Abstract
Host-microbial interactions play a key role during the development of colitis. We have previously shown that chinase 3-like 1 (CHI3L1) is an inducible molecule overexpressed in colonic epithelial cells (CECs) under inflammatory conditions.
In this study, we found that chitin-binding motif (CBM) of CHI3L1 is specifically associated with the CHI3L1-mediated activation of the Akt-signaling in CEC by transfecting the CBM-mutant CHI3L1 vectors in SW480 CECs. Downstream, CHI3L1 enhanced the secretion of IL-8 and TNFα in a dose-dependent manner. We previously show that 325 through 339 amino-acids in CBM are crucial for the biological function of CHI3L1. Here we demonstrated that 325th–339th residues of CBM in CHI3L1 is a critical region for the activation of Akt, IL-8 production, and for a specific cellular localization of CHI3L1.
In conclusion, CBM region of CHI3L1 is critical in activating Akt signaling in CECs, and the activation may be associated with the development of chronic colitis.
Keywords: chitinases, chitin-binding motifs, epithelial cells, signal transduction
1. Introduction
Mammalian chitinases are evolutionarily well conserved proteins and belong to the glycosyl hydrolases 18 family based on structural similarity with other bacterial and plant chitinases [1, 2]. Chitinase 3-like 1 (CHI3L1, also known as YKL-40 or HC-gp39) also belongs to this family, but called as chitinase-like proteins or chitinase-like lectins (chi-lectins) because of lacking chitinase activity, while it still possesses the carbohydrate-binding motif (CBM) in its C-terminus [3]. CBM is a critical region in interacting with chitin, a polymer of β-1,4-linked N-acetylglucosamine and a structural component of the cell walls and coating of many organisms including crustaceans, parasites, fungi, insects, mites, and other pathogens [4]. CHI3L1 is produced by a wide variety of cells including neutrophils, macrophages, synovial cells, fibroblasts, smooth muscle cells, endothelial cells, epithelial cells and tumor cells [5–10]. Increased message and protein levels of CHI3L1 have been detected in patients with chronic inflammation including rheumatoid arthritis, bronchial asthma, inflammatory bowel disease (IBD; Crohn’s disease and ulcerative colitis), breast cancer and colon cancer [11–16]. Approximately 64% of Crohn’s disease patients with extra-intestinal manifestations including arthralgia, erythema nodosum, uveritis, aphthous ulcerations and fistulas showed significantly increased serum levels of CHI3L1, suggesting a strong relationship between serum CHI3L1 and the existence of clinical complications in Crohn’s disease patients [14]. Our group previously identified that the expression of CHI3L1 molecule is highly induced in CECs and macrophages with intestinal inflammation and enhances potentially pathogenic, but not non-pathogenic, bacterial adhesion and invasion on/into CECs [9, 17]. However, the exact biological role of CHI3L1 in host-microbial interactions and in an exacerbation of chronic colitis is still largely unknown.
Physiological concentration of CHI3L1 enhances proliferation of guinea pig chondrocytes, rabbit chondrocytes as well as synovial cells in vitro [18]. Ling et al previously demonstrated that CHI3L1 stimulation in human skin fibroblasts or articular chondrocytes resulted in protein kinase B (Akt)-mediated serine/threonine phosphorylation of the apoptosis signal-regulator kinase, ASK1, suggesting that CHI3L1 elicits anti-catabolic and anti-apoptotic response during tissue remodeling/destruction [19]. In addition, the activation of cytoplasmic signaling cascades with IL-1 and TNFα enhances CHI3L1-mediated interaction with one or several signaling components on the plasma membrane of articular chondrocytes and skin fibroblast (down-regulation of both p38 MAPK and SAPK/JNK phosphorylation) [10, 19]. However, it is unclear whether CHI3L1-mediated signaling cascades are activated in CECs in managing the microbial interface, which seems to be mainly regulated by CBM in CHI3L1 [17].
It has also been previously demonstrated that a chitin-binding domain truncated form of the human chitotriosidase, a chitinolytic enzyme and one of the member of glycoside hydrolase 18 family, can hydrolyze chitotriose but is unable to bind to chitin anymore [6]. In addition, it has been shown that the C-terminal portion of 49 amino acids makes up the minimal sequence required for chitotriosidase chitin-binding activity. There are six cysteine residues within the minimal chitin-binding domain of chitotriosidase, and mutation of any one of these cysteine residues to serine results in a complete loss of the chitin-binding activity [20]. These results strongly suggest that the cysteine-rich character of the chitin-binding domain in chitotriosidase may be a useful probe for identifying and binding with polysaccharides (e.g., chitin, chitin-like substrate) in vertebrate tissue. In spite of a closely related amino acid sequence and structural relationship to chitotriosidase, CHI3L1 lacks apparent glycohydrolase enzymatic activity as well as a complete form of the cysteine-rich chitin-binding domain [1]. However, CHI3L1 still possesses its function as a lectin identifying specific glycan structures in mammalian tissue, and CHI3L1 has the ability to efficiently interact with chitin fragments via the CBM in its C-terminus [1].
In this study, we demonstrated, for the first time, that the CBM in CHI3L1 plays a pivotal role in activating the Akt signaling pathway with enhanced IL-8 production, which presumably is important for maintaining chronic inflammation and the following development of colitis-associated neoplastic change in CECs [21, 22].
2. Materials and Methods
2.1. Cell Culture
SW480 (human colonic epithelial) and Cos 7 (monkey kidney-fibroblast) cells were obtained from American Type Culture Collection (Manassas, VA) and were cultured in Dulbecco’s modified Eagle medium (DMEM) with L-glutamine (Cellgro, Lawrence, KS), supplemented with 10% (vol/vol) fetal calf serum (Atlanta Biological Inc., Norcross, GA) and a mixture of antibiotics (penicillin G and streptomycin) (Cellgro).
2.2. Generation of the truncated form of mouse CHI3L1 expression vector
The protocol for generating the mouse wildtype (WT)-CHI3L1 expression vector has been reported previously [9]. The truncated form of mouse CHI3L1 was generated and cloned into pcDNA4 His/Max-TOPO vector (Invitrogen, Carlsbad, CA) tagged with Xpress® and 6xHis. After transformation into TOP10 competent cells (Invitrogen), the direction of inserted cDNA was screened by PCR. All PCR primers to generate truncated or point mutated CHI3L1 were generated by using the PCR primers as shown in Table 1. The sequences of the wild-type and mutant forms of CHI3L1 were confirmed at the DNA Core at Massachusetts General Hospital. Transient transfection was performed using Lipofectamine 2000 (Invitrogen) in Cos7 and SW480 cells according to the manufacturer’s instruction.
Table 1.
Sequences of sense and antisense primers for CBD mutants
| Mutations | Sense primers | Anti-sense primers |
|---|---|---|
| ΔCBD | 5’-CACACCTCTACTGAAGCCAG | 5’-CTATACTTCAGCTCCTTTGAGG |
| Δ325 | 5’-CACACCTCTACTGAAGCCAG | 5’-GTTGCCCTTGGTAGCGAAGG |
| Δ339 | 5’-CACACCTCTACTGAAGCCAG | 5’-GAACCCAACCTTGTTTTT GA |
2.3. Luciferase assay
The pNFκB-Luc reporter plasmids and pRL plasmids were obtained from Dr. Ramnik J. Xavier (Massachusetts General Hospital, Boston, MA). Human IL-8 promoter which was subcloned into pGL3b luciferase plasmid (Promega, Madison, WI) was obtained from Dr. Hans-Christian Reinecker (Massachusetts General Hospital). SW480 cells were seeded at the density of 2–3 × 105 cells/well in 24-well tissue culture plates (Corning Incorporated, Corning, NY). After seeding the cells for 24 hours, cells were transfected with 0.5 µg/well of wild type or ΔCBM mouse CHI3L1 vector with 10 ng/well of the reporter plasmids (NFκB-Luc or the IL-8 -Luc) and 0.4 ng of Renilla plasmid (control vector) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 24 hours, luciferase activity was measured using the dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer’s instructions and normalized relative to Renilla activity.
2.4. Enzyme-linked Immunosorbent Assay (ELISA)
For ELISA, the procedure was performed following the instruction protocol from R&D systems (Minneapolis, MN). The optical density at 450 nm was read by an Auto-Reader (Bio-Tec Instruments, Burlington, VT). To standardize the assay, recombinant IL-8 was used as a positive control. A standard curve was created with the optical density of 2000 pg/ml to 62.5 pg/ml of recombinant IL-8. The quantity of soluble IL-8 in the culture supernatant was calculated using this standard line. For the inhibition of CHI3L1 activity, 250 µg/mL of affinity purified anti-CHI3L1 antibody (Affinity BioReagents, Golden, CO) [9] was added to the cell culture. As the control, 250 µg/mL of purified normal rabbit IgG (Bethyl Laboratories, Montgomery, TX) was used.
2.5. Indirect Immunofluorescence and confocal microscopic analyses
SW480 cells were seeded on 4 chamber polystyrene vessel tissue culture treated glass slides (BD Biosciences, Bedford, MA) and were transfected with CHI3L1 willdtype or mutant constructs. After 12–15 hours of transfection, cells were fixed with methanol and stained with mouse anti-Xpress monoclonal antibody (Invitrogen) or rabbit anti-calnexin antibody (Enzo Life Sciences, Plymouth Metting, PA) followed by FITC-horse anti-mouse IgG (Vector) or PE-goat anti-rabbit IgG (Vector), respectively, as previously described [9]. The stained sections were examined with a confocal microscope (model Radiance 2000; Bio-Rad, Hercules, CA) using multi-tracking for two-color imaging. Image acquisition was performed with LaserSharp Scanning software (Bio-Rad). The CHI3L1 activity was inhibited same as described above.
2.6. Immunoblot Analysis
Western blot analysis was performed as previously described. Briefly, cells were washed and then homogenized in a lysis buffer containing 50 mM Tris (pH8.0), 0.5% NP-40, 1 mM EDTA, 150 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM, phenylmethylsulfonyl fluoride, and a tablet of protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). After being lysed on ice by 100µL lysis buffer for 30 min, the cell lysates were centrifuged at 10,000 rpm for 10min and the supernatant was collected for measuring protein concentration by using the BCA protein assay kit (Pierce Company, Rockford, IL, USA). Phosphorylation or total of MAPK p42/p44 (Thr202/Tyr204), MAPK p38 (Thr180 /Tyr 182), c-JunNH2-terminal kinase (JNK)/SAPK (Thr183 /Tyr185), and Akt (Thr 308/Ser 473) were detected by antibodies obtained from Cell Signaling Technology. After stripping antiphospho Abs using Western blot stripping buffer (Pierce, Rockford, IL), the membranes were re-probed by anti total antibodies.
2.7. Statistical analysis
Statistical significance was evaluated by the Mann-Whitney U test for non-parametric data or the student t test for parametric data.
3. Results
3.1. Cytoplasmic cellular localization and membrane-association of CHI3L1 protein was mainly regulated by CBM
Although CHI3L1 has no complete chitin-binding domain like chitotriosidase and acidic mammalian chitinase, it still preserves a carbohydrate-binding motif which enables it to interact with chitin and chito-oligisaccharides [1]. In the previous study, we demonstrated that anti-CHI3L1 antibody specifically recognizes CHI3L1 peptide 325–339 amino acid residues within the chitin-binding motif, ameliorated dextran sulfate sodium (DSS)-induced acute colitis [9]. We also showed that inhibition of CHI3L1 by anti-CHI3L1 antibody significantly reduced the adhesion of chitin-binding protein expressing E. coli to CECs [17]. From these results, CBM in CHI3L1 seems to be important in interacting with intestinal bacteria and in maintaining chronic inflammation in the colon, but the biological significance of the CBM in CHI3L1 on CECs remains unknown. To address this issue, we generated three types of CBM-mutant forms in CHI3L1: a truncated form of the whole CBM from the 309–381th residues (ΔCBM mutant), one partially deleted from the 325–381th residues (Δ325 mutant) and one from the 339–381th residues (Δ339 mutant) [Figure 1A]. To determine the cellular localization of the CHI3L1 protein, we examined the subcellular localization of CHI3L1 protein expression by immunofluorescence confocal microscopy. Cos-7 cells, which show at least 80% transfection efficiency, were transiently transfected with pcDNA4His/Max CHI3L1 wild type (WT) expression plasmid [Figure 1B]. The CHI3L1 protein was strongly expressed in the peri-nucleic compartment, which was co-localized with calnexicin, a specific marker for endoplasmic reticulum [Figure 1B]. In addition, CHI3L1 was expressed throughout the cytoplasm with occasional expression near the plasma membrane [Figure 1B]. For the purpose of identifying the protein expression and subcellular localization patterns of the three CHI3L1-mutants (ΔCBM, Δ325 and Δ339), we transfected the mutants- and LacZ control-vectors (pcDNA4His/max backbone) to Cos7 cells as well as SW480 cells. As shown in the Figure 1C, ΔCBM and Δ325 showed restricted protein expression in the peri-nuclear compartment, which were co-localized with calnexin, while WT and Δ325-mutant showed peri-nucleic as well as cytoplasmic protein expressions in COS7 and SW480 cells. The control LacZ protein diffusely expressed in the cytoplasmic compartment with a minor peri-nuclear association pattern [Figure 1C].
Figure 1.
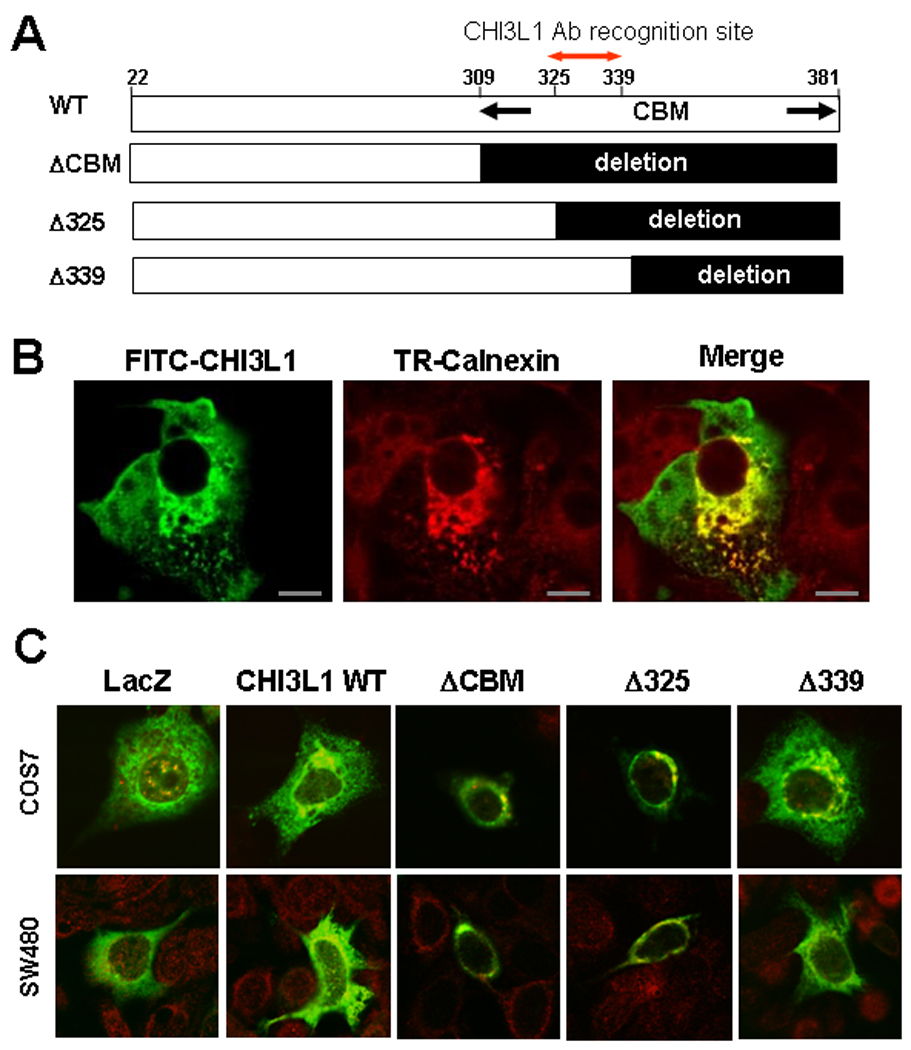
CHI3L1 membrane association and peri-nucreic accumulation is C-terminal chitin-binding domain dependent. A: Schema of CHI3L1 and its deletion mutants in the Ce-terminal chitin-binding motif/domain (CBD). B: COS7 cells were transiently transfected with pCDNA4 HisMax CHI3L1 WT vector, stained with FITC-anti-Xpress antibody (left), Texas Red-calnexin antibody (center), and both as merged image (right), which were analyzed by confocal microscope. Bars, 20 µm. C: COS7 and SW480 cells were transfected with pcDNA HisMax Lacz (control), CHI3L1 WT or CHI3L1 deletion-mutants (ΔCBD, Δ325, and Δ339), were stained with FITC anti- Xpress and Texas Red-anti-calnexin antibodies, and were analyzed by confocal microscope. Magnifications: 240X, objective for COS7 cells, 180×, objective for SW480 cells.
3.2. Chitin-binding motif in CHI3L1 mainly regulates the IL-8 production but not NF-κB activation in CECs
To investigate the biological function of CBM of CHI3L1 in CECs, we transfected SW480 cells with control vector, and CHI3L1 WT- or ΔCBM-containing pcDNA4 vectors. As shown in Figure 2A, CHI3L1 WT and ΔCBM vector transfected cells induced a significant increase of NF-κB activation as compared to non-transfected or control (LacZ) vector transfected cells. In contrast, IL-8 promoter activation was enhanced in CHI3L1 WT- and Δ339- but not in ΔCBM- or Δ325- vector transfected cells as compared to controls [Figure 2B]. The IL-8 release in the culture supernatant within 24 hours after stimulating with purified CHI3L1 (80 ng/ml) and TNFα (25 ng/ml) was analyzed by ELISA. CHI3L1 significantly (p<0.01) enhanced the IL-8 production but was less significant than TNFα (p<0.005) as compared with non-stimulated SW480 cells [Figure 3A]. In addition, the IL-8 production by CHI3L1 is specifically blocked by the optimal concentration (250 µg/ml) of polyclonal anti-CHI3L1 Ab, which specifically recognizes CHI3L1 325–339th amino acid residues [Figure 3B]. In contrast, the same concentration of control rabbit IgG did not alter the effect of CHI3L1 in IL-8 production [Figure 3C]. To further examine whether IL-8 production is specifically regulated by the 325–339th residues of CBM in CHI3L1, the release of IL-8 by SW480 cells transfected with CHI3L1 or mutant forms of CHI3L1 was measured. The amount of IL-8 in the culture supernatant was significantly increased after transfecting with CHI3L1 WT and Δ339-mutant but was not altered with the transfection of ΔCBM- or Δ325-mutants [Figure 3C]. Therefore, 325–339th residues of CBM in CHI3L1 is a critical region in IL-8 promoter activation as well as IL-8 production in CECs. These results suggest that MAPK families may play a role in activating the IL-8 promoter with chitin-binding motif of CHI3L1.
Figure 2.
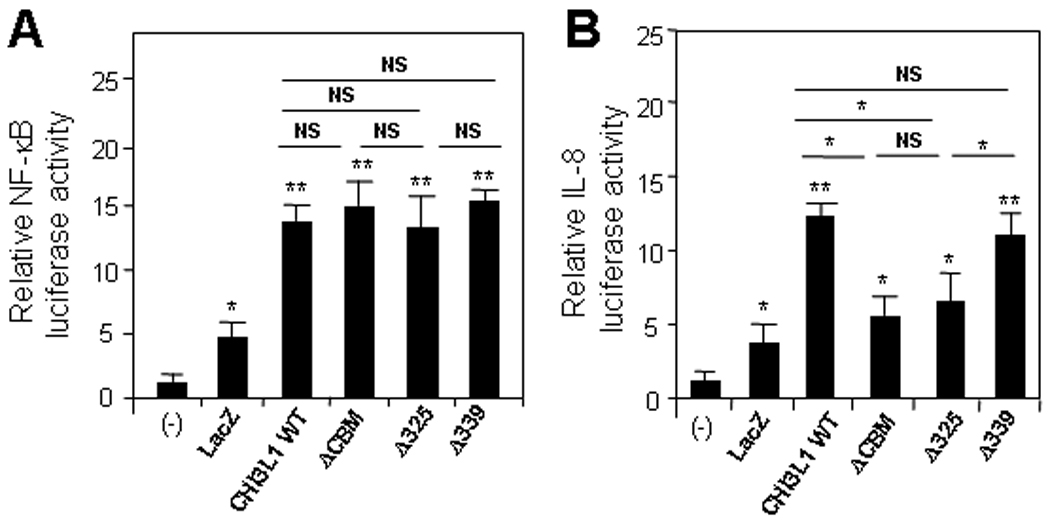
Chitin binding motif/domain of CHI3L1 is necessary for promoter activity of IL-8 but not for NF-κB. SW480 cells were transfected with 0.5 µg/well CHI3L1 expression-, mutants (ΔCBD, Δ325, and Δ339) or control-vector. NF-kB (A) and IL-8-promoter (B) activities were determined by using NF-kB- or IL-8 luciferase reporter assay and were normalized with renilla after 24 hours of transfection. *P<0.05 and **P<0.01 versus non-transfected cells (−) or comparison between the specified groups.
Figure 3.
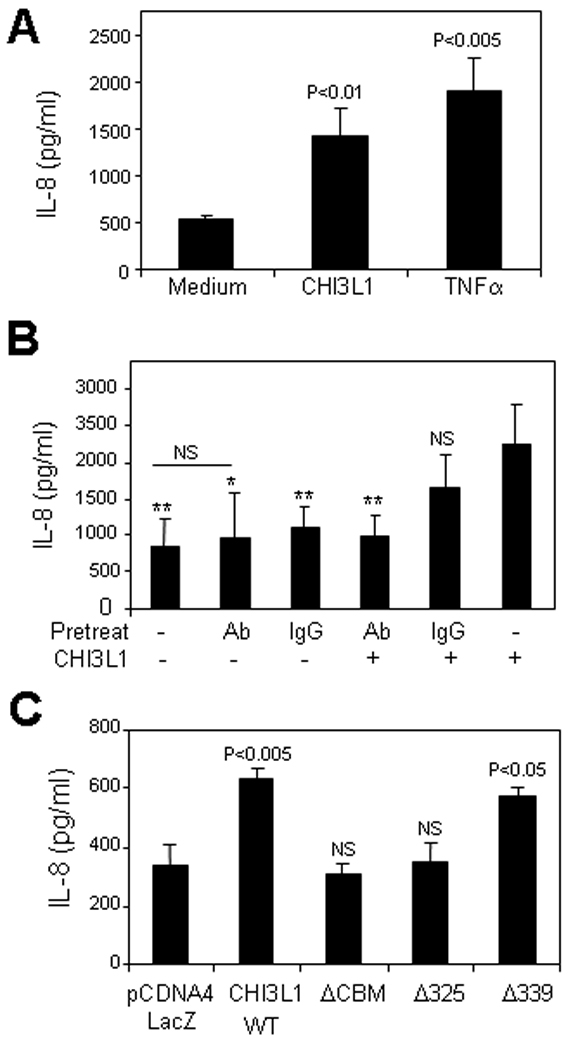
IL-8 secreted in a culture-supernatant of SW480 cells were detected by ELISA after being stimulated with/without purified CHI3L1 (80 ng/ml) or TNFα (25 ng/ml) (A), pretreated with/without rabbit anti-CHI3L1 antibody (250 µg/ml; shown as Ab) or normal rabbit IgG (250 µg/ml; shown as IgG) and transfectec with/without CHI3L1 expression vector (B) or transfected with PCDNA4 LacZ-, CHI3L1 WT- or CHI3L1-deletion mutants (ΔCBD, Δ325, and Δ339)-expressing vectors (C).
*P<0.05 and **P<0.01, versus CHI3L1 WT-vector transfected cells (B) or LacZ-vector transfected cells (C).
3.3. CBM-containing region of CHI3L1 plays a crucial role in AKT Thr308 phosphorylation mediated IL-8 production but not NF-κB activation in SW480 cells
To further examine the involvement of intracellular signaling cascades in CHI3L1 mediated IL-8 activation, we performed phosphor-western blot analysis. The result showed that there is no significant difference between the transfection of the ΔCBM mutant form of CHI3L1 or WT CHI3L1 in phosphorylation of p42/44 MAPK, p38 MAPK, JNK/SAPK, Akt-serine 473 residue [Figure 4A]. In contrast, CHI3L1 WT significantly enhanced the phosphorylation of Akt Thr 308 compared to control-vector transfected or ΔCBM transfected SW480 cells [Figure 4A]. The phosphorylation of Akt-threonine 308 residue is significantly reduced after transiently transfecting with Δ325- or ΔCBM- vector [Figure 4B]. In addition, transfection with Δ339-vector also reduced the level of Akt-thr phosphorylation as compared to the WT CHI3L1-vector transfected SW480 cells [Figure 4B]. The removal of large portions of the CHI3L1 protein in three mutants may affect the protein folding and/or biological effect of this protein. Therefore, we examined whether anti-CHI3L1 antibody, which specially recognize the 325–339 amino acids in CHI3L1, has an influence on the phosphorylation of Akt-thr. We confirmed that anti-CHI3L1 Ab treatment efficiently blocked the Akt-thr phosphorylation to the control level as compared to the same concentration of rabbit IgG-treated SW480 cells [Figure 4C]. In summary, the CBM of CHI3L1 plays a pivotal role in enhancing the activation of Akt pathway in CECs after specifically phosphorylating the Thr 308 residue.
Figure 4.
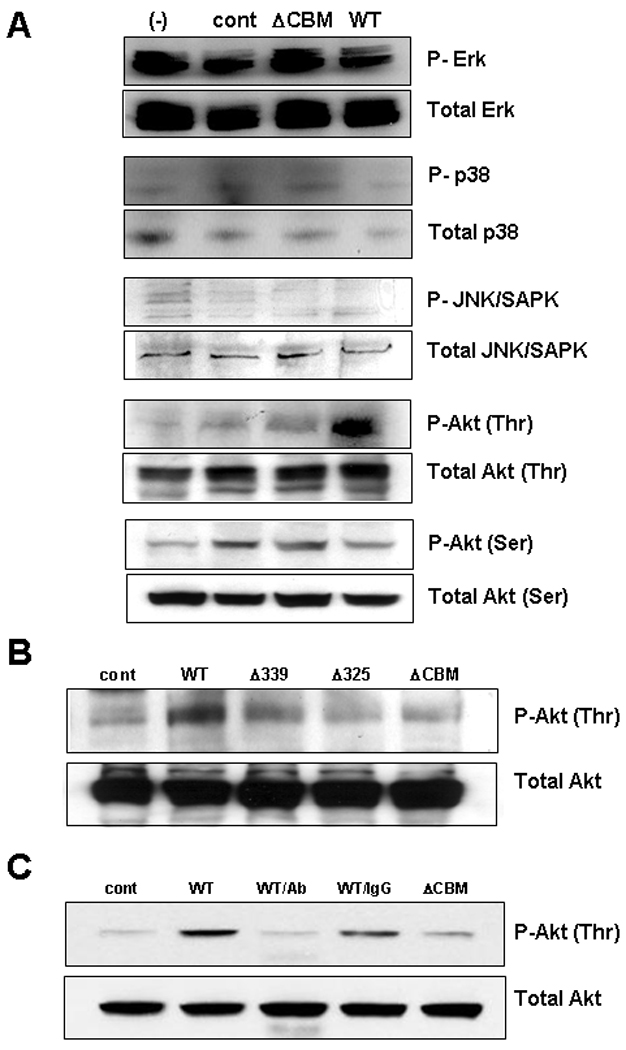
CHI3L1 Chitin-binding motif/domain enhances the specific phosphorylation of Akt Thr 308 in SW480 cells. (A) Phospho- and total-Erk (MAPKp42/p44), MAPKp38, JNK/SAPK, AKT Thr 308, and AKT Ser 473 were analyzed by western blot analysis in SW480 cells which were transiently transfected with/without pcDNA4 LacZ vector (control), CHI3L1 (WT) or ΔCBD-expression vector. Phospho- and total-Akt Thr 308 were detected by western blot after transfected with pcDNA4 Lacz (control), CHI3L1 (WT) or CHI3L1 deletion-mutants (ΔCBD, Δ325, or Δ339) (B) or transfected with pcDNA4 Lacz (cont)-, ΔCBD- or CHI3L1 (WT) or expression vector pre-treating with anti-CHI3L1 Ab (250 µg/ml; shown as WT/Ab) or normal rabbit IgG (250 µg/ml; shown as WT/IgG) (C) in SW480 cells. Proteins were isolated 36–48 hours after the transfection with a lysis buffer as detailed in Materials & Methods.
Discussion
According to the BLAST algorithm, human mammalian chitinases (including acidic mammalian chitinase and chitotriosidase) and non-enzymatic chitinase-like proteins (including CHI3L1, CHI3L2, and stabilin-1 interacting chitinase-like protein), which belong to the glycoside hydrolase 18 family, preserve a relatively high sequential identity with a conserved structure containing three major and distinct domains: a short signal peptide sequence, a catalytic domain and a carbohydrate (chitin)-binding domain or motif [23]. The Chitin-binding motif (CBM) is located at the C-terminal region of human mammalian chitinases and mediates binding to chitin or chito-oligosaccharide. In general, the C-terminal region is required for glycohydrolase activity in insoluble- but not soluble-substrates for mammalian chitinases that have chitinolytic enzymatic activity [6, 20]. CHI3L1 lacks critical six cysteine residues in the C-terminus, but still preserves a CBM and is able to bind chitin and chito-oligosaccharides with high affinity [24]. Several other chi-lectins, including mouse chitinase 3-like 3 (CHI3L3 or Ym 1), a 45 kDa secretory murine protein expressed in alternatively-activated macrophages, human chitinase 3-like-2 (CHI3L2 or YKL-39), a 39 kDa chitinase with a predominant expression in lymphoid cells, and human oviduction, a 43 kDa protein secreted by oviductal epithelial cells, have sequential similarity in CBM and have some close biological function to CHI3L1. These chi-lectins possess a range of biological roles and can interact with restricted carbohydrate ligands including chitin and chito-oligosaccharides [25–28]. Houston et al have revealed clearly that CHI3L1 binds with chitin oligomers as a physiologic ligand by tightly interacting with the tryptophan residues within the C-terminal CBM, and these specific interactions are associated with a significant conformational change in the CHI3L1 protein [29]. In particular, His209 in CHI3L1 seems to play a role as a chitin-sensing residue and enhances the initial innate immune responses at the site of invasion by chitin-containing organisms such as fungi and nematodes. Therefore, the C-terminal CBM seems to have a critical and unique role in interacting with specific sugar components in microorganisms. In addition, Ling’s group extensively analyzed CHI3L1-mediated Akt-pathway activation in human connective-tissue cells in a dose- and time-dependent manner [30]. In the current study, we have generated three mutant forms of C-terminal CBM in CHI3L1 to test the biological significance of the CBM of the CHI3L1 molecule, and we proved that CBM in CHI3L1 plays a critical role in enhancing the activation of the Akt pathway followed by an enhanced production of IL-8 in CECs. It is well known that the functions of Akt in the cell are diverse, but all result in anti-apoptotic effects or pro-cell proliferating effects by phosphorylating a variety of downstream targets including BAD (BCL2 antagonist of cell death), Caspase 9, FKHR (forkhead transcriptional factor), mTOR (mammalian target of Rapmycin), IKK (I-κB kinase), NF-κB (nuclear factor-κB) and GSK3 (glycogen synthase kinase-3) [31]. Here we confirmed that CHI3L1-overexpression on CECs efficiently activated an Akt-signaling pathway. Furthermore, we also revealed that the C-terminal 72 amino acids, in particular from the 325th through the 339th amino acids, are a necessary and sufficient region for the CHI3L1-mediated Akt-signal activation in CECs, which is closely associated with exacerbation and chronicity of IBD.
Our group previously demonstrated that some potentially pathogenic bacteria (e.g., Adherent Invasive Escherichia coli) are strongly associated with the development of IBD by interacting with CHI3L1 molecules on CECs [9, 17]. A blocking experiment utilizing anti-CHI3L1 Ab as well as short interfering RNA (siRNA) clearly demonstrated that potentially pathogenic bacterial adhesion to CECs is CHI3L1 dependent [9, 17]. In addition, our follow-up study has shown that CHI3L1 is involved in the enhancement of the chitin-binding protein expressing bacterial adhesion to CECs [17]. Human and mouse CHI3L1 also have been identified as proteins expressed during conditions of tissue remodeling [32, 33], atherogenesis [34], oncogenesis [35], and cell adhesion/migration [36], and an Akt pathway activation seems to be associated with some of those CHI3L1-mediated biological functions such as cell-proliferation and - survival [30].
CHI3L1 has been used as a sensitive biomarker for early detection of inflammatory disorders including rheumatoid arthritis and IBD [10, 14, 37]. In addition, a high CHI3L1 level in serum was an independent prognostic parameter for short survival of patients with colorectal cancer, and prostate cancer [16, 38], suggesting that CHI3L1 may be a growth factor of cancer cells and/or may protect them from undergoing apoptosis [10]. Furthermore, increased serum levels of CHI3L1 have been reported in patients with several inflammatory disorders including IBD [14], asthma [39] and rheumatoid arthritis [40], while serum CHI3L1 is rarely detectable in healthy individuals. Therefore serum CHI3L1 has been used as a useful prognostic biomarker for IBD in clinical settings [14], and CHI3L1 may play a pathogenic role during the development of IBD. Another group also demonstrated a biological significance of CHI3L1 that this molecule is one of the 31 upregulated cDNAs in Helicobacter felis-infected hypergastrinemic transgenic mice (FVB/N background) as compared to three different control groups detected by the gene expression profile of oligonucleotide cDNA microarray analysis [41]. Of note, CHI3L1 increased 90-fold in the Helicobacter felis-infected hypergastrinemic transgenic mice as compared to uninfected wild-type FVB/N mice. Helicobacter felis-infected hypegastrinemic transgenic mice had the highest change among the entire upregulated genes in the study [41]. These results strongly suggest that CHI3L1 may have a specific affinity with a certain component in some kinds of pathogenic bacteria. Although chitin is not expressed in bacteria, the majority of chitinase-producing pathogenic microorganisms contain a gene encoding for the chitin-binding protein, which potentially interacts with the binding ability between chitinase-producing bacteria and chitin or chitin-like sugar components on CECs [17, 42]. Therefore, CBM in CHI3L1 on CECs must be very important region for interacting with the chitinase-producing bacteria. In addition, in this study, we first demonstrated that the CBM of CHI3L1 is also associated with the continuous activation of Akt-signaling pathway and the following IL-8 production in CECs. This finding may be useful for future development of therapeutic strategies to prevent persistent and chronic epithelial cell proliferation and activation during inflammatory conditions by utilizing anti-CHI3L1 antibody which specifically reacts with the CBM of CHI3L1. In addition, inhibition of CHI3L1 using anti-CHI3L1 antibody, which specifically recognize the 325–339th residues of CBM in CHI3L1 [9], may efficiently block the continuous activation of the phosphorylation of Akt-signaling pathway. In summary, the specific inhibition of CHI3L1 expression on CECs not only blocks the potentially pathogenic bacterial adhesion and invasion on/into CECs, but also may inhibit the following activation of Akt-mediated signaling in CECs and may regulate the chronic inflammation in colon.
Conclusions
The chitin-binding motif of CHI3L1 is specifically associated with activation of the threonine residue of Akt in CECs. Downstream, CHI3L1-mediated signaling enhances the secretion of pro-inflammatory cytokines including IL-8 and TNFα and actively promotes CEC proliferation in a dose-dependent manner. Constitutive activation of Akt pathway at CBM of CHI3L1 may be associated with exacerbation and chronicity of IBD as well as other inflammatory disorders.
Acknowledgement
This work has been supported by National Institute of Health (DK 80070, DK74454, DK64289 and DK43351), and grants from the Eli and Edythe L. Broad Medical Foundation and American Gastroenterological Association Foundation to EM.
The authors are grateful to Drs. Daniel K. Podolsky, Hans-Christian Reinecker, Ramnik J. Xavier, Scott B. Snapper, Atsushi Mizoguchi and Cathryn R. Nagler for helpful discussion and advice. The authors also thank Dr. Carmen Alonso Cotoner for her professional assistance in confocal microscopic analysis. We would thank to Mr. Terry Danford Lott for his excellent secretarial assistance in preparing this manuscript and Mr. A. Mardoniya Moghimi and Ms. Satoko Toei-Shimizu for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Fusetti F, Pijning T, Kalk KH, Bos E, Dijkstra W. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003;278:37753–37760. doi: 10.1074/jbc.M303137200. 2003. [DOI] [PubMed] [Google Scholar]
- 2.Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997;7:637–644. doi: 10.1016/s0959-440x(97)80072-3. [DOI] [PubMed] [Google Scholar]
- 3.van Aalten DM, Synstad B, Brurberg MB, Hough E, Riise BW, Eijsink VG, Wierenga RK. Structure of a two-domain chitotriosidase from Serratia marcescens at 1.9-A resolution. Proc. Natl. Acad. Sci. USA. 2000;97:5842–5847. doi: 10.1073/pnas.97.11.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrera-Estrella A A, Chet I I. Chitinases in biological control. EXS. 1999;87:171–184. doi: 10.1007/978-3-0348-8757-1_12. [DOI] [PubMed] [Google Scholar]
- 5.Volck B, Price PA, Johansen JS, Sorensen O, Benfield TL, Nielsen HJ, Calafat J, Borregarrd N. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc. Assoc. Am. Physicians. 1998;110:351–360. [PubMed] [Google Scholar]
- 6.Krause SW, Rehli M, Kreutz M, Schwarzfischer L, Paulauskis JD, Andreesen R. Differential screening identifies genetic markers of monocyte to macrophage mutations. J. Leukoc. Biol. 1996;60:540–545. doi: 10.1002/jlb.60.4.540. [DOI] [PubMed] [Google Scholar]
- 7.Renkema GH, Boot RG, Strijland A, Donker-Koopman WE, van den Berg M, Muijsers AO, Aerts JM. Synthesis, sorting, and processing into distinct isoforms of human macrophage chitotriosidase. Eur. J. Biochem. 1997;244:279–285. doi: 10.1111/j.1432-1033.1997.00279.x. [DOI] [PubMed] [Google Scholar]
- 8.Nyirkos P P, Golds EE. Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem. J. 1993;269:265–268. doi: 10.1042/bj2690265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterol. 2006;130:398–411. doi: 10.1053/j.gastro.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 11.Kirkpatrick RB, Emery JG, Connor JR, Dodds R, Lysko PG, Rosenberg M. Induction and expression of human cartilage glycoprotein 39 in rheumatoid inflammatory and peripheral blood monocyte-derived macrophages. Exp. Cell Res. 1997;237:46–54. doi: 10.1006/excr.1997.3764. 1997. [DOI] [PubMed] [Google Scholar]
- 12.Johansen JS, Stoltenberg M, Hansen M, Florescu A, Horslev-Petersen K, Lorenzen I, Price PA. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology. 1999;38:618–626. doi: 10.1093/rheumatology/38.7.618. [DOI] [PubMed] [Google Scholar]
- 13.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Eng. J. Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 14.Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2003;38:599–605. doi: 10.1080/00365520310000537. [DOI] [PubMed] [Google Scholar]
- 15.Johansen JS, Cintin C, Jorgensen M, Kamby C, Price PA. Serum YKL-40: a new potential marker of prognosis and location of metastases of patients with recurrent breast cancer. Eur. J. Cancer. 1995;31:1437–1442. doi: 10.1016/0959-8049(95)00196-p. [DOI] [PubMed] [Google Scholar]
- 16.Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br. J. Cancer. 1999;79:1494–1499. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawada M, Chen CC, Arihiro A, Nagatani K, Watanabe T, Mizoguchi E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab. Invest. 2008;88:883–895. doi: 10.1038/labinvest.2008.47. [DOI] [PubMed] [Google Scholar]
- 18.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem. Biophys. Res. Commun. 2001;285:926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 19.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem. J. 2004;380:651–659. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjoelker LW, Gosting L, Frey S, Hunter CL, Trong HL, Steiner B, Brammer H, Gray PW. Structural and functional definition of the human chitinase chitin-binding domain. J. Biol. Chem. 2000;275:514–520. doi: 10.1074/jbc.275.1.514. [DOI] [PubMed] [Google Scholar]
- 21.Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J. Gastroenterol. 2009;15:5249–5259. doi: 10.3748/wjg.15.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J. Biomed. Biotechnol. 2011. 2011:23. doi: 10.1155/2011/342637. Article ID 342637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kzhyshkowska J, Mamidi S, Gratchev A, Kremmer E, Schmuttermaier C, Krusell L, Haus G, Utikai J, Schledzewski K, Scholtze J, Goerdt S. Novel stabilin-1 interacting chitinase-like protein (SI-CLP) is up-regulated in alternatively activated macrophages and secreted via lysosomal pathway. Blood. 2006;107:3221–3228. doi: 10.1182/blood-2005-07-2843. [DOI] [PubMed] [Google Scholar]
- 24.Renkema GH, Boot RG, Au FL, Domker-Koopman WE, Strijland A, Muijsers AO, Hrebicek M, Aerts JM. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosylhydrolases secreted by human macrophages. Eur. J. Biochem. 1998;15:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]
- 25.Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J. Bio. Chem. 2006;281:21082–21095. doi: 10.1074/jbc.M601153200. [DOI] [PubMed] [Google Scholar]
- 26.Rehli M, Krause SW, Andreesen R. Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics. 1997;43:221–225. doi: 10.1006/geno.1997.4778. [DOI] [PubMed] [Google Scholar]
- 27.Chang NC, Hung SI, Hwa KY, Kato I, Chen JE, Liu CH, Chang AC. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J. Bio. Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 28.Malette B, Paquette Y, Merlen Y, Bleau G. Oviductins possess chitinase- and mucin-like domains: a lead in the search for the biological function of these oviduct-specific ZP-associating glycoproteins. Mol. Reprod. Dev. 1995;41:384–397. doi: 10.1002/mrd.1080410315. [DOI] [PubMed] [Google Scholar]
- 29.Houston DR, Recklies AD, Krupa JC, van Aalten DMF. Structure and ligand-induced conformational change of the 39-kDa glycoprotein from human articular chondrocytes. J. Biol. Chem. 2003;278:30206–30212. doi: 10.1074/jbc.M303371200. [DOI] [PubMed] [Google Scholar]
- 30.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem. J. 2002;365:119–126. doi: 10.1042/BJ20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/surviving pathway. J. Biol. Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 32.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J. Biol. Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 33.Hu B, Trinh K, Figueiira WF, Price PA. Isolation and sequence of a novel human chondrocyte protein related to mammalian members of the chitinase protein family. J. Biol. Chem. 1996;271:19415–19420. doi: 10.1074/jbc.271.32.19415. [DOI] [PubMed] [Google Scholar]
- 34.Boot RG, Van Achterberg TAE, van Aken BE, Renkema GH, Jacobs MJHM, Aerts JMFG, de Vries CJ. Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb. Vasc. Biol. 1999;19:687–694. doi: 10.1161/01.atv.19.3.687. [DOI] [PubMed] [Google Scholar]
- 35.Morrison BW, Leder P. neu and ras initiate murine mammary tumors that share genetic markers generally absent in c-myc and int-2-initiated tumors. Oncogene. 1994;9:3417–3426. [PubMed] [Google Scholar]
- 36.Nishikawa KC, Millis AJT. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp. Cell. Res. 2003;287:79–87. doi: 10.1016/s0014-4827(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 37.Peltomma R, Paimela L, Harvey S, Helve T, Leirisalo-Repo M. Increased level of YKL-40 in sera from patients with early rheumatoid arthritis: a new marker for disease activity. Rheumatol. Int. 2001;20:192–196. doi: 10.1007/s002960100115. [DOI] [PubMed] [Google Scholar]
- 38.Brasso K, Christensen IJ, Johansen JS, Teisner B, Garnero P, Price PA, Iversen P. Prognostic value of PINP, bone alkaline phosphatase, CTX-1, and YKL-40 in patients with metastatic prostate carcinoma. Prostate. 2006;66:503–513. doi: 10.1002/pros.20311. [DOI] [PubMed] [Google Scholar]
- 39.Ober C, Tan Z, Sun Y, Possicj JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, Lester LA, Gern JE, Lemanske RF, Jr, Nicolae DL, Elias JA, Chupp GL. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N. Eng. J. Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansen JS, Hvolris J, Hansen M, Becker V, Lorenzen I, Price PA. Serum YK-40 levels in healthy children and adults. Comparison with serum and synovial fluid levels of YKL-40 in patients with osteoarthritis or trauma of the knee joint. Br. J. Rheumatol. 1996;35:553–559. doi: 10.1093/rheumatology/35.6.553. 1996. [DOI] [PubMed] [Google Scholar]
- 41.Takaishi S, Wang STC. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007;98:284–293. doi: 10.1111/j.1349-7006.2007.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaaje-Kolstad G, Horn SJ, van Arlten DM, Synstad B, Eijsink VG. The non-catalytic chitin-binding protein CBP21 from Serratia marcescens is essential for chitin degradation. J. Biol. Chem. 2005;280:28492–28497. doi: 10.1074/jbc.M504468200. [DOI] [PubMed] [Google Scholar]


