Abstract
Sensory afferent transmission and associated spinal reflexes are normally inhibited by serotonin (5-HT) derived from the brain stem. Spinal cord injury (SCI) that eliminates this 5-HT innervation leads to a disinhibition of sensory transmission and a consequent emergence of unusually long polysynaptic excitatory postsynaptic potentials (EPSPs) in motoneurons. These EPSPs play a critical role in triggering long polysynaptic reflexes (LPRs) that initiate muscles spasms. In the present study we examined which 5-HT receptors modulate the EPSPs and whether these receptors adapt to a loss of 5-HT after chronic spinal transection in rats. The EPSPs and associated LPRs recorded in vitro in spinal cords from chronic spinal rats were consistently inhibited by 5-HT1B or 5-HT1F receptor agonists, including zolmitriptan (5-HT1B/1D/1F) and LY344864 (5-HT1F), with a sigmoidal dose-response relation, from which we computed the 50% inhibition (EC50) and potency (−log EC50). The potencies of 5-HT receptor agonists were highly correlated with their binding affinity to 5-HT1B and 5-HT1F receptors, and not to other 5-HT receptors. Zolmitriptan also inhibited the LPRs and general muscle spasms recorded in vivo in the awake chronic spinal rat. The 5-HT1B receptor antagonists SB216641 and GR127935 and the inverse agonist SB224289 reduced the inhibition of LPRs by 5-HT1B agonists (zolmitriptan). However, when applied alone, SB224289, SB216641, and GR127935 had no effect on the LPRs, indicating that 5-HT1B receptors do not adapt to chronic injury, remaining silent, without constitutive activity. The reduction in EPSPs with zolmitriptan unmasked a large glycine-mediated inhibitory postsynaptic current (IPSC) after SCI. This IPSC and associated chloride current reversed at −73 mV, slightly below the resting membrane potential. Zolmitriptan did not change motoneuron properties. Our results demonstrate that 5-HT1B/1F agonists, such as zolmitriptan, can restore inhibition of sensory transmission after SCI without affecting general motoneuron function and thus may serve as a novel class of antispastic drugs.
Keywords: serotonin, muscle spasms, spasticity, motoneurons, synaptic input, triptans
descending brain stem systems innervating the spinal cord, especially those releasing serotonin (5-HT) and norepinephrine (NE), potently inhibit sensory transmission to spinal motoneurons and ascending tracts, ultimately attenuating both segmental reflexes and sensory perception (reviewed by Lundberg 1982; Millan 2002; Schmidt and Jordan 2000; Yoshimura and Furue 2006). Both 5-HT and NE directly inhibit sensory transmission by activating inhibitory Gi protein-coupled receptors, such as 5-HT1A, 5-HT1B, 5-HT1D, and α2-adrenergic receptors, on sensory afferent terminals (including low-threshold group I and II muscle and skin afferents and high-threshold pain afferents) and/or excitatory spinal neurons involved in polysynaptic reflexes and ascending sensory transmission (Clarke et al. 1996, 2002; Di Pasquale et al. 1997; Jankowska et al. 1993, 1994; Jordan et al. 2008; Li et al. 2004b; Lundberg 1982; Manuel et al. 1995; Millan 2002; Rekling et al. 2000; Schmidt and Jordan 2000; Singer et al. 1996; Yoshimura and Furue 2006). 5-HT and NE may also indirectly inhibit sensory transmission by activating excitatory Gq-coupled 5-HT2 and α1-adrenergic receptors located on inhibitory interneurons (but not on afferents), thus facilitating inhibitory interneurons, such as those involved in group Ia reciprocal and Ib nonreciprocal inhibition (Hammar and Jankowska 2003; Jankowska et al. 2000) and pain transmission (Obata et al. 2004; Yoshimura and Furue 2006).
Within this conceptual framework of brain stem-mediated inhibition, it is likely that the hyperreflexia and general spasticity syndrome often seen after spinal cord injury (Ashby and McCrea 1987; Dietz and Sinkjaer 2007; Kuhn and Macht 1948; Maynard et al. 1990; Nielsen et al. 2007; Noth 1991) results partly from a loss of brain stem 5-HT-mediated inhibition (disinhibition), especially if the injury includes the dorsal lateral funiculus (DLF), where most of the 5-HT innervation of the dorsal horn arises (Heckman 1994; Lundberg 1982; Schmidt and Jordan 2000; Taylor et al. 1999). We have been investigating this idea with a rat model of spinal cord injury, where a pronounced muscle spasticity syndrome occurs (Bennett et al. 1999, 2004), with similar characteristics to that seen in human muscles after injury (Kuhn and Macht 1948; Maynard et al. 1990). In this model, spinal cord injury leads to the emergence of unusually long excitatory postsynaptic potentials (long EPSPs) on motoneurons, lasting up to 1 s, that are triggered by low-threshold cutaneous and muscle afferents (groups I and II; Li et al. 2004a) (see also Baker and Chandler 1987), very similar to the exaggerated synaptic transmission seen in spastic humans with spinal cord injury (using motor unit recordings; Norton et al. 2008). In both rats and humans, these long EPSPs initiate long-lasting muscle contractions (defined in this study as spasms), lasting several seconds, whereas before injury, the same stimulation mainly evokes inhibition of ongoing muscle activity (Bennett et al. 1999, 2004; Norton et al. 2008). We know that exogenously applied 5-HT (or NE) can inhibit muscle spasms in these rats (Li et al. 2004b), essentially replacing lost brain stem 5-HT, but we do not know where this inhibition occurs (pre- or postsynaptic) or what receptors are involved, although it is reasonable to suggest that 5-HT1 (or even 5-HT2) receptors could mediate this inhibition (see above). In the current study we addressed these questions by applying selective 5-HT receptor agonists while recording spasms and associated EPSPs, as a step toward developing novel antispastic drugs to replace lost 5-HT innervation.
Brain stem-derived 5-HT and NE normally facilitate motoneuron function (Heckman et al. 2005; Hultborn et al. 2004; Li et al. 2004a; Perrier and Delgado-Lezama 2005; Schmidt and Jordan 2000), in contrast to the inhibition of sensory transmission discussed above. This is mediated by 5-HT2 and α1-adrenergic receptors that lower the sodium spike threshold and facilitate voltage-dependent persistent inward currents (PICs), including both persistent calcium (Ca PIC) and sodium (Na PIC) currents. Together, these currents are essential for normal motoneuron function, including sustained firing in response to synaptic inputs (Gilmore and Fedirchuk 2004; Harvey et al. 2006a; Heckman et al. 2005; Perrier and Hounsgaard 2003). Thus, with spinal cord injury, motoneurons are often rendered acutely unexcitable, in part due to a lack of brain stem-derived 5-HT and NE innervation need for normal motoneuron function (Heckman et al. 2005; Li et al. 2004a), especially if the injury includes the ventral and ventrolateral funiculi that contain most of the 5-HT that innervates the ventral horn (Schmidt and Jordan 2000). The functional consequence of this is that the spinal cord becomes areflexic immediately after injury, despite the exaggerated sensory afferent transmission. However, over weeks after injury (chronic spinal state), motoneurons spontaneously regain their excitability, with the reemergence of large Ca and Na PICs. At this time, the exaggerated sensory transmission, especially the long EPSPs, trigger the PICs, which ultimately produce the many-second-long muscle spasms in humans (Gorassini et al. 2002; Norton et al. 2008) and rats (Bennett et al. 2004; Li et al. 2004a).
Recently, the reasons for the spontaneous recovery of motoneuron function with chronic injury have begun to be understood (Bennett et al. 2004; Button et al. 2008; Gorassini et al. 2004; Harvey et al. 2006b; Hultborn et al. 2004; Murray et al. 2010). Briefly, 5-HT2 and α1-receptors on motoneurons become spontaneously active in the weeks after spinal transection (Harvey et al. 2006b; Murray et al. 2010), due to constitutive receptor activity (activity in the absence of 5-HT or any other ligand) (Murray et al. 2010). This spontaneous receptor activity leads to the reemergence of the large PICs that make the motoneurons permanently excitable (Harvey et al. 2006b; Murray et al. 2010). One goal of the present study was to examine whether similar plasticity (constitutive activity) also occurs in the 5-HT1 receptors that normally inhibit sensory transmission. This seems plausible, because 5-HT1 receptors can exhibit constitutive activity in single-cell cloned receptor systems (Selkirk et al. 1998), but functionally may not be important, because general inhibition is not restored in chronic injury, and in particular, the exaggerated long EPSPs that trigger spasms remain even in chronic injury (Baker and Chandler 1987; Li et al. 2004a).
Finally, although uninjured animals and humans at times have substantial PICs facilitated by brain stem-derived 5-HT and NE (see above and Gorassini et al. 2002; Udina et al. 2010), these PICs do not cause uncontrolled motoneuron firing, because postsynaptic inhibition arising from glycinergic and GABAergic neurons in the spinal cord and brain can directly hyperpolarize motoneurons (Holstege and Bongers 1991; Jankowska 1992; Nielsen et al. 2007; Rekling et al. 2000) to appropriately terminate the voltage-dependent PICs (Bennett et al. 1998; Heckman et al. 2005). In contrast, after spinal cord injury, there is a reduction in such postsynaptic inhibitory currents (Boulenguez et al. 2010; Nielsen et al. 2007), in part due to loss of 5-HT and NE (Jankowska et al. 2000), making motoneuron PICs and firing difficult to voluntarily terminate.
Thus three general factors contribute to spasms after spinal cord injury: 1) unusually long EPSPs and general disinhibition of afferent transmission, 2) large uncontrollable PICs in motoneurons, mediated by 5-HT2 (and α1-adrenergic) receptor activity, and 3) a loss of postsynaptic inhibition over motoneuron activity. In previous articles, we examined how spasms can be controlled by reducing PICs (Murray et al. 2010, 2011). The present article examines how we can control spasms by reducing EPSPs by replacing lost 5-HT innervation with 5-HT1 receptor agonists.
METHODS
Recordings were made from motoneurons and associated ventral roots of the sacrocaudal spinal cord of spastic adult rats with chronic spinal cord injury (3.5–5 mo old). Adult female rats were transected at the S2 sacral level at about 2 mo of age (adult rat), and recordings were made at a time after their affected muscles became spastic (1.5–3 mo after injury), as detailed previously (Bennett et al. 1999, 2004). Recordings were made from the whole sacrocaudal spinal cord that was removed from the rat with an S2 sacral transection and maintained in vitro. This transection was made just rostral to the chronic spinal injury so as not to further damage the sacrocaudal cord. Control, age-matched, normal rats were also studied in vitro. All experimental procedures were approved by the University of Alberta Animal Care and Use Committee, Health Sciences.
In vitro preparation.
Details of the in vitro experimental procedures have been described in previous publications (Harvey et al. 2006c; Li et al. 2004a, 2004b; Murray et al. 2010). Briefly, all the rats were anesthetized with urethane (0.18 g/100 g; with a maximum dose of 0.45 g), and the sacrocaudal spinal cord was removed and transferred to a dissection chamber containing modified artificial cerebrospinal fluid (mACSF). Spinal roots were removed, except the sacral S4 and caudal Ca1 ventral roots and the Ca1 dorsal roots. After 1.5 h in the dissection chamber (at room temperature), the cord was transferred to a recording chamber containing normal ACSF (nACSF) maintained near 23°C and with a flow rate >5 ml/min. A 1-h period in nACSF was given to wash out the residual anesthetic and mACSF before recording, at which time the nACSF was recycled in a closed system with a peristaltic pump (Harvey et al. 2006b).
Ventral root reflex recording and averaging.
Dorsal and ventral roots were mounted on silver-silver chloride wires above the nASCF of the recording chamber and covered with a 1:1 mixture of petroleum jelly and mineral oil (as for intracellular recording) for monopolar stimulation and recording (Li et al. 2004b). We evoked ventral root reflexes with a low-threshold Ca1 dorsal root stimulation [single pulse, 0.1 ms, 0.02 mA, corresponding to 3 times afferent threshold (3×T); afferent and reflex threshold are similar (Bennett et al. 2004)] using a constant current stimulator (Isoflex, Jerusalem, Israel). The stimulation intensity (3×T) is compatible with activation of low-threshold group I and II (Aβ) afferents. Because the Ca1 dorsal root innervates the distal third of the tail, which lacks large muscles (Bennett et al. 2004), this stimulation activates largely cutaneous or joint afferents, although there are small intrinsic muscles in the tail with group Ia and II muscle afferents (Steg 1964), and thus, to a lesser extent, muscle afferents may be activated. The stimulation was repeated 5 times at 10-s intervals for each trial. The ventral root recordings were amplified (×2,000), high-pass filtered at 100 Hz, low-pass filtered at 3 kHz, and recorded with a data-acquisition system sampling at 6.7 kHz (Axonscope 8; Axon Instruments, , Burlingame, CA). Ventral root reflexes were quantified using custom-written software (Matlab, The MathWorks, Natick, MA). That is, data were rectified to allow averaging, and then three components of the ventral root reflexes were quantified: the short-lasting, short-latency polysynaptic reflex (SPR; averaged 10–40 ms poststimulus), the intermediate-latency, longer lasting reflex corresponding to the long EPSP seen in this preparation (termed long-polysynaptic reflex, or LPR; averaged 40–500 ms poststimulus), and the long-latency, long-lasting tonic response associated with the Ca PIC (termed long-lasting reflex, or LLR; 500–4,000 ms poststimulus). Average ventral root activity computed for each trial in a given reflex interval was then averaged for all five stimuli in a trial. Average background ventral root activity before stimulation was measured over the 800 ms preceding the first stimulus and subtracted from the reflex averages to give the final reflex responses (SPR and LPR). This recording procedure was repeated at 15-min intervals, and 5-HT receptor agonists were added immediately after each recording, giving them time to fully act by the next recording session (15 min later). Cumulative dose-response relations were computed by increasing agonist doses at these 15-min intervals (0.003, 0.01, 0.03. 0.1, …, 30 μM doses used). Antagonists took longer to act, and responses reached near steady state typically >30 min after application, at which time responses were averaged. The effect of agonists on the reflexes were reversible on washout of the agonist, but full recovery to baseline only occurred after several hours, likely due to the large size of the whole cord preparation. Thus washout of agonists was not feasible between doses of the agonists used in the construction of dose-response relations.
Intracellular recording.
Sharp intracellular electrodes were made from glass capillary tubes (1.5-mm OD; Warner GC 150F-10) using a Sutter P-87 micropipette puller and filled with either 2 M potassium citrate or a combination of 1 M potassium acetate and 1 M KCl. Electrodes were beveled down from an initial resistance of 40–80 to 26–32 MΩ using a rotary beveller (Sutter BV-10). A stepper-motor micromanipulator (660; Kopf) was used to advance the electrodes through the ventral cord surface into motoneurons. After penetration, motoneuron identification was made with antidromic ventral root stimulation. Data were collected with an Axoclamp 2b intracellular amplifier (Axon Instruments) running in discontinuous current-clamp (DCC; switching rate 4–6 kHz, output bandwidth 3.0 kHz, sample rate of 6.7 kHz) or discontinuous single-electrode voltage-clamp (SEVC; gain 0.8–2.5 nA/mV) modes.
Slow triangular voltage ramps (3.5-mV/s voltage clamp) were applied to the motoneurons to measure their electrical properties (Harvey et al. 2006c). The input resistance (Rm) was measured during the voltage ramps over a 5-mV range near rest and subthreshold to PIC onset. Resting potential was recorded with 0-nA bias current, after the cell had been given about 15 min to stabilize after penetration. During the upward portion of the slow triangular voltage ramp, the current response initially increased linearly with voltage in response to the passive leak conductance. A linear relation was fit in the region just below the PIC onset (5 mV below) and extrapolated to the whole range of the ramp (leak current). At depolarized potentials above the PIC onset threshold, there was a downward deviation from the extrapolated leak current, and the PIC was estimated as the difference between the leak current and the total current (leak-subtracted current). The PIC was quantified as the initial peak amplitude of this downward deviation below the leak line (leak-subtracted current). The PIC onset was estimated as the voltage at which the conductance first went to zero during the upward ramp (Von).
The EPSP and associated reflexes were directly measured in motoneurons by stimulating the Ca1 dorsal roots (at 2–3×T, as in ventral root reflex recording), while applying hyperpolarizing bias currents to block the PICs, in current-clamp mode. We also measured the corresponding excitatory postsynaptic currents (EPSCs) in response to stimulating the dorsal roots while voltage clamping at various potentials to prevent activation of the PIC or motoneuron firing. This allowed synaptic inputs to be assessed at potentials above rest, where EPSPs are normally obscured by firing.
Drugs and solutions.
The mACSF was composed of (in mM) 118 NaCl, 24 NaHCO3, 1.5 CaCl2, 3 KCl, 5 MgCl2, 1.4 NaH2PO4, 1.3 MgSO4, 25 d-glucose, and 1 kynurenic acid. The nACSF was composed of (in mM) 122 NaCl, 24 NaHCO3, 2.5 CaCl2, 3 KCl, 1 MgCl2, and 12 d-glucose. Both types of ACSF were saturated with 95% O2-5% CO2 and maintained at pH 7.4. Drugs were added to the nACSF as indicated in the text, including 5-HT and (−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI; Sigma-Aldrich), 2-methyl-5-HT, 5-carboxyamidotryptamine (5-CT), 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT), α-methyl-5-HT, BW723C86, cisapride, EMD386088, granisetron, isradipine, LP44, LY344864, methylergonovine, methysergide, MK212, RS102221, RS127445, SB216641, SB224289 (all from Tocris), TTX (TTX-citrate; Alomone Labs, Jerusalem, Israel), and zolmitriptan (kindly donated by AstraZeneca, Mississauga, ON, Canada). All drugs were first dissolved as a 10–50 mM stock in water before final dilution in ACSF, with the exception of BW723C86, cisapride, EMD386088, isradipine, LP44, methylergonovine, RS102221, RS127445, and SB224289, which were dissolved in minimal amounts of DMSO (final concentration in ACSF <0.04%; by itself, DMSO had no effect on the LLR in vehicle controls).
Spasms in awake chronic spinal rat.
Tail muscle spasms were evoked with brief electrical stimulation of the skin of the tail and recorded with tail muscle electromyogram (EMG). Percutaneous EMG wires (50-μm stainless steel; Cooner Wire) were inserted in segmental tail muscles at the midpoint of tail, and recordings were made while the rat was in a Plexiglas tube, as detailed previously (Bennett et al. 2004). Muscle spasms were evoked with electrical stimulation of the skin at the distal tip of the tail (cutaneous stimulation; 0.2-ms, 10-mA pulse; 3× reflex T; 6 spasms evoked at 10-s intervals for a trial; trials repeated at 15-min intervals), and the tail was restrained from moving. EMG was sampled at 5 kHz, rectified and averaged over a poststimulus interval of 10–40 ms to quantify the SPR, 40–500 ms to quantify the LPR, and 500–4,000 ms to quantify spasms (LLR) using Axoscope (Axon Instruments) and Matlab (The Mathworks). EMG over the 300 ms preceding stimulation was also averaged (background) and subtracted from the reflex responses.
Zolmitriptan was applied in vivo with intrathecal injections (Mestre et al. 1994). This was done with a direct lumbar puncture under brief isoflurane anesthesia (10- to 30-μl injections). Rats woke up rapidly (within minutes) after removal of anesthesia, and tail spasms were again recorded as detailed above. Control experiments (n = 5) with 30-μl sterile saline injections showed no effect on the spasms, indicating that anesthesia and injection volume had negligible effects on the spasms. Also, three control rats were injected with 10 μl of methylene blue solution and euthanized immediately, to verify that the drug spread to the whole sacral area but not up to the brain stem (Mestre et al. 1994).
Data analysis.
Data were analyzed using Clampfit 8.0 (Axon Instruments) and SigmaPlot (Jandel Scientific). Data are means ± SD. A Student's t-test was used to test for statistical differences before and after drug applications, with a significance level of P < 0.05. A Kolmogorov-Smirnov test for normality was applied to each data set, with a P < 0.05 level set for significance. Most data sets were found to be normally distributed, as is required for a t-test. For those that were not normal, a Wilcoxon signed rank test was instead used with P < 0.05.
Standard sigmoidal curves were fit to the relation between agonist dose and reflex responses, with doses expressed in log units and with a Hill slope of unity. The dose that produced 50% effect (EC50) was measured from the curve, and −log EC50 was used to quantify the drug potency: pEC50 = −log EC50. Also, the maximum drug-induced response (efficacy) was computed from the curve (peak of curve). For comparison with our computed potencies (pEC50), the binding affinity of each drug at the rat 5-HT receptors was also reported, with values taken from the literature (Table 1). The binding of an agonist to a receptor is expressed in terms of its Ki value (in nM), which corresponds to the dose that produces 50% binding to that receptor (Knight et al. 2004). This is typically measured by the agonist's ability to displace a standard radiolabeled ligand, such as [3H]5-HT, from the receptor expressed in isolated cells. Binding affinity is computed as pKi = −log Ki (Knight et al. 2004). When possible, binding affinities of different drugs for a given receptor were taken from large studies or summary reviews (Boess and Martin 1994), usually using isolated cloned receptors. Also, high-affinity agonist-preferring binding sites were always used, measured with radioactive agonists (usually [3H]5-HT), rather than radioactive antagonists that bind to a low-affinity site (Egan et al. 2000; Knight et al. 2004). If rat receptor Ki values were not available, human values were used instead, because these are similar for most receptors (Boess and Martin 1994).
Table 1.
5-HT receptor agonists and receptor binding affinity
| Receptor | Agonist | Ki, nM | pKi, −log Ki | Reference |
|---|---|---|---|---|
| 5-HT1A | 5-CT | 0.35 | 9.46 | Boess and Martin 1994 |
| 5-HT | 1.65 | 8.78 | Hoyer et al. 1985 | |
| 8-OH-DPAT | 0.97 | 9.01 | Boess and Martin 1994 | |
| LP44 | 52.7 | 7.28 | Leopoldo et al. 2007 | |
| 5-HT1B | 5-CT | 3.31 | 8.48 | Boess and Martin 1994 |
| 5-HT | 24.49 | 7.61 | Hoyer et al. 1985 | |
| α-Methyl-5-HT | 85.11 | 7.07 | Ismaiel et al. 1990 | |
| BW723C86 | 125.89 | 6.60 | Baxter 1996 | |
| Zolmitriptan | 5.01 | 8.30 | Martin et al. 1997 | |
| EMD | 179.88 | 6.75 | Mattson et al. 2005 | |
| 5-HT1D | 5-CT | 0.37 | 9.43 | Boess and Martin 1994 |
| 5-HT | 2.5 | 8.60 | Boess and Martin 1994 | |
| BW723C86 | 125.89 | 6.30 | Baxter 1996 | |
| Zolmitriptan | 0.63 | 9.20 | Martin et al. 1997 | |
| EMD386088 | 109.90 | 6.96 | Mattson et al. 2005 | |
| 5-HT1E | 5-HT | 6.16 | 8.21 | Boess and Martin 1994 |
| α-Methyl-5-HT | 120.22 | 6.92 | Boess and Martin 1994 | |
| Methylergonovine | 89.12 | 7.05 | Boess and Martin 1994 | |
| 5-HT1F | 5-HT | 67.60 | 7.17 | Boess and Martin 1994 |
| α-Methyl-5-HT | 181.97 | 6.74 | Boess and Martin 1994 | |
| LY344864 | 6.02 | 8.22 | Phebus et al. 1997 | |
| Methylergonovine | 30.90 | 7.51 | Boess and Martin 1994 | |
| Zolmitriptan | 63.09 | 7.20 | Martin et al. 1997 | |
| 5-HT2A | 5-HT | 5.75 | 8.24 | Boess and Martin 1994 |
| α-Methyl-5-HT | 127.05 | 6.90 | Engel et al. 1986 | |
| BW723C86 | 251.18 | 6.60 | Baxter 1996 | |
| DOI | 0.79 | 9.10 | Boess and Martin 1994 | |
| Methylergonovine | 0.35 | 9.45 | Knight et al. 2004 | |
| 5-HT2B | 2-Methyl-5-HT | 316.23 | 6.50 | Boess and Martin 1994 |
| 5-CT | 151.36 | 6.82 | Boess and Martin 1994 | |
| 5-HT | 10.23 | 7.99 | Boess and Martin 1994 | |
| α-Methyl-5-HT | 10.47 | 7.98 | Boess and Martin 1994 | |
| BW723C86 | 12.59 | 7.90 | Baxter 1996 | |
| DOI | 27.54 | 7.56 | Boess and Martin 1994 | |
| Methylergonovine | 0.50 | 9.30 | Knight et al. 2004 | |
| 5-HT2C | 5-HT | 10.99 | 7.96 | Egan et al. 2000 |
| α-Methyl-5-HT | 2.69 | 8.57 | Knight et al. 2004 | |
| BW723C86 | 125.89 | 6.90 | Baxter 1996 | |
| DOI | 9.30 | 8.03 | Egan et al. 2000 | |
| MK212 | 97.72 | 7.01 | Knight et al. 2004 | |
| Methylergonovine | 4.57 | 8.34 | Knight et al. 2004 | |
| 5-HT3 | 2-Methyl-5-HT | 85.11 | 7.07 | Milbun and Peroutka 1989 |
| BW723C86 | 316.23 | 6.50 | Kennett et al. 1997 | |
| EMD386088 | 34.00 | 7.47 | Mattson et al. 2005 | |
| MK212 | 29.00 | 7.54 | Glennon et al. 1989 | |
| 5-HT4 | 5-HT | 6.31 | 8.20 | Adham et al. 1996 |
| α-Methyl-5-HT | 263.03 | 6.58 | Adham et al. 1996 | |
| Cisapride | 25.00 | 7.60 | Adham et al. 1996 | |
| 5-HT5A | 5-CT | 0.32 | 9.50 | Boess and Martin 1994 |
| 5-HT | 7.94 | 8.10 | Boess and Martin 1994 | |
| 8-OH-DPAT | 50.12 | 7.30 | Boess and Martin 1994 | |
| 5-HT6 | 2-Methyl-5-HT | 52.48 | 7.28 | Boess et al. 1997 |
| 5-CT | 252.93 | 6.60 | Monsma et al. 1993 | |
| 5-HT | 56.23 | 7.25 | Boess and Martin 1994 | |
| BW723C86 | 398.11 | 6.40 | Kennett et al. 1997 | |
| EMD386088 | 7.41 | 8.13 | Mattson et al. 2005 | |
| 5-HT7 | 5-CT | 0.16 | 9.80 | Boess and Martin 1994 |
| 5-HT | 1.51 | 8.82 | Boess and Martin 1994 | |
| 8-OH-DPAT | 34.67 | 7.46 | Boess and Martin 1994 | |
| LP44 | 0.22 | 9.66 | Leopoldo et al. 2007 |
Agonist dose needed to bind to each 5-HT receptor (Ki) and agonist binding affinity (pKi = −log Ki) values were obtained from high-affinity agonist radioligand bindings studies referenced. Each agonists is considered to activate a receptor if Ki < 400 nM and is listed with that receptor. 5-CT, 5-carboxyamidotryptamine; 8-OH-DPAT, 8-hydroxy-2(di-n-propylamino)tetralin; DOI, (−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride.
RESULTS
Polysynaptic reflexes reflect underlying EPSPs.
To study the EPSPs that underlie spasms in chronic spinal rats, we first examined the polysynaptic reflexes mediated by these EPSPs, to allow systematic studies of 5-HT receptor pharmacology not otherwise feasible with direct intracellular recordings of EPSPs (due to limited stability of recordings). When the dorsal roots of chronic spinal rats were stimulated to activate low-threshold sensory afferents, there was a multiphasic reflex response evoked in the motoneurons, as seen both from extracellular ventral root recordings and from single motoneuron intracellular recordings (Fig. 1, in vitro). This reflex response started with a large but transient short-latency reflex that always had a polysynaptic component (short polysynaptic reflex, SPR; central latency 8–15 ms and lasting 10–30 ms; Fig. 1A, inset) and sometimes also had an earlier monosynaptic reflex component (not present in Fig. 1A, but see Li et al. 2004b). This transient SPR arose from a large but transient polysynaptic EPSP (short EPSP) that generally produced only one action potential in intracellularly recorded motoneurons at rest (Fig. 1B). The short EPSP was seen without interference from spiking (or the Ca PIC) when the motoneuron was hyperpolarized with a bias current (Fig. 1B, bottom plot). This short EPSP by itself did not trigger Ca PICs or spasms (see later section), consistent with the previous findings that Ca PICs are slowly activated, requiring >50 ms to substantially activate (Li and Bennett 2007). Nevertheless, we found this SPR useful for studying EPSP modulation in isolation because it was not affected by Ca PICs; that is, the SPR was not inhibited by a block of Ca PICs with isradipine (Fig. 1A, bottom plot; mean change −9.7 ± 41.0%, n = 9, P > 0.05).
Fig. 1.
Polysynaptic reflexes and their underlying excitatory postsynaptic potential (EPSP) in chronic spinal rats. A: a long-lasting reflex triggered by dorsal root stimulation [0.1-ms pulse, 3 times threshold (3×T)] and recorded from the ventral roots, with the reflex components long polysynaptic reflex (LPR) and long-lasting reflex (LLR) quantified during periods indicated by horizontal arrows (top trace). Inset: short polysynaptic reflex (SPR) on expanded time scale. Bottom trace shows elimination of LLR, but not LPR, after blocking the L-type Ca2+ channel with isradipine (15 μM). Bkg, background root activity. B: persistent inward current (PIC)-mediated plateau potential and sustained firing (LLR) evoked by dorsal root stimulation (3×T) in motoneuron at rest (without injected current; top trace). With a hyperpolarizing bias current to prevent PIC activation, the same stimulation only evoked polysynaptic EPSPs, with short and long EPSP components, corresponding to the SPR and the LPR (bottom trace).
After this transient reflex, there was a very long-lasting reflex (lasting seconds) that underlies muscle spasms (Bennett et al. 2004); we broke this down into two components based on their origin. The first half-second of this long reflex was of polysynaptic reflex origin, and we thus refer to it as the long polysynaptic reflex (LPR; Fig. 1A). That is, this LPR was initiated by an unusually long-duration polysynaptic EPSP (long EPSP) and further amplified and prolonged by PICs intrinsic to the motoneuron, as previously described (Fig. 1B) (Li et al. 2004a). The long EPSP underlying this LPR was seen in isolation in motoneurons when the PICs were prevented from activation by hyperpolarizing a motoneuron (PICs are voltage dependent) (Li et al. 2004a). Also, the effects of the long EPSP on the ventral root reflexes (LPR) were seen in isolation when the Ca PICs were blocked with isradipine (Fig. 1A) (Li et al. 2004a). On average, the LPR was reduced by 52.1 ± 39.5% with isradipine (15 μM, n = 9, P < 0.05), consistent with a partial involvement of PICs. Thus, under normal resting conditions (without hyperpolarization or isradipine), the long EPSP activated the PICs, which in turn amplified and prolonged the reflex response, thus producing the mixed PIC- and synaptic-mediated LPR. The remaining portion of the long-lasting reflex (latency >500 ms) was entirely mediated by PICs intrinsic to the motoneuron, because it was eliminated by preventing PIC activation (with hyperpolarization; Fig. 1B) or nearly eliminated by blocking the Ca PICs with isradipine (Fig. 1B; significant 83.9 ± 13.5% reduction, n = 9, P < 0.05). Accordingly, it was called the PIC-mediated long-lasting reflex (or LLR). The remaining LLR in isradipine was likely mediated by the Na PIC, which can produce very slow firing in motoneurons that rest close to threshold (Li et al. 2004a), although this effect appeared small (15%).
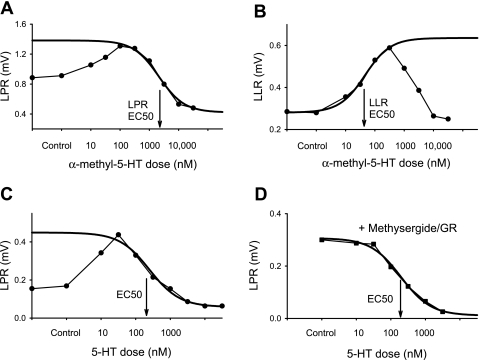
5-HT1B and 5-HT1F receptor activity inhibits the LPR and associated spasms.
Application of the selective 5-HT1B/1D/1F receptor agonist zolmitriptan inhibited the LPR, with increasing doses producing larger responses over about a 100-fold change in dose (Fig. 2 and Table 2). This dose-response relation was well approximated by a sigmoidal curve (Fig. 2C) from which we computed 1) the agonist dose to produce 50% maximal inhibition (EC50; Fig. 2D), 2) agonist potency (pEC50 = −log EC50), and 3) agonist efficacy (maximal inhibition, reported relative to control LPR size; Fig. 2C). For zolmitriptan, the EC50 value was about 100 nM with a corresponding potency of about 7 (−log 100 × 10−9 M; Table 2). Overall, the efficacy of zolmitriptan was so large that the LPR was on average reduced to about 3% of predrug control LPR (97% inhibition in Table 2), suggesting that the associated long EPSP was also reduced. Zolmitriptan also significantly decreased the LLR (Fig. 2D; to 2.15 ± 10.76% of control, n = 12, P < 0.05), consistent with an inhibition of the EPSP that triggers this spasm-related reflex.
Fig. 2.
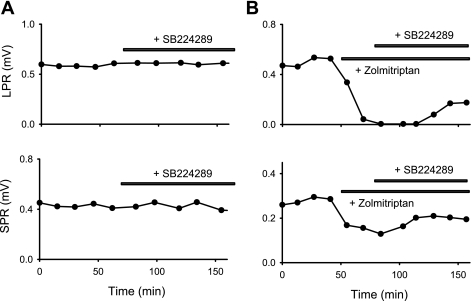
5-HT1B receptor activity inhibits the polysynaptic reflexes in chronic spinal rats. A: long-lasting polysynaptic reflex triggered by dorsal root stimulation (0.1-ms pulse, 3×T) and recorded from the ventral roots, with LPR and LLR components indicated by horizontal bars. B: reduction of LPR and LLR with application of the 5-HT1B/1D/1F agonist zolmitriptan (300 nM; >50% reduction). C and D: reduction of LPR and LLR, respectively, with increasing zolmitriptan dose (decrease over ∼100-fold change in dose; left). Best-fit sigmoidal curves are shown with subsequent estimation of EC50. Prior application of a single blocking dose of the selective 5-HT1B antagonist SB224289 (5 μM) or the 5-HT1B/1D antagonist SB216641 (5 μM) antagonized the inhibitory action of zolmitriptan (shifting EC50 to the right). Each plot shows the typical response from a single rat, with a different rat for each condition, because agonists are not feasible to washout and repeat after antagonist application (taking many hours to wash).
Table 2.
Inhibition of the polysynaptic reflexes by 5-HT1B agonists
| Inhibition of LPR |
Inhibition of SPR |
|||||
|---|---|---|---|---|---|---|
| Agonist | Antagonist Pretreatment | Receptors That Can Be Activated (Ki < 400 nM) | Efficacy, %change | Potency, −log EC50 | Efficacy, %change | Potency, −log EC50 |
| 5-CT | None | 5-HT1A,1B,1D,2B,5–7 | −96.3 ± 65.4* | 7.08 ± 0.50 | −60.4 ± 77.3* | 7.16 ± 0.51 |
| 5-HT | None | 5-HT1–7 | −78.5 ± 23.2* | 6.56 ± 0.17 | −63.4 ± 43.7* | 6.83 ± 0.43 |
| 5-HT | Methys + gran | 5-HT1B,4 | −84.6 ± 14.7* | 6.47 ± 0.38 | −29.7 ± 23.1*† | 6.26 ± 0.22† |
| 5-HT | RSs | 5-HT1,3–7 | −108.1 ± 28.7* | 6.57 ± 0.23 | −74.3 ± 38.4* | 6.36 ± 0.30 |
| 5-HT | RSs + SB216 | 5-HT1A,1E,1F,3–7 | −52.0 ± 22.0*† | 5.70 ± 0.36† | −59.2 ± 23.65 | 5.86 ± 0.32† |
| α-Methyl-5-HT | None | 5-HT1B,1E,1F,2,4 | −57.3 ± 16.5* | 5.75 ± 0.23 | −64.1 ± 29.4* | 5.86 ± 0.53 |
| BW723C86 | None | 5-HT1B,2,3,6 | −38.0 ± 24.8* | 5.89 ± 0.48 | −49.3 ± 43.2* | 5.92 ± 0.33 |
| EMD386088 | None | 5-HT1B,1D,3,6 | −43.6 ± 35.3* | 5.53 ± 0.35 | −51.2 ± 28.8* | 5.76 ± 0.36 |
| EMD386088 | SB216 + gran | 5-HT6 | 12.4 ± 110.6† | ND | 5.7 ± 43.3† | ND |
| LY344864 | None | 5-HT1F | −57.2 ± 25.4* | 7.12 ± 0.17 | −45.9 ± 26.72* | 7.14 ± 0.21 |
| Methylergonovine | None | 5-HT1E,1F,2 | −40.8 ± 21.3* | 6.47 ± 0.25 | −44.0 ± 25.2* | 6.51 ± 0.15 |
| Zolmitriptan | None | 5-HT1B,1D,1F | −99.5 ± 11.3* | 7.07 ± 0.40 | −55.2 ± 20.6* | 7.08 ± 0.41 |
| Zolmitriptan | SB216 or GR127 | 5-HT1F | −41.2 ± 27.6*† | 5.94 ± 0.25† | −38.2 ± 27.1*† | 6.15 ± 0.70† |
| Zolmitriptan | SB224 | 5-HT1D,1F | −26.6 ± 29.0*† | 5.63 ± 0.53† | −31.5 ± 29.9*† | 5.92 ± 0.57† |
Agonists with varying selectivity for the different 5-HT receptors were applied, sometimes after prior application of 5-HT receptor antagonists to effectively make the agonist action more selective (pretreatment). The receptors that can be activated by this agonist-antagonist combination are indicated (Ki < 400 nM; see details in Table 1). The antagonists used, followed by dose and receptors blocked, were as follows: SB224289 (SB224): 5 μM, 5-HT1B; SB216641 (SB216): 3 μM, 5-HT1B/1D; GR127935 (GR127): 3 μM, 5-HT1B/1D; methysergide (methys): 10 μM, all but 5-HT1B/3/4; granisetron (gran): 0.3 μM, 5-HT3; RS127445: 3 μM, 5-HT2B; and RS102221: 3 μM, 5-HT2C (the latter 2 antagonists were applied together and referred to as RSs). The efficacy of the agonists in inhibiting the long (LPR) and short polysynaptic reflex (SPR) are indicated, normalized by the predrug reflex amplitudes (−100% indicates complete elimination of the excitatory reflex by agonist; < −100% indicates inhibitory reflex emerges with agonist). In addition, the agonists 8-OH-DPAT (5-HT1A/5/7 affinity), LP44 (5-HT7/1A), 2-methyl-5-HT (5-HT2B/3/1F), DOI (5-HT2), cisapride (5-HT4), and MK212 (5-HT2C/3) produced no significant inhibition of the LPR or SPR (not shown, doses ≤30 μM; see text). Data are means ± SD; n > 8 per condition.
P < 0.05, significant change in reflex.
P < 0.05, significant decrease in efficacy or potency after application of antagonists (SB224, SB216, or GR127), relative to the inhibitory action of agonists alone (e.g., zolmitriptan; row above antagonist data).
Application of agonists with a relatively high affinity for 5-HT1 receptors, compared with 5-HT2 receptors (5-CT, EMD386088), likewise significantly inhibited the LPR with a simple sigmoidal dose-response relation (significant efficacy; Table 2). Less selective 5-HT1 agonists (including α-methyl-5-HT, BW723C86, methylergonovine, and 5-HT itself) with relatively high affinity for 5-HT2 receptors also inhibited the LPR (Table 2), but this inhibition was partly obscured by their activation of 5-HT2 receptors (Fig. 3), which we have previously shown increases PICs and associated reflexes (Murray et al. 2010, 2011). Fortunately, though, the affinity of these agonists for the 5-HT2B and 5-HT2C receptors was substantially higher than the affinity for 5-HT1 receptors, and thus the effects of each of these receptor types could be observed separately on a dose-response relation as a biphasic response. That is, at low doses, the agonist increased the long-lasting reflexes, including the LPR and LLR (Fig. 3, A–C). This low-dose response was especially prominent in the entirely PIC-mediated LLR (see sigmoid curve fit to ascending phase in Fig. 3B and low EC50), consistent with 5-HT2 receptor-mediated facilitation of the PIC, as described previously (Murray et al. 2010, 2011). As successively higher doses were applied, the reflexes eventually reached a peak (peak reflex), after which they decreased with increasing dose (inhibitory phase), often to the point where the reflex fell well below the reflex before any drug application (control). We fit a sigmoidal curve to this inhibitory phase of the dose-response curve for these agonist actions on the LPR (from peak reflex dose to maximum dose) and from this computed EC50 and efficacy values (Fig. 3, A and C). As shown in Table 2, nonselective agonists with 5-HT1 and 5-HT2 receptor action (e.g., 5-HT) produced a significant inhibition of the LPR (efficacy) after the initial excitatory phase. We confirmed the validity of this estimation of the EC50 and efficacy for reflex inhibition from nonselective agonists by showing that after the confounding 5-HT2 receptor action was blocked with antagonists [methysergide (10 μM) or the selective 5-HT2 antagonists such as RS127445 (3 μM)], 5-HT produced a purely inhibitory action, with a similar dose-response relation to that obtained without the block (Fig. 3D and Table 2). This also shows that the inhibitory action of these nonselective agonists is mediated by 5-HT1 and not 5-HT2 receptors.
Fig. 3.
Mixed 5-HT1 and 5-HT2 receptor agonists have a biphasic response, only inhibiting reflexes at high doses. A–C: dose-response relations for the 5-HT1 and 5-HT2 receptor agonist α-methyl-5-HT and 5-HT itself, with increased reflexes (LPR and LLR) at low doses (5-HT2 mediated) and decreased reflexes at high doses (5-HT1 mediated). In A and C, the heavy line is a sigmoidal curve fit to the inhibitory phase of the dose-response relation and used to estimate the EC50 for the 5-HT1 receptor-mediated inhibitory action. In B, the heavy line is a sigmoidal curve fit to the excitatory phase of the dose-response relation, mediated by 5-HT2 receptors. D: dose-response relation for the 5-HT effect on the LPR after 5-HT2 receptor block with methysergide (10 μM) and 5-HT3 receptor block with granisetron (GR; 0.3 μM), with a similar EC50 to that obtained in C.
Pretreatment with the broad-spectrum antagonist methysergide, as just described, also turned out to be particularly useful, because methysergide has negligible affinity for rat 5-HT1B receptors (Ki > 400 nM), whereas it antagonizes/binds most other 5-HT receptors with high affinity (Ki < 500 nM; except 5-HT3 and 5-HT4 receptors) (Boess and Martin 1994). Thus the inhibition of the LPR by 5-HT seen after pretreatment with methysergide (Fig. 3D and Table 2) suggests that 5-HT1B receptors specifically inhibit the LPR, although this does not rule out additional involvement of other 5-HT1 receptors blocked by methysergide (5-HT1F).
Prior application of the selective 5-HT1B receptor antagonist SB224289 or the selective 5-HT1B/1D receptor antagonist SB216641 significantly reduced the inhibitory action of both selective (zolmitriptan) and nonselective (5-HT; inhibitory phase) 5-HT1 agonists on the LPR (Table 2 and Fig. 2C). These antagonists lowered the efficacy and shifted the agonist dose-response curve by about an order of magnitude to the right (EC50 significantly increased; Table 2), indicating that the 5-HT1B receptor is responsible for a large part of the inhibitory action of these agonists. However, in the presence of these antagonists, there was still significant inhibition of the LPR induced by relatively high doses of both zolmitriptan and 5-HT (Fig. 2C and Table 2). This may be explained by the activation of the 5-HT1F receptor, because this receptor is not blocked by SB224289 or SB216641 (Price et al. 1997; Selkirk et al. 1998) and zolmitriptan and 5-HT have a relatively lower affinity for the 5-HT1F compared with the 5-HT1B receptor (Table 1).
Consistent with the possible involvement of 5-HT1F receptors in regulating the LPR, we found that the selective 5-HT1F agonist LY344864 and nonselective 5-HT1F agonists that have negligible affinity for 5-HT1B receptors (e.g., methylergonovine, α-methyl-5-HT; Table 1) inhibited the LPR (Table 2). However, this does not negate the importance of 5-HT1B receptors, because the agonists with substantial affinity for 5-HT1B receptors but negligible affinity for the 5-HT1F receptors (BW723C86, EMD386088, and 5-CT; Table 1) also inhibited the LPR (Table 2), indicating that both 5-HT1B and 5-HT1F receptors modulate the LPR.
Application of agonists (or agonist-antagonist combinations) relatively selective for 5-HT1A/1E, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors (Table 1) produced no significant inhibition of the LPR (Tables 2 and 3), suggesting that none of these other receptors inhibit the LPR and associated long EPSP. Furthermore, application of the selective 5-HT2 receptor agonist DOI or nonselective 5-HT2 agonists that have negligible affinity for 5-HT1B and 5-HT1F receptors also produced no detectable inhibition in the LPR (Tables 2 and 3). However, these 5-HT2 agonists did produce a significant facilitation of the LPR (by 417.87 ± 346.5, 172.13 ± 70.22, and 79.66 ± 94.82% for 2-methyl-5-HT, DOI, and MK212 respectively, P < 0.05, n > 8 per condition, over a dose range appropriate to activate 5-HT2 receptors; up to 30 μM), due to a facilitation of the underlying Ca PIC, as previously reported for the LLR (Murray et al. 2011). To rule out inhibitory effects of 5-HT2 receptors on the EPSPs that might be masked by their large facilitation of the Ca PIC, we first blocked the Ca PIC with isradipine, giving us a reflex that reflected the polysynaptic EPSP in isolation (see Fig. 1). With this Ca PIC block present, DOI produced no significant change in the LPR (3.90 ± 10.4% change, P > 0.05, n = 8, at 3,000 nM), suggesting that the excitatory action of DOI is mainly on the Ca PIC, and there is no net inhibitory action of 5-HT2 receptors on the EPSP underlying the LPR.
Table 3.
Relative potency of agonists at inhibiting the LPR and SPR
| LPR | SPR | ||
|---|---|---|---|
| Receptor | Agonist | pEC50 − pKi | pEC50 − pKi |
| 5-HT1A | 5-CT | −2.38 ± 0.50 | −2.30 ± 0.51 |
| 5-HT | −2.22 ± 0.17 | −1.95 ± 0.28 | |
| 8-OH-DPAT | ND | ND | |
| LP44 | ND | ND | |
| 5-HT1B | 5-CT | −1.40 ± 0.50* | −1.32 ± 0.51* |
| 5-HT | −1.05 ± 0.17* | −0.78 ± 0.28* | |
| α-Methyl-5-HT | −1.32 ± 0.23* | −1.21 ± 0.53* | |
| BW723C86 | −0.71 ± 0.48* | −0.68 ± 0.33* | |
| EMD386088 | −1.22 ± 0.35* | −0.99 ± 0.36* | |
| Zolmitriptan | −1.23 ± 0.40* | −1.22 ± 0.41* | |
| 5-HT1D | 5-CT | −2.35 ± 0.50 | −2.27 ± 0.51 |
| 5-HT | −2.04 ± 0.17 | −1.77 ± 0.28 | |
| BW723C86 | −0.41 ± 0.48* | −0.38 ± 0.33 | |
| EMD386088 | −1.43 ± 0.35* | −1.20 ± 0.36* | |
| Zolmitriptan (SB224) | −2.13 ± 0.53 | −3.28 ± 0.57 | |
| 5-HT1E | 5-HT (SB216) | −2.51 ± 0.36 | −2.35 ± 0.32 |
| α-Methyl-5-HT | −1.17 ± 0.23* | −1.06 ± 0.53* | |
| Methylergonovine | −0.58 ± 0.25* | −0.54 ± 0.15 | |
| 5-HT1F | 5-HT (SB216) | −1.47 ± 0.17 | −1.31 ± 0.32 |
| α-Methyl-5-HT | −0.99 ± 0.23* | −0.88 ± 0.53* | |
| LY344864 | −1.10 ± 0.17* | −1.08 ± 0.21* | |
| Methylergonovine | −1.04 ± 0.25* | −1.00 ± 0.15* | |
| Zolmitriptan (SB224) | −1.57 ± 0.53* | −1.28 ± 0.57* | |
| 5-HT2A | 5-HT | −1.68 ± 0.17 | −1.41 ± 0.28* |
| α-Methyl-5-HT | −1.15 ± 0.23* | −1.04 ± 0.53* | |
| BW723C86 | −0.71 ± 0.48* | −0.68 ± 0.33* | |
| DOI | ND | ND | |
| Methylergonovine | −2.98 ± 0.25 | −2.94 ± 0.15 | |
| 5-HT2B | 2-Methyl-5-HT | ND | ND |
| 5-CT | +0.26 ± 0.50 | +0.34 ± 0.51 | |
| 5-HT | −1.43 ± 0.17 | −1.16 ± 0.28* | |
| α-Methyl-5-HT | −2.23 ± 0.23 | −2.12 ± 0.53 | |
| BW723C86 | −2.01 ± 0.48 | −1.98 ± 0.33 | |
| DOI | ND | ND | |
| Methylergonovine | −2.83 ± 0.25 | −2.79 ± 0.15 | |
| 5-HT2C | 5-HT | −1.40 ± 0.17 | −1.13 ± 0.28* |
| α-Methyl-5-HT | −2.82 ± 0.23 | −2.71 ± 0.53 | |
| BW723C86 | −1.01 ± 0.48* | −0.98 ± 0.33* | |
| DOI | ND | ND | |
| MK212 | ND | ND | |
| Methylergonovine | −1.87 ± 0.25 | −1.83 ± 0.15 | |
| 5-HT3 | 2-Methyl-5-HT | ND | ND |
| BW723 | −0.61 ± 0.48* | −0.61 ± 0.33* | |
| EMD386088 | −1.94 ± 0.35 | −1.71 ± 0.36 | |
| MK212 | ND | ND | |
| 5-HT4 | 5-HT | −1.64 ± 0.17 | −1.37 ± 0.28* |
| α-Methyl-5-HT | −0.83 ± 0.23* | −0.72 ± 0.53* | |
| Cisapride (RSs) | ND | ND | |
| 5-HT5 | 5-CT | −2.42 ± 0.50 | −2.34 ± 0.51 |
| 5-HT | −1.54 ± 0.17 | −1.27 ± 0.28* | |
| 8-OH-DPAT | ND | ND | |
| 5-HT6 | 2-Methyl-5-HT | ND | ND |
| 5-CT | +0.48 ± 0.50 | +0.56 ± 0.51 | |
| 5-HT | −0.69 ± 0.17* | −0.42 ± 0.28 | |
| BW723C86 | −0.51 ± 0.48* | −0.48 ± 0.33* | |
| EMD386088 (SB216+) | ND | ND | |
| 5-HT7 | 5-CT | −2.72 ± 0.50 | −2.64 ± 0.51 |
| 5-HT | −2.26 ± 0.17 | −1.99 ± 0.28 | |
| 8-OH-DPAT | ND | ND | |
| LP44 | ND | ND |
Relative potency was computed as the difference between potency (Table 2) and affinity (Table 1): pEC50 − pKi. ND, no detected inhibition of the reflex in Table 2.
Relative potency within 2 SD of −1.0, the confidence interval for similarity. Bold type indicates relative potency values for that receptor are all within the confidence interval.
Agonist inhibition potency is correlated with receptor binding affinity at 5-HT1B and 5-HT1F receptors.
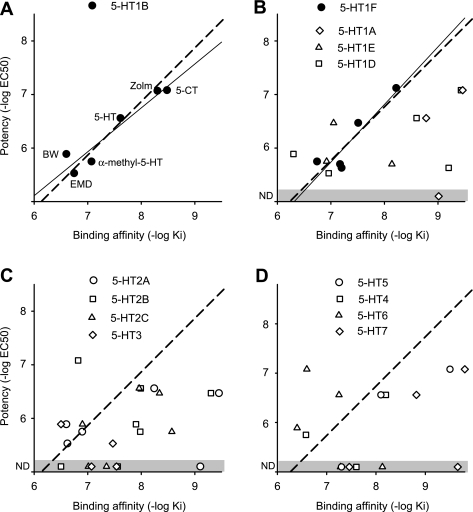
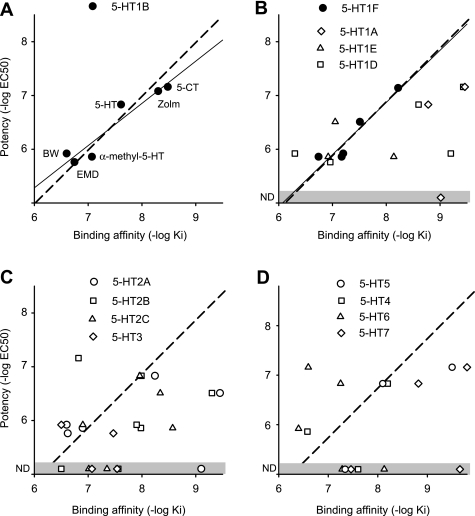
The effective 5-HT1B and 5-HT1F agonist doses that inhibit the LPR (EC50 values and associated potencies, pEC50) varied by orders of magnitude between the different agonists (Table 2), although this variation was largely accounted for by the differing binding affinity of these drugs to 5-HT1B and 5-HT1F receptors (pKi; see description of binding affinity in methods; Table 1). That is, we found that for 5-HT1B agonists, the potency (pEC50) was significantly correlated with the binding affinity (pKi) of the agonist for 5-HT1B receptors and, importantly, very close to a line of unity slope (dashed line; pEC50 = pKi + C) as shown in Fig. 4A. Likewise, the potency was also significantly correlated with the agonist affinity for 5-HT1F receptors (Fig. 4B), with close to a unity slope relation, consistent with an additional involvement of this receptor. The agonist potency was uncorrelated with the agonist binding affinity for other 5-HT receptors (including 5-HT1D; Fig. 4, B–D) with potency scattered widely, far from the linear potency-affinity relation found for the 5-HT1B and 5-HT1F receptors. However, for most of these other receptors, only a few broad-spectrum agonists with affinity to these other 5-HT receptors produced a response (inhibition of LPR), making the correlation analysis statistically weak (n < 5). Thus we sought an independent method of quantifying whether the agonist response potency was attributed to a given receptor, based on quantitatively modeling the expected relation between potency and affinity, as described below.
Fig. 4.
Potency of 5-HT receptor agonists at inhibiting the LPR is only related to binding to 5-HT1B and 5-HT1F receptors. A: 5-HT1B receptor agonist potency (pEC50 = −log EC50) for inhibiting the LPR plotted against the agonist binding affinity to that receptor (pKi). Each agonist is indicated next to its data point: BW, BW723C86; Zolm, zolmitriptan; EMD, EMD386088. Thin line indicates significant linear correlation between potency and affinity (r = 0.96, P < 0.05, n = 6). Dashed line represents the best fit line with unit slope (potency = binding affinity + C, where C ≈ −1). B–D: similar potency-affinity scatter plots for the remaining 5-HT receptors. Thin line indicates significant linear correlation between agonist potency and affinity for 5-HT1F receptors (solid circles; r = 0.91, P < 0.05, n = 5). Dashed lines represent the unit slope line. Other receptors had no significant correlation between potency and affinity (open symbols; P > 0.05). ND and shaded zone indicate no detected effect of agonist on the LPR. Agonists used and affinities are listed in Table 1, with agonists assumed to act at a receptor only if Ki < 400 nM. Potencies are from Table 2. Potencies for 5-HT and zolmitriptan action in the presence of 5-HT1B antagonists were used (plotted) for comparison to 5-HT1D, 5-HT1E, and 5-HT1F receptor binding affinity, because these antagonists removed confounding effects of 5-HT1B receptors. Table 3 also summarizes agonists/antagonists used for each receptor.
Potency of agonist can be quantitatively predicted from its receptor binding affinity.
Ideally, for a receptor to be involved in a particular response, the agonist dose needed to substantially bind to the receptor (Ki) should approximately correspond to the agonist dose needed to produce a functional response (e.g., EC50 for LPR), and thus the agonist binding affinity (pKi) should roughly equal its potency (pEC50) (Selkirk et al. 1998; Wainscott et al. 1993). However, the substantial barriers to drug diffusion in our whole cord preparation (Murray et al. 2011) required higher drug doses (EC50) to get responses, and thus the potency (pEC50 = −log EC50) was higher than the affinity. Furthermore, nonlinearities in the functional receptor response, such as saturation of the EPSP that underlies the LPR and saturation in receptor responses (receptor reserve; Boess and Martin 1994), may have subtly changed the EC50 dose and potency (see discussion). Nevertheless, factors such as drug diffusion and response saturation do not generally depend on the agonist involved (see discussion). Thus we hypothesized that the potency could be predicted from affinity by the following simple relation: pEC50 = pKi + C, where C is a constant that is invariant for all agonist responses at functional receptors that represents drug diffusion barriers, etc. Rearranging, we have pEC50 − pKi = C, and thus determining whether or not a receptor is functional amounts to testing whether the difference between the measured potency and affinity is invariant (C). We call this difference the relative potency (pEC50 − pKi; it reflects all factors that affect potency other than binding affinity). For the 5-HT1B and 5-HT1F receptors that we know are involved in inhibiting the LPR (and associated EPSP), we found that the potency-affinity data significantly fit this simple linear relation (with r = 0.93 and 0.91, respectively, n = 6 and 5, respectively; dashed unity slope lines in Fig. 4, A and B). Also, the difference pEC50 − pKi (relative potency) was, as hypothesized, highly invariant across all agonists tested at these receptors, on average −1.15 ± 0.25 and −1.23 ± 0.27 for 5-HT1B and 5-HT1F receptors, respectively, with each agonist having a relative potency well within two SD of the mean (our confidence interval, SD taken from each agonist potency; see Table 3). Remarkably, this relative potency value of about −1 has been seen for two other functional receptors in our preparation (Murray et al. 2011) and so appears to be an invariant across many or all receptors, in part reflecting the diffusion barriers to drugs reaching the receptors. Thus, in our preparation, if a receptor is functional, then pEC50 − pKi = −1 (constant; dashed line in Fig. 4).
In contrast to the invariant relative potency for 5-HT1B/F receptors, we found that for all other receptors, the relative potency computed from the potency of broad-spectrum agonist response (pEC50) varied widely over a range well outside of our confidence interval (2 SD; Table 3), suggesting than none of these receptors affect the LPR response (pEC50 − pKi not equal to −1). For example, the relative potency computed for zolmitriptan's pEC50 compared with its affinity at the 5-HT1D receptors was less than −2 (Table 3), well outside of the confidence interval, suggesting that its EC50 is too high to be predicted from the Ki for zolmitriptan at the 5-HT1D receptor and thus ruling out this receptor, for which we otherwise had no selective agonist to directly test. Similarly, the potency of 5-HT and 5-CT could not be predicted from their pKi values at the 5-HT1D receptor (relative potency less than −2; Table 3), again suggesting that the 5-HT1D receptor is not involved in modulating the LPR. Sometimes, by chance, a drug (e.g., EMD386088) had a similar affinity for the 5-HT1B receptor and another receptor (e.g., 5-HT1D; Table 3), and in this case the relative potency (pEC50 - pKi) was similar for each receptor and could not be used to distinguish the involvement of these two receptors. Overall, the relative potency varied widely for the action agonists of non-5-HT1B/1F receptors, indicating that no receptor, other than the 5-HT1B/1F receptors, was involved in modulating the LPR.
Another way to interpret the relative potency arises from the law of differences of logarithms: pEC50 − pKi = −log (EC50) − [−log (Ki)] = −log (EC50/Ki). Thus the ratio EC50/Ki equals 10−(pEC50−pKi). For the 5-HT1B receptor, the relative potency was on average −1.15, and thus on average, EC50/Ki = 10.01.15 = 14. This indicates that the EC50 dose needed to affect the LPR in the present whole sacral spinal cord preparation was about 10 times higher than the Ki value, a factor that is most likely due to drug diffusion.
SPR is also inhibited by the 5-HT1B and 5-HT1F receptors.
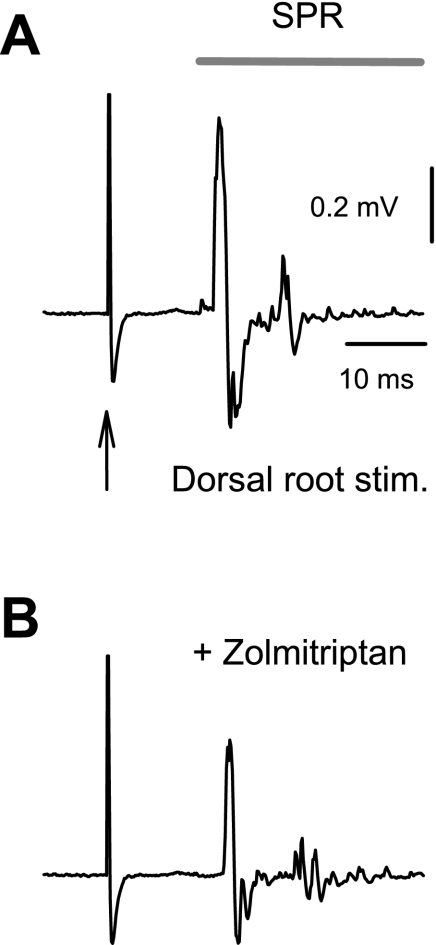
Similar to the LPR, the SPR was inhibited by 5-HT1B and 5-HT1F receptor agonists, including relatively selective agonists (zolmitriptan or agonist-antagonist combinations) and nonselective agonists (5-HT; Fig. 5 and Table 2). Also, the agonist potencies (pEC50) were significantly correlated with the agonist binding affinity at 5-HT1B and 5-HT1F receptors and no other receptor (Fig. 6). In contrast, 5-HT receptor agonists (or agonist-antagonist combinations) that have negligible affinity for 5-HT1B or 5-HT1F receptors did not inhibit the SPR (Table 3). The relative potency computed for each agonist relative to its binding affinity at the 5-HT1B receptor (Table 3) was consistently within 2 SD of −1.0 (our confidence interval), with a mean of −1.03 ± 0.26. Likewise, the relative potency computed for agonists of the 5-HT1F receptor (Table 3) was consistently within 2 SD of −1.0, with a mean of −1.11 ± 018, suggesting that the potency of the agonists on the SPR was well predicted by agonist affinity at the 5-HT1B or 5-HT1F receptor, with an invariant diffusion factor (pEC50 = pKi − 1), just as we found for the LPR. In contrast, the relative potency for other receptors varied widely and for at least one agonist was more than 2 SD from −1 (Table 3). An important example is that the relative potency of zolmitriptan for the 5-HT1D receptor was much too low for this receptor to be involved in the SPR, more than 2 SD below −1.0 (Table 3), ruling out a 5-HT1D action of zolmitriptan.
Fig. 5.
5-HT1B/1D/1F agonist zolmitriptan inhibits the SPR. A: SPR evoked in the ventral root of a chronic spinal rat after dorsal root stimulation (0.1 ms, 3×T), quantified during the period indicated by the horizontal bar. B: inhibition of the SPR by zolmitriptan (300 nM).
Fig. 6.
Potency of 5-HT receptor agonists at inhibiting the SPR is only related to binding to 5-HT1B and 5-HT1F receptors. A: 5-HT1B receptor agonist potency (pEC50) for inhibiting the SPR plotted against the agonist binding affinity to that receptor (pKi). Format is identical to that described in Fig. 4. Thin line indicates significant linear correlation between potency and affinity (r = 0.95, P < 0.05, n = 6). Dashed line represents the best fit line with unit slope. B and C: similar potency-affinity scatter plots for the remaining 5-HT receptors. Thin line indicates significant linear correlation between agonist potency and affinity for 5-HT1F receptors (filled circles; r = 0.94, P < 0.05, n = 5). Dashed lines represent the unit slope line. The remaining receptors had no significant correlation between potency and affinity (open symbols; P > 0.05). ND and shaded zone indicate no detected effect of agonist on the LPR. Agonists used, potencies, and affinities are detailed in Fig. 4. Table 3 also summarizes agonists/antagonists used for each receptor.
The three 5-HT2 receptor agonists tested that have negligible affinity for 5-HT1B receptors produced no inhibition in the SPR (Tables 2 and 3), and only one of these, MK212, significantly increased the SPR (by 70.15 ± 62.4%; n = 8, P <0.05). The remaining two (DOI and 2-methyl-5-HT) had no effect on the SPR (Table 2), unlike the large increase produced by all three of these 5-HT2 agonists on the LPR, suggesting that the PICs controlled by the 5-HT2 receptors do not reliably affect this shorter, transient SPR reflex. Also, when we blocked the PICs with isradipine (as above), the SPR remained unaffected by DOI (0.75 ± 14.87% change, not significant, n = 8, P > 0.05).
Lack of endogenous 5-HT1B receptor activity in chronic spinal rats.
We next examined whether there was any endogenous 5-HT1 receptor activity after chronic spinal cord injury. Without prior agonist application, the selective antagonists SB224289 (5-HT1B selectivity, 3–5 μM; Fig. 7A), SB216641 (5-HT1B/1D, 5 μM), or GR127935 (5-HT1B/1D, 5 μM) produced no significant increase in either the LPR (0.8 ± 13.2, −4.8 ± 40.2, and −8.5 ± 29.8% change, respectively, n = 12 per condition, P > 0.05) or SPR (10.1 ± 34.8, 6.8 ± 31.6, and −3.0 ± 50.0% change, respectively, P > 0.05), suggesting that there is no endogenous 5-HT1B receptor activity inhibiting the reflexes, and consistent with previous findings that there is little functional 5-HT that remains in chronic spinal rats (Murray et al. 2010). SB224289 is unique among these three antagonists because it is classified as an inverse agonist (Price et al. 1997; Selkirk et al. 1998), meaning that it not only blocks agonist-induced activity but also blocks spontaneous activity in the 5-HT1B receptor that occurs in the absence of 5-HT or other agonists (constitutive receptor activity) (Seifert and Wenzel-Seifert 2002). Thus the lack of action of SB224289 indicates that there is not constitutive 5-HT1B receptor activity after injury, unlike what we find with 5-HT2 receptors (Murray et al. 2010, 2011). As a positive control, we applied 5-HT1B agonists (zolmitriptan, 1.0 μM; 5-CT, 1.0 μM; or 5-HT, 0.3 μM) to activate the 5-HT1 receptors, which as expected decreased the LPR and SPR (Table 2), and then applied the antagonists (Fig. 7B). In this situation, the antagonists SB224289 (3–10 μM), SB216641 (5–10 μM), and GR127935 (5 μM) significantly increased the LPR (by 45.8 ± 45.7, 27.7 ± 30.2, and 78.8 ± 87.9%, respectively) and the SPR (by 44.0 ± 41.1, 45.4 ± 40.9, and 66.7 ± 60.3%, respectively, n = 12 each condition, P < 0.05), demonstrating that these antagonists can be used to detect 5-HT1B receptor activity. We did find that the antagonists only partially reversed the inhibition of the reflexes by these 5-HT1 agonists (Fig. 7B), but we attribute this to the agonist activation of 5-HT1F receptors, which our antagonists did not block.
Fig. 7.
5-HT1B receptor is not endogenously active in chronic spinal rats. A: a block of possible endogenous 5-HT1B receptor activity with SB224289 (3 μM, horizontal bar) produced no increase (or change) in the LPR or SPR. Reflexes were measured at about 15-min intervals (●). B: in contrast, SB224289 (3 μM) increased the LPR and SPR after 5-HT1B receptors were exogenously activated by zolmitriptan (1 μM), which initially deceased these reflexes.
Increasing cAMP increases the LPR and SPR.
5-HT1 receptors are coupled to Gi proteins that lead to decreased intracellular cAMP levels. Thus our finding that activating 5-HT1 receptors decreases the LPR and SPR suggests that 5-HT1 receptors may decrease reflexes by decreasing cAMP, and more generally, these reflexes and associated EPSPs may depend on basal cAMP levels. We tested this idea by applying forskolin (1–10 μM), a membrane-permeable drug that increases intracellular cAMP. As expected, forskolin increased both the LPR and SPR (by 116.7 ± 72.0 and 135.7 ± 78.1%, respectively, n = 8, P < 0.05).
EPSPs in motoneurons are inhibited by zolmitriptan.
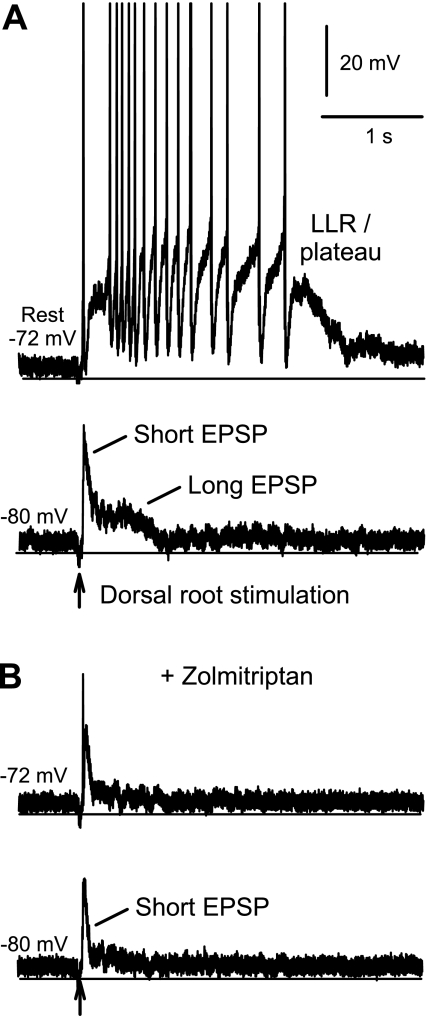
To verify that 5-HT1B/1F receptors inhibit the EPSPs underlying the LPR and SPR, we made intracellular recordings from motoneurons in chronic spinal rats (in vitro) and measured the EPSPs and associated reflexes (firing) evoked by stimulating the dorsal roots (3×T). When a motoneuron was at rest, this stimulation produced a depolarization that activated the large PICs, which in turn produced a many-second-long plateau potential and associated firing (LLR), as previously described (Fig. 8A) (Li et al. 2004a). However, distinguishing the depolarization induced by the EPSPs from the PICs (plateau) was not possible at rest. Thus, to observe the EPSP in isolation, we hyperpolarized the cell with a steady bias current to prevent the activation of PICs (which are voltage dependent; Fig. 8A, −80 mV). At these hyperpolarized potentials, the same dorsal root stimulation evoked an EPSP, typically about 0.5 s long, with two components: the long EPSP, responsible for the LPR, and the short EPSP, responsible for the SPR (as described earlier; both polysynaptic EPSPs). The long EPSP was on average 2.76 ± 1.74 mV (peak, at 200–500 ms poststimulation, n = 10 motoneurons), and the short EPSP was on average larger at 10.85 ± 5.27 mV (peak, at about 5–10 ms), although transient. The 5-HT1B/1D/1F agonist zolmitriptan (1 μM) significantly reduced the long EPSP by 89% (changed by −2.47 ± 2.16 mV) and the short EPSP by 44% (by −4.78 ± 2.49 mV, n = 10, P < 0.05), as shown in Fig. 8B. This near elimination of the long EPSP was accompanied by a loss of activation of PIC-mediated plateaus and LLRs (Fig. 8B), measured with the motoneuron at rest, in all cells tested (n = 8). A substantial short EPSP remained in zolmitriptan (Fig. 3B), and yet there was no plateau or LLR evoked, indicating again that the long EPSP is primarily responsible for triggering the PIC and associated LLR.
Fig. 8.
Zolmitriptan inhibits polysynaptic EPSPs in motoneurons of chronic spinal rats. A: PIC-mediated plateau potential and sustained firing (LLR) evoked by dorsal root stimulation (0.1-ms pulse, 3×T) in a motoneuron at rest (top trace; −72 mV, without injected current; spikes clipped). With a hyperpolarizing bias current to prevent PIC activation, the same stimulation only evoked a polysynaptic EPSP, with short- and long-duration components indicated (bottom trace; motoneuron at −80 mV). B: in the same motoneuron, zolmitriptan (1 μM) eliminated the plateau and LLR evoked by dorsal root stimulation (top trace) and inhibited the short and long EPSPs (hyperpolarized, bottom trace).
PICs and other motoneuron properties are not affected by zolmitriptan.
When we depolarized a motoneuron with a slow voltage ramp (under voltage clamp), a large, persistent inward current (the PIC) was activated about 10 mV above the resting potential and produced a marked downward deflection in the recorded current (inward current, Fig. 9A), relative to the leak current, as previously reported (Li and Bennett 2003). This inward current is what produces the large plateau in Fig. 8A, when the cell is stimulated at rest (in current clamp), and thus underlies the LLR and spasms (synaptic input activates the dendritic PICs more readily than we can activate the PICs with injected electrode current, and thus the threshold is above rest with intracellular current injection) (Bennett et al. 1998; Li et al. 2004a). Zolmitriptan had no significant effect on the PIC amplitude (9.7 ± 20.5% change, n = 8 tested, P > 0.05) or Von (−0.8 ± 0.9%, Fig. 9C). Likewise, zolmitriptan had no significant effect on other motoneuron properties, including Rm (2.7 ± 17.7% change, P > 0.05), resting potential (1.5 ± 4.1%, P > 0.05), and spike threshold (−3.2 ± 6.0%, P > 0.05).
Fig. 9.
Zolmitriptan inhibits excitatory postsynaptic currents but not PICs in motoneurons of chronic spinal rats. A and C: PIC in a motoneuron, activated by slowly increasing the membrane potential under voltage clamp and quantified at its initial peak, where it produced a downward deflection in the recorded current (at arrow) relative to the leak current (thin line). The PIC was unaffected by zolmitriptan application (1 μM). Dashed marks indicate rest (−71 mV) and −50 mV. B: in the same motoneuron, short and long excitatory postsynaptic currents (EPSC; downward current deflections) and inhibitory postsynaptic current (IPSC; upward current deflections) evoked by dorsal root stimulation (0.1-ms pulse, 3×T) in voltage-clamp mode at rest (bottom trace) and above rest (−60 mV). Expanded time scale is shown at right. Note the large IPSC that arises just after the short EPSC at depolarized potentials (−60 mV), which essentially interrupts the EPSCs. D: zolmitriptan (1 μM) reduced the long and short EPSCs (at rest) and revealed a longer and larger IPSC.
Inhibitory glycinergic synaptic currents are revealed by zolmitriptan.
Because of the large PICs and associated firing that was activated just above rest, it was impossible to evaluate the EPSPs at potentials at or above rest. However, by voltage-clamping at a fixed potential, to prevent firing or PIC activity changes, we were able to evaluate the EPSCs at or above rest, as evoked by our standard dorsal root stimulation. At rest there was, as expected, an EPSC (inward, downward current) with short- and long-duration components, the counterparts of the short and long EPSPs described above (Fig. 9B, bottom; seen in n = 9/9 motoneurons tested). However, when we voltage-clamped the motoneurons 10 mV above rest (at about the spike and PIC threshold), the same stimulation evoked an inhibitory postsynaptic current (IPSC; outward current deflection in Fig. 9B, top), in addition to EPSCs, in all motoneurons (n = 9/9). This IPSC started 2–5 ms after the short EPSC, peaked at 20–30 ms, and then decayed slowly. Thus this ISPC was positioned between the short and long EPSCs, essentially interrupting them (Fig. 9B).
Application of zolmitriptan inhibited the EPSCs seen at rest, reducing both the short and long EPSC components in all motoneurons tested (n = 5/5; Fig. 9D, bottom), as expected. Interestingly, once these EPSPs were reduced by zolmitriptan, a long IPSC was revealed (Fig. 9D, top), although the peak of this IPSC was not increased (n = 5/5; Fig. 10). This long IPSC revealed in zolmitriptan suggests that there is a large inhibitory synaptic input that is normally counterbalanced by a simultaneously activated large excitatory synaptic input. To confirm this, we applied strychnine (2 μM) to block inhibitory glycinergic inputs, which produced synaptic responses that were always net excitatory and doubled both the long and short EPSPs (increasing by 5.77 ± 3.22 and 9.70 ± 6.95 mV, respectively, n = 5, P < 0.05; measured at hyperpolarized potentials, as above, not shown; EPSP latency did not change), thus producing very large peak EPSPs of about 15 mV. Furthermore, the EPSPs recorded in strychnine were still significantly reduced by zolmitriptan (reduced by 43.7 ± 34.2 and 23.9 ± 7.8% for long and short EPSPs, respectively, P < 0.05; absolute reduction in EPSP was similar to that without strychnine; thus %change was smaller), suggesting that 5-HT1 receptor activation (with zolmitriptan) directly reduces the EPSPs, and this action is not secondary to changes in large inhibitory inputs that partially mask the EPSPs.
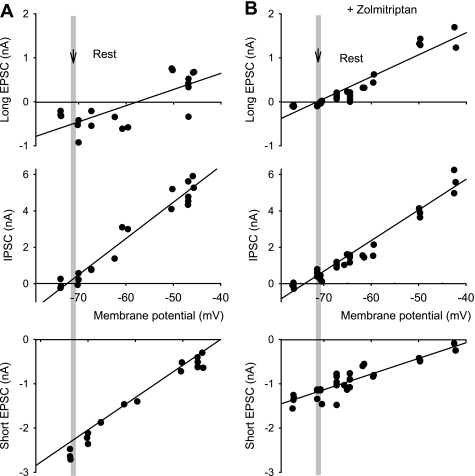
Fig. 10.
Zolmitriptan reduces long EPSC, further revealing IPSCs, with reversal potential at rest. A, top plot: long EPSC (negative currents), measured at 300 ms poststimulation, plotted against the holding potential, for the same motoneuron and stimulation as in Fig. 9 (reversing above −60 mV). Linear regression line, fit to the data, crosses the voltage axis at about −57 mV, the reversal potential for this mixed current. Middle plot: early peak of IPSC, measured at 20–30 ms poststimulation, plotted against holding potential, again during voltage clamp as in Fig. 9. Linear regression line crosses the voltage axis near rest (shaded bar, −71 mV), the reversal potential for this pure IPSC. Bottom plot: short-latency transient EPSP peak, measured at about 5 ms poststimulus, with reversal potential at about −40 mV. B, top plot: zolmitriptan (1 μM) inhibited the long EPSC (negative currents reduced), revealing a pure long-duration IPSC (positive currents; measured again 300 ms poststimulation), with a reversal potential near rest (regression line axis crossing). Middle plot: zolmitriptan did not affect the early peak of the IPSC measured 20–30 ms poststimulation. Bottom plot: zolmitriptan inhibited the short EPSC.
Reversal potential for inhibitory synaptic currents is at the resting membrane potential after injury.
Remarkably, the inhibitory synaptic input always produced negligible potential changes at rest (n = 9/9), even when the opposing EPSCs were largely eliminated with zolmitriptan (n = 5/5; Fig. 9D), suggesting that the reversal potential for these inhibitory glycinergic inputs, and their associated chloride currents, was near rest. To verify this, we estimated the Cl− reversal potential from the reversal potential for the peak of the IPSC, which could generally be measured in isolation because it started abruptly, with a delay relative to short EPSP, and peaked at about 20–30 ms, well after the short EPSC peaked (at 5–10 ms). On average, the reversal potential for the peak IPSC was −73.0 ± 3.8 mV, not significantly different from the mean resting potential of −70.9 ± 7.2 mV in chronic spinal rats (n = 9, P > 0.05) and significantly lower than the spike threshold (by −20.4 ± 4.2 mV, n = 9, P > 0.05; spike threshold −53.3 ± 3.4 mV). The reversal potential for this same IPSC in motoneurons of normal rats was significantly lower (−77.6 ± 2.3 mV) than in chronic spinal rats and significantly lower than the resting potential of 71.8 ± 3.5 mV (P < 0.05, n = 5 normal rats, recorded as in Li et al. 2004a). To independently assess the Cl− reversal potential, we measured the reversal potential for chloride-mediated IPSCs produced by antidromic ventral root activation (Renshaw cell mediated). In chronic spinal rats these Renshaw cell IPSCs had a reversal potential at the resting membrane potential (not significantly different from rest, not shown, n = 8, P > 0.05), confirming that the reversal potential for Cl− was near rest in chronic spinal rats. In contrast, the reversal potentials for the short and the long EPSC in chronic spinal rats were well above rest but below −50 mV (Fig. 10), indicative of mixed excitatory and inhibitory underlying currents.
5-HT2 receptors do not inhibit the EPSPs.
Application of the 5-HT2A/2B/2C receptor agonist DOI did not significantly affect the EPSPs (short EPSP: 10.9 ± 2.0 mV before and 11.5 ± 2.4 mV after DOI; long EPSP: 5.82 ± 5.0 before and 6.48 ± 4.3 mV after DOI; n = 5; P > 0.05). Considering that 5-HT2 agonists like DOI dramatically facilitate the Ca PICs (Harvey et al. 2006a; Murray et al. 2011), these EPSPs were recorded in the presence of isradipine to prevent unclamped activation of large dendritic Ca PICs and, as usual, were recorded at hyperpolarized potentials, in this case to minimize activation of the Na PIC, which is not blocked by isradipine.
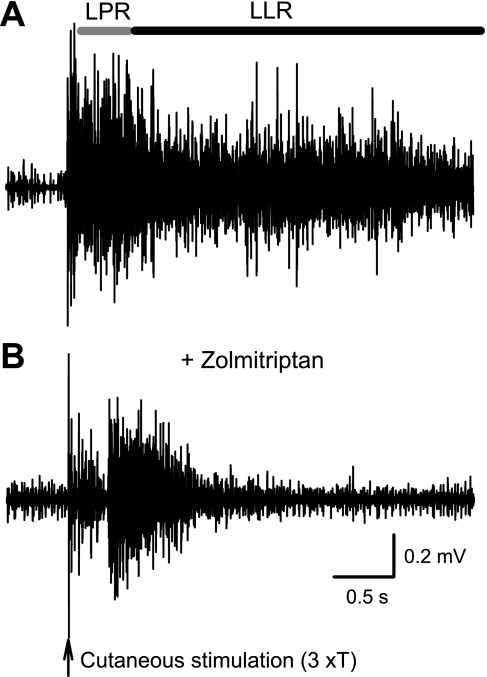
Spasms are reduced in by zolmitriptan in the awake chronic spinal rat.
In the awake chronic spinal rat, electrical cutaneous stimulation of the skin on the tip of the tail evoked many-second-long tail muscle spasms that we recorded with EMG (Fig. 11A). These spasms are the counterpart of the long-lasting reflexes seen in vitro (Fig. 1), and accordingly, we computed the same short and long polysynaptic reflex components mediated by the EPSPs (SPR and LPR), as well as the long-lasting reflex component mediated by the PIC (LLR). Intrathecal application of zolmitriptan (0.1 mM in 30 μl of saline) significantly reduced the SPR and the LPR (by 63.6 ± 8.2 and 63.4 ± 16.0%, respectively, n = 5, P < 0.05), with a clear reduction (notch) in the raw EMG seen during this first half-second period where the EPSPs occur (Fig. 11B). The reflex over the subsequent 4 s (LLR) was also significantly reduced (by 88.2 ± 16.3%, P < 0.05), with only transient rather than sustained activity (Fig. 11B), consistent with a reduction in EPSP, and thus a less effective activation of the PICs that normally produce the spasm. Saline injections had no significant effect on the spasms (n = 5, P > 0.05).
Fig. 11.
Zolmitriptan reduces spasms in the awake chronic spinal rat. A: spasm in chronic spinal rat evoked by electrical-cutaneous stimulation of the tail (3×T) and recorded with electromyogram. B: intrathecal application of zolmitriptan (0.1 mM in 30 μl of saline) reduced the LPR and LLR, quantified at horizontal bars.
DISCUSSION
5-HT1B and 5-HT1F receptor agonists have antispastic action.
Our results demonstrate that the long polysynaptic EPSPs (and associated LPR) that trigger spasms after spinal cord injury are inhibited by 5-HT1B and 5-HT1F receptors. Furthermore, the large transient polysynaptic EPSPs (short EPSPs and associated SPR) are also inhibited by these same receptors. The many-second-long portion of spasms (LLR) is also inhibited by 5-HT1B and 5-HT1F agonists, both in vitro and in vivo, even though this LLR is ultimately produced by Ca PICs intrinsic to the motoneuron (85% isradipine sensitive; Fig. 1A). This is due to an inhibition of the long EPSPs that trigger the Ca PIC, not a reduction in the Ca PIC itself. The short EPSP does not itself trigger the Ca PICs that underlie spasms after spinal cord injury, partly because it is too short to activate Ca PICs (Li and Bennett 2007; Li and Bennett 2003) and partly because it is followed immediately by an inhibitory current that prevents PIC activation (via shunting discussed below). However, the short EPSP may well participate in hyperreflexia and clonus after injury (Ashby and McCrea 1987; Kuhn and Macht 1948), triggered by oscillatory proprioceptive or cutaneous feedback from movement (Bennett et al. 1999). Thus our finding that 5-HT1B and 5-HT1F receptor agonists such as zolmitriptan inhibit short and long EPSPs demonstrates that such agonists may serve as novel antispastic agents in controlling the hyperreflexia, clonus, and spasms following spinal cord injury. We also found that the 5-HT1 receptor agonist zolmitriptan does not affect overall motoneuron excitability or inhibitory synaptic inputs (IPSCs), both of which are important for general coordinated motor output (Heckman et al. 2005; Hultborn et al. 2004; Schmidt and Jordan 2000). Thus zolmitriptan has selective antispastic action that may not affect residual motor function, unlike other antispastic agents such as baclofen that produce weakness and sedation (Dario and Tomei 2004; Li et al. 2004c). Although these conclusions are derived from a sacral spinal rat model of the spasticity syndrome, they are relevant to humans because the spasticity exhibited in the tail muscles in this rat model (slow onset of hyperreflexia, hypertonus, clonus, muscle contractures and spasms; Harris et al. 2006; Bennett 1999, 2004) closely mimics the development of the spastic syndrome in humans (Ashby and McCrea 1987; Dietz and Sinkjaer 2007; Kuhn and Macht 1948; Noth 1991). Furthermore, the long EPSPs in this rat model are remarkably similar to those seen in humans with spinal cord injury (Norton et al. 2008).
Antispastic action of 5-HT1B and 5-HT1F receptors agonists is predicted by their binding affinity.
Although many agonists bind to both 5-HT1B and 5-HT1F receptors with similar affinity, we found that a number of agonists exhibit sufficient selectivity to definitively demonstrate that both these receptors modulate the spastic reflexes (LPR and SPR) and associated EPSPs. In particular, zolmitriptan has a 10 times greater affinity for 5-HT1B receptors than 5-HT1F receptors, and accordingly, its low-dose action is likely mediated by 5-HT1B receptors. Furthermore, after selectively blocking that action of 5-HT1B receptors (with SB224289), zolmitriptan no longer has a low-dose effect on the spastic reflexes but does continue to inhibit the reflexes at a high dose, consistent with activation of 5-HT1F receptors. In contrast, LY344864 and methylergonovine are relatively selective to 5-HT1F receptors, exhibiting negligible affinity at 5-HT1B receptors, and thus their inhibitory action on the spastic reflexes demonstrates that the 5-HT1F receptor also modulates these reflexes and associated EPSPs. Finally, agonists with negligible affinity for 5-HT1B/1F receptors, such as the 5-HT1/5/7 receptor agonist 8-OH-DPAT, exhibit no inhibitory effects on the polysynaptic reflexes (SPR and LPRs), ruling out the involvement of other receptors.
As further evidence for the involvement of just 5-HT1B and 5-HT1F receptors, we found that the binding affinity of agonists to 5-HT1B and 5-HT1F receptors is highly correlated with our measured potencies of these agonists at inhibiting the spastic reflexes, whereas the binding affinity at other receptors is not related to the potency. The lack of correlation between affinity and potency for 5-HT1D receptors is especially important in ruling out the involvement of the 5-HT1D receptor because this receptor is so similar to the 5-HT1B receptor, activated by many of the same agonists (Table 1). We also showed that the reflex potencies can be quantitatively predicted from receptor binding affinity for 5-HT1B and 5-HT1F receptors, whereas this was not the case for other receptors, including 5-H1D and 5-HT1E receptors. Specifically, we found that reflex potencies are consistently one log unit less than the agonist affinity for 5-HT1B and 5-HT1F receptors, which means that the dose (EC50) needed to inhibit the reflexes is 10 times the dose at which agonists bind to these receptors (Ki) in isolated cell systems (see results). Remarkably, both the 5-HT1B and 5-HT1F receptors exhibit the same factor of 10 (1 log unit) greater effective dose (EC50) compared with the Ki value as we have also shown for the 5-HT2B and 5-HT2C receptors in their modulation of the PICs. Thus this factor of 10 is not only independent of the agonists tested but is also independent of the receptor type, or the response system (EPSPs vs. PICs). We therefore suggest that it in part reflects diffusion barriers in our whole spinal cord preparation that prevent the full applied dose from reaching the receptors, over the time used between doses (see details in Murray et al. 2011). Other factors may affect the functional potency (EC50) vs. receptor binding affinity relation (Boess and Martin 1994; Egan et al. 2000; Porter et al. 1999), including the receptor saturation, intracellular signaling (cAMP), and saturation in EPSPs, although these are specific to each receptor and signaling system and thus are less likely to be involved in the invariant potency-affinity relation.
Possible location of 5-HT1B and 5-HT1F receptors on group II afferents and interneurons?
Considering that we found no action of zolmitriptan on PICs or basic membrane properties of motoneurons, the functional 5-HT1B and 5-HT1F receptors that inhibit the EPSPs that trigger spasms are not likely to be on motoneurons. There remains a possibility that these receptors could be on motoneurons and only modulate postsynaptic glutamate receptors involved in the EPSPs. However, it is more likely that these receptors are on low-threshold group II and I type afferents terminals, which are the main afferents we stimulated (including cutaneous and muscle afferents; see methods), and consistent with previous reports of 5-HT1B and 5-HT1F receptors on various sensory afferents (Millan 2002). An intriguing possibility is that these 5-HT1 receptors that inhibit EPSPs/spasms may specifically act by inhibiting transmission to spinal interneurons that receive group II afferent input and are modulated by 5-HT1 receptors (pre- or postsynaptically; Dougherty et al. 2005; Jankowska and Hammar 2002). These interneurons could include commissural interneurons with group II input that have been implicated in coordinated left-right movements, such as rhythmic locomotion (Hammar et al. 2007; Jankowska 2008; Schmidt and Jordan 2000), consistent with the prominent left-right coordination of the spasms we have previously reported in the sacral spinal rat (Bennett et al. 2001, 2004; Li et al. 2004b). However, it remains to be resolved why we failed to detect effects of 5-HT1A, 5-HT2A, 5-HT3, and 5-HT7 receptors on reflex transmission in the spastic rat, even though these receptors have previously been suggested to play a prominent role in modulating spinal interneuronal circuits, including interneurons involved in group II sensory transmission (see Introduction and Dougherty et al. 2005; Hammar et al. 2007; Jankowska et al. 1994; Jordan et al. 2008; Schmidt and Jordan 2000).
Gi protein-coupled receptors that decrease cAMP have antispastic action.
5-HT1-type receptors activate Gi-coupled proteins that inhibit intracellular cAMP production by inhibiting adenylate cyclase activity (Boess and Martin 1994) and accordingly in many systems are inhibitory, although there are some excitatory actions of these receptors, even on motoneurons (Perrier et al. 2003). The EPSPs in our chronic spinal rats are facilitated by raising cAMP with forskolin, and thus the EPSPs are regulated by cAMP, and our observed antispastic action of 5-HT1B and 5-HT1F receptors (inhibiting EPSPs) is likely mediated by inhibiting cAMP. The involvement of this Gi-coupled pathway in regulating spasms is also consistent with the known antispastic action of other Gi-coupled receptors, including α2-adrenergic receptors and GABAB receptors that are respectively activated by tizanidine and baclofen, two classic antispastic drugs (Dario and Tomei 2004; Li et al. 2004c).
Not all 5-HT receptors exhibit constitutive activity after spinal cord injury.
The 5-HT1B receptor is known to exhibit substantial activity in the absence of 5-HT (constitutive receptor activity and associated cAMP production), and this is potently inhibited by the inverse agonist SB224289 in isolated cloned receptor cell systems (Selkirk et al. 1998). Thus our finding that SB224289 has no effect by itself is somewhat unexpected and suggests that the native 5-HT1B receptor exhibits less constitutive activity than predicted from the cloned system. As a positive control, we found that SB224289 antagonized 5-HT1B receptor agonist action, and thus we used it at an appropriate dose, and our negative finding is likely due to a genuine lack of constitutive activity. We could not test for constitutive activity in 5-HT1F receptors due to a lack of availability of selective inverse agonists to this receptor. The other 5-HT1B antagonists tested also had no detectable effect by themselves, although these are not inverse agonists (Price et al. 1997), and their lack of action simply confirms our previous finding that there is no functional residual 5-HT in the chronic spinal rat (Murray et al. 2010).
In contrast to the 5-HT1B receptor, over time after injury 5-HT2C receptors become constitutively active and help produce the dramatic increase in the PIC that leads to motor recovery (Murray et al. 2010). It is intriguing that one 5-HT receptor compensates for lost 5-HT, whereas others do not (5-HT1B). A common underlying functional pattern that emerges is that both the adaptation in the 5-HT2 receptor and lack of adaptation in the 5-HT1 receptor lead to increased spinal cord excitability (larger PICs and EPSP) and associated activity in motoneurons (spasms). Thus it could be that the receptors are regulated in an activity-dependent manner; although the mechanisms for this remain unknown, it may explain why intensive treadmill training (activity) can reduce spastic activity during walking (Gorassini et al. 2009).
Simultaneous activation of excitatory and inhibitory synaptic inputs.
Our finding that cutaneous reflexes result from the simultaneous activation of large excitatory and inhibitory synaptic inputs may help further explain the changes in these reflexes after injury. We suggest that only subtle changes in the balance of these large synaptic inputs may contribute to the shift to net excitatory reflexes after injury compared with before; these changes could result from loss of descending inhibition (5-HT; see Introduction), as well as cellular changes in inhibitory currents (Boulenguez et al. 2010). In normal intact humans and rats, cutaneous stimulation, like we used, predominantly evokes long-duration inhibitory reflexes and decreases ongoing muscle activity (Bennett et al. 2004; Norton et al. 2008; Schmidt and Jordan 2000). This stimulation does evoke a transient excitation (like SPR), but this is interrupted by a long period of inhibition, which can be followed by a further excitatory reflex, consistent with there being EPSPs that are interrupted by an overriding IPSP (Norton et al. 2008). After spinal cord injury, the net synaptic responses are excitatory (at rest), but there still remains an inhibitory synaptic input to motoneurons that 1) is seen at depolarized potentials (Fig. 9), 2) peaks shortly after the EPSP onset (interrupting the excitation), 3) is enhanced by blocking opposing EPSCs with zolmitriptan, and 4) is reduced by eliminating glycine-mediated chloride currents with strychnine, revealing very large net EPSPs (∼15 mV). Although at the most depolarized levels we tested (−50 mV), the long-duration synaptic responses are outward (net IPSCs; Fig. 10), motoneurons do not on average ever reach such depolarized levels, because the potential is limited to being well below the spike threshold (about −53 mV) by the spike afterhyperpolarization during firing (Li X et al. 2007; Li Y et al. 2004a). Thus the mixed EPSC and IPSCs seen just above rest are likely most relevant to motor function. For example, the slightly delayed onset of the inhibitory synaptic input likely explains the pause in firing often seen after the first spike at the start of spasms (Fig. 8A) (Li et al. 2004a), with an IPSP interrupting otherwise depolarizing EPSPs and PICs.
Shunting limits EPSPs and spasms.
Functionally, the action of mixed inhibitory and excitatory synaptic inputs is to substantially increase the overall membrane conductance, thus limiting (shunting) the action of other currents, including intrinsic PICs (Bennett et al. 1998; Berg et al. 2007; Berg and Hounsgaard 2009; Rekling et al. 2000), making them relatively negligible during the synaptic input. Such synaptic shunting may explain why PICs (and spasms) take up to a second to turn on fully when activated by synaptic inputs (Fig. 9A) (Gorassini et al. 2004; Li et al. 2004a), even though Ca PICs can be turned on much more rapidly with intracellular current injection (Li and Bennett 2007): the shunting from the EPSPs/IPSPs (lasting up to 1 s) may prevent the full PIC activation until the synaptic input ends. Furthermore, synaptic shunting may explain why the polysynaptic reflex inputs, especially the SPR, are so resistant to the large increases in Ca and Na PICs induced by 5-HT2 receptor agonists (Harvey et al. 2006a) or even calcium channel blockers (isradipine). Possibly, the two distinct short and long EPSPs may actually be mediated by the same synaptic input but appear separated because they are interrupted by the large inhibitory synaptic input that is activated just after the short EPSP; this inhibitory input must limit the EPSPs by shunting, especially at rest where these inhibitory inputs produce no net hyperpolarization (reversal potential).
Reversal potential for chloride-mediated IPSC is near rest after injury.
Recently, it has been shown that inhibitory chloride currents (IPSCs) are reduced over time after injury (in chronic injury) due to a reduction in the potassium-chloride cotransporter (KCC2; Boulenguez et al. 2010). Such a reduction in the chloride currents might further explain the large net excitatory synaptic inputs we observed by shifting the balance more in favor of EPSPs over IPSPs, although this does not itself explain the large EPSPs seen acutely after injury (Li et al. 2004a). On the basis of data from neonatal rats, Boulenguez et al. (2010) suggested that the KCC2 is so impaired following spinal cord injury that the reversal potential for Cl− is depolarized from about −75 mV to well above rest (above −70 mV), and thus at rest, there are net depolarizing responses to synaptic chloride current inputs that normally produce inhibitory hyperpolarizing responses (reversal of IPSPs to depolarizing). Boulenguez et al. (2010) also found that the KCC2 was impaired in adult rats after spinal cord injury, although they did not examine the impact on the Cl− reversal potential. We did not find evidence for outright reversed (depolarizing) IPSPs at rest in adult rats after injury. However, our data do suggest that after injury in adult rats, the reversal potential for Cl− is depolarized by about 5 mV, from below the resting potential in normal rats to levels similar to the resting potential in chronic spinal rats. Interestingly, blockage of glycine receptors with strychnine reveals a very large EPSP at rest, suggesting that although no net change in potential is induced by glycine receptor activation at rest (at reversal potential), the receptor still induces a marked shunting of the EPSP that limits its size, by preventing excitatory current from reaching the soma (or our electrode).
Summary and clinical implications.
In summary, activity in 5-HT1 receptors inhibits reflex transmission, and thus a loss of 5-HT with injury contributes to an acute loss of inhibition over reflex transmission, and this contributes to the classic disinhibition of reflexes observed acutely after injury. After injury, the 5-HT1B and 5-HT1F receptors are still capable of inhibiting the reflexes when activated by exogenously applied agonists, but these receptors remain inactive because of a lack of endogenous 5-HT in transected rats and a lack of observed constitutive activity. In contrast, other receptors, such as the 5-HT2C receptor, become constitutively active after injury, leading to a recovery of their normal function (facilitating motoneuron PICs). Thus reflex transmission remains chronically elevated (disinhibited) after injury, with large net EPSPs. These EPSP are of adequate duration (seconds) to trigger the PICs in motoneurons that ultimately cause the sustained motoneuron firing that underlies spasms. Thus reactivating 5-HT1B and 5-HT1F receptors after injury offers a new means of selectively controlling spasticity, by reducing EPSPs, without affecting PICs that are critical for motor function. This could be done with clinically available 5-HT1B/1F agonists like the triptans (zolmitriptan), which are currently a first-line treatment for migraines (Millan 2002). However, improved 5-HT1B/1F agonists would be necessary, because triptans are only clinically used intermittently and continuous use is not without adverse effects. In the long run, understanding why 5-HT1B receptors do not become constitutively active after injury, unlike the 5-HT2C receptors, may further help with antispastic therapy and general recovery of motor function after injury, especially if this involves activity-dependent receptor plasticity that can be modulated by intensive rehabilitative training.
GRANTS
Funding was provided by National Institute of Neurological Disorders and Stroke Grants NS-047567 and NS-048170, Canadian Foundation for Innovation, the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We especially thank Leo Sanelli and Jeniffer Nevett-Duchcherer for expert technical assistance and AstraZeneca Canada for donating zolmitriptan.
REFERENCES
- Adham N, Gerald C, Schechter L, Vaysse P, Weinshank R, Branchek T. [3H]5-hydroxytryptamine labels the agonist high affinity state of the cloned rat 5-HT4 receptor. Eur J Pharmacol 304: 231–235, 1996 [DOI] [PubMed] [Google Scholar]
- Ashby P, McCrea DA. Neurophysiology of spinal spasticity. In: Handbook of the Spinal Cord, edited by Davidoff RA. New York: Dekker, 1987, p. 119–143 [Google Scholar]
- Baker LL, Chandler SH. Characterization of postsynaptic potentials evoked by sural nerve stimulation in hindlimb motoneurons from acute and chronic spinal cats. Brain Res 420: 340–350, 1987 [DOI] [PubMed] [Google Scholar]
- Baxter GS. Novel discriminatory ligands for 5-HT2B receptors. Behav Brain Res 73: 149–152, 1996 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma 16: 69–84, 1999 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke C, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- Berg RW, Hounsgaard J. Signaling in large-scale neural networks. Cogn Process 10, Suppl 1: S9–S15, 2009 [DOI] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology 33: 275–317, 1994 [DOI] [PubMed] [Google Scholar]
- Boess FG, Monsma FJ, Jr, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ. Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacology 36: 713–720, 1997 [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16: 302–307, 2010 [DOI] [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF. Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586: 529–544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RW, Eves S, Harris J, Peachey JE, Stuart E. Interactions between cutaneous afferent inputs to a withdrawal reflex in the decerebrated rabbit and their control by descending and segmental systems. Neuroscience 112: 555–571, 2002 [DOI] [PubMed] [Google Scholar]
- Clarke RW, Harris J, Houghton AK. Spinal 5-HT-receptors and tonic modulation of transmission through a withdrawal reflex pathway in the decerebrated rabbit. Br J Pharmacol 119: 1167–1176, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dario A, Tomei G. A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf 27: 799–818, 2004 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Lindsay A, Feldman J, Monteau R, Hilaire G. Serotonergic inhibition of phrenic motoneuron activity: an in vitro study in neonatal rat. Neurosci Lett 230: 29–32, 1997 [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6: 725–733, 2007 [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Bannatyne BA, Jankowska E, Krutki P, Maxwell DJ. Membrane receptors involved in modulation of responses of spinal dorsal horn interneurons evoked by feline group II muscle afferents. J Neurosci 25: 584–593, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse 35: 144–150, 2000 [DOI] [PubMed] [Google Scholar]
- Engel G, Gothert M, Hoyer D, Schlicker E, Hillenbrand K. Identity of inhibitory presynaptic 5-hydroxytryptamine (5-HT) autoreceptors in the rat brain cortex with 5-HT1B binding sites. Naunyn Schmiedebergs Arch Pharmacol 332: 1–7, 1986 [DOI] [PubMed] [Google Scholar]
- Gilmore J, Fedirchuk B. The excitability of lumbar motoneurones in the neonatal rat is increased by a hyperpolarization of their voltage threshold for activation by descending serotonergic fibres. J Physiol 558: 213–224, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Ismaiel AE, McCarthy BG, Peroutka SJ. Binding of arylpiperazines to 5-HT3 serotonin receptors: results of a structure-affinity study. Eur J Pharmacol 168: 387–392, 1989 [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang JF. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol 101: 969–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Jankowska E. Modulatory effects of alpha1-,alpha2-, and beta-receptor agonists on feline spinal interneurons with monosynaptic input from group I muscle afferents. J Neurosci 23: 332–338, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Stecina K, Jankowska E. Differential modulation by monoamine membrane receptor agonists of reticulospinal input to lamina VIII feline spinal commissural interneurons. Eur J Neurosci 26: 1205–1212, 2007 [DOI] [PubMed] [Google Scholar]
- Harris RL, Bobet J, Sanelli L, Bennett DJ. Tail muscles become slow but fatigable in chronic sacral spinal rats with spasticity. J Neurophysiol 95: 1124–1133, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ. Alterations in synaptic input to motoneurons during partial spinal cord injury. Med Sci Sports Exerc 26: 1480–1490, 1994 [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Holstege JC, Bongers CM. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res 566: 308–315, 1991 [DOI] [PubMed] [Google Scholar]
- Hoyer D, Engel G, Kalkman HO. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (−)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol 118: 13–23, 1985 [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004 [DOI] [PubMed] [Google Scholar]
- Ismaiel AM, Titeler M, Miller KJ, Smith TS, Glennon RA. 5-HT1 and 5-HT2 binding profiles of the serotonergic agents alpha-methylserotonin and 2-methylserotonin. J Med Chem 33: 755–758, 1990 [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992 [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev 57: 46–55, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev 40: 19–28, 2002 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci 12: 701–714, 2000 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lackberg ZS, Dyrehag LE. Effects of monoamines on transmission from group II muscle afferents in sacral segments in the cat. Eur J Neurosci 6: 1058–1061, 1994 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS, Skoog B, Noga BR. Gating of transmission to motoneurones by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol 461: 705–722, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57: 183–191, 2008 [DOI] [PubMed] [Google Scholar]
- Kennett GA, Ainsworth K, Trail B, Blackburn TP. BW 723C86, a 5-HT2B receptor agonist, causes hyperphagia and reduced grooming in rats. Neuropharmacology 36: 233–239, 1997 [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol 370: 114–123, 2004 [DOI] [PubMed] [Google Scholar]
- Kuhn RA, Macht MB. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp 84: 43–75, 1948 [PubMed] [Google Scholar]
- Leopoldo M, Lacivita E, Contino M, Colabufo NA, Berardi F, Perrone R. Structure-activity relationship study on N-(1,2,3,4-tetrahydronaphthalen-1-yl)−4-aryl-1-piperazinehexanamides, a class of 5-HT7 receptor agents. J Med Chem 50: 4214–4221, 2007 [DOI] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97: 1236–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes in the chronic spinal rat, studied in vitro. J Neurophysiol 91: 2236–2246, 2004b [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92: 2694–2703, 2004c [DOI] [PubMed] [Google Scholar]
- Lundberg A. Inhibitory control from the brainstem of transmission from primary afferents to motoneurons, primary afferent terminals and ascending pathways. In: Brainstem Control of Spinal Mechanisms, edited by Sjolund B, Bjorklund A. New York: Elsevier, 1982, p. 179–224 [Google Scholar]
- Manuel NA, Wallis DI, Crick H. Ketanserin-sensitive depressant actions of 5-HT receptor agonists in the neonatal rat spinal cord. Br J Pharmacol 116: 2647–2654, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Robertson AD, MacLennan SJ, Prentice DJ, Barrett VJ, Buckingham J, Honey AC, Giles H, Moncada S. Receptor specificity and trigemino-vascular inhibitory actions of a novel 5-HT1B/1D receptor partial agonist, 311C90 (zolmitriptan). Br J Pharmacol 121: 157–164, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson C, Sonesson C, Sandahl A, Greiner HE, Gassen M, Plaschke J, Leibrock J, Bottcher H. 2-Alkyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indoles as novel 5-HT6 receptor agonists. Bioorg Med Chem Lett 15: 4230–4234, 2005 [DOI] [PubMed] [Google Scholar]
- Maynard FM, Karunas RS, Waring WP., 3rd Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 71: 566–569, 1990 [PubMed] [Google Scholar]
- Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods 32: 197–200, 1994 [DOI] [PubMed] [Google Scholar]
- Milburn CM, Peroutka SJ. Characterization of [3H]quipazine binding to 5-hydroxytryptamine3 receptors in rat brain membranes. J Neurochem 52: 1787–1792, 1989 [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol 66: 355–474, 2002 [DOI] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 43: 320–327, 1993 [PubMed] [Google Scholar]
- Murray K, Nakae A, Stephens MJ, Rank M, D'Amico J, Harvey P, Li X, Harris L, Ballou EW, Anelli R, Heckman CJ, Mashimo T, Vavrek R, Sanelli L, Gorassini MA, Bennett DJ, Fouad K. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med 16: 694–700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Stephens MJ, Ballou EW, Anelli R, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol 105: 731–278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity—from a basic science point of view. Acta Physiol (Oxf) 189: 171–180, 2007 [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131: 1478–1491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth J. Trends in the pathophysiology and pharmacotherapy of spasticity. J Neurol 238: 131–139, 1991 [DOI] [PubMed] [Google Scholar]
- Obata H, Saito S, Sakurazawa S, Sasaki M, Usui T, Goto F. Antiallodynic effects of intrathecally administered 5-HT2C receptor agonists in rats with nerve injury. Pain 108: 163–169, 2004 [DOI] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol 548: 485–492, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci 25: 7993–7999, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89: 954–959, 2003 [DOI] [PubMed] [Google Scholar]
- Phebus LA, Johnson KW, Zgombick JM, Gilbert PJ, Van Belle K, Mancuso V, Nelson DL, Calligaro DO, Kiefer AD, Jr, Branchek TA, Flaugh ME. Characterization of LY344864 as a pharmacological tool to study 5-HT1F receptors: binding affinities, brain penetration and activity in the neurogenic dural inflammation model of migraine. Life Sci 61: 2117–2126, 1997 [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128: 13–20, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GW, Burton MJ, Collin LJ, Duckworth M, Gaster L, Gothert M, Jones BJ, Roberts C, Watson JM, Middlemiss DN. SB-216641 and BRL-15572—compounds to pharmacologically discriminate h5-HT1B and h5-HT1D receptors. Naunyn Schmiedebergs Arch Pharmacol 356: 312–320, 1997 [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000 [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366: 381–416, 2002 [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Scott C, Ho M, Burton MJ, Watson J, Gaster LM, Collin L, Jones BJ, Middlemiss DN, Price GW. SB-224289—a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br J Pharmacol 125: 202–208, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Bellingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol 76: 799–807, 1996 [DOI] [PubMed] [Google Scholar]
- Steg G. Efferent muscle innervation and rigidity. Acta Physiol Scand Suppl 225: 1–53, 1964 [PubMed] [Google Scholar]
- Taylor J, Munson J, Vierck C., Jr Effects of dorsolateral spinal lesions on stretch reflex threshold and stiffness in awake cats. Eur J Neurosci 11: 363–368, 1999 [DOI] [PubMed] [Google Scholar]
- Udina E, D'Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol 103: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainscott DB, Cohen ML, Schenck KW, Audia JE, Nissen JS, Baez M, Kursar JD, Lucaites VL, Nelson DL. Pharmacological characteristics of the newly cloned rat 5-hydroxytryptamine2F receptor. Mol Pharmacol 43: 419–426, 1993 [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 101: 107–117, 2006 [DOI] [PubMed] [Google Scholar]