Abstract
Background:
The purpose of this study was to clarify important risk factors for distant recurrence of hepatocellular carcinoma in patients positive for hepatitis C and without local recurrence.
Methods:
A total of 212 patients (145 males and 67 females) underwent radiofrequency ablation and transcatheter arterial embolization or transcatheter arterial chemoembolization at initial development of hepatocellular carcinoma. All patients were positive for hepatitis C. Child–Pugh classification was A in 115 and B in 97. The indication for radiofrequency ablation was the presence of up to three tumors ≤ 3 cm. The distant recurrence rate was analyzed using the Kaplan–Meier method and tested by Wilcoxon’s method.
Results:
Cumulative distant recurrence rates at years 1, 3, and 5 were 19%, 62%, and 79%, respectively. On univariate analysis, a ≥ 3 cm tumor, ≥ 50 ng/mL α-fetoprotein level, and < 3.6 g/dL serum albumin level were significant risk factors for distant recurrence, but only a serum albumin level < 3.6 g/dL (P = 0.004) was identified as significant on multivariate analysis. In the group with a pretreatment albumin level ≥ 3.6 g/dL, the distant recurrence rate was compared between patients in whom the albumin level rose, remained unchanged, or decreased by < 0.3 g/dL, and those in whom the level decreased by ≥ 0.3 g/dL. The rate was significantly higher in the latter, with a one-year recurrence rate of 7% versus 15% (P = 0.04).
Conclusion:
Distant recurrence was significantly decreased in patients with a high serum albumin level. Distant recurrence was more likely to occur in patients with a decreased albumin level, although the pretreatment level was high. Thus, strict follow-up after treatment for hepatocellular carcinoma is necessary in patients with low serum albumin levels.
Keywords: distant recurrence, hepatocellular carcinoma, radiofrequency ablation
Introduction
Since radiofrequency ablation (RFA) for locally treatable hepatocellular carcinoma became the first-choice for internal medical treatment, favorable local control rates have been achieved.1 However, distant recurrence occurs at a high rate even among patients for whom local treatment is adequately performed, and no method to prevent recurrence has been established. It is very important to clarify the mechanism and risk factors for distant recurrence, and many papers have been published on this subject.2–8 These papers have reported a combination of percutaneous ethanol injection and RFA or percutaneous microwave coagulation therapy, included mixed hepatitis C (HCV) or hepatitis B cases, or considered only a few patients. In this study, we investigated risk factors for long-term distant recurrence in HCV patients with hepatocellular carcinoma treated by RFA.
Methods and materials
The subjects were 312 patients (HCV-positive and hepatitis B surface antigen-negative) who underwent RFA for hepatocellular carcinoma at our hospital between January 2001 and December 2008, in whom complete coagulation was achieved and follow-up was complete for at least one year. Twelve patients with extrahepatic metastasis were excluded. A further 15 patients were excluded because distant recurrence developed within six months. Esophageal varices ruptured in three patients and one patient died from liver failure within one year of RFA therapy, so they were also excluded. In 69 patients, local recurrence in the same subsegment as the primary nodule was found over two years by enhanced computed tomography (CT).
The remaining 212 patients (145 males and 67 females) underwent RFA for hepatocellular carcinoma at our hospital between November 2001 and December 2008 and achieved complete coagulation without local recurrence or recurrence in the same subsegment as the primary nodule. The diagnosis of hepatocellular carcinoma was established by typical hypervascular findings on dynamic CT scans. The patients underwent transarterial embolization or transarterial chemoembolization, which was performed prior to RFA as often as possible (198 cases underwent RFA with transarterial embolization or transarterial chemoembolization and 14 cases underwent RFA only). Transarterial chemoembolization was performed by injecting mitomycin C (Kyowa Hakko, Tokyo, Japan), epirubicin hydrochloride (Kyowa Hakko), lipiodol (Lipiodol Ultra Fluid, Mitsui, Tokyo, Japan), and a mixture of gelatin sponge particles (Gelfoam, Upjohn Pharmacia, Tokyo, Japan) into the feeding subsegmental artery through either a 4.0 French catheter (Selecon PA, Clinical Supply, Gifu, Japan) or a 2.9 French microcoaxial catheter (Hepa Slider, Solution, Yokohama, Japan) preloaded with a 0.016 inch core guide wire (Run and Run, Solution) inserted through the 4.0 French catheter.
Recurrence in the proximity or the same subsegment of the ablated lesion was regarded as local recurrence, and that in other regions as ectopic recurrence. Patient background factors, including age, gender, cause of hepatitis, tumor size, number of tumors, α-fetoprotein, serum albumin, total bilirubin levels, prothrombin time, platelet count, and serum aspartate aminotransferase and alanine aminotransferase levels, were investigated.
RFA treatment
For ablation, hooked electrodes (LeVeen needle electrodes, Radiotherapeutics, Mountain View, CA) with an RTC 2000 generator (Boston Scientific Co, Tokyo, Japan), or cooled electrodes (Radionics, Burlington, MA) with a radiofrequency generator (Radionics) were used. When a LeVeen needle was used, it was expanded after insertion into the target position. Radiofrequency energy was then applied to the tissue and increased to a maximum power of 130 W. After reaching maximum power, it was sustained until “roll-off ” occurred. For the cool-tip method, radiofrequency energy delivery was applied and increased to a maximum power of 130 W, and continued until impedance increased beyond the limit of the generator.
After the radiofrequency electrode was inserted into the tumor under ultrasound imaging, a peristaltic pump (Watson-Marlow, Wilmington, MA) was used to infuse 0°C normal saline solution into the lumen of the electrodes to maintain the tip temperature below 20°C. The initial output was set to 40 W or 60 W, and the output was then increased by 10 W or 20 W every 60 seconds until peak power of 130 W was attained. Ablation was maintained at peak power for at least 12 minutes unless a rapid rise in impedance stopped the current flow and ablation.9 Multiple electrode placements were required for a large tumor. The radiofrequency generator was activated at each tumor site.
Response to RFA therapy was assessed by contrast-enhanced CT or magnetic resonance imaging. Basically, the endpoint of therapy was judged when the presence of the nonenhanced area was greater than that of the treated tumors in the arterial and portal phases of enhanced CT. If nodular peripheral enhancement of the ablation area was shown after therapy, the residual part of the tumor was treated with additional RFA within a few days post treatment. Distant recurrence in the liver was defined as a new tumor appearing in the liver separate from the ablated area and in a different segment of the liver, so when follow-up CT scans showed an enhanced area within the same segment as the primary tumor, this was designated as local tumor recurrence.4,10
Monitoring of therapeutic efficacy and follow-up
After initial curative RFA therapy, each patient was followed up at three-monthly intervals with blood tests, including liver function and tumor markers, and by enhanced CT scan. If contrast-enhanced CT showed an enhanced area within or at the periphery of the ablated lesion within the follow-up period, this was designated as local tumor recurrence.
Statistical analysis
Baseline data are presented as the mean ± standard deviation for quantitative variables. The Wilcoxon rank-sum test was used to evaluate differences in the number of tumors, tumor diameter, serum albumin level, serum bilirubin level, prothrombin time, platelet count, white cell count, red cell count, alanine transaminase, aspartate transaminase, and α-fetoprotein. Cumulative recurrence curves were analyzed using the Kaplan–Meier method and tested by Wilcoxon’s method. The Cox proportional hazard regression model was used to examine the efficacy of initial curative RFA therapy. The results were reported as hazard ratios with 95% confidence intervals (CI). P < 0.05 was considered to be statistically significant. All statistical analyses were performed using JMP version 7.2 software (SAS Institute, Cary, NC).
Results
Patient characteristics
The clinical features of the patients are summarized in Table 1. There were 145 male and 67 female patients, of mean age 67.2 ± 16.8 years. Child–Pugh classification was A in 115 patients and B in 97 patients. The indication for RFA was the presence of up to three tumors ≤ 3 cm. At the start of treatment, there were 170 single nodular and 42 multinodular cases (35 cases of two lesions and five cases of three lesions). The median nodule diameter was 20 (range 5–34) mm.
Table 1.
Patient profiles at the time of initial treatment for HCC (n = 212)
| Characteristics | Ranges | |
|---|---|---|
| Host-related factors | Age (years) | 67.2 ± 16.8 (43–84) |
| Sex (male:female) | 145:67 | |
| Child–Pugh group (A:B:C) | 115:97:0 | |
| Alb (g/dL) | 3.66 ± 1.46 (2.8–4.5) | |
| T-Bil (mg/dL) | 1.1 ± 0.6 (0.7–2.6) | |
| PT (%) | 80.2 ± 33.1 (50.9–144.4) | |
| Platelet count (×104/μL) | 11.2 ± 20.1 (3.1–26.6) | |
| AST (U/L) | 65.2 ± 169.7 (21–179) | |
| ALT (U/L) | 57.1 ± 159.2 (15–131) | |
| Tumor-related factors | Size of tumor (mm) | 20.2 ± 8.0 (5–34) |
| Number (single: more than 2) | 170:42 | |
| AFP (ng/mL) | 100.2 ± 212.8 (2–1360) | |
| Treatment-related factors | RFA with TAE or TACE:RFA only | 198:14 |
Abbreviations: Alb, albumin; AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate amino transferase; HCC, hepatocellular carcinoma; PT, prothrombin time; RFA, radiofrequency ablation; TAE, transcatheter arterial embolization T-Bil, total bilirubin; TACE, transarterial chemoembolization.
Overall distant recurrence rate
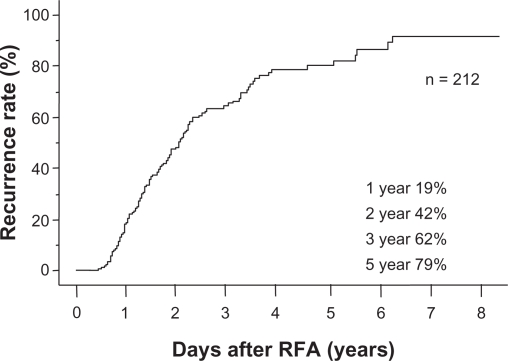
The cumulative survival rate was 100%, 78.3%, and 50.9% at years 1, 3, and 5, respectively. Baseline patient characteristics are shown in Table 1. Tumor size was 5–34 mm, with a mean size of 20.2 (median 20.0) mm. Only 14 patients were treated by RFA only and 198 patients were treated by RFA and transarterial embolization or transarterial chemoembolization. The median follow-up period until distant recurrence occurred was 1.9 (range 0.5–8.1) years (25% follow-up time, 1.0 year; 75% follow-up time, 2.2 years). Cumulative distant recurrence rates at years 1, 2, 3, and 5 estimated by the Kaplan–Meier method for all patients were 19%, 42%, 62%, and 79%, respectively (Figure 1).
Figure 1.
Overall cumulative distant recurrence rates in all patients. The median follow-up period until distant recurrence occurred was 1.9 (range 0.5–8.1) years. Cumulative distant recurrence rates estimated by the Kaplan–Meier method for all patients were 19%, 42%, 62%, and 79%, at years 1, 2, 3, and 5, respectively.
Abbreviation: RFA, radiofrequency ablation.
Prognostic factors
On univariate analysis, a ≥ 3 cm tumor (P = 0.048), α-fetoprotein level ≥ 50 ng/mL (P = 0.005), and serum albumin level < 3.6 g/dL (P = 0.0004) were significant risk factors for recurrence. However, only a serum albumin level < 3.6 g/dL (P = 0.041) was identified as an independent risk factor on multivariate analysis (see Table 2).
Table 2.
Risk factors contributing to distant recurrence of HCC
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Risk ratio (95% CI) | P | Risk ratio (95% CI) | P | |
| Age ≧ 70 | 1.2 (0.98–1.62) | 0.0714 | ||
| Size of tumor ≧ 3 cm | 1.1 (1.08–1.29) | 0.0487 | 1.05 (0.65–1.23) | 0.092 |
| Number of tumors ≧ 2 | 1.1 (0.89–1.50) | 0.3059 | ||
| RBC (/μL) < 400 | 1.1 (0.90–1.34) | 0.8605 | ||
| WBC (/μL) < 3000 | 1.0 (0.78–1.36) | 0.5336 | ||
| plt (/μL) < 10 | 1.0 (0.85–1.26) | 0.6889 | ||
| PT (%) < 80 | 1.1 (0.87–1.31) | 0.3538 | ||
| Alb (g/dl) < 3.6 | 1.4 (1.18–1.77) | 0.0004 | 1.85 (1.04–2.79) | 0.004 |
| AST (U/L) ≧ 80 | 1.0 (0.83–1.27) | 0.4176 | ||
| ALT (U/L) ≧ 80 | 1.0 (0.85–1.31) | 0.9092 | ||
| T.Bil (mg/dl) ≧ 1.5 | 1.1 (0.86–1.39) | 0.7055 | ||
| AFP (ng/ml) ≧ 50 | 1.6 (1.04–3.27) | 0.0050 | 1.03 (0.75–1.42) | 0.114 |
Abbreviations: Alb, albumin; AFP, α-fetoprotein; ALT, alanine aminotransferase; AST, aspartate amino transferase; CI, confidence interval; HCC, hepatocellular carcinoma; PT, prothrombin time; WBC, white blood cells; RBC, red blood cells; plt, platelet; T-Bil, total bilirubin.
Relationship between recurrence rate and serum albumin
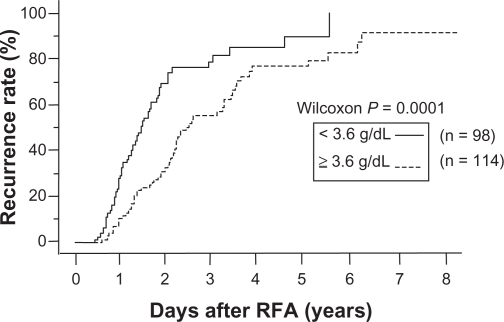
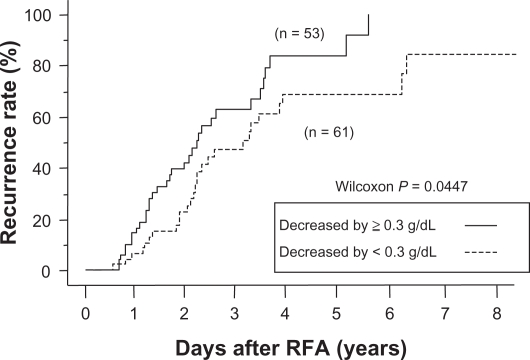
The recurrence rate in patients with a serum albumin level < 3.6 g/dL was significantly higher than in those with an albumin level ≥ 3.6 g/dL (Figures 2 and 3). In the group with a pretreatment albumin level ≥ 3.6 g/dL (n = 114), the distant recurrence rate was compared between patients in whom the albumin level rose, remained unchanged, or decreased by < 0.3 g/dL, and in those in whom the level decreased by ≥ 0.3 g/dL. The recurrence rate was significantly higher in the latter group (one-year recurrence rate 7% versus 15%, P = 0.04).
Figure 2.
Relationship between cumulative distant recurrence rates and serum albumin level. Recurrence rates with a serum albumin level < 3.6 g/dL were significantly higher than those with an albumin level ≥ 3.6 g/dL (P = 0.0001). After years 1, 3, and 5, cumulative distant recurrence rates were 28%, 72%, and 88%, respectively, for patients whose albumin level was < 3.6 g/dL, and 10%, 56%, and 76%, respectively, for patients whose albumin level was ≥ 3.6 g/dL.
Abbreviation: RFA, radiofrequency ablation.
Figure 3.
Relationship between cumulative distant recurrence rates and changes in serum albumin levels after treatment. In the group with a pretreatment albumin level < 3.6 g/dL, the recurrence rates in patients in whom the albumin level rose remained unchanged or decreased by < 0.3 g/dL were significantly lower than those in whom the level decreased by ≥ 0.3 g/dL (P = 0.0447). After years 1, 3, and 5, cumulative distant recurrence rates were 7%, 47%, and 69%, respectively, for patients in whom the albumin level rose, remained unchanged, or decreased by < 0.3 g/dL, and 15%, 62%, and 83%, respectively, for patients in whom the level decreased by ≥ 0.3 g/dL.
Abbreviation: RFA, radiofrequency ablation.
Discussion
The albumin level is an index of reserve capacity and nutritional condition of the liver. The incidence of distant recurrence following the combination of RFA and transcatheter arterial embolization or transcatheter arterial chemoembolization was correlated with the albumin level, and the distant recurrence rate was significantly lower in patients with a high serum albumin level. We wondered how strongly serum albumin level influenced the survival rate. The cumulative survival rate in patients with a serum albumin level < 3.6 g/dL was significantly lower than in those with an albumin level ≥ 3.6 g/dL (P = 0.02). We therefore investigated the patients who died without distant recurrence after RFA. Four in the group with a serum albumin level ≥ 3.6 g/dL (n = 114) and six patients in the group with a serum albumin level < 3.6 g/dL (n = 98) died without recurrence during the study period. There were no significant differences between these two groups using Fisher’s Exact probability test (P = 0.52). Therefore, we concluded that there is no relationship between distant recurrence rates, patient mortality, and serum albumin level. In addition, distant recurrence was more likely to occur in patients with a decreased albumin level, although the pretreatment level was high. Therefore, close follow-up after treatment for liver cancer is necessary in patients with a decreased serum albumin level.
Several earlier reports have identified risk factors for recurrence of hepatocellular carcinoma, but these considered both local and distant recurrence or factors presented by microwave coagulation therapy, percutaneous ethanol injectionm, or RFA combination.7,11 RFA is now considered superior to ethanol injection for small hepatocellular carcinoma and we chose to use only RFA,1 so the treatment efficacy was different. This study was restricted to only distant recurrences and RFA treatments. Many reports included several etiologies and some stated that being HCV-positive is an independent risk factor7 for recurrence. In Japan, the predominant background is HCV-positive liver injury, so we restricted our study to HCV-positive patients. This study included a large number of patients and a long observation period. We defined distant recurrence as nodules appearing in other segments, and hepatocellular carcinoma nodules that were seen in the same liver segment were considered as local recurrence.4,10
Curative treatment of first hepatocellular carcinoma is important. Arimura et al demonstrated that local recurrence is an important prognostic factor in hepatocellular carcinoma.5 Okuwaki et al reported that serum α-fetoprotein and plasma des-γ-carboxy levels, as well as an ablative margin < 5 mm were correlated with multiple intrahepatic distant recurrences.8 Local recurrence occasionally depends on technical problems related to RFA treatment and should be a concern with a margin < 5 mm.
We did not investigate the effects of inadequate treatment of local hepatocellular carcinoma because local recurrence is due to procedural skill, and a tumor appearing close to the first treatment or in the same segment within two years after treatment is considered as local recurrence. In this study, we therefore examined the prognostic factors independent of these technical and local factors.
Several reports have shown that serum albumin is an independent prognostic factor for intrahepatic recurrence after resection2,3,12 or RFA6 of the liver. In our study, the albumin level is of concern for distant recurrence of hepatocellular carcinoma, and a relationship between hepatocellular carcinoma and albumin level have been reported before.13 The serum albumin rose after administration of branched-chain amino acids, and hepatocellular carcinoma development was blocked after administration.14 However, the mechanism of preventing hepatocellular carcinoma by the administration of branched-chain amino acids has been only partly elucidated.15,16 It is possible that administration of branched-chain amino acids increased serum albumin levels, and progression of hepatocellular carcinoma seemed to be prevented as a result.
Interpretation of our results regarding albumin levels is difficult. Albumin transiently decreased immediately after RFA and was subsequently restored. One of the main reasons for this was not only a reduction in hepatocytes but also an increase in inflammatory cytokines, such as interleukin-6 and tumor necrosis factor alpha. The proportion of amino acids in liver cirrhosis is changed, such as a lower branched-chain amino acid to tyrosine ratio.17 Leucine stimulated the growth of hepatocytes and induced release of hepatic growth factor from stellate cells, perhaps in advanced liver injury, and reduction of leucine caused insufficient recovery from liver damage following the delayed recovery of serum albumin.18
The proportion of oxidative albumin is increased in advanced liver disease.19 The high proportion of oxidized human serum albumin impaired its function and had the potential to increase fibrosis and the risk of carcinogenesis.20 Changes in the structure of albumin similar to oxidant human serum albumin had a short half-life, occurring ever earlier by stress,20,21 and the liver and spleen uptake clearance of oxidized human serum albumin was significantly greater than that of normal human serum albumin.22 In addition, truncated albumin in a critically ill patient had rapid clearance,23 so the serum albumin in more advanced liver disease may fall more rapidly than with less liver damage. One possible theory is that the population of structurally changed albumin was higher in patients with reduced serum albumin after RFA and the states of such patients possibly led to early distant recurrence. Further investigations are needed and it is necessary to study the recurrence rate from this aspect, including the rapid reduction of serum albumin and the proportion of oxidant human serum albumin in patients after RFA treatment. In general, more detailed follow-up of treatment is needed in the group in whom the albumin level decreased by ≥ 0.3 g/dL after RFA treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 4.Komorizono Y, Oketani M, Sako K, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 5.Arimura E, Kotoh K, Nakamuta M, Morizono S, Enjoji M, Nawata H. Local recurrence is an important prognostic factor of hepatocellular carcinoma. World J Gastroenterol. 2005;11:5601–5606. doi: 10.3748/wjg.v11.i36.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka Y, Shiraki K, Miyashita K, et al. Risk factors for the recurrence of hepatocellular carcinoma after radiofrequency ablation of hepatocellular carcinoma in patients with hepatitis C. World J Gastroenterol. 2005;11:2174–2178. doi: 10.3748/wjg.v11.i14.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izumi N, Asahina Y, Noguchi O, et al. Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation. Cancer. 2001;91:949–956. [PubMed] [Google Scholar]
- 8.Okuwaki Y, Nakazawa T, Shibuya A, et al. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: risk factors and patterns. J Gastroenterol. 2008;43:71–78. doi: 10.1007/s00535-007-2123-z. [DOI] [PubMed] [Google Scholar]
- 9.McGhana JP, Dodd GD., 3rd Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 10.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006;59:432–441. doi: 10.1016/j.ejrad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Hirohashi K, Shuto T, Kubo S, et al. Prognostic factors after recurrence of resected hepatocellular carcinoma associated with hepatitis C virus. J Hepatobiliary Pancreat Surg. 2001;8:81–86. doi: 10.1007/s005340170054. [DOI] [PubMed] [Google Scholar]
- 13.Marchesini G, Bianchi G, Merli M, et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 14.Muto Y, Sato S, Watanabe A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 15.Kawaguchi T, Taniguchi E, Itou M, et al. Branched-chain amino acids improve insulin resistance in patients with hepatitis C virus-related liver disease: report of two cases. Liver Int. 2007;27:1287–1292. doi: 10.1111/j.1478-3231.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michitaka K, Hiraoka A, Kume M, et al. Amino acid imbalance in patients with chronic liver diseases. Hepatol Res. 2010;40:393–398. doi: 10.1111/j.1872-034X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 18.Tomiya T, Inoue Y, Yanase M, et al. Leucine stimulates the secretion of hepatocyte growth factor by hepatic stellate cells. Biochem Biophys Res Commun. 2002;297:1108–1111. doi: 10.1016/s0006-291x(02)02339-2. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe A, Matsuzaki S, Moriwaki H, Suzuki K, Nishiguchi S.Problems in serum albumin measurement and clinical significance of albumin microheterogeneity in cirrhotics Nutrition 2004; 20351–357. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami A, Kubota K, Yamada N, et al. Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions. FEBS J. 2006;273:3346–3357. doi: 10.1111/j.1742-4658.2006.05341.x. [DOI] [PubMed] [Google Scholar]
- 21.Iwao Y, Anraku M, Yamasaki K, et al. Oxidation of Arg-410 promotes the elimination of human serum albumin. Biochim Biophys Acta. 2006;1764:743–749. doi: 10.1016/j.bbapap.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Iwao Y, Anraku M, Hiraike M, et al. The structural and pharmacokinetic properties of oxidized human serum albumin, advanced oxidation protein products (AOPP) Drug Metab Pharmacokinet. 2006;21:140–146. doi: 10.2133/dmpk.21.140. [DOI] [PubMed] [Google Scholar]
- 23.Bar-Or R, Rael LT, Bar-Or D. Dehydroalanine derived from cysteine is a common post-translational modification in human serum albumin. Rapid Commun Mass Spectrom. 2008;22:711–716. doi: 10.1002/rcm.3421. [DOI] [PubMed] [Google Scholar]