Abstract
Background
This prospective, randomized controlled clinical trial compared changes in exercise performance and daily ambulatory activity in peripheral artery disease (PAD) patients with intermittent claudication following a home-based exercise program, a supervised exercise program, and usual care control.
Methods and Results
Of 119 patients randomized, 29 completed home-based exercise, 33 completed supervised exercise, and 30 completed usual care control. Both exercise programs consisted of intermittent walking to near maximal claudication pain for 12 weeks. Patients wore a step activity monitor during each exercise session. Primary outcome measures included claudication onset time (COT) and peak walking time (PWT) obtained from a treadmill exercise test, and secondary outcome measures included daily ambulatory cadences measured during a 7-day monitoring period. Adherence to home-based and supervised exercise was similar (p = 0.712) and exceeded 80%. Both exercise programs increased COT (p < 0.001) and PWT (p < 0.01), whereas only home-based exercise increased daily average cadence (p < 0.01). No changes were seen in the control group (p > 0.05). The changes in COT and PWT were similar between the two exercise groups (p > 0.05), whereas the change in daily average cadence was greater with home-based exercise (p < 0.05).
Conclusions
A home-based exercise program, quantified with a step activity monitor, has high adherence and is efficacious in improving claudication measures similar to that seen with a standard supervised exercise program. Furthermore, home-based exercise appears more efficacious in increasing daily ambulatory activity in the community setting than supervised exercise.
Keywords: Claudication, Exercise, Peripheral Vascular Disease
INTRODUCTION
A primary therapeutic goal for patients with peripheral artery disease (PAD) and intermittent claudication is to regain lost physical function through exercise rehabilitation.1 Medically supervised exercise programs are efficacious for clinical management of intermittent claudication,2 as improvements are noted for claudication onset time (COT) and peak walking time (PWT).3 As such, supervised exercise has been given a Class I recommendation by the American College of Cardiology (ACC) and the American Heart Association (AHA) indicating general agreement for effectiveness of treatment, supported by Level A evidence derived from multiple randomized controlled trials and meta-analyses.4
Unfortunately, reimbursement is not provided for supervised exercise programs, and of the few programs that exist in research centers, only a small percentage of eligible patients can feasibly attend regularly. The majority of PAD patients could, however, benefit from an exercise program transported to the community setting (i.e., home-based walking). To date, the efficacy of home-based exercise has been poorly studied and has suffered from methodological flaws,5–15 most notably the inability to accurately quantify the volume of exercise performed. As such, home-based, unsupervised exercise has been given a Class IIb recommendation by the ACC and AHA indicating conflicting evidence for efficacy, supported only by Level B evidence derived from non-randomized trials.4
We have recently used a step activity monitor to quantify daily ambulatory activity in patients with intermittent claudication,16 and we further explored its utility to address the primary flaw of home-based exercise programs by directly measuring exercise adherence and exercise volume performed. This prospective, randomized controlled clinical trial compared changes in exercise performance and daily ambulatory activity in PAD patients with intermittent claudication following a home-based exercise rehabilitation program, a supervised exercise program, and a usual care control group. We hypothesized that home-based exercise utilizing step activity monitor technology will result in greater changes in our primary outcome measures (COT and PWT) and secondary outcomes (daily ambulatory cadences) than usual care control, and that these changes will be similar to those obtained with standard, supervised exercise.
METHODS
PATIENTS
Recruitment
Patients participated at the General Clinical Research Center (GCRC), University of Oklahoma Health Sciences Center (HSC) from September, 2004 to April, 2007. Patients were recruited by HSC vascular clinic referrals, as well as by newspaper advertisements. Procedures used were approved by the Institutional Review Board at the University of Oklahoma HSC. Written informed consent was obtained from each patient prior to investigation.
Screening
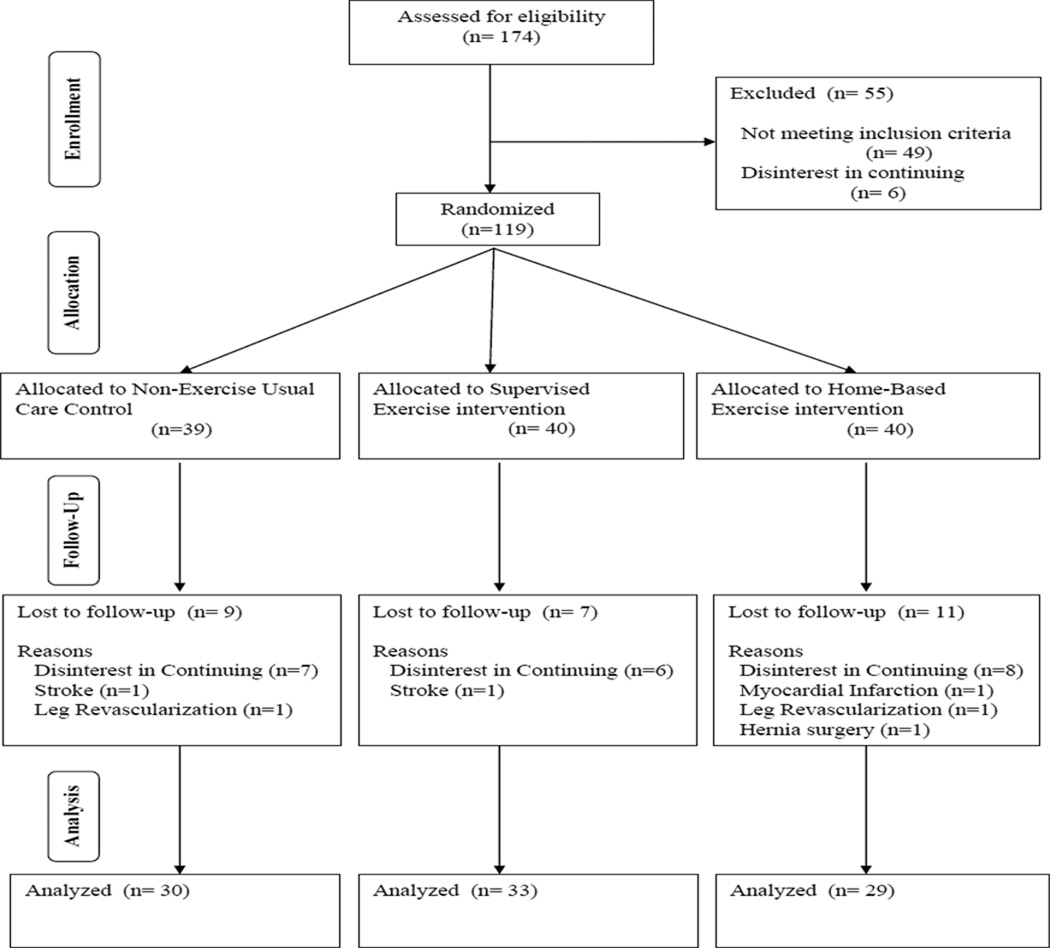
Patients who had intermittent claudication secondary to vascular insufficiency were included if they met the following criteria: (a) a history of any type of exertional leg pain, (b) ambulation during a graded treadmill test limited by leg pain consistent with intermittent claudication,17 and (c) an ankle-brachial index (ABI) ≤ 0.90 at rest4 or an ABI ≤ 0.73 after exercise.18 Patients were excluded for the following conditions: (a) absence of PAD (ABI > 0.90 at rest and ABI > 0.73 after exercise), (b) inability to obtain an ABI measure due to non-compressible vessels, (c) asymptomatic PAD determined from the medical history and verified during the graded treadmill test, (d) use of cilostazol and pentoxifylline initiated within three months prior to investigation, (e) exercise tolerance limited by factors other than leg pain, and (f) active cancer, renal disease, or liver disease. Patient flow in the study is shown in Figure 1.
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow diagram of patients through each stage of the trial.
INTERVENTION AND CONTROL GROUPS
Home-Based Exercise Rehabilitation Program
Exercise sessions in our home-based exercise program were rigorously quantified with a step activity monitor (StepWatch3™, Cyma Inc., Mountlake Terrace, WA) to accurately record the duration and cadence of ambulation. Home-based exercise program was designed to be as similar to the supervised exercise program as possible, and consisted of 12 weeks of intermittent walking to near maximal claudication pain three days per week at a self-selected pace. Walking duration began at 20 minutes for the first two weeks, and progressively increased five minutes biweekly until a total of 45 minutes of walking was accomplished during the final two weeks of the program. These exercise durations were five minutes longer throughout the program than in the supervised program in an attempt to better match the programs on total volume of exercise, determined by multiplying the intensity and the duration of walking. Intensity of walking, expressed as metabolic equivalents (MET’s), was determined by first converting cadence of each home-based exercise session to an average speed of walking, and then estimating oxygen uptake from walking speed. Speed was calculated by comparing cadence of home-based exercise sessions with cadence obtained at several speeds during overground walking trials prior to the intervention. Oxygen uptake of walking was then estimated by the following equation from the American College of Sports Medicine: VO2 (ml/kg/min) = 3.5 + (0.1 × speed in m/min).19 MET’s were calculated by dividing oxygen uptake value by 3.5, and MET-minutes were calculated by multiplying the MET value by duration of exercise sessions to yield a measure of exercise volume.
Patients were given a step activity monitor and were instructed to wear it on the right ankle during each exercise session, and then to remove the monitor at the completion of each session. Additionally, they received an exercise logbook to record their walking sessions. Patients returned their step activity monitors and logbooks to the research staff at the end of week 1, 2, 4, 6, 8, 10, and 12 of the program, and data from the monitor was downloaded. During these brief 15-minute meetings, patients discussed their progress with an exercise physiologist, were given feedback about the data from the step activity monitor, and were given new instructions regarding changes in exercise duration. No exercise was performed by the patients in our facility during these meetings with the research staff.
Supervised Exercise Rehabilitation Program
The supervised program was designed to elicit increases in COT and PWT according to our previous studies,2.3, 20 This standardized program consisted of three months of supervised, intermittent treadmill walking, three days per week at a speed of approximately two mph. Walking duration began at 15 minutes for the first two weeks of the program, and progressively increased by 5 minutes biweekly until a total of 40 minutes of walking was accomplished during the final two weeks of the program. Because we have previously shown that changes in COT and MWT are similar for patients who train at a relatively high exercise intensity (80% of peak work load) and patients who train at a lower intensity (40% of peak work load) for longer duration,21 we selected the lower intensity program for the patients in the current study because it is well tolerated. Thus, patients walked at a grade equal to 40% of the final work load from the baseline maximal treadmill test to the point of near maximal claudication pain, at which point they stopped to relieve their leg pain. Patients then repeated the intermittent walking and rest periods until the prescribed total number of minutes of exercise was attained for the training session. During each exercise session, patients wore a step activity monitor on the right ankle to quantify the cadence and time of ambulation. To quantify volume of exercise performed in the supervised exercise program, expressed as MET-minutes, intensity of each exercise training session was determined from objectively measured oxygen uptake during the baseline maximal treadmill test of each patient. Oxygen uptake corresponding to the training grade was then divided by 3.5 to convert to MET’s, and this value was multiplied by duration of each supervised exercise session to yield a MET-minute value. Patients in the supervised program were not given advice or instructions to perform additional exercise away from our research center.
Non-Exercise, Usual Care Contol Group
Patients randomized to this group were encouraged to walk more on their own but they did not receive specific recommendations regarding an exercise program during the study. This approach is similar to advice typically given by clinicians during routine follow-up vascular appointments. No other risk factor management, lifestyle modification, or educational programming interventions were provided to any of the three groups.
MEASUREMENTS
Medical History and Physical Examination
Patients arrived in the morning fasted, but were permitted to take their usual morning medication regimen. Demographic information, height, weight, cardiovascular risk factors, co-morbid conditions, claudication history, blood samples, a list of current medications, and ABI were obtained from a medical history and physical examination.22
Gardner Maximal Treadmill Test
Patients performed a progressive, graded treadmill protocol on two separate days. The first test was to determine study eligibility by assessing whether exercise performance was limited by claudication, whereas the second test was done on another day to obtain the outcome measures of COT, PWT, and peak oxygen uptake. COT was defined as the walking time at which the patient first experienced pain, and PWT was defined as the time at which ambulation could not continue due to maximal pain. Peak oxygen uptake was measured as previously described 23 The final grade attained during this test at baseline was used to calculate training intensity of patients in the supervised exercise group.
Walking Economy Test
Oxygen uptake was measured during a constant, submaximal work rate at a treadmill speed of 2 mph and a grade of 0% until maximal claudication pain, or for a maximum of 20 minutes at baseline.24 The walking economy test at follow up was performed for the same duration as at baseline. Walking economy was measured as oxygen uptake during the final minute of exercise. Fractional utilization was calculated as walking economy oxygen uptake / peak oxygen uptake.
Ambulatory Activity Monitoring
Ambulatory activity was measured with a step activity monitor during seven consecutive days before and after the intervention period.16 None of the exercise interventions were included in the monitoring period. The monitor was attached to the right ankle above the lateral malleolus using elastic Velcro straps, and continuously recorded the number of strides taken on a minute-to-minute basis. Variables are expressed as the average daily cadence, maximum cadence for 60, 30, 20, and 5 continuous minutes of ambulation each day. These daily measures are recorded and then are averaged over the seven-day monitoring period.
Walking Impairment Questionnaire (WIQ)
Self-reported ambulatory ability was obtained using a validated questionnaire for PAD patients that assesses ability to walk at various speeds and distances, and to climb stairs.25
Baltimore Activity Scale for Intermittent Claudication (BASIC)
Self-reported physical activity level was assessed using the BASIC questionnaire for patients with PAD.26
Health-Related Quality of Life
Self-reported physical function was assessed with the Medical Outcomes Study Short-Form 36 (MOS SF-36) General Health Survey.27
STATISTICAL ANALYSES
Patients were randomized to three groups using off site NCSS random number program with blocking to assure that group sizes never differed by more than two at any time during allocation. Study personnel were allowed access to allocation list only after subject eligibility determined and baseline data completed. A one way ANOVA examined group means for baseline measurement variables. A 2x3 Chi Square examined group proportions for dichotomous variables. Independent t tests tested for differences in means between the two exercise groups for the exercise rehabilitation measures. For each response variable an ANOVA appropriate for a two factor study design with repeated measures on one factor was used as initial procedure. This was followed by two procedures that directly address issues likely to be of clinical interest.. Within each group the simple effect of change from baseline was tested for difference from zero using a paired t test. A more detailed analysis was made of the interaction component using comparisons among the change means by a one way ANOVA followed by a Tukey-Kramer test for pairwise comparisons. To address the issue that some differences might be attributed to differences in dropout patterns across groups, all ANOVA’s were repeated as intent-to-treat analysis using imputed data and the results compared with initial.. All analyses and imputing multiple regression routine were performed using the NCSS statistical package.
RESULTS
Randomization resulted in similar (p > 0.05) baseline clinical characteristics among groups (Table 1). Ninety-two patients completed the study, whereas 27 did not (Figure 1). Baseline characteristics remained similar among the groups (p > 0.05) after only including the 92 patients who completed the trial (data not shown). Of the 27 patients who did not complete, no significant difference among the groups was noted for total number of dropouts (p = 0.56), dropouts due to disinterest (p = 0.845), and dropouts due to adverse events (p = 0.592). Furthermore, no group difference (p = 0.896) was found for total number of adverse events (4 in controls, 3 in supervised group, and 4 in home-exercise group).
Table 1.
Baseline clinical characteristics. Values are means (standard deviation) or percentage of patients.
| Variables | Control Group (n = 39) |
Supervised Exercise Group (n = 40) |
Home-Based Exercise Group (n = 40) |
P Value |
|---|---|---|---|---|
| Age (years) | 65 (10) | 66 (12) | 65 (11) | 0.902 |
| Weight (kg) | 83.8 (18.4) | 82.2 (21.5) | 85.2 (17.6) | 0.789 |
| Body Mass Index (kg/m2) | 29.7 (6.9) | 29.2 (7.1) | 29.9 (5.6) | 0.885 |
| Ankle/Brachial Index | 0.76 (0.22) | 0.71 (0.25) | 0.72 (0.23) | 0.613 |
| Race (% Caucasian) | 62 | 45 | 65 | 0.155 |
| Current Smoking (% yes) | 10 | 10 | 10 | 0.999 |
| Sex (% men) | 54 | 45 | 45 | 0.663 |
| Diabetes (% yes) | 31 | 43 | 43 | 0.467 |
| Hypertension (% yes) | 79 | 88 | 88 | 0.519 |
| Dyslipidemia (% yes) | 85 | 88 | 90 | 0.771 |
| Abdominal Obesity (% yes) | 49 | 45 | 55 | 0.665 |
| Metabolic Syndrome Components (number) | 3.1 (1.3) | 3.4 (1.4) | 3.5 (1.1) | 0.372 |
| Metabolic Syndrome (% yes) | 69 | 73 | 83 | 0.366 |
| Obesity (% yes) | 41 | 43 | 48 | 0.830 |
Adherence to home-based exercise and supervised exercise was similar (p = 0.712) (Table 2). Patients in the home-based exercise program walked for a longer duration per exercise session than patients in supervised exercise (p < 0.001), but at a slower cadence (p = 0.019), resulting in a similar total exercise volume, expressed as MET-minutes (p = 0.150)
Table 2.
Exercise intervention measures. Values are means (SD).
| Variables | Supervised Exercise Group (n = 33) |
Home-Based Exercise Group (n = 29) |
P Value |
|---|---|---|---|
| Exercise Sessions Completed (%) | 84.8 (20.9) | 82.5 (27.7) | 0.712 |
| Total Exercise Time (min) | 795 (249) | 1218 (577) | < 0.001 |
| Total Exercise Strides (strides) | 40376 (7403) | 46357 (25757) | 0.386 |
| Total Volume of Exercise (MET-minutes)* | 2394 (818) | 2832 (1491) | 0.150 |
| Average Exercise Time (min / exercise session) | 25.4 (3.0) | 41.6 (21.4) | < 0.001 |
| Average Exercise Strides (strides / exercise session) | 1170 (142) | 1529 (703) | 0.058 |
| Average Exercise Cadence (strides/min) | 43.3 (4.7) | 37.4 (8.6) | 0.019 |
MET = metabolic equivalent.
MET-minutes of exercise determined by multiplying estimated intensity of walking (expressed in MET’s) and total exercise time for each session.
The groups were similar (p > 0.05) at baseline on each treadmill measure (Table 3). Home-based exercise increased the primary outcome measures of COT (p < 0.001) and PWT (p < 0.01), which were significantly different (p < 0.05) than changes in the control group. Supervised exercise increased COT (p < 0.001) and PWT (p < 0.001). These changes were significantly different (p < 0.05) than changes in controls, but were not different (p > 0.05) than changes in home-based exercise.
Table 3.
Exercise performance measures in patients completing usual care control (n = 30), supervised exercise (n = 33), and home-based exercise (n = 29). Values are means (SD).
| Variables | Pre-Test | Post-Test | Change Score | ANOVA p values Observed |
ANOVA p values ITT |
||||
|---|---|---|---|---|---|---|---|---|---|
| G | T | GxT | G | T | GxT | ||||
| Claudication Onset Time (seconds) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
225 (157) 196 (144) 204 (137) |
209 (168) 361 (264) 337 (250) |
−16 (125) 165 (173)‡§ 134 (197)‡§ |
ns | <.01 | .01 | ns | <.01 | <.01 |
| Peak Walking Time (seconds) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
505 (216) 325 (169) 402 (285) |
494 (240) 540 (281) 526 (374) |
−10 (176) 215 (207) ‡§ 124 (193) †§ |
ns | <.01 | <.01 | ns | <.01 | <.01 |
| Peak Oxygen Uptake (ml.kg−1.min−1) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
13.7 (3.7) 11.4 (2.5) 11.8 (3.8) |
12.8 (3.5) 11.7 (2.9) 12.4 (3.8) |
−1.00 (1.9) † 0.3 (1.9) § 0.6 (2.0) § |
ns | ns | <.01 | ns | ns | <.05 |
| Walking Economy (ml.kg−1.min−1) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
10.3 (1.9) 10.6 (2.0) 9.4 (2.0) |
10.5 (2.2) 9.5 (1.9) 9.3 (1.9) |
0.2 (1.4) −1.1 (1.6)‡§|| −0.1 (1.2) |
ns | ns | <.01 | ns | ns | <.05 |
| Fractional Utilization (%) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
80 (19) 94 (11) 83 (18) |
87 (21) 82 (14) 78 (16) |
8 (20) * −12 (10) ‡§ −5 (12) *§ |
ns | <.05 | <.01 | ns | <.01 | <.01 |
| WIQ Distance Score (%) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
43 (34) 25 (26) 32 (29) |
44 (31) 38 (31) 42 (33) |
1 (34) 13 (28) * 10 (25) * |
ns | <.05 | ns | ns | <.01 | ns |
| WIQ Speed Score (%) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
39 (25) 27 (19) 30 (22) |
44 (20) 36 (24) 41 (22) |
4 (25) 9 (15) † 11 (22) * |
ns | <.01 | ns | ns | <.01 | ns |
| WIQ Stair Climbing Score (%) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
47 (32) 32 (32) 38 (26) |
50 (29) 44 (34) 48 (27) |
3 (25) 12 (15) † 10 (22) * |
ns | <.01 | ns | ns | <.01 | ns |
| Physical Function Score (%) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
55 (19) 37 (17) 40 (22) |
54 (18) 46 (21) 48 (23) |
−1 (17) 9 (16) †§ 8 (15) † |
<.01 | <.01 | <.05 | <.05 | <.01 | ns |
Change from pre-test (p < .05),
(p < 0.01),
(p < 0.001).
Different than Control Group (p < 0.05).
Different than Home-Based Exercise Group (p < 0.05).
Walking economy = oxygen uptake obtained during final minute of a constant, submaximal treadmill test.
Fractional utilization = walking economy oxygen uptake / peak oxygen uptake.
ITT = Intent-to-Treat, G = Group effect, T = Test effect, GxT = Group by Test interaction.
Groups were similar (p > 0.05) at baseline on each daily ambulatory activity measure (Table 4). Home-based exercise increased maximal cadences for 20, 30, and 60 minutes of ambulation (p < 0.01), average daily cadence (p < 0.01), and the BASIC questionnaire score (p < 0.001). These changes were significantly different (p < 0.05) than changes in controls, and the increases in maximal cadence for 30 minutes and average daily cadence were significantly greater (p < 0.05) than changes with supervised exercise. When each variable in Table 3 and 4 was re-analyzed using an intent-to-treat approach, all of the significant group by time interactions found in the original ANOVA analyses remained significant except for physical function score (Table 3). These finding indicate that attrition patterns across groups had minimal influence on study results.
Table 4.
Daily ambulatory activity measures in patients completing usual care control (n = 30), supervised exercise (n = 33), and home-based exercise (n = 29). Values are means (SD).
| Variables | Pre-Test | Post-Test | Change Score | ANOVA p values Observed |
ANOVA p values ITT |
||||
|---|---|---|---|---|---|---|---|---|---|
| G | T | GxT | G | T | GxT | ||||
| Total Strides (strides/day) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
3778 (1723) 2912 (1358) 3139 (1860) |
3819 (1590) 3153 (1553) 3464 (1805) |
51 (1265) 169 (1120) 324 (1256) |
ns | ns | ns | ns | ns | ns |
| Total Activity Time (min/day) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
301 (98) 255 (98) 266 (104) |
312 (94) 270 (101) 269 (100) |
4 (81) 11 (69) 3 (76) |
ns | ns | ns | ns | ns | ns |
| Maximum 5-minute cadence (strides/min) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
32.7 (8.4) 26.2 (4.7) 28.0 (8.2) |
31.5 (7.6) 28.2 (7.2) 30.1 (10.2) |
−1.2 (8.1) 2.0 (6.0) 2.1 (6.0) |
<.05 | ns | ns | <.01 | ns | ns |
| Maximum 20-minute cadence (strides/min) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
20.4 (7.6) 14.9 (4.2) 16.5 (7.3) |
18.5 (7.1) 15.3 (5.4) 19.8 (10.9) |
−1.9 (6.9) 0.4 (5.5) 3.3 (6.1) †§ |
<.05 | ns | <.01 | <.05 | ns | <.01 |
| Maximum 30-minute cadence (strides/min) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
17.1 (6.3) 12.7 (3.9) 14.1 (6.8) |
15.3 (5.6) 12.8 (4.3) 17.5 (10.3) |
−1.8 (5.3) 0.1 (4.3) 3.4 (5.7)†§|| |
<.05 | ns | <.01 | ns | ns | <.01 |
| Maximum 60-minute cadence (strides/min) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
12.4 (4.9) 9.2 (3.0) 10.4 (5.2) |
11.1 (4.0) 9.3 (3.3) 12.9 (7.8) |
−1.3 (4.2) 0.1 (2.8) 2.5 (4.7)†§ |
<.05 | ns | <.01 | ns | ns | <.01 |
| Average Cadence (strides/min) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
12.3 (3.2) 11.6 (2.5) 11.3 (2.7) |
12.0 (2.6) 11.5 (2.3) 12.4 (3.2) |
−0.3 (1.8) −0.1 (1.2) 1.1 (2.0)†§|| |
ns | ns | <.01 | ns | ns | <.01 |
| BASIC (score) | |||||||||
| Control Group Supervised Exercise Group Home-Based Exercise Group |
3.5 (2.2) 2.2 (1.6) 2.1 (1.7) |
2.9 (1.7) 2.9 (1.9) 3.5 (1.9) |
−0.6 (1.9) 0.6 (1.9)§ 1.4 (1.5) ‡§ |
ns | <.05 | <.01 | ns | <.01 | <.01 |
Change from pre-test (p < 0.05),
(p < 0.01),
(p < 0.001).
Different than Control Group (p < 0.05).
Different than Supervised Exercise Group (p < 0.05).
ITT = Intent-to-Treat, G = Group effect, T = Test effect, GxT = Group by Test interaction.
DISCUSSION
Home-base exercise program: Adherence and exercise volume
The most unique feature of this investigation was directly measuring adherence to home-based exercise, and quantifying exercise time, cadence, and total ambulatory volume during the program. The relatively high adherence rate in home-based exercise was similar to that found with supervised exercise. No other randomized controlled trial has reported adherence to home-based exercise, whereas five non-controlled trials determined adherence via exercise diaries and telephone contact.6, 8, 10, 12, 13
Home-based exercise is inherently different from supervised exercise, and it was not the intent of this project to duplicate a supervised program in the community setting because this would simply compare treadmill training at various exercise facilities in the community to treadmill training at our university-based program. Instead, the home-based group walked at their freely-chosen pace on level ground around their homes and neighborhoods. We attempted to match the two programs on total volume of exercise, expressed as MET-minutes. Because training intensity of home-based exercise is limited by speed of ambulation that patients are willing or capable of sustaining, which is not the case for supervised training typically performed at various treadmill inclines, we increased the duration of home-based exercise by approximately five minutes per session to achieve a similar exercise volume. Thus, by design, we anticipated that average time per training session would be five minutes longer in the home-based program. In fact, the home-based group chose to walk for 11 additional minutes beyond that prescribed per training session, but they accomplished this by ambulating at a slower freely-chosen cadence than the supervised group, yielding similar exercise volumes between groups. Overall, the home-based exercise program was characterized by ambulating longer at a slower pace than the supervised exercise program. This is the first study to provide direct and accurate measurements of the exercise stimulus in a home-based exercise program.
It is likely that monitoring ambulation, meeting periodically with staff, and providing feedback to patients in home-based exercise enhanced their motivation to adhere to the program. Another possibility is that patients noticed improvements in their ambulation during the program, encouraging them to continue. Our home-based exercise program consisted of seven brief office-based meetings with an exercise physiologist interspersed throughout the program in which data from the step activity monitor was downloaded and reviewed along with the exercise log books. At the end of the 15-minute meeting, new instructions were given about increasing exercise duration and overcoming any challenges that might exist until their next visit. It is difficult to speculate whether adherence would be similar without these visits, but it is reasonable to guess that adherence would be equal or worse without them. Furthermore, the quality of the program would probably suffer without periodic meetings, as appropriate increases in exercise duration and problem solving might not occur.
Overall attrition rate of study participation was 23%, but this was not different among groups. Nearly 80% of dropouts were due to disinterest in continuing, and this typically occurred early in both home-based and supervised exercise programs. Careful medical screening and appropriate inclusion and exclusion criteria for study participation were factors contributing to relatively low occurrence of 11 patients (9%) having an adverse event, forcing only 6 patients (5%) to discontinue. We believe there is no systematic effect of attrition on study results among the groups. Further, it is possible that home-based training can be done as safely, if not more so, than supervised exercise due to slower ambulatory cadences that patients self-select during exercise.
Efficacy of Home-base exercise program vs. Usual Care Control
Another unique aspect was the randomized, controlled design comparing efficacy of home-based exercise with usual care control. Home-based exercise resulted in a 65% increase in COT and a 31% increase in PWT, and these improvements were greater than changes seen in the controls. Two other studies directly compared home-based exercise and control, but neither used a randomized design.14, 15 One study reported increases in COT and PWT following unsupervised exercise,15 whereas the other did not evaluate these measures.14 Additionally, we are the first to report that home-based exercise was efficacious in improving fractional utilization during treadmill exercise, daily ambulatory cadence for durations between 20 and 60 minutes, and average daily ambulatory cadence. Improvement in fractional utilization was due to the combination of small changes in walking economy and peak oxygen uptake, indicating that relative exercise intensity of ambulation was lower following rehabilitation. Thus, daily ambulation performed at a given pace can be done with less exertion following a program of home-based exercise.
Home-based exercise is efficacious in improving claudication measures, exercise performance, and daily ambulatory activity in patients with intermittent claudication. Consequently, participating in a home-based exercise program with minimal, periodic feedback may be a viable alternative for most patients compared to participating in a structured, on-site, supervised exercise program. This has significant clinical implications of managing many more patients with home-based exercise compared to the relatively small, select group who are candidates for supervised exercise.
Efficacy of Home-base exercise program vs. Supervised Exercise
Previous studies have compared home-based exercise with supervised exercise,5–7, 9–11 which was recently summarized.28 In contrast to the current randomized controlled trial, supervised exercise resulted in significantly greater increases in COT and PWT.28 However, none of the home-based programs objectively quantified amount of exercise accomplished, thus making comparison with supervised exercise difficult to interpret. Our study also demonstrated that home-based exercise increases daily ambulatory cadences at sustained exercise durations between 20 and 60 minutes, and average daily cadence, indicating that home exercise is efficacious in increasing community-based ambulation apart from the home exercise program. These increases in daily ambulatory cadences were greater than changes in the control and supervised exercise groups. Thus, home-based exercise may be superior to supervised exercise to increase community-based ambulation.
Limitations
Although this trial supports the efficacy of home-based and supervised exercise rehabilitation for PAD patients, several limitations exist. First, patients who participated in this trial were volunteers and therefore may represent those more interested in exercise, those who had better access to transportation to the program, and those in relatively better health than patients who did not volunteer. Second, although patients were randomized into one of the groups prior to intervention, the possibility that those who participated in home-based exercise were more motivated than other patients cannot be ruled out.
Conclusions and Clinical Implications
A home-based exercise program, quantified with a step activity monitor, has high adherence and is efficacious in improving claudication measures similar to that seen with a standard supervised exercise program. Furthermore, home-based exercise appears more efficacious in increasing daily ambulatory activity in the community setting than supervised exercise. The clinical implication is that home-based exercise programming, with patient monitoring and periodic feedback, may serve as a new model for improving claudication measures in more patients with less effort and fewer resources.
Clinical Perspective.
A primary therapeutic goal for patients with peripheral artery disease (PAD) and intermittent claudication is to regain lost ambulatory function through exercise rehabilitation. Medically supervised exercise programs are efficacious for improving claudication onset time (COT) and peak walking time (PWT), but more patients could benefit from an exercise program transported to the community setting (ie, home-based walking). However, home exercise has been poorly studied. This prospective, randomized controlled clinical trial compared changes in COT, PWT, and daily ambulatory activity in PAD patients with intermittent claudication following home-based exercise, supervised exercise, and usual care control. Both exercise programs consisted of intermittent walking to near maximal claudication pain for 12 weeks. We used a step activity monitor to address the primary flaw of previous home exercise programs by objectively measuring ambulatory cadence during home exercise sessions. Patients in home-based exercise completed 83% of their exercise sessions, averaging 42 minutes per session at a cadence of 37 strides per minute, and they increased COT, PWT, and daily ambulatory cadences apart from the exercise sessions. The changes in COT and PWT following home-based exercise were similar to supervised exercise, whereas the change in daily ambulatory cadences was greater. The clinical implication is that a home-based exercise program consisting of ambulatory monitoring, biweekly 15-minute meetings with staff, and feedback motivated patients to adhere with the program, and may serve as a new model for improving claudication measures in more patients with less effort and fewer resources than a traditional supervised exercise program.
Acknowledgments
Funding Sources
Supported by National Institute on Aging (R01-AG-24296; AWG), Oklahoma Center for the Advancement of Science and Technology grant (HR09-035; AWG), and OUHSC General Clinical Research Center grant (M01-RR-14467) sponsored by National Center for Research Resources (NCRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The final peer-reviewed version of this manuscript is subject to the NIH Public Access Policy, and will be submitted to PubMed Central.
Disclosures
None.
References
- 1.Hiatt WR, Hirsch AT, Regensteiner JG, Brass EP. Clinical trials for claudication. Assessment of exercise performance, functional status, and clinical end points. Vascular Clinical Trialists. Circulation. 1995;92:614–621. doi: 10.1161/01.cir.92.3.614. [DOI] [PubMed] [Google Scholar]
- 2.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 3.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001;49:755–762. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 5.Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. Eur J Vasc Endovasc Surg. 2004;27:17–23. doi: 10.1016/j.ejvs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Degischer S, Labs KH, Hochstrasser J, Aschwanden M, Tschoepl M, Jaeger KA. Physical training for intermittent claudication: a comparison of structured rehabilitation versus home-based training. Vasc Med. 2002;7:109–115. doi: 10.1191/1358863x02vm432oa. [DOI] [PubMed] [Google Scholar]
- 7.Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. Eur J Vasc Endovasc Surg. 2005;30:164–175. doi: 10.1016/j.ejvs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Mouser MJ, Zlabek JA, Ford CL, Mathiason MA. Community trial of home-based exercise therapy for intermittent claudication. Vasc Med. 2009;14:103–107. doi: 10.1177/1358863X08098596. [DOI] [PubMed] [Google Scholar]
- 9.Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. J Vasc Surg. 1997;25:312–318. doi: 10.1016/s0741-5214(97)70352-5. [DOI] [PubMed] [Google Scholar]
- 10.Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300. doi: 10.1177/000331979704800402. [DOI] [PubMed] [Google Scholar]
- 11.Savage P, Ricci MA, Lynn M, Gardner A, Knight S, Brochu M, Ades P. Effects of home versus supervised exercise for patients with intermittent claudication. J Cardiopulm Rehabil. 2001;21:152–157. doi: 10.1097/00008483-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Tan KH, Cotterrell D, Sykes K, Sissons GR, de CL, Edwards PR. Exercise training for claudicants: changes in blood flow, cardiorespiratory status, metabolic functions, blood rheology and lipid profile. Eur J Vasc Endovasc Surg. 2000;20:72–78. doi: 10.1053/ejvs.2000.1137. [DOI] [PubMed] [Google Scholar]
- 13.Wullink M, Stoffers HE, Kuipers H. A primary care walking exercise program for patients with intermittent claudication. Med Sci Sports Exerc. 2001;33:1629–1634. doi: 10.1097/00005768-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald DE, Keates JS, MacMillan D. Angiographic and plethysmographic assessment of graduated physical exercise in the treatment of chronic occlusive arterial disease of the leg. Angiology. 1971;22:99–106. doi: 10.1177/000331977102200208. [DOI] [PubMed] [Google Scholar]
- 15.Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Lancet. 1966;19(2):1093–1096. doi: 10.1016/s0140-6736(66)92191-x. [DOI] [PubMed] [Google Scholar]
- 16.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg. 2007;46:1208–1214. doi: 10.1016/j.jvs.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–408. [PubMed] [Google Scholar]
- 18.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, Hamman RF. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]
- 19.ACSM's guidelines for exercise testing and prescription. 6th. Philadelphia: Lippincott Williams & Wilkins; 2000. American College of Sports Medicine. [Google Scholar]
- 20.Gardner AW, Killewich LA, Montgomery PS, Katzel LI. Response to exercise rehabilitation in smoking and nonsmoking patients with intermittent claudication. J Vasc Surg. 2004;39:531–538. doi: 10.1016/j.jvs.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Gardner AW, Montgomery PS, Flinn WR, Katzel LI. The effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. J Vasc Surg. 2005;42:702–709. doi: 10.1016/j.jvs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 22.Gardner AW, Killewich LA, Katzel LI, Womack CJ, Montgomery PS, Otis RB, Fonong T. Relationship between free-living daily physical activity and peripheral circulation in patients with intermittent claudication. Angiology. 1999;50:289–297. doi: 10.1177/000331979905000404. [DOI] [PubMed] [Google Scholar]
- 23.Gardner AW. Reliability of transcutaneous oximeter electrode heating power during exercise in patients with intermittent claudication. Angiology. 1997;48:229–235. doi: 10.1177/000331979704800305. [DOI] [PubMed] [Google Scholar]
- 24.Womack CJ, Sieminski DJ, Katzel LI, Yataco A, Gardner AW. Improved walking economy in patients with peripheral arterial occlusive disease. Med Sci Sports Exerc. 1997;29:1286–1290. doi: 10.1097/00005768-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Regensteiner JG, Steiner JF, Panzer RL, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 26.Gardner AW, Montgomery PS. The Baltimore activity scale for intermittent claudication: a validation study. Vasc Endovascular Surg. 2006;40:383–391. doi: 10.1177/1538574406288575. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 28.Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. Eur J Vasc Endovasc Surg. 2007;34:1–9. doi: 10.1016/j.ejvs.2006.12.030. [DOI] [PubMed] [Google Scholar]