Abstract
Escitalopram is the S-enantiomer of the racemic compound citalopram, a selective serotonin reuptake inhibitor (SSRI) widely used for the treatment of depression. This review describes the current body of pharmacologic and clinical evidence supporting the use of escitalopram for the treatment of depression and anxiety. Preclinical studies have confirmed that it is primarily this molecule that provides the inhibition of serotonin reuptake responsible for the antidepressant effect of citalopram, with minimal-to-nonexistent affinity for other receptor sites. Clinical trials of escitalopram in depressed patients indicate that escitalopram, 10 mg/day, is as effective as 40 mg/day of its parent compound, citalopram, with an excellent safety and tolerability profile. Because of its increased selectivity, escitalopram represents a refinement in SSRI therapy for symptoms of depression and anxiety. This article also explores the implications of a more selective SSRI on the management of depressed patients in the primary care clinical practice.
Escitalopram is the S-isomer of the racemic compound citalopram, a selective serotonin reuptake inhibitor (SSRI) that is widely used in both psychiatric and primary care practices for the treatment of depression. The theoretical rationale for separating a single isomer from its parent compound and synthesizing it into a new therapeutic agent is 2-fold. First, it may capture the molecule that is actually responsible for the desired therapeutic action. At the same time, it may isolate and remove the molecule that is not only therapeutically unproductive but may be the source of undesirable pharmacologic activity. The result is a “purer” agent, one that can potentially offer an improved safety and efficacy profile versus the parent compound.1
In the case of citalopram, the molecules in question are a set of enantiomers, that is, a pair of stereoisomers that are non-superimposable, mirror images of one another. This review examines 2 issues: first, that escitalopram represents a refinement in SSRI therapy for symptoms of depression and, possibly, for various anxiety disorders as well; second, that increased selectivity and improved tolerability are clinically important for the management of depressed patients in primary care.
AFFINITY AND SELECTIVITY FOR SEROTONIN TRANSPORT SITES
All SSRIs inhibit reuptake of serotonin into presynaptic serotonergic neurons, an action that increases the availability of serotonin at the synapse and, ultimately, enhances serotonergic function in the central nervous system. This mechanism of action depends on the binding of drug to the serotonin transporter protein.2
In a radioligand binding study of cells expressing human serotonin transporters, escitalopram proved to be approximately 30 times more potent than its enantiomer, R citalopram, in its capacity to bind to the serotonin transporter receptor site.2 This finding confirms preclinical studies demonstrating that the antidepressant effect of citalopram resides primarily within the S enantiomer rather than the R-enantiomer.3,4 Furthermore, a microdialysis study performed in rat brain demonstrated that a subcutaneous injection of 2 mg/kg of escitalopram increases serotonin levels (i.e., blocks serotonin reuptake) significantly more than an injection of 4 mg/kg of the racemate, citalopram (2 mg/kg of escitalopram + 2 mg/kg of R-citalopram). These data suggest that R-citalopram may actually interfere with the activity of escitalopram.5
Escitalopram is also extremely selective for serotonergic transport proteins relative to noradrenergic or dopaminergic binding sites, particularly when compared directly with other antidepressants such as fluoxetine, paroxetine, fluvoxamine, or sertraline.2 In fact, escitalopram had little or no binding affinity for more than 100 receptor or binding sites tested in vitro, including α-adrenergic (α1) receptors, muscarinic (M1) receptors, and histamine (H1) receptors.6
Selectivity for serotonergic, rather than muscarinic, histaminergic, or adrenergic, receptors suggests a lower potential for causing dry mouth, sedation, or cardiovascular side effects.1,2 Patients who have switched from fluoxetine, sertraline, paroxetine, or citalopram to escitalopram (10–20 mg) due to adverse events do indeed experience a lower incidence of side effects while taking escitalopram versus their prior SSRI.7
DRUG-DRUG INTERACTIONS
Escitalopram is metabolized in humans by 3 cytochrome P450 (CYP) hepatic enzymes that each offer relatively comparable contributions to intrinsic clearance of the drug: CYP2C19 (36%), CYP2D6 (30%), and CYP3A4 (34%).8 With 3 parallel routes of biotransformation, a drug interaction that interferes with any one of these isoforms is unlikely to affect overall clearance rates.8 Furthermore, because escitalopram and its 2 metabolites have only weak-to-negligible inhibitory effects on CYP enzymes, they are unlikely to be involved in clinically significant drug interactions mediated through these pathways. In contrast, antidepressants such as fluoxetine, fluvoxamine, and paroxetine cause moderate-to-strong inhibition of several CYP enzymes and, as such, carry a greater potential for these types of drug interactions.9 For example, fluoxetine and paroxetine strongly inhibit CYP2D6, which is responsible for about 50% of drug metabolism in the liver.10,11 Escitalopram is only 55% bound to human plasma proteins, further reducing its potential for producing drug-drug interactions.12 Protein binding increases the possibility of displacement of other highly protein bound drugs.
CLINICAL EFFICACY IN DEPRESSION
The efficacy and safety of escitalopram have been demonstrated in several large, controlled trials of depressed patients.
Primary Care Settings
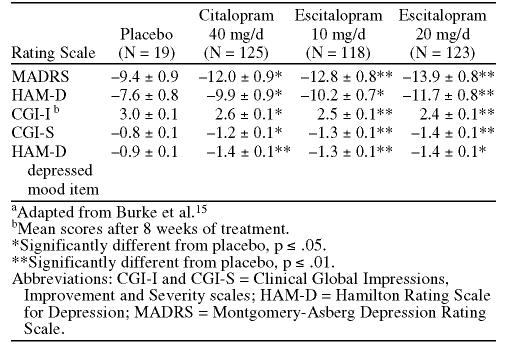
In a randomized, double-blind, placebo-controlled trial conducted in primary care centers in Canada, Europe, and the United Kingdom, patients treated with escitalopram (10 mg/day) for 8 weeks experienced a significantly greater decline in mean scores on the Montgomery-Asberg Depression Rating Scale (MADRS) than did placebo-treated patients (Figure 1).13 Scores for escitalopram-treated patients were significantly lower from the second week of treatment forward.
Figure 1.
Mean Changes From Baseline MADRS Score by Week of Treatment With Escitaloprama
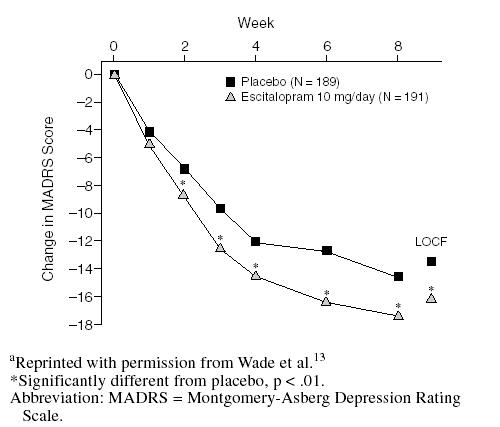
At week 8, 55% of patients receiving escitalopram achieved a clinical response (i.e., ≥ 50% reduction from baseline in MADRS score).13 A total of 85% of these responders also achieved remission (i.e., a MADRS score ≤ 12). Rates of response and remission associated with escitalopram were significantly higher than those in placebo-treated patients (Figure 2) and were particularly noteworthy in light of the moderate-to-severe levels of depression at the start of the study (mean baseline MADRS score of 29).
Figure 2.
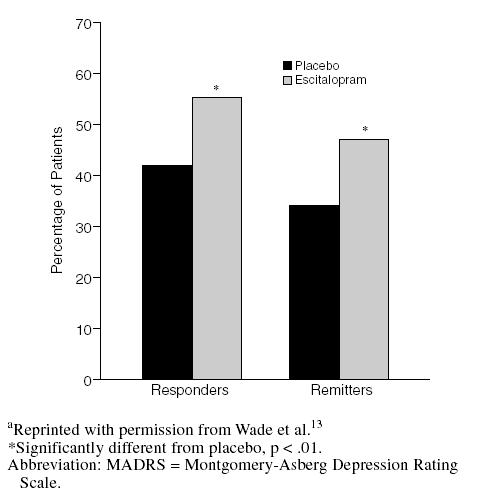
Proportion of Responders (≥ 50% reduction in MADRS score) and Remitters (MADRS score ≤ 12) at Week 8a
Escitalopram was well tolerated in this trial, with rates of withdrawal due to adverse events of less than 5% and not significantly different from placebo (4.7% vs 1.1%, respectively). The only treatment-related adverse event reported with significantly greater frequency than it was for placebo was nausea (8.9% vs. 3.7%, for escitalopram and placebo, respectively); this difference diminished by week 2.
A second, similarly designed trial in Canada, Europe, and the United Kingdom compared the efficacy and safety of escitalopram (10–20 mg/day) to those of placebo and to those of citalopram (20–40 mg/day) as an active comparator.14 For the first 4 weeks of this study, the doses were fixed at escitalopram, 10 mg/day, and citalopram, 20 mg/day. As shown in Figure 3, escitalopram had a faster onset of action than citalopram, indicating the effectiveness of the 10-mg dose. Significant differences in MADRS scores for escitalopram versus placebo were sustained throughout the entire study period. Withdrawal rates due to adverse events were equal for escitalopram and placebo (both 2.6%); for citalopram, the incidence was 3.8%. Adverse events occurring in > 10% of patients in any treatment groups were headache and nausea. Headache was more frequent in patients taking placebo.
Figure 3.
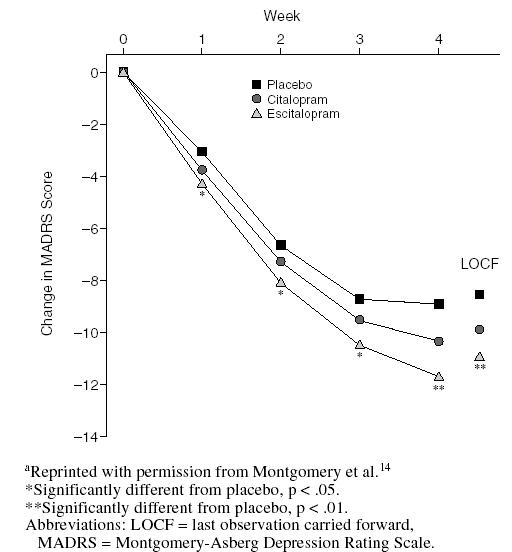
Adjusted Mean Change from Baseline in MADRS Total Score by Treatment Weeka
Additional Escitalopram Clinical Trials
In a randomized, double-blind, placebo-controlled U.S. clinical trial among depressed outpatients, 8 weeks of treatment with 10 mg/day or 20 mg/day of escitalopram proved significantly more effective than placebo in all 5 major efficacy endpoints (Table 1).15 Onset of therapeutic efficacy occurred rapidly: a significantly greater therapeutic effect relative to placebo was evident at 2 weeks in both escitalopram treatment groups for MADRS and Hamilton Rating Scale for Depression (HAM-D) outcomes and after 1 week of treatment for Clinical Global Impressions-Improvement scale (CGI-I) and HAM-D depressed mood item scores.
Table 1.
Mean Change (± SEM) From Baseline Efficacy Endpoint Values at 4 Weeks of Treatmenta
While this trial was not statistically powered to compare escitalopram with citalopram, scores on key efficacy endpoints in patients suggested that the efficacy of 10 mg/day of escitalopram was at least as effective as treatment with 40 mg/day of citalopram in alleviating symptoms of depression. Rates of clinical response, defined prospectively as the number of patients achieving 50% improvement at endpoint from baseline MADRS score, were also comparable among patients receiving escitalopram, 10 mg/day (50%); escitalopram, 20 mg/day (51.2%); or citalopram, 40 mg/day (45.6%).
Efficacy advantages for escitalopram relative to citalopram were also apparent when the results of this 8-week trial were pooled with 2 other clinical trials that used citalopram as an active comparator in identical study protocols.16 These 3 randomized, multicenter trials were designed specifically to determine the efficacy of escitalopram relative to citalopram. In a meta-analysis of these trials, Gorman et al.16 reported that escitalopram may have a faster onset of therapeutic efficacy and significantly greater antidepressant effect than citalopram. For example, while both agents were significantly superior to placebo in alleviating symptoms of depression, statistically significant differences first appeared at week 1 for escitalopram versus week 6 for citalopram (Figure 4). Escitalopram was also significantly more effective than citalopram in lowering MADRS scores at multiple evaluation points in the trial. Both escitalopram and citalopram treatments led to significantly greater proportions of responders than placebo treatment (Figure 5).
Figure 4.
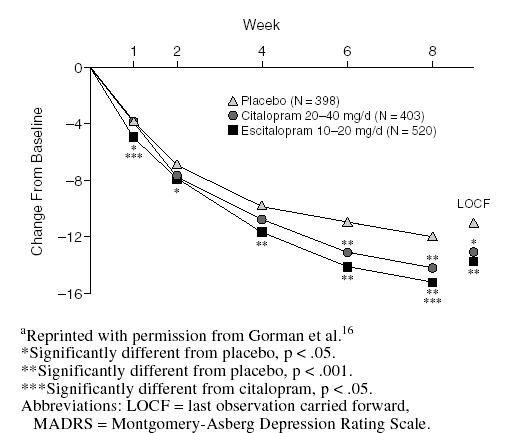
Mean Change From Baseline in MADRS Total Score by Treatment Week in Meta-Analysis of U.S. Clinical Trialsa
Figure 5.
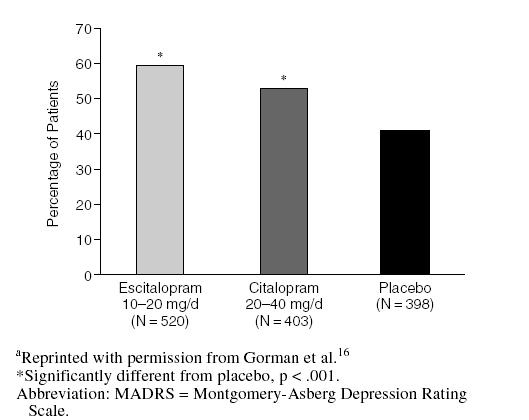
Proportion of Responders (> 50% improvement from baseline in MADRS score) at Week 8 in Meta-Analysis of U.S. Clinical Trialsa
A total of 715 depressed patients received escitalopram (10–20 mg) in acute clinical trials. Only 1 adverse effect (nausea) occurred in more than 10% of escitalopram-treated depressed patients and more frequently than in 592 placebo-treated depressed patients.17 The discontinuation rate (6%) was also low.12
EFFICACY IN ANXIETY
Because primary care physicians are just as likely as psychiatrists to be consulted by patients with anxiety disorders,18 the efficacy of escitalopram in anxiety is an especially relevant clinical attribute. The percentage of patients with depression who experience comorbid anxiety is extremely high.19 Approximately 60% of depressed patients have some anxiety symptoms and 68% have comorbid anxiety disorders.20,21 Identifying such psychiatric comorbidity is helpful in determining treatment, evaluating response, and managing the patient over the long term. Animal models of anxiety suggest the anxiolytic activity of citalopram is due to its S-enantiomer22; escitalopram clinical trials in various anxiety disorders defined by DSM-IV criteria support this observation.
Generalized anxiety disorder (GAD) consists of physical and psychological symptoms of persistent anxiety that compromise the patient's ability to function.23 In a randomized, double-blind, placebo-controlled study, escitalopram (flexibly dosed from 10–20 mg/day) for 8 weeks significantly improved symptoms of GAD. Significant changes from baseline in mean Hamilton Rating Scale for Anxiety (HAM-A), HAM-A anxiety subscale, CGI, and Hospital Anxiety and Depression (HAD) anxiety subscale scores were observed, prior to up-titration at the end of week 4. This supports the efficacy of the 10-mg/day dose in treating GAD.24 Using a quality-of-life scale, mean changes from baseline to endpoint were significantly superior for the escitalopram group versus the placebo group. Quality-of-life measurements included assessments of life satisfaction and contentment, ability to function, mood, and sense of well-being.
Social anxiety disorder is an excessive, irrational, and unrelenting fear of social interaction that disrupts academic or occupational performance in up to 85% of patients diagnosed with it.25 It is the third most common psychiatric disorder, trailing depression and alcohol abuse.26 Its prevalence in primary care has been reported to range from 2.9% to 7%.27 In a 12-week trial, treatment with escitalopram (10–20 mg/day) significantly improved symptoms of severe social anxiety disorder relative to placebo in both primary and secondary efficacy outcome parameters. The mean decline in the Liebowitz Social Anxiety Scale (LSAS) score from baseline to week 12 of treatment was significantly greater in the escitalopram group versus the placebo group. At 12 weeks, patients treated with escitalopram also experienced significantly greater improvement versus placebo in LSAS fear/anxiety and avoidance subscale scores, CGI-Severity of Illness scale (CGI-S) and CGI-I scores, as well as Sheehan Disability Scale work and social life scores.28
Family practitioners also are likely to encounter panic disorder, which consists of recurrent and unexpected panic attacks coupled with persistent worry or behavioral changes related to these episodes.29 According to the National Comorbidity Survey, 56% of individuals with lifetime major depression will experience lifetime panic disorder.30 SSRIs have become first-line therapeutic options for this potentially devastating disorder, particularly since they are better tolerated and have minimal lethality even in large overdoses compared with tricyclic antidepressants.29
When compared with placebo, 10 weeks of treatment with escitalopram (10–20 mg/day) significantly improved multiple symptoms of panic disorder and overall quality of life. Significantly greater improvement at study endpoint for all efficacy assessments was measured in the escitalopram group versus the placebo group. This included the HAM-A; CGI-I, CGI-S, and CGI-phobic avoidance; patient global evaluation; a panic and agoraphobia scale; a quality of life measurement; and the duration of anticipatory anxiety. Over half of patients treated with escitalopram had no panic attacks at 10 weeks, which was a significantly higher proportion than in placebo-treated patients.31 More than 30% of patients treated with escitalopram remitted at 10 weeks, significantly more than those treated with placebo.
Escitalopram was safe and well-tolerated in all 3 trials.24,28,31 Of note, in the panic disorder trial, the rate of discontinuation due to adverse events was lower in patients receiving escitalopram versus placebo (6% for escitalopram and 8% for placebo).28,31
IMPLICATIONS FOR PRIMARY CARE PATIENTS
Despite what often seems to be an overwhelming surfeit of antidepressant agents, pharmacologic treatment of the depressed patient in primary care practice remains problematic. Kroenke et al.32 studied the efficacy of 3 SSRIs in primary care patients, using criteria designed to emulate actual practice. That is, patients had been diagnosed as candidates for antidepressant therapy by their physicians rather than through the use of diagnostic research instruments, they were not excluded for having comorbid or other complicating disorders, and their treatment choices and dosages used were selected, and changed, as their physicians deemed appropriate. In this trial, about 40% of patients stopped or switched their antidepressant at 9 months. This rate of discontinuation did not differ among SSRI treatment groups (paroxetine, fluoxetine, or sertraline). This study was designed before citalopram was in use in the United States.32 Forty-three percent (paroxetine), 39% (fluoxetine), and 45% (sertraline) stopped or switched the initially prescribed medication; of these, 56% (paroxetine), 44% (fluoxetine), and 46% (sertraline) did so due to adverse effects, and 33% (paroxetine), 29% (fluoxetine), and 33% (sertraline) did so because the medication was not helping. Thus, despite a large number of therapeutic options, improvement in efficacy and tolerability is still very much needed.
At 10 mg/day, escitalopram is extremely well tolerated. In clinical trials, the overall incidence of adverse events and the rate of discontinuation for this dose were statistically comparable to placebo. Escitalopram's efficacy and tolerability profile has particular meaning in primary care, where comorbid conditions are a clinical fact of life. Patients with diabetes, for example, have approximately twice the rate of depression as nondiabetic patients, and the presence of depression is associated with an increased risk of diabetic complications.33,34 Other chronic conditions, such as coronary artery disease, also tend to exist comorbidly with depression. For many chronic medical illnesses, major depression both increases the likelihood of their onset and is increased several fold among those with chronic medical conditions.35–38 For instance, the Institute of Medicine concluded that depression is strongly associated with the occurrence of myocardial infarctions and death following myocardial infarctions.39 The low potential for drug interactions between escitalopram and other CYP-metabolized drugs the primary care patient may be taking for these conditions is therefore an obvious benefit.
The low potential for drug interactions with escitalopram is also important even when the patient is not currently taking any other medications. Since it is quite reasonable to assume that the primary care patient receiving chronic treatment for depression will at some point develop other acute or chronic medical illnesses, the fact that the medications these illnesses necessitate are not expected to interact adversely with escitalopram could simplify patient management well into the future.
CONCLUSION
Numerous studies have documented that primary care physicians provide care for patients with psychological disorders more frequently than do mental health providers.18 Results of numerous clinical studies of escitalopram in the management of patients with moderate-to-severe depression bear out the promise inherent in the development of single-isomer drugs. Escitalopram is efficacious and safe and shows particularly good tolerability at the usual dose of 10 mg, which is also an effective starting dose. The increased efficacy and safety of this single isomer relative to its parent compound represent a refinement in SSRI pharmacology that translates into much-needed benefits in the management of the primary care patient with depression.
Drug names: citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), paroxetine (Paxil), sertraline (Zoloft).
Footnotes
Dr. Culpepper has served as a consultant to Forest, Pfizer, Abbott, Lilly, Janssen, and Wyeth-Ayerst.
This article was supported by an unrestricted educational grant from IntraMed, New York, N.Y.
REFERENCES
- Owens MJ, Rosenbaum JF. Escitalopram: a second-generation SSRI. CNS Spectrums. 2002;7(suppl 1):34–39. doi: 10.1017/s1092852900028583. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Hytell J, Bøgesø KP, Perregaard J, et. al. The pharmacological effect of citalopram resides in the (S)-(+)-enantiomer. J Neural Transm. 1992;88:157–160. doi: 10.1007/BF01244820. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hogg S. The antidepressant effect of citalopram resides in the S-enantiomer (Lu 26-054) Biol Psychiatry. 2000;47:88S. [Google Scholar]
- Mork A, Kreilgaard M, Sanchez C, and et. al. Escitalopram: a comparative study of in vitro and in vivo 5-HT uptake inhibitory activity [poster]. Presented at the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologium; June 23–27, 2002; Montreal, Canada. P.1.E.054. [Google Scholar]
- Sanchez C, Tøttrup Brennum L. The S-enantiomer of citalopram (Lu 26-054) is a highly selective and potent serotonin reuptake inhibitor [poster]. Presented at the annual meeting of the Society for Biological Psychiatry; May 11–13, 2000; Chicago, Ill. [Google Scholar]
- Rosenthal MH, Li D. Efficacy and tolerability of escitalopram in patients intolerant of other SSRIs [poster]. Presented at the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologium; June 23–27, 2002; Montreal, Canada. P.3.E.038. [Google Scholar]
- von Moltke LL, Greenblatt DJ, Giancarlo GM, et. al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29:1102–1109. [PubMed] [Google Scholar]
- Greenblatt DJ, von Moltke LL, Harmatz JS, et. al. Drug interactions with newer antidepressants: role of human cytochromes P450. J Clin Psychiatry. 1998;59(suppl 15):19–27. [PubMed] [Google Scholar]
- Preskorn SH. Debate resolved: there are differential effects of serotonin selective reuptake inhibitors on cytochrome P450 enzymes. J Psychopharmacol. 1998;12(3, suppl B):S89–S97. doi: 10.1177/0269881198012003051. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- Lexapro Prescribing Information. New York, NY: Forest Pharmaceuticals, Inc. 2002 [Google Scholar]
- Wade A, Lemming MO, Hedegaard BK. Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. Int Clin Psychopharmacol. 2002;17:95–102. doi: 10.1097/00004850-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Loft H, Sanchez C, et. al. Escitalopram (S-enantiomer of citalopram): clinical efficacy and onset of action predicted from a rat model. Pharmacol Toxicol. 2001;88:282–286. doi: 10.1034/j.1600-0773.2001.d01-118.x. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J Clin Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Korotzer A, Su G. Efficacy comparison of escitalopram and citalopram in the treatment of major depressive disorder: pooled analysis of placebo-controlled trials. CNS Spectrums. 2002;7(suppl 1):40–44. doi: 10.1017/s1092852900028595. [DOI] [PubMed] [Google Scholar]
- Gorman J. Comparison of efficacy in placebo-controlled trials of escitalopram and citalopram [poster]. Presented at the 154th annual meeting of the American Psychiatric Association; May 5–10, 2001; New Orleans, La. [Google Scholar]
- Shear MK, Schulberg HC. Anxiety disorders in primary care. Bull Menninger Clin. 1995;59(2, suppl A):A73–A85. [PubMed] [Google Scholar]
- Keller MB, Hanks DL. The natural history and heterogeneity of depressive disorders: implications for rational antidepressant therapy. J Clin Psychiatry. 1994 55(9, suppl A):25–31.discussion 32–33, 98–100. [PubMed] [Google Scholar]
- Keller MB, Hanks DL. Anxiety symptom relief in depression treatment outcomes. J Clin Psychiatry. 1995;56(suppl 6):22–29. [PubMed] [Google Scholar]
- Wittchen H-U, Essau CA. Comorbidity and mixed anxiety-depressive disorders: is there epidemiologic evidence? J Clin Psychiatry. 1993;54(1, suppl):9–15. [PubMed] [Google Scholar]
- Sanchez C. Escitalopram has potent anxiolytic effects in rodent anxiety models [poster]. Presented at the 41st annual meeting of the New Clinical Drug Evaluation Unit; May 28–31, 2001; Phoenix, Ariz. [Google Scholar]
- Gliatto MF. Generalized anxiety disorder. Am Fam Physician. 2000;62:1591–1600. 1602. [PubMed] [Google Scholar]
- Davidson J, Bose A, and Su G. Escitalopram in the treatment of generalized anxiety disorder [poster]. Presented at the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologium; June 23–27, 2002; Montreal, Canada. [Google Scholar]
- Bruce TJ, Saeed SA. Social anxiety disorder: a common, underrecognized mental disorder. Am Fam Physician. 1999;60:2311–2322. [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, et. al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Stein MB. Social phobia: prevalence and diagnostic threshold. J Clin Psychiatry. 2001;62(suppl 1):5–10. [PubMed] [Google Scholar]
- Kasper S, Loft H, and Smith RJ. Escitalopram is efficacious and well tolerated in the treatment of social anxiety disorder [poster]. Presented at the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologium; June 23–27, 2002; Montreal, Canada. [Google Scholar]
- Saeed SA, Bruce TJ. Panic disorder: effective treatment options. Am Fam Physician. 1998;57:2405–2412. 2419–2420. [PubMed] [Google Scholar]
- Roy-Byrne PP, Stang P, Wittchen HU, et. al. Lifetime panic-depression comorbidity in the National Comorbidity Survey. Association with symptoms, impairment, cause, and help-seeking. Br J Psychiatry. 2000;176:229–235. doi: 10.1192/bjp.176.3.229. [DOI] [PubMed] [Google Scholar]
- Stahl S, Gergel I, and Li D. Escitalopram in the treatment of panic disorder [poster]. Presented at the 23rd Congress of the Collegium Internationale Neuro-Psychopharmacologium; June 23–27, 2002; Montreal, Canada. [Google Scholar]
- Kroenke K, West SL, Swindle R, et. al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA. 2001;286:2947–2955. doi: 10.1001/jama.286.23.2947. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, et. al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ, Henry JH, Buki VMV. Treatment of depression in patients with diabetes mellitus. J Clin Psychiatry. 1995;56:128–136. [PubMed] [Google Scholar]
- Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes: an epidemiological evaluation. Diabetes Care. 1993;16:1167–1178. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- Egberts AC, Leufkens HG, Hofman A, et. al. Incidence of antidepressant drug use in older adults and association with chronic diseases: the Rotterdam Study. Int Clin Psychopharmacol. 1997;12:217–223. doi: 10.1097/00004850-199707000-00006. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, Barer D, Nouri F. Affective illness after stroke. Br J Psychiatry. 1987;151:52–56. doi: 10.1192/bjp.151.1.52. [DOI] [PubMed] [Google Scholar]
- Dwight MM, Kowdley KV, Russo JE, et. al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49:311–317. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US). Committee on Health and Behavior: Research, Practice, and Policy. Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences. Washington, DC: National Academy Press. 2001 66–67. [PubMed] [Google Scholar]