors in a model of painful diabetic neuropathy in the rat. Neuroscience 102: 185–192, 2001 [DOI] [PubMed] [Google Scholar]
- Christianson J. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. Pain 4: 493–504, 2003 [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience 145: 303–313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courteix C, Eschalier A, Lavarenne J. Streptozotocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain 53: 81–88, 1993 [DOI] [PubMed] [Google Scholar]
- DCCT Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- Drel VR, Pacher P, Vareniuk I, Pavlov IA, Ilnytska O, Lyzogubov VV, Bell SR, Groves JT, Obrosova IG. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int J Mol Med 20: 783–792, 2007 [PMC free article] [PubMed] [Google Scholar]
- Fedele D, Giugliano D. Peripheral diabetic neuropathy. Current recommendations and future prospects for its prevention and management. Drugs 54: 414–421, 1997 [DOI] [PubMed] [Google Scholar]
- Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol 12: 553–563, 1999 [DOI] [PubMed] [Google Scholar]
- Greene DA, Stevens MJ, Feldman EL. Diabetic neuropathy: scope of the syndrome. Am J Med 107: 2S–8S, 1999 [DOI] [PubMed] [Google Scholar]
- Griffin JW, McArthur JC, Polydefkis M. Assessment of cutaneous innervation by skin biopsies. Curr Opin Neurol 14: 655–659, 2001 [DOI] [PubMed] [Google Scholar]
- Harati Y. Diabetic neuropathies: unanswered questions. Neurol Clin 25: 303–317, 2007 [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32: 77–88, 1988 [DOI] [PubMed] [Google Scholar]
- Johnson MS, Ryals JM, Wright DE. Early loss of peptidergic intraepidermal nerve fibers in an STZ-induced mouse model of insensate diabetic neuropathy. Pain 140: 35–47, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Johnson T. Quantitation of epidermal nerves in diabetic neuropathy. Neurology 47: 1042–1048, 1996 [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophys 78: 1841–1850, 1997 [DOI] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29: 4808–4819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 12: 747–758, 2005 [DOI] [PubMed] [Google Scholar]
- Lennertz RC, Tsunozaki M, Bautista DM, Stucky CL. Physiological basis of tingling paresthesia evoked by hydroxyl-alpha-sanshool. J Neurosci 30: 4353–4361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Drel VR, Szabó C, Stevens MJ, Obrosova IG. Low-dose poly(ADP-ribose) polymerase inhibitor-containing combination therapies reverse early peripheral diabetic neuropathy. Diabetes 54: 1514–1522, 2005 [DOI] [PubMed] [Google Scholar]
- Love S, Cruz-Höfling MA, Duchen LW. Morphological abnormalities in myelinated nerve fibres caused by Leiurus, Centruroides and Phoneutria venoms and their prevention by tetrodotoxin. Q J Exp Physiol 71: 115–122, 1986 [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature 445: 858–865, 2007 [DOI] [PubMed] [Google Scholar]
- Maeda K, Fernyhough P, Tomlinson DR. Regenerating sensory neurones of diabetic rats express reduced levels of mRNA for GAP-43, gamma-preprotachykinin and the nerve growth factor receptors, trkA and p75NGFR. Mol Brain Res 37: 166–174, 1996 [DOI] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science 324: 1580–1582, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology 45: 1848–1855, 1995 [DOI] [PubMed] [Google Scholar]
- Mohiuddin L, Fernyhough P, Tomlinson DR. Reduced levels of mRNA encoding endoskeletal and growth-associated proteins in sensory ganglia in experimental diabetes. Diabetes 44: 25–30, 1995 [DOI] [PubMed] [Google Scholar]
- Mohiuddin L, Tomlinson DR. Impaired molecular regenerative responses in sensory neurones of diabetic rats: gene expression changes in dorsal root ganglia after sciatic nerve crush. Diabetes 46: 2057–2062, 1997 [DOI] [PubMed] [Google Scholar]
- Myers RR, Powell HC. Galactose neuropathy: impact of chronic endoneurial edema on nerve blood flow. Ann Neurol 16: 587–594, 1984 [DOI] [PubMed] [Google Scholar]
- Obrosova I, Xu W, Lyzogubov VV, Ilnytska O, Mashtalir N, Vareniuk I, Pavlov IA, Zhang J, Slucher B, Drel VR. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med 44: 972–981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova I. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeautics 6: 638–647, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A, Wei H, Kalmari J, Ruotsalainen M. Pain behavior and response properties of spinal dorsal horn neurons following experimental diabetic neuropathy in rat: modulation by nitecapone, a COMT inhibitor with antioxidant properties. Exp Neurol 167: 425–434, 2001 [DOI] [PubMed] [Google Scholar]
- Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 [author's translation]. Diabete Metab 3: 97–107, 1977 [PubMed] [Google Scholar]
- Powell HC, Myers RR. Schwann cell changes and demyelination in chronic galactose neuropathy. Muscle Nerve 6: 218–227, 1983 [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett 66: 141–146, 1986 [DOI] [PubMed] [Google Scholar]
- Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 127: 1593–1605, 2004 [DOI] [PubMed] [Google Scholar]
- Sima AA, Lattimer SA, Yagihashi S, Greene DA. Axo-glial dysjunction. A novel structural lesion that accounts for poorly reversible slowing of nerve conduction in the spontaneously diabetic bio-breeding rat. J Clin Invest 77: 474–484, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AA, Lorusso AC, Thibert P. Distal symmetric polyneuropathy in the spontaneously diabetic BB-wistar rat. An ultrastructural and teased fiber study. Acta Neuropathol 58: 39–47, 1982 [DOI] [PubMed] [Google Scholar]
- Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M. Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci 18: 7040–7046, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci 19: 8509–8516, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Sato J, Kawanishi M, Mizumura K. Lowered response threshold and increased responsiveness to mechanical stimulation of cutaneous nociceptive fibers in streptozotocin-diabetic rat skin in vitro–correlates of mechanical allodynia and hyperalgesia observed in the early stage of diabetes. Neurosci Res 43: 171–178, 2002 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Sato J, Kawanishi M, Mizumura K. Tissue glucose level modulates the mechanical responses of cutaneous nociceptors in streptozotocin-diabetic rats but not normal rats in vitro. Pain 99: 475–484, 2002 [DOI] [PubMed] [Google Scholar]
- Talbot S, Théberg-Turmel P, Liazogli D, Sénécal J, Gaudreau P, Couture R. Cellular localization of kinin B1 receptor in the spinal cord of streptozotocin-diabetic rats with a fluorescent [Nalpha-Bodipy]-des-Arg9-bradykinin. J Neuroinflammation 16: 11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareniuk I, Pavlov IA, Obrosova IG. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia 51: 2126–2133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Dohle C, Friemann J, Green BS, Gleichmann H. Prevention of high- and low-dose STZ-induced diabetes with d-glucose and 5-thio-d-glucose. Diabetes 42: 420–428, 1993 [DOI] [PubMed] [Google Scholar]
- Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care 32, Suppl 2: S414–S419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW. Is early diabetic neuropathy a disorder of the dorsal root ganglion? A hypothesis and critique of some current ideas on the etiology of diabetic neuropathy. J Periph Nerv Syst 1: 119–130, 1996 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.