Abstract
Tobacco use is a major public health problem, and although many smokers report that they want to quit, only a small percentage succeed. Side effects associated with nicotine withdrawal, including depression, anxiety, and restlessness, certainly contribute to the low success rate. The dorsal raphe nucleus (DRN) is a serotonergic center with many functions, including control of mood and emotional state. We investigated the effect of nicotine on DRN neurons that project to the nucleus accumbens (NAc), an area involved in reward-related behaviors. Using a retrograde labeling method, we found that 75% of DRN-NAc projection neurons are serotonergic. In coronal slices that include the DRN, whole cell recordings were conducted on neurons identified by fluorescent backlabeling from NAc or randomly selected within the nucleus. Nicotine increased action potential firing rates in a subset of DRN neurons. Voltage-clamp recording revealed nicotinic acetylcholine receptor (nAChR)-mediated inward currents that contribute to the nicotine-induced excitation. Nicotinic receptors also indirectly affect excitability by modulating synaptic inputs to these neurons. Nicotine enhanced excitatory glutamatergic inputs to a subset of DRN-NAc projection neurons, while inhibitory γ-aminobutyric acid (GABA)ergic inputs were modulated either positively or negatively in a subset of these neurons. The net effect of nAChR activation is enhancement of serotonergic output from DRN to the NAc, which may contribute to the effects of nicotine on mood and affect.
Keywords: nicotine, dorsal raphe nucleus, addiction, serotonin, nicotine withdrawal
tobacco is one of the most commonly abused substances in the world and the leading cause of preventable illness in the United States (Centers for Disease Control and Prevention 2004). A large percentage of smokers attempt to quit each year, but remarkably few succeed. Withdrawal symptoms, including anxiety, irritability, restlessness, craving, and depression, contribute to the low success rate (Kenny and Markou 2001; Koob 2000). In addition, there is a strong association between tobacco use and psychiatric disturbances (Anda et al. 1990; Killen et al. 2003).
Serotonin contributes to mood, depression, and psychiatric illness, and several animal studies suggest that changes in serotonin transmission may also contribute to the affective symptoms of nicotine withdrawal (Cheeta et al. 2001; Kenny and Markou 2001; Seth et al. 2002). Serotonin reuptake inhibitors (SSRIs) can diminish the anhedonic effects of nicotine withdrawal when administered with a blocker of 5-HT1A receptors (Harrison et al. 2001). While humans do not experience significantly improved smoking cessation in SSRI clinical trials, subjects in the treatment group report significantly lower withdrawal symptoms than control subjects (Hughes et al. 2007; Saules et al. 2004).
The rewarding effects of many drugs, including nicotine, are mediated through dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (Di Chiara and Imperato 1988). Serotonergic projections from the dorsal raphe nucleus (DRN) to the NAc may also contribute to addiction (Tork 1990) (Fig. 1A). In the case of nicotine, at least some of the behavioral reinforcement derives from the anxiolytic effects of the drug, which are mediated by serotonin (Cheeta et al. 2001). It has been hypothesized that a nicotine-induced alteration in the balance between serotonin and dopamine transmission in brain reward areas is important in establishing the addicted phenotype (Olausson et al. 2002). The cellular mechanisms that underlie nicotinic modulation of serotonin signaling and its ultimate role in reward and withdrawal are unclear.
Fig. 1.
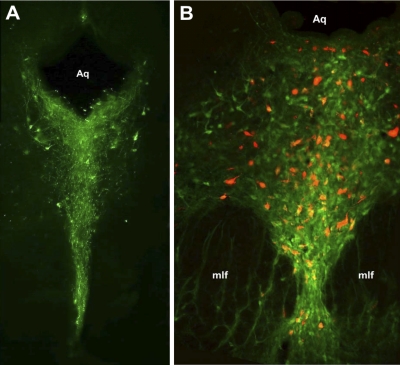
The dorsal raphe nucleus (DRN) projects to the nucleus accumbens (NAc). A: simplified diagram of parasagittal section illustrating DRN and ventral tegmental area (VTA) projections to NAc. PAG, periaqueductal gray. B: diagram of coronal section at the level of the NAc, bregma +1.00. C: light micrograph showing example of bilateral Fluoro-Red injection sites in NAc. Most injections occurred in both core and shell subregions. D: diagram of coronal section at the level of the PAG, bregma −7.80. E: fluorescent image showing retrograde labeling of neurons in the DRN that project to the NAc. Aq, aqueduct; mlf, medial longitudinal fasciculus. Diagrams in B and D are adapted from Paxinos and Watson (1998), Copyright Elsevier.
Electrophysiology studies have revealed the influence of nicotine on serotonin release from the DRN. Serotonergic DRN cells express functional nicotinic acetylcholine receptors (nAChRs) and receive cholinergic input from the pedunculopontine tegmentum (Galindo-Charles et al. 2008). The majority of serotonergic DRN neurons increase their action potential (AP) firing in response to nicotine, and this leads to increased serotonin release (Li et al. 1998; Mihailescu et al. 2001, 2002). Here we have confirmed earlier findings demonstrating an anatomic connection between the mood and affect control centers of the DRN and the reward-associated NAc (Li et al. 1989). Physiological recordings from DRN neurons were conducted to assess the cellular mechanisms underlying nicotinic excitation of putative serotonergic neurons projecting to the NAc. Exploring the effects of nicotine on the serotonergic inputs to the NAc may provide insight into the link between nicotine and mood, which may influence both the addictive and withdrawal effects of the drug.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (>P60, weight 180–250 g at delivery; Harlan, Indianapolis, IN) were housed one or two per cage with a reverse light-dark cycle and free access to water and food. All animal experiments were approved by the University of Chicago Institutional Animal Care and Use Committee.
Survival surgery.
To fluorescently label DRN-NAc projections, rats were anesthetized with intraperitoneal injections of 100 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 10 mg/kg xylazine (Ben Venue Laboratories, Bedford, OH). Bilateral stereotaxic (David Kopf Instruments, Tujunga, CA) NAc injections of 0.25 μl of Fluoro-Red dye (Tombow Pencil, Tokyo, Japan) (Dong et al. 1996; Suzuki et al. 2004) were performed with bregma coordinates A/P: +1.5, L: +2.6, D/V: −8.1, angle 10° off the vertical (Paxinos and Watson 1998). The animals were allowed to recover for a minimum of 96 h before anatomic or electrophysiological investigation.
Electrophysiology.
Rats were anesthetized with isoflurane (Abbott Labs, North Chicago, IL) and rapidly decapitated. The brains were dissected in a solution of ice-cold sucrose-artificial cerebrospinal fluid (aCSF) (in mM: 250 sucrose, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 20 glucose, 1 NaH2PO4, 25 NaHCO3, 10 ascorbic acid) and bubbled with 95% O2-5% CO2 during dissection and slicing. Coronal slices (250 μm thick) including the DRN were prepared on a vibratome and then transferred to a chamber with 32°C aCSF (in mM: 125 NaCl, 2.5 KCl, 1 MgCl2, 2.5 CaCl2, 20 glucose, 1 NaH2PO4, 25 NaHCO3, 1 ascorbic acid), which was bubbled constantly with 95% O2-5% CO2 and perfused at a rate of 20 ml/min.
For recording, the slices were transferred to a chamber continuously perfused with aCSF without 1 mM ascorbic acid and bubbled with 95% O2-5% CO2. To label recorded neurons, in some experiments biocytin was included in the internal solution (0.1% wt/vol). All physiology experiments were performed at room temperature, on both backlabeled and randomly chosen unlabeled neurons that were distributed across all subdivisions of the DRN. Neurons were visualized under infrared illumination with an upright Zeiss Axioskop FS fixed-stage microscope (Carl Zeiss Microimaging, Thornwood, NY) equipped with a charge-coupled device (CCD) camera (Hamamatsu C2400, Bridgewater, NJ). Retrogradely labeled neurons were visualized with a fluorescent light source (Cy3 filter set). Infrared light was then used to identify the same neuron and guide the electrode placement. Whole cell patch-clamp recordings used microelectrodes (3–8 MΩ) pulled on a Flaming/Brown micropipette puller (model P-97, Sutter Instrument, Novato, CA). Data were acquired with an Axopatch 200B with a Digidata 1200 Interface and pCLAMP 8 software (Molecular Devices, Sunnyvale, CA).
To measure APs, recording electrodes were filled with potassium gluconate internal solution (in mM: 154 K-gluconate, 1 KCl, 1 EGTA, 10 HEPES, 10 glucose, 5 ATP, 0.1 GTP, pH 7.4 with KOH). Slices were bathed with normal aCSF or aCSF with 12 μM phenylephrine. Both whole cell and on-cell recording techniques were used. On-cell recordings involved forming a “loose patch” on the cell membrane, with seal resistance close to 100 MΩ. Voltage fluctuations were recorded in current clamp with no current applied. Criteria for regular and frequent spontaneous AP firing were defined as baseline firing that was at least 0.05 Hz and an interspike interval that varied no more than 20-fold.
For the acetylcholine (ACh)-induced current measurements, rapid focal application of ACh was accomplished with a Picospritzer II (General Valve, Fairfield, NJ; 200-ms duration) and antagonists were applied by bath perfusion controlled with a BPS-4 valve system (ALA Scientific Instruments, Westbury, NY). One millimolar ACh, 1 μM prazosin, 100 μM mecamylamine (MEC), 10 nM methyllycaconitine (MLA), and 1 μM dihydro-β-erythroidine (DHβE) solutions were prepared daily from frozen aliquots. All external aCSF included 1 μM tetrodotoxin (TTX; Alamone Laboratories, Jerusalem, Israel) and 1 μM atropine. The microelectrodes were filled with a cesium internal solution [in mM: 140 CsSO3CH3, 10 HEPES, 1 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 2 ATP, 0.1 GTP, 10 glucose, 5 tetraethylammonium-HCl, pH to 7.35 with CsOH].
For the synaptic modulation experiments measuring excitatory postsynaptic currents (EPSCs), 20 μM bicuculline was added to the aCSF to eliminate γ-aminobutyric acid (GABA)ergic transmission and a potassium gluconate internal solution was used. To measure inhibitory postsynaptic currents (IPSCs), 10 μM 6,7-dinitroquinoxaline-2,3-dione (DNQX; Tocris, Ellisville, MO) was used to block AMPA-mediated currents and an internal solution with a higher chloride concentration was used (in mM: 78 K-gluconate, 77 KCl in the K-gluconate internal solution). One micromolar nicotine was prepared fresh daily and applied to the slice via bath perfusion. All measurements of postsynaptic currents were performed in voltage clamp with a holding potential of −70 mV. Cells were eliminated from these investigations if the input resistance or series resistance varied >15% during the course of the recordings.
Data analysis and statistics.
Spontaneous EPSCs and IPSCs were analyzed with Mini-Analysis (Synaptosoft, Decatur, GA). Determination of “responsive” cells involved comparing the baseline frequency for a 1-min period immediately prior to nicotine application with a 1-min period centered on the peak nicotine effect by Student's t-test. In the absence of a clear nicotine-induced change in frequency, the data were sampled from a 1-min window centered 1 min after the start of the nicotine application. This time point most commonly corresponds to the maximal effect of nicotine on synaptic transmission (Mansvelder et al. 2002; Mansvelder and McGehee 2000). The change in synaptic transmission was quantified as the difference between the baseline and the average of 30 s of frequency data centered around the peak nicotine response.
All results are expressed as means ± SE. Graphs were produced with SigmaPlot version 10.0 (Systat Software, Richmond, CA).
AP frequency was analyzed with Mini-Analysis. Identification of responsive cells and response magnitudes used the same statistical methods described for assessing synaptic modulation. A χ2-test was used to compare the prevalence of nicotine responses between different populations of cells or between different experimental groups.
Immunohistochemistry.
Tissue preparation for histology involved induction of deep anesthesia with isoflurane followed by perfusion with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) and 15% saturated picric acid (LabChem, Pittsburgh, PA) in 0.2 M phosphate buffer via the ascending aorta. Brains were removed and immersed in fixative solution (described above for perfusion) for 2 h and then cryoprotected in 30% sucrose for 72 h. Coronal sections (60 μm thick) were made with a cryostat from the NAc through the DRN. The NAc slices were immediately slide mounted for verification of injection sites with antifade mountant. For immunohistochemistry, DRN slices were washed, blocked with normal goat serum (NGS), permeabilized with 1% Triton X, and then exposed to a solution containing a tryptophan hydroxylase (TPH) antibody (1:400) (Chemicon, Temecula, CA), followed by an Alexa Fluor 488-conjugated secondary antibody (1:50) (Molecular Probes, Eugene, OR). Control experiments were conducted with secondary antibody alone, which did not yield cellular staining at this dilution. To visualize biocytin-filled cells after electrophysiological recording, sections were treated with Cy5-conjugated streptavidin (1:100) (Jackson Immuno, West Grove, PA). Images were obtained with Fluoview 200 software on an Olympus IX70 confocal microscope (Olympus America, Melville, NY) or with an Olympus IX81 inverted microscope using Metamorph software (Universal Imaging, Downingtown, PA).
Drugs.
All reagents and chemicals were obtained from Sigma (St. Louis, MO), unless specified otherwise.
RESULTS
Fluoro-Red dye was injected bilaterally into the NAc (Fig. 1, B and C) and retrogradely transported to cell bodies located in the DRN (Fig. 1, D and E). Histology confirmed that injection cannula placement was in the NAc (Fig. 1C). Control studies performed by injecting dye dorsal to the NAc did not demonstrate any labeling of DRN neurons (data not shown).
Because the DRN is primarily a serotonergic nucleus, we investigated the percentage of backlabeled neurons that express the serotonin synthetic enzyme TPH. As expected, the majority of DRN neurons stained positively for TPH (Fig. 2A). In double-labeling experiments 74.5% of retrogradely labeled NAc projection neurons also expressed TPH (n = 1,378 backlabeled neurons counted in sections from 6 different rats; Fig. 2B).
Fig. 2.
The majority of cells in the DRN that project to the NAc are serotonergic. A: coronal section of the PAG stained for tryptophan hydroxylase (TPH; green), demonstrating TPH-positive cells clustered in the DRN at approximately bregma −8.6. B: overlay image of retrogradely labeled DRN neurons that project to the NAc (red) and TPH-positive cells (green) at approximately bregma −7.6. In the population of backlabeled DRN-NAc projection neurons, 74.5% were colabeled for TPH (n = 1,378 backlabeled neurons from 6 rats).
To assess whether nAChRs contribute to excitability of DRN neurons, AP firing rate was measured with both whole cell and on-cell recording techniques. These experiments assessed nAChR effects only on neurons that displayed regular spontaneous AP firing (see materials and methods for criteria). When recorded with normal aCSF, 4 of 15 neurons demonstrated a significant increase in firing rate in response to 1 μM nicotine to 190.66 ± 38.63% of baseline (Fig. 3). These data include seven cells that were backlabeled, and nicotine increased firing in two of these (250.3 ± 42.0% of baseline) and decreased firing in one (2.8% of baseline). There was no difference in the prevalence of either response to nicotine between backlabeled and randomly chosen neurons (P > 0.05, χ2-test).
Fig. 3.
Nicotine increases DRN neuronal firing rate. A: example frequency histogram from a DRN neuron that responded to bath application of 1 μM nicotine with an increase in action potential (AP) frequency. Inset: representative traces before (Control) and after perfusion with nicotine. B: mean change in firing frequency following application of 1 μM nicotine. Responsive cells were identified by comparing firing frequency before and during nicotine application (see materials and methods). Black bars represent recordings in artificial cerebrospinal fluid (aCSF), while gray bars represent recordings in aCSF + 12 μM phenylephrine. Bars represent means ± SE. C: response prevalence of the effects of 1 μM nicotine on AP frequency for cells recorded in normal aCSF (black bars) and aCSF + 12 μM phenylephrine (gray bars). B and C: numbers inside bars indicate n for each condition.
Previous studies indicate that adrenergic input to the DRN affects baseline excitability of these neurons in vivo (Li et al. 1998). As these inputs are eliminated in our slices, the majority of neurons tested did not meet our criteria for regular spontaneous AP firing and were not tested with nicotine. To mimic endogenous adrenergic tone, and to increase the pool of testable cells, we repeated the above experiments in the presence of the α1-adrenergic agonist phenylephrine (12 μM), which resulted in a significant increase in the prevalence of spontaneously active neurons (phenylephrine: 14/16 neurons, aCSF: 5/46 neurons; P < 0.0001, χ2-test). In phenylephrine, nicotine induced an increase in firing rate in 5 of 14 cells to 194.37 ± 37.21% of baseline, a decrease in 2 cells (to 38.55 ± 29.85% of baseline), and no effect on the remaining neurons (Fig. 3, B and C). Of the eight backlabeled cells tested under these conditions, nicotine increased the firing rate in two cells (to 143.08 ± 8.39% of baseline) and decreased the firing in two cells. There was not a difference in the nicotine response prevalence in aCSF- versus phenylephrine-treated groups (P > 0.05, χ2-test).
To test mechanisms underlying the excitatory effects of nicotine on DRN neurons, we tested functional nAChR expression with focal application of 1 mM ACh. The average of the ACh-induced inward currents was 171.2 ± 36 pA in 60.7% (51/84) of the neurons tested. These included recordings from 17 backlabeled NAc-projecting DRN neurons, and 12 of these also responded. The prevalence of the ACh-responding DRN neurons was similar between randomly chosen (39/67) and backlabeled (12/17) neurons (P = 0.4145, χ2-test).
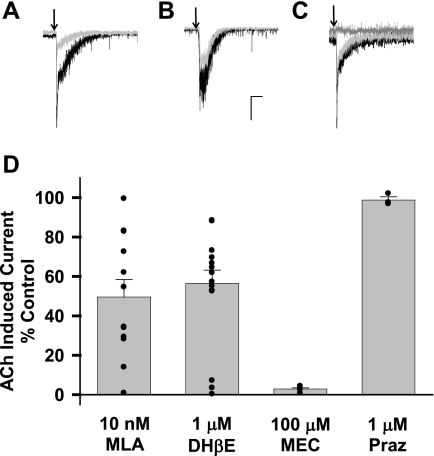
Next, we explored the pharmacology of the ACh effects on DRN neurons. The contribution of nAChR subtypes was tested by pretreating the slice with either the α7-selective antagonist MLA or the β2-nAChR antagonist DHβE. On average, MLA (10 nM) reduced the ACh current to 49.6 ± 8.9% of control (n = 12; Fig. 4, A and D). In the presence of DHβE (1 μM), the ACh current decreased to 56.4 ± 6.8% of control (n = 14; Fig. 4, B and D). The effects of both DHβE and MLA varied considerably between cells, suggesting that no one nAChR subtype dominates. These responses were entirely mediated by nAChRs, as the nonselective nAChR antagonist MEC (100 μM) blocked the responses completely (0 ± 0.9% of control, n = 5; Fig. 4, C and D). Consistent with this, the ACh response was completely inhibited when tested in the presence of both MLA and DHβE (not shown). Presynaptic nAChRs can modulate release of a number of different neurotransmitters including norepinephrine, which was shown to mediate nAChR-induced excitatory effects in one study (Li et al., 1998). Under our recording conditions, pretreatment with the α1-adrenergic receptor antagonist prazosin (1 μM) had no effect on ACh-induced currents (98.8 ± 1.6% of control, n = 3; Fig. 4, C and D). Together, these data demonstrate that focal ACh application onto DRN neurons results in direct activation of nAChR-mediated excitatory inward currents, through activation of a mixture of α7- and β2-containing receptor subtypes.
Fig. 4.
DRN neurons express functional nicotinic acetylcholine receptors (nAChRs). A: focal application of 1 mM ACh (arrows) induced rapid inward currents in 60.7% of DRN neurons tested (n = 84, black traces). In a subset of responsive neurons, pretreatment with 10 nM methyllycaconitine (MLA) inhibited ACh-induced currents (gray trace). B: in a subset of responsive neurons, pretreatment with 1 μM dihydro-β-erythroidine (DHβE) inhibited ACh-induced currents (gray trace). C: pretreatment of the slice with 100 μM mecamylamine (MEC, dark gray trace) blocked the ACh-induced current (black trace), but 1 μM prazosin (Praz, light gray trace) had no effect. D: average ACh-induced current in the presence of the antagonists tested as % of control. Bars represent means ± SE. Filled circles represent individual current amplitudes. Scale: A and B, 50 pA and 1 s; C: 200 pA, 0.5 s.
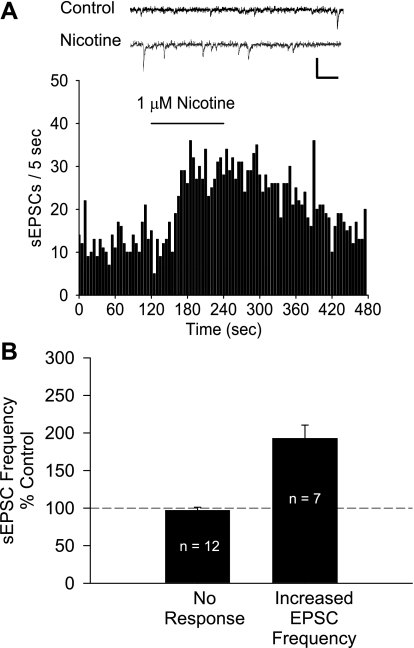
Our lab has previously shown that nicotine enhances glutamate inputs to VTA dopamine neurons (Mansvelder and McGehee 2000). To test for this phenomenon in the DRN, we recorded the effects of nicotine on the frequency of spontaneous EPSCs (sEPSCs). Inhibitory GABAergic transmission was blocked with 20 μM bicuculline in these experiments. Bath application of 1 μM nicotine increased sEPSC frequency in 7 of 19 neurons (192.47 ± 18.01% of baseline; Fig. 5) and had no effect in the other 12 neurons tested. Neither the prevalence nor the magnitude of this increase was significantly different between randomly chosen (4/10, 190.34 ± 19.01% of baseline) and backlabeled (3/9, 195.3 ± 39.26% of baseline) cells (P > 0.05, χ2-test, Student's t-test, respectively). Under these conditions, the excitatory synaptic inputs to these neurons were glutamatergic, as all synaptic currents were eliminated when the preparation was exposed to 10 μM DNQX (data not shown).
Fig. 5.
Nicotine modulates excitatory synaptic inputs to DRN neurons. A: effect of 1 μM nicotine on spontaneous excitatory postsynaptic current (sEPSC) frequency in a DRN neuron. Inset: representative traces before (Control) and after perfusion with nicotine. Scale: 50 pA, 25 ms. B: nicotine increased the sEPSC frequency in 58.3% of DRN neurons. Responsive cells were identified by comparing sEPSC frequency before and during nicotine application (see materials and methods). Bars represent means ± SE.
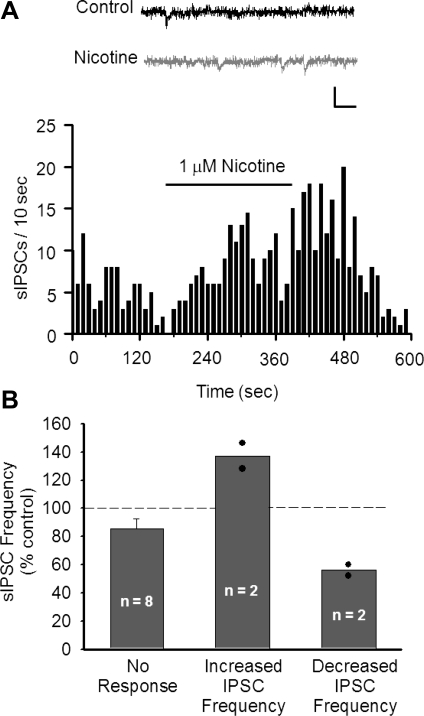
Nicotine also modulates inhibitory GABAergic synaptic transmission in other areas of the midbrain (Mansvelder et al. 2002), so we investigated whether DRN neurons have similar effects. Spontaneous GABAergic IPSCs (sIPSCs) were recorded from DRN neurons by using an elevated intracellular chloride concentration to enhance their resolution. DNQX (10 μM) was included in the aCSF to inhibit AMPA receptor-mediated excitatory currents. A small percentage of DRN neurons (2 of 12) responded to 1 μM nicotine with an increase in sIPSC frequency (137.13 ± 9.02% of prenicotine baseline; Fig. 6). Furthermore, in 2 of 12 DRN neurons 1 μM nicotine decreased the sIPSC frequency (56.07 ± 3.93% of prenicotine baseline; Fig. 6B).
Fig. 6.
Nicotine induces weak modulation of inhibitory inputs to DRN neurons. A: example frequency histogram demonstrating increased spontaneous inhibitory postsynaptic current (sIPSC) frequency after application of 1 μM nicotine. Inset: representative traces before (Control) and after perfusion with nicotine. Scale: 25 pA, 25 ms. B: effect of 1 μM nicotine application on sIPSC frequency in DRN neurons. Responsive cells were identified by comparing sIPSC frequency before and during nicotine application (see materials and methods). Nicotine increased sIPSC frequency in 16.7% of cells tested and decreased sIPSC frequency in 16.7% of cells tested. Bars represent means ± SE. Filled circles represent individual observations.
DISCUSSION
We explored the contribution of nAChRs to the excitability of DRN neurons, including a subset of DRN neurons that project to the NAc. We demonstrate that nicotine can increase the firing rate of DRN neurons and that this increased excitability is mediated directly, via nAChRs expressed on the DRN neurons themselves, and indirectly by modulation of synaptic inputs to these neurons. In the subset of backlabeled DRN neurons that project to the NAc, we found similar physiological effects of nicotine. These findings support our hypothesis that nicotine may affect serotonergic signaling to the NAc.
Previous anatomic studies demonstrated the presence of α4-, α7-, and β2-nAChR subunits in the DRN (Bitner and Nikkel 2002; Bitner et al. 2000; Cucchiaro et al. 2005; Cucchiaro and Commons 2003). The nAChR α7-subunit was found on large-diameter DRN neurons (15–25 μm in diameter) that colocalized with TPH and small-diameter neurons (5–10 μm) that were GABAergic (Bitner and Nikkel 2002). Our investigation of the neurochemical makeup of DRN neurons that project to the NAc indicates that almost 75% were TPH positive. This indicates a strong serotonergic projection from DRN to the NAc, where serotonin may modulate reward-related signaling. Taken together, these data further support the influence of nicotine on serotonin signaling.
Our results are consistent with previous studies demonstrating nicotine-induced increases in DRN neuron firing rate and serotonin release in vitro (Mihailescu et al. 2001, 2002). In those experiments, nicotine increased excitability in 70% of DRN neurons, with a maximal effect at 2.15 μM. In our experiments, the slightly lower prevalence and magnitude of the excitatory effects may be due to a lower concentration of nicotine tested (1 μM), which is close to the arterial blood concentrations achieved after smoking (Henningfield et al. 1993).
Interestingly, in vivo systemic nicotine was reported to decrease the firing rate in the majority of DRN neurons and increased firing in only a small subset (Engberg et al. 2000). However, nicotine was without effect when micro-iontophoresed locally into the DRN. This does not agree with our results, but it is important to note that serotonergic neurons are strongly influenced by the animal's arousal state (sleep-waking) (Ranade and Mainen 2009). Thus it is possible that the effect of nicotine on DRN neuron firing rate in vivo was influenced by anesthesia. Other groups have demonstrated nicotine-induced increases in firing rates in vivo in awake as well as anesthetized animals (Evrard and Changeux 2008; Huang et al. 2010; Schilstrom et al. 2003).
To assess the direct excitation of DRN neurons by nicotine, we tested nAChR expression with focal application of a saturating concentration of ACh in the presence of blockers of synaptic transmission. The nAChR responses had a mixed pharmacology, suggesting expression of α7- and β2-containing nAChR subtypes. In addition, there was variability in the antagonist sensitivity of the nAChR responses between individual neurons, suggesting considerable diversity of nAChR expression within the DRN. These findings are consistent with recent reports of nAChR expression in the DRN (Cucchiaro et al. 2005; Cucchiaro and Commons 2003; Galindo-Charles et al. 2008).
One study reports that the excitatory effects of nicotinic agonists in DRN neurons are dependent upon presynaptic modulation of norepinephrine release (Li et al. 1998). The recording conditions used by that group facilitated resolution of the indirect nAChR modulation of monoamine transmission. In contrast, our on-cell recording method provided assessment of firing rate, but not nicotine-induced changes in membrane potential. In our whole cell voltage-clamp recordings from DRN neurons that project to the NAc the average input resistance was 273 ± 27 MΩ, indicating that the 2- to 5-mV changes in membrane potential reported by Li et al. (1998) are mediated by currents that are at or below the limit of detection in voltage clamp. The slow time course of metabotropic receptor-mediated changes in holding current can also confound resolution of those currents. The lack of evidence for direct activation of nAChRs in that study could be attributed to differences in rat age or strain, or a slower drug application system, which could desensitize postsynaptic nAChRs. However, their data did show a nicotine-induced enhancement of serotonin release in DRN, which indirectly supports nAChR expression on serotonin neurons.
Nicotine modulates the release of various neurotransmitters via presynaptic nAChRs (Mansvelder et al. 2002), and we sought to extend previous studies by examining the influence of nicotine on glutamatergic and GABAergic input to DRN-NAc projection neurons. We observed that nAChR activation increased excitatory glutamatergic input, while the effects on inhibitory GABAergic input were minimal. These data taken together support a net excitatory role for nAChRs in the DRN through presynaptic modulation, as well as direct excitation.
The somatodendritic localization of nAChRs on DRN neurons does not guarantee that they mediate fast synaptic transmission (McGehee and Role 1996). However, the DRN is one of the few brain loci where synaptic currents mediated by nAChRs have been demonstrated by stimulating brain stem cholinergic nuclei (Galindo-Charles et al. 2008). Thus, in addition to the acute effects of exogenous nicotine, fast cholinergic synaptic transmission contributes to the excitability of serotonergic DRN neurons that project to the NAc.
Thus, in the context of previous work, our data suggest that acute exposure to nicotine excites DRN neurons by direct depolarization and indirect synaptic modulation. These effects were seen with high prevalence in DRN neurons backlabeled from NAc, and the majority of these projection neurons express serotonin. Therefore, we conclude that these effects likely contribute to nicotine-induced increases in serotonin release in the NAc.
This relationship between nicotine and serotonin release may have important implications in nicotine addiction, as serotonin modulates excitability of many brain areas, including those that are involved in reward and mood. Abundant serotonin receptor expression has been demonstrated in the NAc (Clemett et al. 2000) and activation of these receptors can enhance DA release in vivo (Benloucif et al. 1993). Serotonin receptor antagonists administered locally into the NAc inhibit cocaine-induced increases in locomotion (McMahon and Cunningham 2001). Furthermore, activating NAc serotonin receptors modulates the facilitation of reward by systemic cocaine in an intracranial self-stimulation paradigm (Katsidoni et al. 2011). Finally, a recent human PET scan study demonstrated lower levels of certain serotonin receptors in the NAc of patients with major depressive disorder compared with control subjects (Murrough et al. 2011), suggesting a contribution of dysfunctional serotonin signaling to that condition. Together these data support the idea that NAc serotonin contributes to mood and reward-associated behavior.
Given the excitatory effects of nicotine on DRN neurons, serotonin likely contributes to the rewarding effects of nicotine. In support of that idea, behavioral sensitization to repeated nicotine exposure was enhanced when inhibitory 5-HT1A autoreceptors were blocked or desensitized, and this correlated with increased release of serotonin (Lanteri et al. 2009). Intracranial self-stimulation studies indicate that chronic nicotine lowers the brain reward thresholds, suggesting increased reward, whereas withdrawal from nicotine has the opposite effect (Harrison and Markou 2001). That study also showed reversal of withdrawal by SSRIs combined with a 5-HT1A receptor antagonist, suggesting that decreased serotonergic tone is a key aspect of nicotine withdrawal.
In summary, we have shown that the serotonergic neurons arising in the DRN and projecting to the NAc express nAChRs. Furthermore, acutely administered nicotine increases the excitatory input to these neurons and ultimately increases their firing rate. The fact that nicotine increases DRN serotonergic output may help explain some of its influence on mood and affect, which ultimately contributes to the high relapse rate in smokers who are attempting to quit. Withdrawal from chronic systemic nicotine increases anxiety, which is relieved by nicotine in the DRN (Cheeta et al. 2001; Irvine et al. 2001). This anxiogenic effect of withdrawal is thought to be caused by a persistent increase in excitability of DRN neurons and increased serotonergic tone. Therefore, relief from nicotine withdrawal may involve changes in serotonergic tone selectively in brain regions mediating reward, such as the NAc.
GRANTS
This project was supported by funding from the National Institute on Drug Abuse Grants DA-07255 to B. Chang and C. Daniele, DA-07255 and DA-21458 to R. D. Mitchum, DA-015918 to D. S. McGehee, and DA-019695 to D. S. McGehee and P. Vezina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Chau Chau Tu Pham for help with stereotaxic surgeries, Vytas Bindokas for imaging assistance, and Jenny Shen for graphic design.
REFERENCES
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking. A national perspective. JAMA 264: 1541–1545, 1990 [PubMed] [Google Scholar]
- Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther 265: 373–377, 1993 [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL. Alpha-7 nicotinic receptor expression by two distinct cell types in the dorsal raphe nucleus and locus coeruleus of rat. Brain Res 938: 45–54, 2002 [DOI] [PubMed] [Google Scholar]
- Bitner RS, Nikkel AL, Curzon P, Donnelly-Roberts DL, Puttfarcken PS, Namovic M, Jacobs IC, Meyer MD, Decker MW. Reduced nicotinic receptor-mediated antinociception following in vivo antisense knock-down in rat. Brain Res 871: 66–74, 2000 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention The Health Consequences of Smoking: a Report of the Surgeon General. Atlanta, GA: U. S. Department of Health and Human Services, 2004 [PubMed] [Google Scholar]
- Cheeta S, Irvine EE, Kenny PJ, File SE. The dorsal raphe nucleus is a crucial structure mediating nicotine's anxiolytic effects and the development of tolerance and withdrawal responses. Psychopharmacology (Berl) 155: 78–85, 2001 [DOI] [PubMed] [Google Scholar]
- Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39: 123–132, 2000 [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Chaijale N, Commons KG. The dorsal raphe nucleus as a site of action of the antinociceptive and behavioral effects of the alpha4 nicotinic receptor agonist epibatidine. J Pharmacol Exp Ther 313: 389–394, 2005 [DOI] [PubMed] [Google Scholar]
- Cucchiaro G, Commons KG. Alpha4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse 49: 195–205, 2003 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K, Qu T, Ahmed FA, Zhang L, Yamada K, Guison NG, Miller M, Yamadori T. Fluoro-Green and Fluoro-Red: two new fluorescent retrograde tracers with a number of unique properties. Brain Res 736: 61–67, 1996 [DOI] [PubMed] [Google Scholar]
- Engberg G, Erhardt S, Sharp T, Hajos M. Nicotine inhibits firing activity of dorsal raphe 5-HT neurones in vivo. Naunyn Schmiedebergs Arch Pharmacol 362: 41–45, 2000 [DOI] [PubMed] [Google Scholar]
- Evrard A, Changeux JP. Abnormal response of dopaminergic neurons to nicotine without perturbation of nicotinic receptors in alphaCGRP knock-out mice. Brain Res 1228: 89–96, 2008 [DOI] [PubMed] [Google Scholar]
- Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduno J, Frias-Dominguez C, Drucker-Colin R, Mihailescu S. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse 62: 601–615, 2008 [DOI] [PubMed] [Google Scholar]
- Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology 25: 55–71, 2001 [DOI] [PubMed] [Google Scholar]
- Harrison AA, Markou A. Serotonergic manipulations both potentiate and reduce brain stimulation reward in rats: involvement of serotonin-1A receptors. J Pharmacol Exp Ther 297: 316–325, 2001 [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend 33: 23–29, 1993 [DOI] [PubMed] [Google Scholar]
- Huang LT, Sherwood JL, Sun YJ, Lodge D, Wang Y. Activation of presynaptic alpha7 nicotinic receptors evokes an excitatory response in hippocampal CA3 neurones in anaesthetized rats: an in vivo iontophoretic study. Br J Pharmacol 159: 554–565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007: CD000031, 2007 [DOI] [PubMed] [Google Scholar]
- Irvine EE, Cheeta S, File SE. Development of tolerance to nicotine's anxiogenic effect in the social interaction test. Brain Res 894: 95–100, 2001 [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Apazoglou K, Panagis G. Role of serotonin 5-HT2A and 5-HT2C receptors on brain stimulation reward and the reward-facilitating effect of cocaine. Psychopharmacology (Berl) 213: 337–354, 2011 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70: 531–549, 2001 [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A. Onset of major depression during treatment for nicotine dependence. Addict Behav 28: 461–470, 2003 [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann NY Acad Sci 909: 170–185, 2000 [DOI] [PubMed] [Google Scholar]
- Lanteri C, Hernandez Vallejo SJ, Salomon L, Doucet EL, Godeheu G, Torrens Y, Houades V, Tassin JP. Inhibition of monoamine oxidases desensitizes 5-HT1A autoreceptors and allows nicotine to induce a neurochemical and behavioral sensitization. J Neurosci 29: 987–997, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci 18: 1904–1912, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Rao ZR, Shi JW. Serotoninergic projections from the midbrain periaqueductal gray to the nucleus accumbens in the rat. Neurosci Lett 98: 276–279, 1989 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33: 905–919, 2002 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27: 349–357, 2000 [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Presynaptic ionotropic receptors. Curr Opin Neurobiol 6: 342–349, 1996 [DOI] [PubMed] [Google Scholar]
- McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther 297: 357–363, 2001 [PubMed] [Google Scholar]
- Mihailescu S, Guzman-Marin R, Dominguez Mdel C, Drucker-Colin R. Mechanisms of nicotine actions on dorsal raphe serotoninergic neurons. Eur J Pharmacol 452: 77–82, 2002 [DOI] [PubMed] [Google Scholar]
- Mihailescu S, Guzman-Marin R, Drucker-Colin R. Nicotine stimulation of dorsal raphe neurons: effects on laterodorsal and pedunculopontine neurons. Eur Neuropsychopharmacol 11: 359–366, 2001 [DOI] [PubMed] [Google Scholar]
- Murrough JW, Henry S, Hu J, Gallezot JD, Planeta-Wilson B, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology (Berl) 213: 547–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Engel JA, Soderpalm B. Involvement of serotonin in nicotine dependence: processes relevant to positive and negative regulation of drug intake. Pharmacol Biochem Behav 71: 757–771, 2002 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998 [Google Scholar]
- Ranade SP, Mainen ZF. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J Neurophysiol 102: 3026–3037, 2009 [DOI] [PubMed] [Google Scholar]
- Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. Am J Addict 13: 438–446, 2004 [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol 6: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- Seth P, Cheeta S, Tucci S, File SE. Nicotinic-serotonergic interactions in brain and behaviour. Pharmacol Biochem Behav 71: 795–805, 2002 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Abe Y, McGehee DS, Keath JR, Yajima H, Sharma K, Brorson JR. Long-lived retrograde fluorescent labeling of corticospinal neurons in the living animal. Brain Res Brain Res Protoc 13: 183–188, 2004 [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann NY Acad Sci 600: 9–34, 1990 [DOI] [PubMed] [Google Scholar]