The presence of stable multiprotein complexes containing adrenergic receptors is verified in live H9c2 cardiomyocyte-like cells by single-particle tracking. The immobilization of β-adrenergic receptors presumably contributes to the specificity of cardiac adrenergic responses.
Abstract
The sympathetic nervous system regulates cardiac output by activating adrenergic receptors (ARs) in cardiac myocytes. The predominant cardiac ARs, β1- and β2AR, are structurally similar but mediate distinct signaling responses. Scaffold protein–mediated compartmentalization of ARs into discrete, multiprotein complexes has been proposed to dictate differential signaling responses. To test the hypothesis that βARs integrate into complexes in live cells, we measured receptor diffusion and interactions by single-particle tracking. Unstimulated β1- and β2AR were highly confined in the membrane of H9c2 cardiomyocyte-like cells, indicating that receptors are tethered and presumably integrated into protein complexes. Selective disruption of interactions with postsynaptic density protein 95/disks large/zonula occludens-1 (PDZ)–domain proteins and A-kinase anchoring proteins (AKAPs) increased receptor diffusion, indicating that these scaffold proteins participate in receptor confinement. In contrast, modulation of interactions between the putative scaffold caveolae and β2AR did not alter receptor dynamics, suggesting that these membrane domains are not involved in β2AR confinement. For both β1- and β2AR, the receptor carboxy-terminus was uniquely responsible for scaffold interactions. Our data formally demonstrate that distinct and stable protein complexes containing β1- or β2AR are formed in the plasma membrane of cardiomyocyte-like cells and that selective PDZ and AKAP interactions are responsible for the integration of receptors into complexes.
INTRODUCTION
G-protein–coupled receptors (GPCRs) constitute the largest family of membrane-bound receptors, initiate diverse signal processes, and are the targets for many drugs (Pierce et al., 2002; Lagerström and Schiöth, 2009). In the heart, GPCRs in the adrenergic receptor (AR) family bind catecholamines to convert sympathetic nervous system stimulation into cardiovascular responses. Cardiac myocytes express nine ARs; however, the β1- and β2AR subtypes have been demonstrated to largely regulate contractile function (Xiang and Kobilka, 2003; Xiao et al., 2006). Although structurally very similar, β1- and β2AR initiate different signaling cascades and undergo different trafficking processes in cardiac myocytes (Rockman et al., 2002; Xiang and Kobilka, 2003; Xiao et al., 2006). For instance, β1AR stimulation is manifest by an increase in contraction rate, whereas β2AR activation produces a biphasic response, with an initial increase followed by a sustained decrease in contraction rate (Xiang and Kobilka, 2003; Xiao et al., 2006). Activated β1AR is retained at the cell surface, whereas activated β2AR is internalized to mediate desensitization (Xiang et al., 2002a; Xiang and Kobilka, 2003). Furthermore, chronic stimulation of β1AR is proapoptotic, whereas persistent β2AR activation is cardioprotective (Zheng et al., 2005; Xiao et al., 2006).
Compartmentalization of βARs in plasma membrane microdomains containing specific proteins with scaffolding and catalytic activities has been proposed to explain subtype-specific cardiac adrenergic signaling (Hall and Lefkowitz, 2002; Xiang and Kobilka, 2003; Malbon et al., 2004; Steinberg, 2004; Xiao et al., 2006). Scaffold proteins associate with two or more proteins to increase the efficiency or specificity of a signaling pathway and usually contain at least one protein–protein interaction domain (Wong and Scott, 2004; Zeke et al., 2009). In various cell types, association between β2AR and scaffolds, including caveolin-3 (CAV3), A-kinase anchoring protein 5 (AKAP5; alternative names human AKAP79 and murine AKAP150), AKAP12 (human gravin/AKAP250 or murine SSeCKS), and postsynaptic density protein 95/disks large/zonula occludens-1 (PDZ)–domain proteins, including ezrin-binding phosphoprotein of 50 kDa (EBP50; alternative names NHERF1 or Slc9a3r1), have been demonstrated by biochemical and functional assays (Hall et al., 1998a, 1998b; Fraser et al., 2000; Rybin et al., 2000; Fan et al., 2001; Xiang et al., 2002b; Tao et al., 2003; Balijepalli et al., 2006). Similarly, functional and biochemical assays have demonstrated association between β1AR and AKAP5 and PDZ domain proteins, including synapse-associated protein of 97 kDa (SAP97; alternative name Dlg1) and postsynaptic density protein 95 (PSD-95) (Gardner et al., 2006; He et al., 2006). In addition, interaction with the cytoskeleton may influence receptor compartmentalization, as many putative receptor scaffolds, including PDZ-domain proteins, AKAPs, and caveolae, interact directly or indirectly with the actin cytoskeleton (Wu et al., 1998; Bretscher et al., 2000; Gomez et al., 2002; Pelkmans and Zerial, 2005).
Understanding the physical nature of adrenergic receptor complexes has important implications on a basic level and for the design of therapeutic approaches for cardiomyopathies (Negro et al., 2008). Although a large body of data supports the concept of compartmentalized signaling in cardiac myocytes, no information is available to describe receptor dynamics in the plasma membrane of cardiac myocytes or the contribution of potential scaffolds to complex integrity and stability. In addition, studies that support the concept that receptors are compartmentalized are not without caveats. For instance, biochemical approaches that demonstrate protein interactions generally provide no information regarding the degree or dynamics of protein association and are typically performed in dilute, nonphysiological solutions. Similarly, studies that implicate caveolae in receptor scaffolding often use pharmacological maneuvers that alter many cellular processes (Hancock, 2006). In this study, single-particle tracking (SPT) was applied to test the hypothesis that βARs integrate into stable, discrete protein complexes in live cardiomyocyte-like H9c2 cells. The involvement of PDZ-domain proteins, AKAPs, caveolae, and actin in receptor dynamics was systematically investigated using maneuvers that modulated interactions between these scaffolds and receptors.
RESULTS
β1AR and β2AR are confined in the plasma membrane of H9c2 cells
The diffusion and interactions of unstimulated β1- and β2ARs were studied in H9c2 cardiomyocyte-like cells by SPT of engineered receptors containing amino-terminal hemagglutinin (HA) tags. These receptor analogues recapitulate the signaling and trafficking properties of untagged receptors in a variety of cell systems, including cardiac myocytes (Cao et al., 1999; Luttrell et al., 1999; Xiang et al., 2002a; Shcherbakova et al., 2007). H9c2 cells express endogenous β1- and β2AR, and transfection conditions were initially established such that overexpression of epitope-tagged receptors did not result in substantially increased levels of either receptor. In HA-β1AR–transfected cells, the total levels of β1AR (β1AR plus HA-β1AR) increased only ∼1.5-fold, and similar increases in β2AR levels were observed in HA-β2AR–transfected cells (levels were characterized by quantitative immunohistochemistry, as described in Materials and Methods). Using these transfection conditions, HA-tagged adrenergic receptors expressed in H9c2 cells demonstrate similar properties as in neonatal cardiac myocytes; for instance, HA-β1AR was retained at the cell surface upon isoproterenol stimulation, whereas HA-β2AR was internalized (Supplemental Figure S1; Xiang et al., 2002a). For SPT, epitope-tagged receptors were labeled by successive incubation of transfected cells with anti-HA antibody, biotin-conjugated Fab fragments, and streptavidin conjugated, 655 nm emitting quantum dots (Qdots). Qdot attachment was selective such that individual fluorophores with intermittent emissions could be observed on the surface of HA-β1– or HA-β2AR–expressing cells, whereas neighboring, untransfected cells showed near-zero nonspecific labeling (Supplemental Figure S2).
To characterize the diffusive properties of Qdot-labeled receptors, image sequences were acquired at 30 frames per second (fps) over 20 s. Figure 1A shows frames from representative image sequences for Qdot-labeled HA-β1AR (top) and HA-β2AR (bottom) (see Supplemental Movies S1 and S2; note that intermittent fluorescent emissions confirm that individual Qdots were imaged). Visual inspection of image sequences indicated that both receptors were confined over the data acquisition period. Time-lapse imaging acquired at 1 fps qualitatively confirmed that receptor confinement persisted over a time scale of minutes (Figure 1B). To quantify receptor diffusion, individual trajectories were reconstructed from SPT image sequences. As expected, trajectories for HA-β1AR (Figure 1C, left) and HA-β2AR (Figure 1C, right) were highly confined, typically being restricted to membrane domains of approximately 200 nm diameter. Trajectories derived from Qdot-labeled receptors in live cells were more mobile than those derived from fixed cells or from Qdots immobilized on a glass surface (Figure 1C, bottom) indicating that trajectories in live cells, albeit highly confined, report actual receptor diffusion. Individual trajectories were analyzed to generate mean-squared displacement (MSD) versus time plots, and values of short-range diffusion coefficient (D) and range (MSD1/2, a representation of how far receptors move in 2 s) determined (Haggie et al., 2006; Crane and Verkman, 2008). Figure 1D summarizes values of D (left) and range (right) for all trajectories derived from HA-β1AR–expressing (blue) or HA-β2AR–expressing (red) H9c2 cells as cumulative distribution functions (the probability distribution of the variables D and range for complete data sets). The continuous nature of cumulative distributions for both receptor populations indicates that similar diffusive behavior presumably describes the motion of HA-β1AR and of HA-β2AR. The median values of D and range for both receptors were ∼0.001 μm2/s and ∼0.03 μm, respectively, similar to values obtained for other proteins that demonstrate confined diffusion, including the cystic fibrosis transmembrane conductance regulator (CFTR; Haggie et al., 2006) and orthogonal array–associated aquaporin-4 (Crane et al., 2008). To verify that the Qdot-labeling strategy was not responsible for receptor confinement, experiments were performed in which receptors were labeled with monomeric anti-HA Fab fragments and DyLight 649–conjugated secondary Fab fragments. On the basis of continuous image acquisition (10 fps, 100 ms exposure), HA-β1AR (Figure 1E) and HA-β2AR (Supplemental Figure S3) were qualitatively confined. Finally, experiments performed at a higher frame rate (60 fps) established that 30 fps data sampling adequately describes receptor diffusion (unpublished data).
Figure 1:
Single-particle tracking of fluorescently labeled adrenergic receptors in H9c2 cardiomyocyte-like cells. (A) Individual frames from SPT image sequences acquired continuously at 30 fps for Qdot-labeled HA-β1AR (top) and HA-β2AR (bottom). Scale bar, 2 μm. (B) Individual frames from time-lapse image sequences acquired at 1 fps for Qdot-labeled HA-β1AR (top) and HA-β2AR (bottom). Scale bar, 5 μm. (C) Representative trajectories of HA-β1AR (left) and HA-β2AR (right) diffusion in the plasma membrane derived from SPT experiments. Trajectories from paraformaldehyde fixed cells and immobilized Qdots are also shown (bottom). Scale bar (200 nm) applies to all trajectories. (D) Cumulative distributions of diffusion coefficient (D, left) and range at 2 s (right) for HA-β1AR (blue) and HA-β2AR (red) expressed in H9c2 cells and HA-β1AR (black) and HA-β2AR (gray) expressed in A549 alveolar epithelial cells. (E) Individual frames from image sequences of HA-β1AR labeled with anti-HA Fab fragment and DyLight 649–conjugated secondary Fab fragment acquired continuously at 10 fps with 100 ms exposures. Labeled receptors are highlighted with colored circles; photobleaching during image acquisition reduced fluorescence associated with the green- and orange-highlighted receptors. Scale bar, 2 μm.
HA-β1AR and HA-β2AR were expressed in alternative cell types to determine whether confinement of these receptors in H9c2 cells was a common finding. When expressed in A549 alveolar epithelial cells at levels similar to those studied in H9c2 cells, β1AR (Figure 1D, black; see Supplemental Movie S3) and β2AR (Figure 1D, gray; see Supplemental Movie S4) showed relatively unhindered diffusion. Similar receptor mobility was observed in COS7 kidney fibroblasts (unpublished data). The median values of D and range for βAR diffusion in A549 and COS7 cells were ∼0.02 μm2/s and ∼0.3 μm, respectively (p < 0.005 for D and range for both HA-βARs in H9c2 cells vs. when expressed in A549 or COS7 cells), similar to prior measurements of β2AR diffusion in A549 cells (Hegener et al., 2004) and to freely diffusing transmembrane proteins such as aquaporin-1 (Crane and Verkman, 2008).
PDZ domain interactions contribute to the confinement of β1AR and β2AR
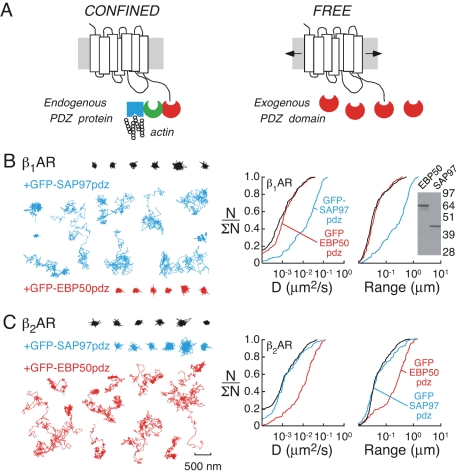
The confined diffusion of HA-β1AR and HA-β2AR is consistent with the concept that receptors stably associate with relatively static protein complexes in the plasma membrane. As such, a series of experimental maneuvers were performed to investigate the roles of putative scaffold proteins in receptor tethering. The carboxy-termini of β1AR (residues Glu-Ser-Lys-Val) and β2AR (residues Asp-Ser-Leu-Leu) constitute class I PDZ-binding motifs (with the consensus X-[Ser/Thr]-X-[Val/Ile/Leu], where X is any amino acid) that selectively interact with PDZ-domain proteins. Scaffold proteins with PDZ domains are modular and typically contain domains that mediate protein complexation and/or cytoskeletal interaction (Hall and Lefkowitz, 2002; Feng and Zhang, 2009). If PDZ interactions are involved in βAR confinement, we reasoned that overexpressed PDZ domains (lacking protein/cytoskeleton–interacting domains) that selectively bind to β1- or β2AR would compete with endogenous PDZ-domain proteins to release receptors from complexes and reduce receptor confinement (see Figure 2A for schematic representation of this strategy). A similar experimental strategy was previously used to investigate the role of EBP50 in the confinement of CFTR (Haggie et al., 2006).
Figure 2:
Role of PDZ-interactions in βAR confinement in H9c2 cells. (A) Schematic representation of the experimental strategy used to investigate the role of PDZ interactions in βAR confinement. Left, the carboxy-terminus of βAR interacts with a (hypothetical) endogenous scaffold protein that contains an interacting PDZ domain (red), an additional protein–protein interaction domain (green), and an actin-interacting domain (blue), which governs receptor confinement. Right, isolated PDZ domains (red) compete for the βAR carboxy-terminus and reduce receptor confinement (increased diffusion) if PDZ-type interactions are involved in receptor confinement. (B, C) Single-particle trajectories (left) and cumulative distributions (right) for D and range for (B) HA-β1AR under control conditions (black) and in the presence of an interacting PDZ domain (SAP97pdz, blue) and a noninteracting PDZ domain (EBP50pdz, red) and (C) HA-β2AR under control conditions (black) and in the presence of a noninteracting PDZ domain (SAP97pdz, blue) and an interacting PDZ domain (EBP50pdz, red). Scale bar (500 nm) applies to all trajectories. Integrity of GFP-EBP50pdz and GFP-SAP97pdz was confirmed by anti-GFP immunoblot of transfected H9c2 cell lysates (B, inset).
To probe β1AR PDZ interactions, the third PDZ domain of SAP97, which interacts with the carboxy-terminus of β1AR but not β2AR (He et al., 2006), was expressed as a green fluorescent protein (GFP) chimera (GFP-SAP97pdz) together with HA-tagged receptors. GFP-SAP97pdz distributed through the cytoplasm, as would be predicted for a soluble protein. Coexpression of GFP-SAP97pdz with HA-β1AR reduced receptor confinement such that many trajectories covered micron distances (Figure 2B, left, blue trajectories; see Supplemental Movie S5) and cumulative distributions represented higher values of D and range (Figure 2B, right, blue curves, median values of D and range were 0.018 μm2/s and 0.34 μm, respectively, p < 0.005, relative to control data). In a similar manner, the PDZ domains of EBP50 fused to GFP (GFP-EBP50pdz; Haggie et al., 2006), which interacts with β2AR but not β1AR (Hall et al., 1998a; He et al., 2006), was expressed to investigate β2AR-specific PDZ interactions. Expression of GFP-EBP50pdz, which also distributed throughout the cytoplasm, together with HA-β2AR decreased receptor confinement (Figure 2C, red trajectories and curves; median values of D and range were 0.013 μm2/s and 0.25 μm, respectively, p < 0.005; see Supplemental Movie S6). In contrast, coexpression of GFP-EBP50pdz with HA-β1AR (Figure 2B, red trajectories and curves) or GFP-SAP97pdz with HA-β2AR (Figure 2C, blue trajectories and curves) produced no apparent change in the diffusive properties of either receptor, indicating that PDZ-domain interactions are specific.
To confirm this observation, the carboxy-terminal amino acids of HA-β1AR and HA-β2AR were mutated to alanines to obviate association between βARs and interacting scaffold proteins that contain PDZ domains (Hall et al., 1998a; He et al., 2006). The diffusion of both HA-β1AR-V477L and HA-β2AR-L413A was increased relative to that of the wild-type receptors (Supplemental Figure S4; p < 0.005 for both receptor populations). Taken together, these experiments indicate that selective PDZ–βAR interactions contribute to the tethering of βAR receptors in live H9c2 cells.
AKAP interactions contribute to the confinement β1AR and β2AR
Cardiac myocytes express numerous AKAPs (Ruehr et al., 2004); however, a variety of experimental approaches have suggested that AKAP5 and AKAP12 interact with βARs (Nauert et al., 1997; Fraser et al., 2000; Fan et al., 2001; Tao et al., 2003; Gardner et al., 2006; Shcherbakova et al., 2007). Sequences within AKAP5 and AKAP12 have been identified that are responsible for receptor interaction (Fraser et al., 2000; Tao et al., 2003). Therefore, as for PDZ-type interactions, we reasoned that overexpressed receptor-interacting AKAP domains would disrupt receptor–AKAP association and release receptors from complexes if AKAPs participate in βAR confinement.
The amino-terminus of human AKAP5 (residues 1–107) interacts with β2AR and, in addition, contains basic amino acid–rich regions that mediate plasma membrane association (Dell'Acqua et al., 1998; Fraser et al., 2000). Confocal imaging indicated that a chimera of the amino-terminus of murine AKAP5 (residues 1–105) and GFP (AKAP5at-GFP) also predominantly localized to the plasma membrane of H9c2 cells (unpublished data), as expected, given the high degree of similarity between the human and murine protein sequences. The amino-terminus of AKAP12 (residues 1–920, AKAP12at) also contains residues that mediate interaction with β2AR and membrane localization (Tao et al., 2003; Yan et al., 2009) and a GFP chimera of AKAP12at (AKAP12at-GFP) localized to the plasma membrane of H9c2 cells (unpublished data). Given the membrane localization of AKAP5at-GFP and AKAP12at-GFP (as opposed to the cytoplasmic localization of PDZ-domain GFP fusion proteins), the mobility of these chimeras was investigated by photobleaching. Fluorescence recovery after bleaching was imaged by laser-scanning confocal microscopy at the plane between cells and coverslips over a large area (∼10 μm diameter) of the plasma membrane. Fluorescence recovery for AKAP5at-GFP (Figure 3A, top) and AKAP12at-GFP (Figure 3A, bottom) was observed with a recovery half-time of ∼10–20 s. For both AKAP5at-GFP and AKAP12at-GFP, fluorescence recovery was ∼75% complete within 2 min (Figure 3B). Therefore, as studied, the plasma membrane–associated AKAP domains cover micron distance scales within seconds, indicating that their diffusion is unhindered relative to that of βARs.
Figure 3:
Role of AKAP interactions in βAR confinement in H9c2 cells. (A) Diffusion of AKAP amino-terminal domains measured by photobleaching with laser scanning confocal image detection of GFP chimeras. Representative image sequences are shown for AKAP5at-GFP (top) and AKAP12at-GFP (bottom); bleached regions are highlighted by red circles. Scale bar, 10 μm. (B) Fluorescence recovery of AKAP amino-terminal domain–GFP chimeras at 2 min. (C, D) Single-particle trajectories (left) and cumulative distributions (right) for D and range are shown for (C) HA-β1AR in control conditions (black) and in the presence of AKAP5at-GFP (blue) and AKAP12at-GFP (red) and (D) HA-β2AR under control conditions (black) and in the presence of AKAP5at-GFP (blue) and AKAP12at-GFP (red). Scale bar (500 nm) applies to all trajectories.
Expression of AKAP5at-GFP together with HA-β1AR increased receptor mobility such that many trajectories covered micron distance scales (Figure 3C, left, blue trajectories) and cumulative distributions represented higher values of D and range (Figure 3C, right, blue curves; median values of D and range were 0.014 μm2/s and 0.14 μm, respectively, p < 0.01). Similarly, expression of AKAP12at-GFP with HA-β2AR decreased receptor confinement (Figure 3D, red trajectories and curves; median values of D and range were 0.013 μm2/s and 0.21 μm, respectively, p < 0.01). In contrast, coexpression of AKAP12at-GFP with HA-β1AR (Figure 3C, red trajectories and curves) or AKAP5at-GFP with HA-β2AR (Figure 3D, blue trajectories and curves) did not change the diffusive properties of either receptor, suggesting that AKAP domain interactions were selective. A soluble β2AR-interacting domain from AKAP12 (residues 554–938; Tao et al., 2003) fused to GFP also increased β2AR but not β1AR diffusion (unpublished data). As such, selective AKAP–βAR interactions contribute to the tethering of βAR receptors in live H9c2 cells.
Caveolae do not participate in the confinement of β2AR
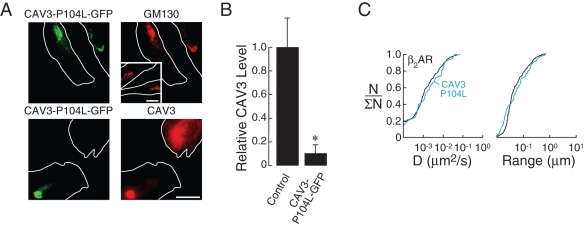
In cardiac myocytes, caveolae have been reported to compartmentalize signal transduction pathways mediated by β2AR but not β1AR (Rybin et al., 2000; Steinberg, 2004). Myocyte caveolae are composed of the structural protein CAV3, which forms high-order oligomeric structures (Steinberg, 2004; Parton and Simons, 2007). In contrast to PDZ-domain and AKAP proteins, specific CAV3 sequences (or specific intermediary proteins) that interact with β2AR have not been identified. Maneuvers were designed to modulate the surface levels of CAV3, and the effects of these perturbations on β2AR dynamics were characterized by SPT.
Although various small inhibitory RNA approaches were attempted, expression of CAV3 in H9c2 cells could not be reduced reliably. However, expression of CAV3-P104L, a dominant-negative CAV3 mutant that is retained in the Golgi and causes limb girdle muscular dystrophy (Galbiati et al., 1999), dramatically reduced surface expression of CAV3 (unpublished data). In H9c2 cells, a chimera of CAV3-P104L and GFP (CAV3-P104L-GFP) was also observed in perinuclear regions that colocalized with the Golgi marker GM130 (Figure 4A, top) and retained essentially all endogenous CAV3 in the Golgi (Figure 4A, bottom; see Supplemental Figure S5B for additional images). Quantitative image analysis of CAV3-P104L-GFP–expressing cells immunostained for CAV3 indicated that ∼90% of CAV3 was retained in the Golgi (Figure 4B), a value that is comparable to many knockdown approaches. Expression of CAV3-P104L-GFP did not result in Golgi retention of HA-β2AR (Supplemental Figure S5C). In contrast, expression of CAV3-P104L-mCherry resulted in Golgi retention of dysferlin (Supplemental Figure S5D), a protein previously shown to interact with CAV3 (Hernández-Deviez et al., 2006).
Figure 4:
Reduced levels of caveolin-3 at the plasma membrane do not alter β2AR confinement. (A) Fluorescence micrographs of H9c2 cells expressing CAV3-P104L-GFP (left) immunostained for GM130 (right, top) or CAV3 (which identifies endogenous CAV3 and CAV3-P104L-GFP; right, bottom). Inset (right, top), shows untransfected cells immunostained for GM130, indicating that CAV3-P104L-GFP expression did not alter Golgi morphology. Green fluorescence represents GFP fluorescence from the CAV3-P104L-GFP moieties. Cell outlines (white) were traced from transmission images. Scale bar, 10 μm. (B) Quantitative image analysis of CAV3 levels (assessed by immunocytochemistry) in non-Golgi regions of control cells and cells expressing CAV3-P104L-GFP; *p < 0.001. (C) Cumulative distributions of D (left) and range (right) for β2AR under control conditions (black) and in the presence of CAV3-P104L-GFP (blue).
Single-particle tracking experiments to measure HA-β2AR mobility in cells expressing CAV3-P104L-GFP reveal that receptor diffusion was not apparently altered by dramatic removal of surface caveolae (Figure 4C, blue). As such, surface levels of CAV3 can be significantly reduced without altering the confinement of β2AR.
A prerequisite for caveolar-mediated β2AR confinement is that caveolae would also be largely immobile. A prior study in HeLa cells, which express caveolin-1 (CAV1), indicated that CAV1-GFP–labeled caveolae are relatively static and that increased CAV1-GFP expression levels could increase caveolar mobility (Pelkmans and Zerial, 2005). Therefore, the plasma membrane dynamics of caveolae and the effect of CAV3 expression levels on caveolar dynamics were characterized in H9c2 cells. To visualize caveolae in live cells, a CAV3-GFP fusion was generated, similar to an extensively studied and functional CAV1-GFP fusion (Pelkmans and Zerial, 2005).
Initially, studies were conducted in which cells were transfected to express low levels of CAV3-GFP. Transfected cells were visualized using total internal reflection fluorescence (TIRF) imaging that selectively illuminates the plasma membrane and cortical cytoplasm (imaging depth is ∼150–200 nm). Caveolae labeled with CAV3-GFP could be visualized as discrete, diffraction-limited fluorescent punctae (Figure 5A), consistent with a reported size of ∼100–200 nm (Parton and Simons, 2007). The appearance of CAV3-GFP–labeled caveolae was similar to that of endogenous immunostained CAV3 in H9c2 cells visualized by TIRF, suggesting that the GFP moiety did not interfere with CAV3-GFP localization (unpublished data). To verify that CAV3-GFP was associated with cholesterol-rich membrane domains by standard biochemical criteria, Triton X-100–soluble (S) and Triton X-100–insoluble (P) cellular fractions were prepared (Song et al., 1997; Galbiati et al., 1999). SDS–PAGE–resolved extracts from untransfected cells immunoblotted for CAV3 (Figure 5C, lanes 1 and 2) verified that CAV3 (∼17 kDa) was insoluble in Triton X-100, as previously described (Galbiati et al., 1999). In cells expressing low levels of CAV3-GFP, both CAV3 and CAV3-GFP (∼44 kDa) were also largely isolated in Triton X-100–insoluble fractions (Figure 5C, lanes 3 and 4), indicating that CAV3 and CAV3-GFP were present in the same membrane environment. TIRF image sequences acquired at 10 fps demonstrated that the majority of labeled caveolae (∼80%) were essentially static in the plasma membrane over time scales of tens of seconds (Figure 5A and Supplemental Movie S7). A small number of caveolae (∼20%), however, associated with (Figure 5B, top) or dissociated from (Figure 5B, bottom) the plasma membrane (see also Supplemental Movies 8 and 9). As such, the diffusive behavior of caveolae labeled with CAV3-GFP expressed at low levels in H9c2 cells was similar to that reported for CAV1-GFP–labeled caveolae in HeLa cells (Pelkmans and Zerial, 2005) and consistent with a possible role in β2AR scaffolding.
Figure 5:
Caveolar dynamics and the role of caveolae in β2AR confinement in H9c2 cells. Individual frames from TIRF image sequences are shown for cells expressing low levels of CAV3-GFP to illustrate (A) caveolar dynamics and (B) caveolae association (top) and dissociation (bottom) events. In A, stable caveolae are highlighted with circles, and in B, the positions of membrane association (top) and dissociation (bottom) events are highlighted. (C) Biochemical analysis of caveolin-3 solubility. Triton X-100–soluble (S) and Triton X-100–insoluble (P) cellular fractions were prepared, resolved by SDS–PAGE, and immunoblotted with anti-CAV3 antibodies (top) and anti-GFP antibodies (bottom). With anti-CAV3 antibodies, both CAV3 (∼17 kDa) and CAV3-GFP (∼44 kDa) were identified, whereas with anti-GFP antibodies only CAV3-GFP was identified. (D) Individual frames from TIRF image sequences are shown for a cell expressing high levels of CAV3-GFP. Contrast enhancement was applied to highlight membrane-associated caveolae, although membrane fluorescence was increased relative to cells expressing low levels of CAV3-GFP. (E) Individual frames from photobleaching experiments for cells expressing high levels of CAV3-GFP. Images were collected using confocal image detection from plasma membrane proximal to the coverslip. The bleached region is highlighted by a red circle. (F) Cumulative distributions of D (left) and range (right) for β2AR under control conditions (black) and in the presence of high levels of CAV3-GFP (red). Scale bars, 1 μm in A, B, and D and 15 μm in E.
When H9c2 cells were transfected to express high levels of CAV3-GFP, the behavior of caveolae was very different. CAV3-GFP–labeled caveolae were still visible by TIRF imaging as bright fluorescent punctae (Figure 5D); however, significant fluorescence was also observed diffusely throughout the plasma membrane. CAV3-GFP was also detected in both Triton X-100 –soluble and Triton X-100–insoluble fractions (Figure 5C, lanes 5 and 6), confirming that excess CAV3-GFP was incompletely incorporated into cholesterol-rich membrane domains. An approximately threefold increase in Triton X-100–soluble endogenous CAV3 was observed in cells with high CAV3-GFP levels, indicating that endogenous CAV3 was also mistargeted. An anti-GFP immunoblot confirmed the presence of CAV3-GFP in Triton X-100–soluble fractions and the absence of soluble GFP moieties that could result from proteolytic cleavage of CAV3-GFP (Figure 5C, bottom). With the use of TIRF imaging, rapid exchange of caveolae between intracellular sites and the plasma membrane and movement of caveolae in the plane of the membrane were observed (Figure 5D and Supplemental Movie S10). In addition, photobleaching experiments using laser-scanning confocal microscopy were performed in cells expressing high levels of CAV3-GFP (Figure 5E). Bleaching was applied over a large area (∼15 μm diameter) of the plasma membrane, and relatively rapid (t1/2 ∼20 s) and complete (∼90% at 1 min) recovery of CAV3-GFP fluorescence was observed in all cells studied. Given that multiple processes (including lateral diffusion of caveolar and noncaveolar CAV3-GFP and trafficking events) contribute to the recovery of fluorescence, no formal characterization of CAV3-GFP diffusion was attempted. However, the general conclusion of these experiments is that caveolar and noncaveolar CAV3-GFP are mobile over time scales of seconds and distance scales of microns when expressed at high levels and unhindered relative to the diffusion of β2AR.
Given the dependence of caveolar dynamics and membrane targeting on CAV3-GFP expression level, we reasoned that high levels of CAV3-GFP could also be used to assess the role of caveolae in β2AR confinement by reducing the number of stable caveolae in the plasma membrane (and/or by competing for as-yet-unidentified proteins involved in caveolae–β2AR interactions). The mobility of HA-β2AR was not, however, altered by high-level expression of CAV3-GFP (Figure 5F, red). Taken together, these studies suggest that stable plasma membrane caveolae are not required for β2AR confinement.
The actin cytoskeleton contributes to β1AR and β2AR confinement, and the binding capacity of β1AR and β2AR scaffold proteins can be saturated
The studies so far indicate that selective PDZ and AKAP interactions are involved in the confinement of βARs, and further experiments were performed to characterize these interactions. Several PDZ-domain proteins and AKAPs that associate with βARs also interact with the actin cytoskeleton, including SAP97, EBP50 (via ezrin), AKAP5, and AKAP12 (Wu et al., 1998; Bretscher et al., 2000; Gomez et al., 2002; Malbon et al., 2004). To assess the role of cytoskeletal interactions in receptor confinement, cells were treated with the actin-disrupting agent latrunculin using conditions that did not grossly alter cell morphology. The confinement of HA-β1AR (Figure 6A, red curves and trajectories; median values of D and range were 0.012 μm2/s and 0.20 μm, respectively, p < 0.01) and HA-β2AR (Figure 6B, red curves and trajectories; median value of D and range were 0.006 μm2/s and 0.12 μm, respectively, p < 0.05) was reduced by latrunculin treatment. Frick et al. (2007) demonstrated that latrunculin-mediated actin fragmentation does not alter the diffusive properties of proteins that do not interact with actin (thus confirming that this experimental maneuver specifically identifies proteins that interact with actin). We confirmed this finding in H9c2 cells by demonstrating that the diffusive properties of CD4-GFP, a chimeric transmembrane protein that contains no sites of actin interaction, were not altered by latrunculin treatment (Supplemental Figure S6). As such, our data indicates that the actin cytoskeleton participates in the tethering of βARs.
Figure 6:
Role of the actin cytoskeleton and receptor concentration in βAR confinement. Single-particle trajectories (left) and cumulative distributions (right) for D and range are shown for (A) HA-β1AR and (B) HA-β2AR in the presence of latrunculin (red trajectories and curves) and under conditions in which receptor is overexpressed relative to control conditions (blue trajectories and curves). Scale bar (500 nm) applies to all trajectories.
A prior study that investigated the plasma membrane dynamics of CFTR indicated that scaffold protein–binding capacity was saturable, such that overexpressed CFTR was not coupled to EBP50, ezrin, and the actin cytoskeleton (Haggie et al., 2006). To test whether the binding capacity of βAR-specific scaffold interactions could also be saturated, receptors levels were increased by altering transfection conditions (see Materials and Methods). In the presence of high levels of HA-tagged receptor (>25-fold overexpression of receptor), the diffusive mobility of both HA-β1AR (Figure 6A, blue curves and trajectories; median values of D and range were 0.012 μm2/s and 0.3 μm., respectively, p < 0.01) and HA-β2AR (Figure 6B, blue curves and trajectories; median value of D and range were 0.009 μm2/s and 0.18 μm, respectively, p < 0.05) was increased, indicating that a finite number of receptors can be appropriately coupled to scaffold proteins in H9c2 cells. To determine the relative capacity of receptor scaffold in H9c2 cells, increasing levels of epitope-tagged receptors were expressed and receptor mobility was determined (Supplemental Figure S7). For both HA-β1AR and HA-β2AR, cells were able to effectively tether receptors up to at least about threefold endogenous level.
The carboxy-terminal domains of β1AR and β2AR are responsible for confinement
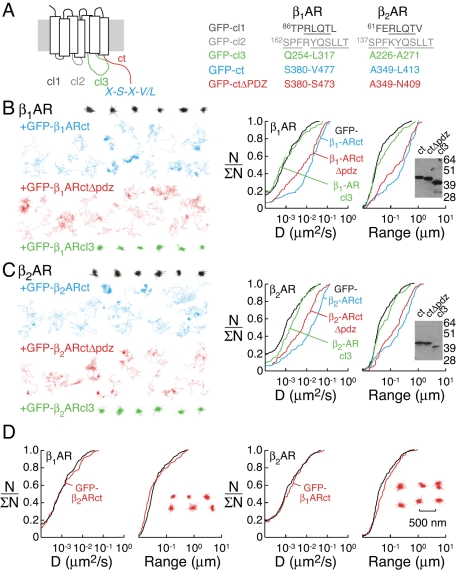
Finally, the domains within βARs that interact with scaffold proteins in live cells were identified. Prior biochemical analysis has indicated that the carboxy-terminal domain and the third intracellular loop of βARs associate with PDZ-domain proteins and AKAPs, as well as with other proteins, including potassium channels and endophilin (Tang et al., 1999; Fraser et al., 2000; Fan et al., 2001; Lui et al., 2004). Therefore, intracellular receptor domains were expressed as soluble GFP fusions to compete with receptors for association with cytoplasmic scaffold proteins (see Figure 7A for description of the receptor regions that were expressed as GFP chimeras). Isolated cytoplasmic regions of GPCRs have been used in a variety of experimental strategies, including biochemical approaches to identify βAR-binding partners (Fraser et al., 2000; Fan et al., 2001), in functional assays to inhibit desensitization (Krupnick et al., 1994; Mukherjee et al., 1999), and in whole-cell systems to disrupt signal transduction (Borroto-Escuela et al., 2010). As such, the strategy applied here to investigate βAR interactions is analogous to prior approaches, albeit with receptor diffusion used as a readout of interactions.
Figure 7:
Identification of βAR domains involved in receptor confinement. (A) Schematic representation of receptor domains (left) and the specific protein sequences (right) that were expressed as soluble GFP chimeras to investigate cytoplasmic receptor domains required for scaffold interactions. Representative single-particle trajectories (left) and cumulative distribution functions (right) for D and range are shown for (B) HA-β1AR under control conditions (black), coexpressed with a GFP chimera of the β1AR carboxy-terminus (GFP-β1ARct, blue), coexpressed with a GFP chimera of the β1AR carboxy-terminus without a PDZ-binding motif (GFP-β1ARctΔpdz, red), and coexpressed with a GFP chimera of the β1AR cytoplasmic loop 3 region (GFP-β1ARcl3, green) and (C) HA-β2AR under control conditions (black), coexpressed with a GFP chimera of the β2AR carboxy-terminus (GFP-β2ARct, blue), coexpressed with a GFP chimera of the β2AR carboxy-terminus without the PDZ-binding motif (GFP-β2ARctΔpdz, red), and coexpressed with a GFP chimera of the β2AR cytoplasmic loop 3 region (GFP-β2ARcl3, green). (D) Cumulative distribution functions and trajectories (insets) for HA-β1AR coexpressed with a GFP chimera of the β2AR carboxy-terminus (GFP-β2ARct; left, red) and HA-β2AR coexpressed with a GFP chimera of the β1AR carboxy-terminus (GFP-β1ARct; right, red). Scale bar (500 nm) applies to all trajectories. Integrity of soluble GFP-β1/β2AR domain chimeras was confirmed by anti-GFP immunoblot of transfected H9c2 cell lysates (B and C, insets).
We initially focused on the carboxy-terminal domains of βARs, which contain PDZ-binding domains and AKAP interaction sites (Hall et al., 1998a, 1998b; Fraser et al., 2000; Fan et al., 2001; He et al., 2006). As expected, coexpression of HA-β1AR with the soluble β1AR carboxy-terminus domain fused to GFP (GFP-β1ARct) resulted in reduced confinement of HA-β1AR (Figure 7B, blue trajectories and curves; median values of D and range were 0.018 μm2/s and 0.30 μm, respectively, p < 0.01), presumably in part due to competition for PDZ-binding partners. Expression of a soluble GFP chimera containing the C-terminus of β1AR with no PDZ-binding domain (GFP-β1ARctΔPDZ) also increased the diffusion of HA-β1AR (Figure 7B, red; median values of D and range were 0.009 μm2/s and 0.15 μm, respectively, p < 0.01), confirming that AKAP–β1AR interactions mediate receptor confinement. In a similar manner, coexpression of HA-β2AR with the β2AR carboxy-terminus (GFP-β2ARct; Figure 7C, blue; median values of D and range were 0.019 μm2/s and 0.16 μm, respectively, p < 0.01) and the β2AR carboxy-terminus with no PDZ-binding domain (GFP-β2ARctΔPDZ; Figure 7C, red; median values of D and range were 0.007 μm2/s and 0.16 μm, respectively, p < 0.01) resulted in reduced confinement of HA-β2AR, confirming that both PDZ and AKAP interactions participate in β2AR tethering. The cytoplasmic loop 3 region of β2AR has been shown to interact with AKAP5 in biochemical assays (Fraser et al., 2000). However, the confined diffusion of HA-β2AR was not altered by coexpression of the β2AR cytoplasmic loop 3 regions, suggesting that this interaction is not relevant for receptor tethering in live cells (GFP-β2ARcl3; Figure 7C, green). Similarly, coexpression of the β1AR cytoplasmic loop 3 regions (GFP-β1ARcl3) did not effect the confinement of HA-β1AR (Figure 7B, green). The cytoplasmic loop 1 and 2 regions of β1- and β2AR show high degrees of sequence similarity (Figure 7A), and neither region would thus be predicted to constitute a potential site of receptor-specific scaffold interactions. Therefore, the roles of these loops in receptor confinement were assessed only for β1AR; however, neither region altered receptor diffusion (unpublished data). Coexpression of GFP-β2ARct with HA-β1AR (Figure 7D, left, red) or GFP-β1ARct with HA-β2AR (Figure 7D, right, red) did not alter the diffusion of receptors, confirming the specificity of interactions in receptor confinement. Taken together, these studies indicate that specific interactions with the cytoplasmic-exposed, carboxy-terminal regions of βARs, but not other receptor regions, mediate receptor tethering in H9c2 cells.
DISCUSSION
The primary conclusion of this study is that unstimulated β1- and β2ARs are confined in the plasma membrane of live H9c2 cardiomyocyte-like cells expressing relevant concentration of proposed receptor complex constituents. Interactions with PDZ-domain–containing proteins, AKAPs, and the actin cytoskeleton were largely responsible for the immobilization of receptors. For instance, the expression of dominant-negative PDZ domains was able to disrupt receptor complexes, indicating that PDZ-type interactions tether receptors. PDZ interactions that mediated β1- and β2AR confinement were selective, as predicted from biochemical approaches (Hall et al., 1998a, 1998b; He et al., 2006). In a similar manner, overexpression of AKAP domains shown to interact with βARs was able to disrupt receptor complexes, suggesting that AKAPs participate in receptor complexation. In cardiac myocytes, AKAP interactions that confined β1 and β2ARs were selective, such that β1AR interacted with AKAP5, and β2AR associated with AKAP12. This finding contrasts with prior functional and biochemical studies that demonstrated AKAP5 association with β2AR, albeit in different cell types (Fraser et al., 2000). Treatment of cells with the actin-disrupting agent latrunculin also reduced βAR confinement, consistent with association of receptors with the actin cytoskeleton presumably via PDZ or AKAP interactions. The carboxy-terminal domain of receptors was shown to be largely responsible for receptor tethering, whereas other receptor domains were of much less or no importance in confining receptors. Prior biochemical studies indicated that the intracellular loop 3 region of β2AR interacts with AKAP5; however, in H9c2 cells no evidence was found to support such an interaction. Our study indicates that β1- and β2AR are compartmentalized in live H9c2 cells at the level of their interactions with PDZ-domain proteins, AKAPs, and the actin cytoskeleton.
Previous studies implicated caveolae in the compartmentalization of β2AR. In live H9c2 cells, caveolae were found to be largely immobile, consistent with a possible role in β2AR tethering. Reduction of surface caveolae (by ∼90%) by overexpression of a dominant-negative CAV3 mutant had no effect on β2AR dynamics. Furthermore, the mobility and cellular distribution of caveolin-3 were dramatically altered when a CAV3-GFP fusion was overexpressed; however, this perturbation did not alter β2AR confinement. Taken together, these findings suggest that in unstimulated H9c2 cells confinement of β2AR does not depend on caveolae. A number of caveats exist with the interpretation of prior studies that reported β2AR–caveolin association. Biochemical approaches generally provide no information regarding the degree or dynamics of protein association. Similarly, techniques such as isopycnic centrifugation offer limited resolution and in many instances actually demonstrate that both β1- and β2AR exist in overlapping membrane fractions with CAV3 (Rybin et al., 2000; Balijepalli et al., 2006). Functional assays in which caveolae are proposed to be disrupted selectively use compounds (e.g. filipin, cyclodextrin) that alter many cellular properties and processes and interrogate multiple steps in adrenergic signaling pathways (Hancock, 2006; Pontier et al., 2008). The diffusive properties of raft-associated and non–raft-associated membrane proteins are reduced by cyclodextrin treatment, further indicating that this treatment nonselectively alters plasma membrane properties (Kenworthy et al., 2004). Finally, in a study using superresolution imaging of βARs in H9c2 cells, the vast majority of β2ARs (∼85%) were not associated with caveolae, although these data were interpreted to support the hypothesis that β2AR–caveolae association promotes specificity in adrenergic signaling (Ianoul et al., 2005). The present study does not rule out the participation of caveolae in adrenergic signaling responses upon receptor stimulation.
Increased efficiency and specificity are proposed outcomes of compartmentalized signal transduction processes; however, only a limited number of studies have directly studied spatial organization within signaling pathways. Several studies have indicated that receptors and scaffolds demonstrate relatively unhindered diffusion, inconsistent with their participation in stable multiprotein complexes. Endogenous and transfected β2AR has been shown to be largely mobile in several cell types (Barak et al., 1997; Hegener et al., 2004; Yudowski et al., 2006), in addition to the COS7 and A549 cells used in this study. Other GPCRs (Sergé et al., 2002; Suzuki et al., 2005; Hern et al., 2010) and the epidermal growth factor (EGF) receptor (Lidke et al., 2005; Chung et al., 2010) also demonstrate relatively unhindered diffusion or short periods of immobilization (seconds) in the plasma membrane. Several neuronal receptors and PDZ scaffolds have been shown to be mobile over seconds to minutes (Choquet and Triller, 2003; Gray et al., 2006; Zheng et al., 2010). Protein kinase A (PKA) catalytic subunits in neurons are mobile over seconds despite AKAP-mediated confinement to dendritic shafts, suggesting low affinity of PKA–AKAP association in live cells (Zhong et al., 2009). Conversely, a number of studies support the concept of compartmentalized signal transduction, particularly in activated pathways. Imaging of cAMP and Ca2+ transients with fluorescence resonance energy transfer (FRET)–based probes has demonstrated that second messenger concentrations can increase in localized regions of cardiac myocytes and other cell types (Zaccolo et al., 2002). Live-cell imaging studies of immune cells have provided evidence that signaling molecules become clustered and immobilized upon cellular activation (Huppa and Davis, 2003; Douglass and Vale, 2005). Similarly, fluorescence imaging studies have demonstrated that activation of signaling pathways initiated by Ras, a lipidated G protein, and CD59, a glycosylphosphatidylinositol–anchored receptor, proceeds via transient immobilization (seconds) and nanoscale clustering of signaling molecules (Murakoshi et al., 2004; Kholodenko et al., 2010). Together, these studies provide evidence that compartmentalization of signaling is a physiologically relevant phenomenon, but that confinement of signaling components is not a default mechanism. These studies also suggest that dynamic analysis of receptors and scaffolds in live cells, in addition to biochemical and functional studies, may be necessary to formally conclude that confinement mechanisms occur within signaling pathways.
Spatial confinement of proteins can be mediated by several mechanisms. Interactions with actin-associated PDZ and/or AKAP scaffolds have been shown to confine the diffusion of CFTR (Haggie et al., 2006), in addition to the βARs studied here. The actin cytoskeleton has also been shown to participate in the confinement of caveolae and nanoscale protein clusters such as those observed during Ras activation (Murakoshi et al., 2004; Pelkmans and Zerial, 2005). The M23 form of the aquaporin-4 water channel forms largely immobile oligomeric complexes; however, confinement of these structures (over minutes) is not influenced by actin but does involve PDZ-type interactions (Crane et al., 2008). In activated T-cells, the confinement of protein clusters containing CD2 (a non–raft-associated coreceptor) is mediated by protein–protein association with scaffold proteins and does not involve actin (Douglass and Vale, 2005). Lipid microdomains have been proposed to mediate protein confinement; however, several biophysical approaches such as FRET and photobleaching have provided evidence against this hypothesis (Glebov and Nichols, 2004; Kenworthy et al., 2004; Douglass and Vale, 2005). In the case of β1- and β2AR, the present study suggests that receptor confinement is determined by the affinities, number, and association/dissociation rates of specific scaffold proteins. Overexpression of β1- and β2AR in H9c2 cardiomyocyte-like cells resulted in increased receptor diffusion, presumably due to saturation of the binding capacity of scaffold proteins. This observation suggests that receptor–scaffold interactions are unlikely to be fully recapitulated in cell systems in which receptors are highly overexpressed. When studied in A549 and COS7 cells, β1- and β2ARs demonstrated relatively unhindered mobility, most likely due to the absence or reduced concentration of scaffold proteins. For instance, COS7 and A549 cells express EBP50 and SAP97 but lack many AKAPs (Naren et al., 2003; Oliveria et al., 2003; Pieretti et al., 2003; Bossard et al., 2006).
In summary, our study provides direct evidence that unstimulated β1- and β2AR are immobilized in the plasma membrane of H9c2 cardiomyocyte-like cells. Complexation is mediated by carboxy-terminal PDZ domain and AKAP interactions and not by association with caveolae. Immobilization of βARs presumably contributes to the spatial segregation of signal transduction components and consequent specificity of cardiac adrenergic pathways.
MATERIALS AND METHODS
Plasmid constructs
cDNA constructs containing β1AR and β2AR with amino-terminal HA-epitope tags have been used extensively and previously described (Cao et al., 1999; Xiang et al., 2002a). Receptor mutants in which the C-terminal residue was mutated to an alanine residue were generated using standard PCR-based techniques, and the complete sequence of mutants was confirmed. The GFP-EBP50pdz construct has been previously described (Haggie et al., 2006) and consists of GFP ligated to the two PDZ domains of EBP50 (residues 1–325) but lacks the C-terminal ezrin-binding domain of EBP50 (residues 326–355). To generate GFP-SP97pdz, the third PDZ domain of SAP97 (residues 459–607, provided as a 6-his-tagged domain by Randy Hall, Emory University School of Medicine, Atlanta, GA; He et al., 2006) was PCR amplified and inserted in pEGFP-C1 (Clontech, Mountain View, CA). To generate AKAP5at-GFP, the N-terminus of AKAP5 (residues 1–105) was PCR amplified (using a plasmid containing full-length AKAP5-GFP as template, a generous gift from Brigitte Blackman, University of California, San Francisco, San Francisco, CA) and inserted in pEGFP-N1 (Clontech). The AKAP12at-GFP plasmid (AKAP12 residues 1–920 fused to the N-terminus of GFP) was a generous gift from Bryon Grove (University of North Dakota, Grand Forks, ND) and has been described (Yan et al., 2009). cDNA for murine CAV3 was purchased from the American Type Culture Collection (ATCC, Manassas, VA), PCR amplified, and ligated into pEGFP-N1 (Clontech) to generate CAV3-GFP. The CAV3-P104L and CAV3-P104L-GFP mutants of CAV3 were generated using the QuikChange II Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA). To generate soluble GFP chimeras of cytoplasmic-facing regions of β1AR and β2AR, appropriate receptor sequences were identified (based on crystallographic data and literature precedents), PCR amplified, and ligated into pEGFP-C1 (Clontech). The location of these regions, which represent the three intracellular loop regions, the full carboxy-terminus, and the carboxy-terminus lacking the PDZ-binding domain (four residues), within the βARs and the precise protein sequences that were considered are presented in Figure 7A. CD4-GFP was a generous gift from Gergely Lukacs (McGill University, Montreal, Canada) and contains the extracellular and transmembrane domains of CD4 ligated to GFP. A construct encoding GFP fused to the amino-terminus of dysferlin was a generous gift from Kate Bushby (Newcastle University, Newcastle upon Tyne, United Kingdom; Hernández-Deviez et al., 2006).
Cell culture, transfection, and treatments
Rat H9c2(2-1) cells, which show myoblast morphology and were originally isolated from embryonic heart myocardium, were purchased from the ATCC (ATCC number CRL-1446). This cell type shares several phenotypic properties with cardiac myocytes and was used in several prior studies that investigated adrenergic signaling complexes or processes in heart cells (for example, see Ianoul et al., 2005). Cells were cultured on 18-mm coverglasses in 12-well plates for experiments and were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with 1 μg of plasmid. Expression levels of HA-βARs and CAV3-GFP were controlled by using defined amounts of receptor (0.01–0.02 μg) or CAV3-GFP (0.05 μg) encoding plasmids together with pcDNA3.1 or plasmids encoding GFP chimeras (as described in Results) in a total of 1 μg of DNA. To overexpress HA-βARs or CAV3-GFP, cells were transfected with 50-fold more plasmid encoding HA-tagged receptor or 20-fold more plasmid encoding CAV3-GFP in a total of 1 μg of plasmid. To fragment the actin cytoskeleton, cells were treated with 0.5 μM latrunculin B for 10 min before and during tracking experiments. A549 human alveolar epithelial cells and COS7 monkey kidney fibroblast cells were acquired from the University of California, San Francisco, Cell Culture Facility and cultured using standard conditions.
Immunostaining and biochemistry
To establish levels of adrenergic receptor overexpression in H9c2 cells expressing either HA-β1AR or HA-β2ARs, a two-step immunostaining protocol was established. For cells expressing HA-β1AR, transfected cells were identified by immunostaining with anti-HA antibody (16B12; Covance, Berkeley, CA) and Alexa Fluor 555–conjugated secondary antibody and β1AR levels were assessed with anti-β1AR antibody (ab3546; Abcam, Cambridge, MA) and Alexa Fluor 488–labeled secondary antibody. For HA-β2AR–expressing cells, transfected cells were also identified with anti–HA antibody/Alexa Fluor 555–conjugated secondary antibody staining, and total β2AR was assessed with anti-β2AR antibody (ab13989; Abcam) and Alexa Fluor 488–labeled secondary antibody. Given that overall transfection efficiencies were relatively low in H9c2 cells, this protocol allowed comparison of adrenergic receptor levels in adjacent transfected (containing HA-β1 or 2AR and endogenous β1 or 2AR [Alexa Fluor 555 and Alexa Fluor 488 fluorescence]) and nontransfected (endogenous β1 or 2AR only [Alexa Fluor 488 fluorescence only]) cells. Standard image analysis methods were applied to quantify receptor levels in transfected and nontransfected cells by comparison of background-corrected Alexa Fluor 488 intensities, and data were derived from 30–40 cells. Images were acquired using a Nikon TE2000E microscope equipped with an EXFO X-Cite, Nikon 60× numerical aperture (NA)–1.45 TIRF objective lens and high-sensitivity QuantEM:512SC charge-coupled device (CCD) camera (Photometrics, Tucson, AZ). Given the low levels of HA-tagged receptors and high levels of cellular autofluorescence, the ∼50% increase (1.5-fold total receptor levels in transfected cell) represented a minimal level that could be accurately quantified.
Cells transiently transfected to express CAV3-P104L-GFP were fixed, permeabilized, and immunostained with anti-CAV3 antibody (ab30750; Abcam) and Alexa Fluor 555–conjugated secondary antibody to quantitatively determine the effects of CAV3-P104L-GFP expression on non-Golgi CAV3 levels. Analysis of images acquired by wide-field fluorescence microscopy was used to calculate background-corrected, area-integrated Alexa Fluor 555 intensities in non-Golgi regions (areas 6–10 μm away from the nucleus; see Supplemental Figure S5A) of cells that expressed and did not express CAV3-P104L-GFP. Given that wide-field fluorescence imaging was used and that CAV3-associated fluorescence could be detected from all cellular compartments (including the plasma membrane, trafficking vesicles, and compartments in which CAV3 is synthesized), the ∼90% reduction in CAV3 levels in non-Golgi regions of CAV3-P104L-GFP–expressing cells potentially represents an overestimate of the CAV3 levels present at the cell surface.
Triton X-100–soluble and Triton X-100–insoluble cell fractions were prepared as described (Song et al., 1997), and equal amounts of soluble and insoluble proteins were resolved on Bis-Tris PAGE gels (Invitrogen). To verify the integrity of GFP chimeras used in experiments, transfected cells were lysed using RIPA buffer and resolved on Bis-Tris PAGE gels. CAV3 was immunoblotted using antibody ab30750 (Abcam). GM130 was immunostained using antibody ab52649 (Abcam). GFP was immunoblotted with polyclonal antibody ab290 (Abcam).
Live-cell imaging experiments and analysis
For all live-cell imaging, cells were mounted in custom fabricated chambers, bathed in phosphate-buffered saline supplemented with 6 mM glucose and 1.1 mM pyruvate, and maintained at 37°C with a microincubator (Harvard Apparatus, Holliston, MA) and objective lens heater (Bioptechs, Butler, PA). SPT experiments with Qdot) labeling were performed using a Nikon TE2000U microscope equipped with an EXFO X-Cite light source, Nikon 100× NA-1.45 TIRF objective lens, and Hamamatsu (Hamamatsu City, Japan) C9100 EM-CCD camera. Data were typically acquired at 30 fps, and the lateral resolution of the system, defined as the standard deviation of centroid positions derived from immobilized Qdots, was ∼14 nm. HA-β1AR–, HA-β2AR–, HA-β1AR-V477A–, and HA-β2AR-L413A–expressing cells were labeled with anti-HA antibody (HA.11 Clone 16B12; Covance), biotin-conjugated goat anti–mouse Fab fragment (Jackson ImmunoResearch Laboratories, West Grove, PA), and streptavidin-conjugated Qdots emitting at 655 nm (Invitrogen). CD4-GFP–expressing cells were labeled with anti–human CD4 antibody (Invitrogen), biotin-conjugated goat anti–mouse Fab fragment (Jackson ImmunoResearch), and streptavidin-conjugated Qdots emitting at 655 nm (Invitrogen). Trajectories describing receptor motions were reconstructed from image sequences using IDL algorithms, and quantitative analysis of trajectories was performed using LabView algorithms (Haggie et al., 2006; Crane and Verkman, 2008). For all SPT experiments, data presented in this study were derived from two to six independently transfected sets of cells, and cumulative distributions are compiled from 62–315 individual trajectories generated from >10 cells (for HA-β1AR–expressing cells, the average number of trajectories per maneuver was 173, and for HA-β2AR–expressing cells, the average number of trajectories per maneuver was 132). In all instances, cumulative distribution plots represent complete data sets. Statistical analysis was performed using the nonparametric Kolmogorov–Smirnov test. For maneuvers that produced statistically significant data relative to control conditions, D and range were always both significant (and often identical); as such, p values of lowest significance are quoted for either D or range for all significant data sets. Time-lapse imaging was performed at 1 fps using 100 ms acquisitions and shuttered illumination, and experiments were performed on >12 cells. Imaging of receptor labeled with anti-HA Fab fragment (generated from HA.11 antibody using Fab Preparation Kit; Pierce, Thermo Fisher Scientific, Rockford, IL) and DyLight 649–conjugated secondary Fab (Jackson ImmunoResearch) was performed using a Nikon TE2000E microscope equipped with an EXFO X-Cite, Nikon 100× NA-1.49 TIRF objective lens, Cy5 filter set (Chroma Technology, Bellows Falls, VT), QuantEM:512SC CCD (Photometrics), and autofocus system. Data were acquired continuously at 10 fps (100 ms acquisitions) over 20 s from 7–10 cells. As was observed with Qdot-labeling strategies, nonspecific labeling of untransfected cells with anti-HA Fab fragments was near zero. Total internal reflection fluorescence was performed using a Nikon TE2000E microscope with a 488 nm argon-ion laser, 60× NA-1.49 TIRF objective lens, Z488/10X, Z488RDC, and ET525/50M filters (Chroma), QuantEM:512SC CCD, and autofocus system. The point spread function of this microscope system was determined using 40 nm FluoSpheres emitting at 515 nm (Invitrogen). Data for caveolae dynamics were acquired from 10–12 cells. Laser scanning confocal microscopy was performed using a Nikon FN-S2N microscope equipped with a Nikon D-Eclipse CI confocal module, 488 nm argon-ion laser, and Nikon 100× NA-1.49 TIRF objective lens. Data for AKAP diffusion were derived from 8–10 individual cells.
Supplementary Material
Acknowledgments
We thank Randy Hall, Kate Bushby, Bryon Grove, Brigette Blackman, Gergely Lukacs, and Mark von Zastrow for supplying plasmid constructs. We thank A. S. Verkman, Florian Baumgart, Jonathan Crane, and Tobias Moritz for their comments and helpful discussions regarding the manuscript. This study was supported by National Institutes of Health Grant DK 081355 and a Beginning Grant-in-Aid from the American Heart Association (0765070Y).
Abbreviations used:
- AKAP
A-kinase anchoring protein
- AR
adrenergic receptor
- CFTR
cystic fibrosis transmembrane conductance regulator
- PDZ
postsynaptic density protein 95/disks large/zonula occludens-1
- Qdots
quantum dots
- SPT
single-particle tracking
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-01-0034) on June 16, 2011.
REFERENCES
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak LS, Ferguson SG, Zhang J, Martenson C, Meyer T, Caron MG. Internal trafficking and surface mobility of a functionally intact β2-adrenergic receptor–green fluorescent protein conjugate. Mol Pharmacol. 1997;51:177–184. doi: 10.1124/mol.51.2.177. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Correia PA, Perez Alea M, Narvaez M, Garriga P, Fuxe K, Ciruela F. Impaired M3 muscarinic acetylcholine receptor signal transduction through blockade of binding of multiple proteins to its third intracellular loop. Cell Physiol Biochem. 2010;25:397–408. doi: 10.1159/000303044. [DOI] [PubMed] [Google Scholar]
- Bossard F, Robay A, Toumaniantz G, Dahimene S, Becq F, Merot J, Gauthier C. NHE-RF1 protein rescues ΔF508-CFTR function. Am J Physiol Lung Cell Mol Physiol. 2006;292:L1085–L1094. doi: 10.1152/ajplung.00445.2005. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Ngyuyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci. 2003;4:251–265. doi: 10.1038/nrn1077. [DOI] [PubMed] [Google Scholar]
- Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464:783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- Crane JM, Van Hoek AN, Skach WR, Verkman AS. Aquaporin-4 dynamics in orthogonal arrays in live cells visualized by quantum dot single particle tracking. Mol Bio Cell. 2008;19:3369–3378. doi: 10.1091/mbc.E08-03-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JM, Verkman AS. Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys J. 2008;94:702–713. doi: 10.1529/biophysj.107.115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phospatidylinositol-4,5-bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass A, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Shumay E, Wang HH, Malbon CC. The scaffold protein gravin (AKAP250) binds β2-adrenergic receptor via the receptor cytoplasmic R329-L413 domain and provides a mobile scaffold during desensitization. J Biol Chem. 2001;276:24005–24014. doi: 10.1074/jbc.M011199200. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Fraser IDC, Cong M, Kim JY, Rollins EN, Daaka Y, Lefkowitz RJ, Scott JD. Assembly of an A kinase-anchoring protein–β2-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Frick M, Schmidt K, Nichols BJ. Modulation of lateral diffusion in the plasma membrane by protein density. Curr Biol. 2007;17:462–467. doi: 10.1016/j.cub.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonté D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy. J Biol Chem. 1999;274:25632–25641. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Travalin SJ, Goehring AS, Scott JD, Bahouth SW. AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the β1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J Biol Chem. 2006;281:33537–33553. doi: 10.1074/jbc.M601809200. [DOI] [PubMed] [Google Scholar]
- Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol. 2004;6:238–243. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggie PM, Kim JK, Lukacs GL, Verkman AS. Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol Biol Cell. 2006;17:4937–4945. doi: 10.1091/mbc.E06-08-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Lefkowitz RJ. Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res. 2002;91:672–212. doi: 10.1161/01.res.0000037000.74258.03. [DOI] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998a;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, et al. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998b;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bellini M, Inuzuka H, Xu J, Xiong Y, Yang X, Castleberry AM, Hall RA. Proteomic analysis of β1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem. 2006;281:2820–2827. doi: 10.1074/jbc.M509503200. [DOI] [PubMed] [Google Scholar]
- Hegener O, Prenner L, Runkel F, Baader SL, Kappler J, Häberlein H. Dynamics of β2-adrenergic receptor-ligand complexes on living cells. Biochemistry. 2004;43:6190–6199. doi: 10.1021/bi035928t. [DOI] [PubMed] [Google Scholar]
- Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JET, Lazareno S, Molloy JE, Birdsall NJM Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci USA. 2010;107:2693–2698. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Deviez DJ, Martin S, Laval SH, Lo HP, Cooper ST, North KN, Bushby K, Parton RG. Aberrant dysferlin trafficking in cells lacking caveolin or expressing dystrophy mutants of caveolin-3. Hum Mol Gen. 2006;15:129–142. doi: 10.1093/hmg/ddi434. [DOI] [PubMed] [Google Scholar]
- Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- Ianoul A, Grant DD, Rouleau Y, Bani-Yaghoub M, Johnston LJ, Pezacki JP. Imaging nanometer domains of β-adrenergic receptor complexes on the surface of cardiac myocytes. Nat Chem Biol. 2005;1:196–202. doi: 10.1038/nchembio726. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Gurevich VV, Schepers T, Hamm HE, Benovic JL. Arrestin-rhodopsin interactions. Multi-site binding delineated by peptide inhibition. J Biol Chem. 1994;269:3226–3232. [PubMed] [Google Scholar]
- Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discovery. 2009;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Lidke KA, Reiger B, Jovin TM, Arndt-Jovin D. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of Ca2+-dependent BK channel signaling complex by binding to β2 adrenergic receptor. EMBO J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, et al. β-Arrestin dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Malbon CC, Tao J, Wang H-Y. AKAPs (A-kinase anchoring proteins) and molecules that compose their G-protein-coupled receptor signalling complexes. Biochem J. 2004;379:1–9. doi: 10.1042/BJ20031648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Palczewski K, Gurevich VV, Hunzicker-Dunn M. β-Arrestin-dependent desensitization of lutenizing hormone/choriogonadotropin receptor is prevented by a synthetic peptide corresponding to the third intracellular loop of the receptor. J Biol Chem. 1999;274:12984–12989. doi: 10.1074/jbc.274.19.12984. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Iino R, Kobayahi T, Ohshima C, Yoshimura A, Kusumi A. Single-molecule imaging analysis of Ras activation in living cells. Proc Natl Acad Sci USA. 2004;101:7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naren AP, et al. A macromolecular complex of β2 adrenergic receptor, CFTR and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci USA. 2003;100:342–346. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauert JB, Klauck TM, Langeberg LK, Scott JD. Gravin, and autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- Negro A, Dodge-Kafka K, Kapiloff MS. Signalosomes as therapeutic targets. Prog Pediatr Cardiol. 2008;25:51–56. doi: 10.1016/j.ppedcard.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Gomez LL, Dell'Acqua ML. Imaging kinase–AKAP79–phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. J Cell Biol. 2003;160:101–112. doi: 10.1083/jcb.200209127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Pieretti F, Depez-Beauclair P, Bonardo B, Aubert H, Juhan-Vague I, Nalbone G. Identification of SAP97 as an intracellular binding partner of TACE. J Cell Sci. 2003;116:1949–1957. doi: 10.1242/jcs.00415. [DOI] [PubMed] [Google Scholar]
- Pontier SM, Percherancier Y, Galadrin S, Breit A, Galés C, Bouvier M. Cholesterol-dependent separation of the β2-adrenergic receptor from its partners determines signaling efficacy. J Biol Chem. 2008;283:24659–24672. doi: 10.1074/jbc.M800778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- Ruehr ML, Russell MA, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol. 2004;37:653–665. doi: 10.1016/j.yjmcc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J Biol Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- Sergé A, Fourgeaud L, Hémar A, Choquet D. Receptor activation and Homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci. 2002;22:3910–3920. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbakova OG, Hurt CM, Xiang Y, Dell'Acqua ML, Zhang Q, Tsien RW, Kobilka BK. Organization of β-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007;176:521–533. doi: 10.1083/jcb.200604167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Tang Z, Li S, Lisanti MP. Mutational analysis of the properties of caveolin-1. A novel role for the C-terminal domain in mediating homo-typic caveolin-caveolin interactions. J Biol Chem. 1997;272:4398–4403. doi: 10.1074/jbc.272.7.4398. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. β2-Adrenergic receptor signaling complexes in cardiomyocyte caveolae/lipid rafts. J Mol Cell Cardiol. 2004;37:407–415. doi: 10.1016/j.yjmcc.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ritchie K, Kajikawa E, Fujiwara T, Kusumi A. Rapid hop diffusion of a G-protein-coupled receptor in the plasma membrane as revealed by single-molecule techniques. Biophys J. 2005;88:3659–3680. doi: 10.1529/biophysj.104.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Hu LA, Miller WE, Ringstad N, Hall RA, Pitcher JA, DeCamilli P, Lefkowitz RJ. Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the β1-adrenergic receptor. Proc Natl Acad Sci USA. 1999;96:12559–12564. doi: 10.1073/pnas.96.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Wang H-Y, Malbon CC. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the 2-adrenergic receptor. EMBO J. 2003;22:6419–6429. doi: 10.1093/emboj/cdg628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signaling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2378. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Devic E, Kobilka BK. The PDZ binding motif of the β1-adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002a;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Rybin VO, Steinberg SF, Kobilka BK. Caveolar localization dictates physiologic signaling of β2-adenoreceptors in neonatal cardiac myocyte. J Biol Chem. 2002b;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific α1- and β-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Yan X, Walkiewicz M, Carlson J, Leiphon L, Grove B. Gravin dynamics regulates the subcellular distribution of PKA. Exp Cell Res. 2009;315:1247–1259. doi: 10.1016/j.yexcr.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat Neurosci. 2006;9:622–627. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Magalhães P, Pozzan T. Compartmentalization of cAMP and Ca2+ signals. Curr Opin Cell Biol. 2002;14:160–166. doi: 10.1016/s0955-0674(02)00316-2. [DOI] [PubMed] [Google Scholar]
- Zeke A, Lukacs M, Lim WA, Remenyi A. Scaffolds: interaction platforms for cellular signalling circuits. Trends Cell Biol. 2009;19:364–374. doi: 10.1016/j.tcb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C-Y, Petralia RS, Wang Y-X, Kacher B, Wenthold RJ. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 2010;30:4757–4766. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Zhu W, Han Q, Xiao R-P. Emerging concepts and therapeutic implications of β-adrenergic receptor subtype signaling. Pharmacol Ther. 2005;108:257–268. doi: 10.1016/j.pharmthera.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Zhong H, Sia G-M, Sato TR, Gray NW, Mao T, Khuchua Z, Huganir RL, Svoboda KL. Subcellular dynamics of type II PKA in neurons. Neuron. 2009;62:363–374. doi: 10.1016/j.neuron.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.