Abstract
Cytokines play a central role in the pathogenesis of many diseases, including HIV infection. However, the role of the cytokine network in early HIV infection is only now starting to be elucidated. A number of studies conducted in recent years have indicated that cytokines of the acute/early stages of HIV and SIV infection can impact viral set-point months later, and this is of critical importance since viral set-point during chronic HIV infection affects virus transmission and disease progression. This raises the question whether modulating the cytokine environment during acute/early HIV infection can be a target for novel approaches to develop a vaccine and therapeutics. In this review we focus on the kinetics and function of cytokines during acute HIV and SIV infection and how these may impact viral set-point. We also discuss unresolved questions that are essential for our understanding of the role of acute infection cytokines in HIV infection and that, if answered, may suggest novel therapeutic and vaccine strategies to control the worldwide HIV pandemic.
Introduction
Early events during acute HIV infection may determine progression and pathogenesis of infection, as the immunological milieu of the initial antigen encounter appears critical in dictating the long-term equilibrium between the host and the pathogen [1]. This early period, which includes the eclipse phase before viremia is detected and the viremic phase before viral set-point is reached, is critical for target cell availability, seeding of latent reservoirs, and the initiation and expansion of antiviral immune responses by the host. While such events have been difficult to assess in humans [2], [3], animal models such as rodent and the non-human primate model of AIDS have afforded us the opportunity to address such seminal questions. Thus, chronic immune stimulation [4], [5], immunosuppression [6], partial virus-specific immunity [7], and/or the use of cytokines [8]–[11] or inhibitors of cell death [12] have all been shown to alter not only the viral replication dynamics and quality of immune responses, but more importantly also the kinetics of disease progression. Among these immunomodulatory approaches, cytokines provide one of the most targeted factors to investigate alterations of the viral kinetics, the recruitment of viral targets, and the development of anti-viral immunity.
Cytokine Milieu in Early/Acute HIV/SIV Infection
The complexity of the role of the cytokine milieu in acute HIV and SIV infection has only partially been addressed. The first reports examining cytokines in acute HIV infection were conducted in patients with symptomatic acute infection [13], [14]. However, very early events during the first days and weeks could not be assessed since the exact time of infection was unknown and the symptomatic phase can occur several weeks after initial viral exposure [3]. A more recent study analyzed plasma cytokines in HIV infection after the eclipse phase in patients with detectable viral load (at least 100 HIV RNA copies/ml) [15]. This examination of systemic plasma cytokines revealed that IFNα and IL-15 were the first cytokines elevated within 5 days after detection of viremia, followed by TNFα, CXCL10, and IFNγ, and then by IL-12 [15]. As expected for the anti-inflammatory cytokine IL-10, increased IL-10 mRNA and protein levels are detected rather late in HIV infection, after the increased expression of proinflammatory cytokines [15], [16]. Another well-known inhibitory cytokine upregulated in the majority of acutely HIV infected individuals is IL-1R antagonist (IL-1Rα) [15]. In vitro, IL-1Rα inhibits IL-1-mediated HIV replication [17], suggesting that IL-1Rα would suppress viral replication during acute infection. Similar to IL-10, however, IL-1Rα may also affect anti-viral immunity. A major caveat in all of these human studies is the estimated time point of infection.
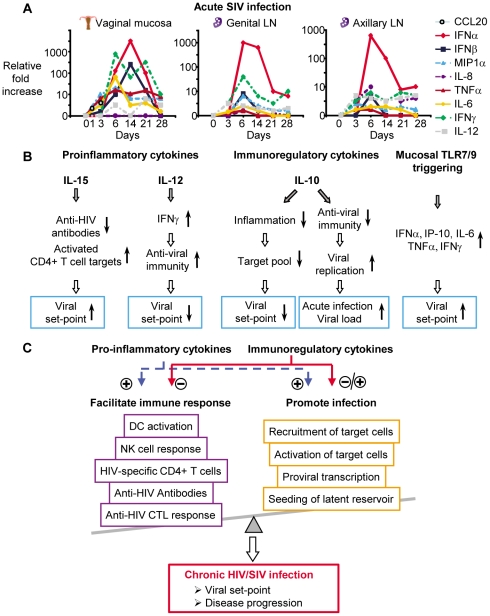
A more precise timing of cytokine kinetics, however, can be done in SIV-infected non-human primates. Several such studies have been conducted of very early SIV infection in non-human primates to analyze the cytokine production during the first weeks of infection and compare differences between non-pathogenic and pathogenic infections [2], [18]–[25]. Some of the first studies examined cytokine mRNA after intravenous (i.v.) SIV infection in tissues as the viral dynamics evolved. By day 7 post i.v. infection, IL-10 mRNA was detected in bronchial lavage cells but not lymph nodes (LNs) or peripheral blood mononuclear cells while IFNγ mRNA was detected later [26], [27]. Another study, however, indicated that IFNγ mRNA is upregulated in LNs at day 7 while IL-2 and IL-12 mRNA increase after day 14 [28]. When plasma cytokines were measured, IL-12 and IL-18 were found to be induced after 2 weeks of infection, whereas IFNα/β was detected already by week 1 [29]. While these studies provided seminal observations, the i.v. route of infection used does not mirror the predominant route of infection in humans where virus infection and cytokine production start at mucosal tissues and spread distally. Within 24 hours following mucosal infection, endocervical epithelium produces MIP-3α (CCL20) [22], a chemokine involved in recruitment of plasmacytoid dendritic cells (pDCs). Such subepithelial pDCs are recruited and produce IFNα, IFNβ, MIP-1α (CCL3), and MIP-1β (CCL4) at day 1 of infection, which in turn promote inflammation and recruit T cell targets for infection [22]. Subsequently, IL-8 and RANTES are detected in mucosal sites and these further enhance local inflammation [22]. After the initial mucosal infection, the virus spreads within days into the local draining LNs and then subsequently to more distal LNs [30], [31]. When cytokine mRNA expression was evaluated in vaginal mucosal tissue, genital tract draining LNs, and distal LNs, MIP-1α, TNFα, and IL-6 were detected at days 3–5 in all tissues. IFNβ was only detected in vaginal mucosa at these early time points [18] (Figure 1A). IFNα was observed in all tissues at days 6–10, whereas IL-12 and IFNγ were detected in draining and distal LNs at days 3–5 and later at days 6–10 in the vaginal mucosa. Cytokine mRNA declined in parallel with the decrease of viral replication after day 14, suggesting that the virus directly or indirectly drives much of the cytokine production [18]. Similar to the human studies above, IL-15 is detected systemically in plasma during acute SIV infection within a few days of i.v. infection and peaks at day 10 [32]. Limited data is available for kinetics of immunosuppressive cytokines during acute SIV infection. Such cytokines can act as a double-edged sword, as they can dampen virus-specific immunity but also inhibit infection by reducing T cell recruitment and activation. Immunosuppressive cytokines such as IL-10 and transforming growth factor-β1 (TGFβ1) are also present during acute SIV infection. In SIV-infected rhesus macaques, IL-10 production in LNs is already detected at day 7 and increases further by day 28 post-infection [33]. Increased TGFβ1 in LNs is also observed early in SIV-infected rhesus macaques by day 7, with production peaking on day 12 [33].
Figure 1. Early cytokines determine pathogenesis of HIV/SIV infection.
(A) Several cytokines are produced during acute SIV infection, either at the site of initial viral exposure or in draining or distal lymphoid tissue (cytokine mRNA kinetics are adapted from [18], [22]; scale depicts fold changes over time for each individual cytokine). (B) Cytokines either increase (↑) or decrease (↓) immune parameters or target availability and therefore directly influence viral replication and viral set-point (effects of cytokines are adapted from [8], [10], [23], [66], [70]). (C) Pro-inflammatory and immunoregulatory cytokines either negatively or positively modulate immune responses and viral replication and this determines viral set-points and disease progression during chronic HIV/SIV infection.
Comparison of cytokine gene expression profiles and kinetics in the non-pathogenic SIV infection of natural hosts (African green monkeys and sooty mangabeys) with pathogenic SIV infection of non-natural hosts (rhesus macaques and pigtailed macaques) can be used to link cytokine production with pathogenesis. IFNα, TGFβ, and IL-10 mRNA and protein expression is increased earlier in non-pathogenic SIV infection compared to pathogenic SIV infection [20], [21], [34], and this is accompanied by transient IFNγ expression only in non-pathogenic SIV infection [20]. These data suggested that although natural hosts of SIV upregulate cytokines like IFNα and IFNγ, these cytokines are downregulated much faster. This is paralleled by a much earlier production of regulatory cytokines, indicating an important role of these cytokines in controlling SIV pathogenesis. Comparison of non-pathogenic and pathogenic SIV infection has suggested a possible role in HIV/SIV pathogenesis for IL-17. IL-17 is produced by several cell types, including Th17 cells, natural killer T (NKT) cells, and γδ T cells (reviewed in [35]). Th17 cells are depleted during acute SIV infection from peripheral blood in pathogenic but not in non-pathogenic SIV infection [36]. During chronic infection, Th17 cells are significantly reduced in pathogenic SIV infection in lymphoid tissue [36] and gastrointestinal track [37], [38], which likely impairs control of intestinal flora and pathogens [37]. In contrast, increased numbers of IL-17-producing NKT cells are detected at day 14 in LNs of pathogenic but not non-pathogenic SIV infection [39]. Since IL-17 overproduction can promote inflammation [40], increased IL-17 secretion in LNs during acute HIV/SIV infection could lead to increased recruitment of target cells, viral dissemination, and malabsorption. Clearly, IL-17 may have an important impact on the pathogenesis of infection and further studies are required to address this. Although comparisons of non-pathogenic and pathogenic SIV infection may reveal important aspects of how cytokines contribute to pathogenesis, they are of limited use for studying the role of cytokines on viral set-point as non-pathogenic SIV infection of natural hosts is accompanied by high viral loads.
Source of Cytokines during Acute HIV/SIV Infection
The source and location of cytokine production during acute HIV/SIV infection is only partially understood. Cytokines in the very early phase of HIV and SIV infection are most likely produced locally, whereas after virus dissemination, cytokines are produced by more generalized and distant sites such as genital draining and distal LNs and gut-associated lymphoid tissue [30]. The human studies have relied on measuring cytokines in plasma [15], and their kinetics suggest they reflect systemically produced cytokines after dissemination beyond the site of entry. In SIV infection, several cell types present in the vaginal mucosa and LNs have been shown previously to produce cytokines during acute infection. Type I IFN is primarily produced by plasmacytoid DCs [41], IL-12 by conventional DCs and macrophages [42], [43], and IL-15 by many different cell types, including DCs and macrophages [44]–[46]. As mentioned above, the epithelium at the local port of entry produces MIP-3α (CCL20), and recruits/activates pDCs to produce IFNα, IFNβ, MIP-1α (CCL3), and MIP-1β (CCL4) [22]. Once the virus spreads to the draining LNs, T cells, monocytes, and pDCs produce IFNα, IL-12, IFNγ, IL-10, and TGFβ1 in draining and distal LNs [18], [33]. Despite the recent progress at dissecting cytokine production during acute infection, much more is needed to identify the cell types and respective location of cytokine production and how these relate to plasma levels during the disseminating acute infection. This latter point is critical for our understanding of the human studies that cannot easily access tissues and rely on plasma measurements.
Viral Set-Point and Cytokines during Acute HIV/SIV Infection
A central question regarding cytokines during acute HIV/SIV infection is whether these cytokines are beneficial or detrimental for the host. Cytokines can upregulate or accelerate the anti-viral immune response, and this could contribute to viral control [47] . However, at the same time, these cytokines can also increase the target cell pool for HIV/SIV by recruiting and activating CD4+ T cells, the primary targets for HIV/SIV infection. Immunosuppressive cytokines can dampen anti-viral immunity but also reduce inflammation and decrease the target cell pool of activated CD4+ T cells. Most cytokines exert pleiotropic and sometimes contrasting effects on the immune response and viral replication as suggested by a number of studies. Examples of these are pro-inflammatory cytokines such as TNFα that are produced during acute HIV/SIV infection [15], [18] and increase anti-viral immunity but also induce NF-κB [48], which enhances proviral transcription and drives HIV replication [49]–[51]. IL-15 can enhance NK cell and CD8+ T cell responses in HIV and SIV [52]–[54] but also correlates with increased viral replication [32].
Recent studies have suggested that cytokines during acute HIV/SIV infection are important determinants of the viral set-point of chronic infection [8], [10], [15], [39], [42], [55]. Viral set-points are stable in anti-retroviral naïve, asymptomatic, chronically HIV-infected patients but can vary greatly between patients. In patients on highly active antiretroviral therapy (HAART) for a prolonged time, viral set-points seen after discontinuation of HAART match pre-treatment set-points [56]–[58]. Experimental depletion of CD4+ T cells in monkeys only temporarily lowered the set-point [59], indicating a remarkable individual stability. Viral set-points correlate with disease progression with higher viral set-points being associated with a more rapid progression of HIV [60], [61]. Reduced viral set-point during chronic HIV infection is associated with reduced heterosexual and maternal–infant transmission of HIV [60]–[65]. Untreated HIV-infected women with a viral set-point below 1,500 HIV-1 RNA copies/ml plasma fail to sexually transmit the virus [64]. The risk of maternal–infant HIV-1 transmission is reduced in pregnant HIV-infected individuals with low viral set-point [62], [63], [65]. Therefore, understanding how cytokines of acute HIV infection can affect viral set-points may suggest novel approaches to decrease this important determinant of disease progression and transmission.
An association of acute infection cytokines with viral set-point was recently suggested in HIV infection. Higher plasma IL-7 and IL-15 levels during acute/early HIV infection appear detrimental and correlate with higher viral set-points, while conversely, plasma IL-12 and IFNγ levels appear beneficial and associate with lower viral set-points [55]. A protective role of early IL-10 upregulation was also indicated in studies, which showed that individuals with polymorphisms resulting in increased IL-10 production have lower viral set-points [23], [66]. These human studies have suggested that cytokine profiles during acute infection may determine viral set-point, and non-human primate studies have provided further support for this.
One prediction from the above human studies would be that addition of cytokines during acute SIV infection would alter viral set-point months later. Such studies have been conducted and remarkably agree with these predictions based on plasma level correlations in HIV infection. Treatment of acute SIV infection with simian IL-15 resulted in a dramatic 1,000-fold increase in viral set-point [10], [67] (Figure 1B). IL-15 treatment of acute SIV infection accelerated disease progression [10] while treatment during chronic infections did not affect viral set-points or disease progression [52]. In contrast, when IL-12 was administered to rhesus macaques acutely infected with SIV, it lowered viral set-point by 100-fold [8] (Figure 1B) and inhibited disease progression [8]. Treatment of chronic SIV-infection with IL-12 did not affect viral set-point or disease progression [68], [69].
That cytokines present early during acute infection can affect viral set-point is further supported by a study testing whether the induction of antiviral cytokines at the vaginal mucosa would prevent SIV infection. The basic premise for this study was that Toll-like receptor (TLR) 7 and 9 agonists applied intravaginally would stimulate antiviral cytokines and induce an antiviral state in the vaginal mucosa. As expected, these agonists upregulated mRNA and protein for several cytokines in vaginal secretions, including IFNα, TNFα, IL-6, IP-10, and IFNγ [70]. Despite the induction of such cytokines, animals treated with TLR agonists were readily infected, exhibiting increased viral set-points compared to untreated animals [70] (Figure 1B). Peak viremia post-infection, however, was not affected, and this is similar to IL-15 and IL-12 administration during acute infection [8], [10], [67].
The potential mechanisms by which acute HIV/SIV infection cytokines affect viral set-point are suggested by the above studies. Although the precise mechanism by which IL-15 is affecting viral set-point is not known, effects on host immunity and/or infection of target cells are suspected, as IL-15 decreases anti-SIV antibody responses but enhances the homeostatic proliferation of predominantly memory CD4+ T cells during acute infection, and both correlate with increased viral set-point [10]. IL-15’s effect on viral set-point occurred despite an increase in virus-specific CD8+ T cell and NK cell numbers in these animals [10]. The IL-15-mediated increase in CD4+ T cell proliferation may accelerate or enhance the loss of SIV-specific CD4+ T cells and this would explain the reduced anti-SIV antibody levels. On the other hand, increased CD4+ T cell proliferation could augment the seeding of infected cells. Of interest, IL-12 treatment during acute infection markedly promoted the recruitment and activation of NK cells similar to IL-15. IL-12 however, also induced a rapid production of IFNγ in these animals [8], and/or prevention of apoptosis of SIV-specific CD4+ T cells [71], which prevented contraction of effector T cells and enhanced anti-viral immunity [12], [72]. The preservation of newly minted antiviral CD4+ and CD8+ T cells may be key to downstream control of viremia and disease progression [6], [8], [12], [72]. The intravaginal application of TLR agonists induced the host to produce cytokines that have both anti-viral and pro-inflammatory activity, including IFNγ [70]. This cytokine induction was accompanied by the markedly enhanced recruitment of activated CD4+ T cells, macrophages, and DCs in animals treated with TLR agonists fueling the initial foyer of infection [70]. These seemingly contradicting results with overlapping profiles of cytokines highlight our lack of understanding about cytokine profiles and kinetics that are ultimately beneficial versus those that are deleterious to the newly infected host during acute HIV/SIV infection
Can Knowledge of the Cytokines Network in Acute HIV/SIV Infection Enable Us to Design New Therapeutic and/or Vaccine Strategies?
From the data discussed above, it is clear that without a better understanding of the complex interactions between cytokines and the host during acute HIV/SIV infection, the accurate prediction of the effect of cytokine interventions will be difficult (Figure 1C). A number of critical questions remain unanswered. Thus, what are the precise cytokine profiles of acute infection as the infection spreads from mucosal sites into lymphoid tissue? From what cell types and tissues are these cytokines being made during acute infection? What is the source of systemically detected cytokines? What triggers their production? Are some cytokines induced by pattern recognition receptors directly triggered by virus and others by virus-specific immunity? What is the window of opportunity during acute HIV/SIV infection for cytokine manipulations to affect viral set-point? How are acute infection cytokines affecting viral set-point? Are cytokines affecting viral set-point by altering innate and adaptive immunity, availability and infectivity of target cells, or evolution and fitness of the virus?
Some of these questions can now be answered, as assays to measure multiple cytokines in non-human primates are being developed and validated. To investigate the cytokine profile in plasma and tissues, the cell type source and the production location will require a concerted effort of multiple laboratories specializing in non-human primate studies. Importantly, understanding the role and function of individual cytokines in determining viral set-point will require the inhibition or addition of individual cytokines during acute SIV infection. These types of experiments require significant resources for the production and validation of cytokines and blocking reagents. Finally, both extensive immunological and virological studies need to be conducted in the setting of cytokine administration or inhibition during acute SIV infection to determine the mechanism by which viral set-points are altered.
Findings from studies above can help identify cytokines that can be targeted to alter HIV viral set-point, transmission, and disease progression. This could lead to cytokines or cytokine inhibitors being used as pharmacological agents in acute/early infection with the goal of altering the course of infection and lowering viral set-points. Cytokine hierarchies have been exploited therapeutically in patients with rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) where blocking TNFα, a key driver of the inflammatory cytokine network in RA and IBD, has a major impact on inflammation [73]–[75]. Blocking cytokines is clearly feasible but its applicability in HIV infection will depend on the window of opportunity after initial exposure that still enables the reduction of the viral set-point. Another strategy would be to develop vaccines that imprint the cytokine network. Such vaccines would be non-sterilizing as the host would acquire infection but could potentially lower viral set-points below critical levels required for HIV transmission. In addition, such vaccines could be combined with vaccines designed to prevent acquisition at the mucosal surface [76], thereby providing a double barrier. Adjuvants can dictate the development of Th1 or Th2 immunity [77]. Toll-like receptor ligands, for example, can direct the cytokine profile of immunity against pathogens [78]. When Leishmania and Mycobacterium tuberculosis are formulated in oil-in-water emulsions they primarily induce Th2 responses, whereas the addition of TLR4 agonists such as monophosphoryl lipid A or glycopyranosyl lipid [79], [80] directed their response to a Th1 profile [78]. Several recent studies utilized cytokines as vaccine adjuvants with some promising results, such as IL-15-leading to increased levels of CD8+ T cell responses [81]–[83]. Such adjuvant or cytokine approaches could be used to develop vaccines which imprint an immune response to HIV that results in a beneficial cytokine profile or network during acute HIV infection of the host.
Conclusion
We have reviewed above the evidence that viral set-point can be manipulated by cytokine interventions during acute HIV/SIV infection. This raises the question whether we can target cytokines during acute HIV infection to alter viral set-point through immunotherapeutics and potentially vaccines. Treating with cytokines or blocking cytokines is clearly feasible but will depend on the window of opportunity after initial HIV exposure, during which such treatments are still effective at lowering viral set-point. Non-sterilizing vaccine strategies that alter the cytokines produced during acute infection will be challenging, but the reward of a vaccine that reduces viral set-point below transmission levels would be tremendous. Further understanding of the cytokine network and the potential cytokine hierarchies during acute HIV/SIV infection may uncover critical cytokines that control viral set-point. This may not only provide fundamental insight into the poorly understood mechanisms that control viral set-point but could instruct the development of novel immunotherapeutics or vaccine adjuvants against HIV.
Acknowledgments
The authors would like to thank Dr. Jim Burns, Drexel University College of Medicine, for helpful suggestions.
Footnotes
The authors have declared that no competing interests exist.
This review was supported by grants R01AI046719 and R21AI082680 to PDK and R24 RR169188 to FV from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weissman D, Barker TD, Fauci AS. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J Exp Med. 1996;183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosinger SE, Hosiawa KA, Cameron MJ, Persad D, Ran L, et al. Gene expression profiling of host response in models of acute HIV infection. J Immunol. 2004;173:6858–6863. doi: 10.4049/jimmunol.173.11.6858. [DOI] [PubMed] [Google Scholar]
- 3.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folks T, Rowe T, Villinger F, Parekh B, Mayne A, et al. Immune stimulation may contribute to enhanced progression of SIV induced disease in rhesus macaques. J Med Primatol. 1997;26:181–189. doi: 10.1111/j.1600-0684.1997.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwiebert R, Fultz PN. Immune activation and viral burden in acute disease induced by simian immunodeficiency virus SIVsmmPBj14: correlation between in vitro and in vivo events. J Virol. 1994;68:5538–5547. doi: 10.1128/jvi.68.9.5538-5547.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garber DA, Silvestri G, Barry AP, Fedanov A, Kozyr N, et al. Blockade of T cell costimulation reveals interrelated actions of CD4+ and CD8+ T cells in control of SIV replication. J Clin Invest. 2004;113:836–845. doi: 10.1172/JCI19442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staprans SI, Barry AP, Silvestri G, Safrit JT, Kozyr N, et al. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci U S A. 2004;101:13026–13031. doi: 10.1073/pnas.0404739101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansari AA, Mayne AE, Sundstrom JB, Bostik P, Grimm B, et al. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J Virol. 2002;76:1731–1743. doi: 10.1128/JVI.76.4.1731-1743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giavedoni LD, Velasquillo MC, Parodi LM, Hubbard GB, Hodara VL. Expression of IL-18 by SIV does not modify the outcome of the antiviral immune response. Virology. 2002;303:327–337. doi: 10.1006/viro.2002.1647. [DOI] [PubMed] [Google Scholar]
- 10.Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, et al. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol. 2008;180:350–360. doi: 10.4049/jimmunol.180.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawai ET, Hamza MS, Ye M, Shaw KE, Luciw PA. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J Virol. 2000;74:2038–2045. doi: 10.1128/jvi.74.4.2038-2045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvato MS, Yin CC, Yagita H, Maeda T, Okumura K, et al. Attenuated disease in SIV-infected macaques treated with a monoclonal antibody against FasL. Clin Dev Immunol. 2007:93462. doi: 10.1155/2007/93462. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biglino A, Sinicco A, Forno B, Pollono AM, Sciandra M, et al. Serum cytokine profiles in acute primary HIV-1 infection and in infectious mononucleosis. Clin Immunol Immunopathol. 1996;78:61–69. doi: 10.1006/clin.1996.0009. [DOI] [PubMed] [Google Scholar]
- 14.Graziosi C, Gantt KR, Vaccarezza M, Demarest JF, Daucher M, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson J, Kinloch-de-Loes S, Granath A, Sonnerborg A, Goh LE, et al. Early immune activation in gut-associated and peripheral lymphoid tissue during acute HIV infection. AIDS. 2007;21:565–574. doi: 10.1097/QAD.0b013e3280117204. [DOI] [PubMed] [Google Scholar]
- 17.Poli G, Kinter AL, Fauci AS. Interleukin 1 induces expression of the human immunodeficiency virus alone and in synergy with interleukin 6 in chronically infected U1 cells: inhibition of inductive effects by the interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1994;91:108–112. doi: 10.1073/pnas.91.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naicker DD, Werner L, Kormuth E, Passmore JA, Mlisana K, et al. Interleukin-10 promoter polymorphisms influence HIV-1 susceptibility and primary HIV-1 pathogenesis. J Infect Dis. 2009;200:448–452. doi: 10.1086/600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benveniste O, Vaslin B, Le Grand R, Cheret A, Matheux F, et al. Comparative interleukin (IL-2)/interferon IFN-gamma and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SICmac251 virus. Proc Natl Acad Sci U S A. 1996;93:3658–3663. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou W, Lackner AA, Simon M, Durand-Gasselin I, Galanaud P, et al. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheret A, Le Grand R, Caufour P, Dereuddre-Bosquet N, Matheux F, et al. Cytokine mRNA expression in mononuclear cells from different tissues during acute SIVmac251 infection of macaques. AIDS Res Hum Retroviruses. 1996;12:1263–1272. doi: 10.1089/aid.1996.12.1263. [DOI] [PubMed] [Google Scholar]
- 27.Cheret A, Le Grand R, Caufour P, Neildez O, Matheux F, et al. RANTES, IFN-gamma, CCR1, and CCR5 mRNA expression in peripheral blood, lymph node, and bronchoalveolar lavage mononuclear cells during primary simian immunodeficiency virus infection of macaques. Virology. 1999;255:285–293. doi: 10.1006/viro.1998.9558. [DOI] [PubMed] [Google Scholar]
- 28.Khatissian E, Chakrabarti L, Hurtrel B. Cytokine patterns and viral load in lymph nodes during the early stages of SIV infection. Res Virol. 1996;147:181–189. doi: 10.1016/0923-2516(96)80233-0. [DOI] [PubMed] [Google Scholar]
- 29.Giavedoni LD, Velasquillo MC, Parodi LM, Hubbard GB, Hodara VL. Cytokine expression, natural killer cell activation, and phenotypic changes in lymphoid cells from rhesus macaques during acute infection with pathogenic simian immunodeficiency virus. J Virol. 2000;74:1648–1657. doi: 10.1128/jvi.74.4.1648-1657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 31.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberly MD, Kader M, Hassan W, Rogers KA, Zhou J, et al. Increased IL-15 production is associated with higher susceptibility of memory CD4 T cells to simian immunodeficiency virus during acute infection. J Immunol. 2009;182:1439–1448. doi: 10.4049/jimmunol.182.3.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, et al. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703–712. doi: 10.1086/500368. [DOI] [PubMed] [Google Scholar]
- 34.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 36.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. [Google Scholar]
- 38.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, et al. AIDS progression is associated with the emergence of IL-17-producing cells early after simian immunodeficiency virus infection. J Immunol. 2010;184:984–992. doi: 10.4049/jimmunol.0902316. [DOI] [PubMed] [Google Scholar]
- 40.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, et al. Pillars article: development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. 1993. Science 260(5107): 547-549. J Immunol. 2008;181:4437–4439. [PubMed] [Google Scholar]
- 43.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 44.Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, et al. Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-gamma by natural killer cells in vitro. J Clin Invest. 1995;96:2578–2582. doi: 10.1172/JCI118321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 46.Jonuleit H, Wiedemann K, Muller G, Degwert J, Hoppe U, et al. Induction of IL-15 messenger RNA and protein in human blood-derived dendritic cells: a role for IL-15 in attraction of T cells. J Immunol. 1997;158:2610–2615. [PubMed] [Google Scholar]
- 47.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 49.Alcami J, Lain de Lera T, Folgueira L, Pedraza MA, Jacque JM, et al. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen BK, Feinberg MB, Baltimore D. The kappaB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J Virol. 1997;71:5495–5504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, et al. Interleukin-15 increases effector memory CD8+ t cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol. 2005;79:4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mueller YM, Bojczuk PM, Halstead ES, Kim AH, Witek J, et al. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 54.Petrovas C, Mueller YM, Dimitriou ID, Bojczuk PM, Mounzer KC, et al. HIV-specific CD8+ T cells exhibit markedly reduced levels of Bcl-2 and Bcl-xL. J Immunol. 2004;172:4444–4453. doi: 10.4049/jimmunol.172.7.4444. [DOI] [PubMed] [Google Scholar]
- 55.Roberts L, Passmore JA, Williamson C, Little F, Bebell LM, et al. Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS. 2010;24:819–831. doi: 10.1097/QAD.0b013e3283367836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS. 1999;13:F59–62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 57.Hatano H, Vogel S, Yoder C, Metcalf JA, Dewar R, et al. Pre-HAART HIV burden approximates post-HAART viral levels following interruption of therapy in patients with sustained viral suppression. AIDS. 2000;14:1357–1363. doi: 10.1097/00002030-200007070-00008. [DOI] [PubMed] [Google Scholar]
- 58.Neumann AU, Tubiana R, Calvez V, Robert C, Li TS, et al. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. Comet Study Group. AIDS. 1999;13:677–683. doi: 10.1097/00002030-199904160-00008. [DOI] [PubMed] [Google Scholar]
- 59.Klatt NR, Villinger F, Bostik P, Gordon SN, Pereira L, et al. Availability of activated CD4+ T cells dictates the level of viremia in naturally SIV-infected sooty mangabeys. J Clin Invest. 2008;118:2039–2049. doi: 10.1172/JCI33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Wolf F, Spijkerman I, Schellekens PT, Langendam M, Kuiken C, et al. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11:1799–1806. doi: 10.1097/00002030-199715000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, et al. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 62.Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 63.Mayaux MJ, Dussaix E, Isopet J, Rekacewicz C, Mandelbrot L, et al. Maternal virus load during pregnancy and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohort studies. SEROGEST Cohort Group. J Infect Dis. 1997;175:172–175. doi: 10.1093/infdis/175.1.172. [DOI] [PubMed] [Google Scholar]
- 64.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 65.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335:1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 66.Shin HD, Winkler C, Stephens JC, Bream J, Young H, et al. Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc Natl Acad Sci U S A. 2000;97:14467–14472. doi: 10.1073/pnas.97.26.14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, et al. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med. 2009;206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villinger F, Bucur S, Chikkala NF, Brar SS, Bostik P, et al. In vitro and in vivo responses to interleukin 12 are maintained until the late SIV infection stage but lost during AIDS. AIDS Res Hum Retroviruses. 2000;16:751–763. doi: 10.1089/088922200308756. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe N, Sypek JP, Mittler S, Reimann KA, Flores-Villanueva P, et al. Administration of recombinant human interleukin 12 to chronically SIVmac-infected rhesus monkeys. AIDS Res Hum Retroviruses. 1998;14:393–399. doi: 10.1089/aid.1998.14.393. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Abel K, Lantz K, Krieg AM, McChesney MB, et al. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. J Virol. 2005;79:14355–14370. doi: 10.1128/JVI.79.22.14355-14370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, et al. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)- mediated apoptosis of CD4+ T cells from human immunodeficiency virus- infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poonia B, Salvato MS, Yagita H, Maeda T, Okumura K, et al. Treatment with anti-FasL antibody preserves memory lymphocytes and virus-specific cellular immunity in macaques challenged with simian immunodeficiency virus. Blood. 2009;114:1196–1204. doi: 10.1182/blood-2009-02-202655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Feldman M, Taylor P, Paleolog E, Brennan FM, Maini RN. Anti-TNF alpha therapy is useful in rheumatoid arthritis and Crohn's disease: analysis of the mechanism of action predicts utility in other diseases. Transplant Proc. 1998;30:4126–4127. doi: 10.1016/s0041-1345(98)01365-7. [DOI] [PubMed] [Google Scholar]
- 75.Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19. doi: 10.1111/j.1600-065X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 76.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Silva DG, Cooper PD, Petrovsky N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol Cell Biol. 2004;82:611–616. doi: 10.1111/j.1440-1711.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- 78.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev. 2011;239:178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–5963. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 80.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, et al. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 81.Kutzler MA, Robinson TM, Chattergoon MA, Choo DK, Choo AY, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 82.Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci U S A. 2007;104:18648–18653. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubie RA, Maksaereekul S, Shacklett BL, Lemongello D, Cole KS, et al. Co-immunization with IL-15 enhances cellular immune responses induced by a vif-deleted simian immunodeficiency virus proviral DNA vaccine and confers partial protection against vaginal challenge with SIVmac251. Virology. 2009;386:109–121. doi: 10.1016/j.virol.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]