Abstract
OBJECTIVE
Cerebrospinal fluid (CSF) biomarkers of Alzheimer’s disease (AD) are currently being considered for inclusion in revised diagnostic criteria for research and/or clinical purposes to increase the certainty of ante-mortem diagnosis. Establishing biomarker validity requires demonstration that the assays are true markers of underlying disease pathology (e.g., amyloid plaques and/or neurofibrillary tangles) in living individuals.
DESIGN
We compared the performances of the two most commonly used platforms, INNOTEST® ELISA and INNO-BIA AlzBio3 for measurement of CSF amyloid-beta (Aβ) and tau(s), for identifying the presence of amyloid plaques in a research cohort (n=103). Values obtained for CSF Aβ1-42, total tau and phosphorylated tau181 (p-tau181) using the two assay platforms were compared to brain amyloid load as assessed by positron emission tomography using the amyloid imaging agent, Pittsburgh Compound B (PIB).
SUBJECTS
Research volunteers who are cognitively normal or have very mild to moderate AD dementia.
RESULTS
The two assay platforms yielded different (~2–6-fold) absolute values for the various analytes, but relative values were highly correlated. CSF Aβ1-42 correlated inversely, and tau and p-tau181 correlated positively, with the amount of cortical PIB binding, albeit to differing degrees. Both assays yielded similar patterns of CSF biomarker correlations with amyloid load. The ratios of total tau/Aβ1-42 and p-tau181/Aβ1-42 outperformed any single analyte, including Aβ1-2, in discriminating individuals with versus without cortical amyloid.
CONCLUSIONS
The INNOTEST® and INNO-BIA CSF platforms performed equally well in identifying individuals with underlying amyloid plaque pathology. Differences in absolute values, however, point to the need for assay-specific diagnostic cut-point values.
Keywords: Alzheimer’s disease; amyloid; biomarkers; cerebrospinal fluid; imaging (PET, MRI) in dementias; Pittsburgh Compound B
Introduction
Alzheimer’s disease (AD), the most common cause of dementia in the elderly, currently affects an estimated 35.6 million individuals world-wide, with numbers expected to triple and reach epidemic proportions by the year 2050 (http://www.alz.co.uk/research/statistics.html). Such an aggressive disease trajectory will have a devastating impact on patients, their caregivers, as well as health care resources and public health burden. Appreciation of the societal and economic implications of this projection and disappointing AD clinical trial results to date make it imperative that the AD research, clinical, and pharmaceutical communities work together to better identify those at the very earliest stages of the disease in concert with designing more effective clinical trial strategies. Converging evidence suggests that AD pathology (amyloid plaques and neurofibrillary tangles) begins to develop years, if not decades, prior to the diagnosis of clinical dementia 1–3. This disease state, AD pathology prior to the development of dementia symptoms, has been termed “preclinical” or “presymptomatic” AD. With this in mind, the AD field is experiencing a paradigm shift in the way therapeutic strategies are viewed, from “cure” to “prevention,” with the ultimate goal of intervening very early in the disease process (perhaps even at the initial preclinical stage) in order to prevent neurodegeneration and its clinical sequela, dementia.
Although a definitive diagnosis still requires histopathologic evaluation of the brain at autopsy, AD currently is diagnosed clinically according to criteria published in 1984 4. These criteria (i.e., National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA)), have been reliable for the diagnosis of “Probable AD,” with mean reported sensitivities and specificities of 81% and 70%, respectively 5. However, research published over the past 25 years has led to a recent effort, co-sponsored by the Alzheimer’s Association and the National Institute on Aging, to propose revision of these criteria to expand the scope of what is now considered to be AD (to more accurately reflect the full continuum of the disease), as well as incorporate newly discovered biomarkers of underlying AD pathology that would add to the certainty of ante-mortem diagnosis.
Cerebrospinal fluid (CSF) levels of amyloid-β 1-42 (Aβ1-42) and tau (the primary constituents of plaques and tangles, respectively) have been shown to have diagnostic utility for discriminating AD dementia cases from cognitively normal controls but with a wide range of reported sensitivities and specificities 6. Such discrepancies likely reflect (i) methodologic variables, including the clinical characteristics of the cohorts studied; (ii) biologic variables, such as possible misdiagnosis of cognitively impaired individuals (especially at the earliest clinical stages) and the inevitable inclusion of preclinical cases in the cognitively normal control group (estimated to be 25–35% in these elderly cohorts) 1–2; (iii) the specific CSF collection and processing protocols employed, and (iv) the various CSF assays used. The advent of in vivo amyloid imaging techniques now permits evaluation of the extent to which these CSF measures are reflective of underlying AD pathology in living individuals. Several recent studies have demonstrated that low CSF Aβ1-42 is a good indicator of the presence of cortical amyloid 7–13. However, no one has reported a comparison of the head-to-head performance of the different CSF assays in identifying amyloid status within the same cohort. Since efforts are currently underway to standardize CSF collection, processing, and analytic protocols, we compared the performance of the two most commonly used commercial AD CSF assay platforms (INNOTEST® Enzyme-linked Immunosorbant Assay and INNO-BIA AlzBio3 xMAP technology) for classifying amyloid status in a large cohort of research participants. Endorsement of a given assay platform for standardization purposes will depend upon the extent to which the assay(s) truly mark underlying disease pathology.
Materials and Methods
Participants
Participants were community-dwelling research volunteers enrolled in longitudinal studies of healthy aging and dementia through the Knight Alzheimer’s Disease Research Center at Washington University in St. Louis (WU-ADRC). Participants were in good general health, having no neurological, psychiatric, or systemic medical illnesses that could contribute importantly to dementia, nor medical contraindication to lumbar puncture (LP) for CSF collection or positron emission tomography (PET) with the amyloid imaging agent Pittsburgh Compound B (PIB) 14. One hundred and three participants who had an LP and a PET PIB scan within two years of each other (between January 2004 and November 2008) were included in the analysis. The scan could be before or after the LP, but the majority of subjects (80%) had LP prior to PET PIB (Table 1). The cohort included both demented and cognitively normal participants (see below) in order to obtain a wide range of PIB and CSF biomarker levels. Although clinical status is not a variable of interest in the present study, cognitive status at the annual clinical assessment just prior to LP was determined in accordance with standard protocols and criteria 15–16. A Clinical Dementia Rating (CDR) 17 of 0 indicates cognitively normal, whereas a CDR of 0.5, 1 or 2 indicates very mild, mild or moderate dementia, respectively. All but one dementia diagnosis in this cohort was considered by clinicians to be due to AD; an etiologic diagnosis could not be determined for the one remaining CDR 0.5 participant, who was diagnosed with uncertain dementia. All studies were approved by the Human Research Protection Office at Washington University, and written informed consent was obtained from all participants. Genotyping for apolipoprotein E (APOE) was performed by the WU-ADRC Genetics Core.
Table 1.
Study participant demographics
| Characteristics | |
|---|---|
| Number | 103† |
| Mean age at LP, years (SD) | 67.8 (9.9) |
| Age range, years | 46–89 |
| Gender, % female | 68% |
| APOE genotype, % with at least one ε4 allele | 44% |
| Median LP/PIB interval, days (25th, 75th percentile) | 97 (35, 236) |
| Mean LP/scan interval, days (SD) | 161.7 (166.5) |
| LP/PIB range, days | 0–719 |
| % LP same day or prior to PIB (range, days) | 80% (0–719) |
| % LP after PIB (range, days) | 20% (1–400) |
CDR 0 (n=89), CDR 0.5 (n=11), CDR 1 (n=1), CDR 2 (n=2)
Abbreviations: APOE, apolipoprotein E; CDR, Clinical Dementia Rating; LP, lumbar puncture; PIB, Pittsburgh Compound B scan
CSF collection and processing
CSF (20–30 ml) was collected via standard, sterile technique at 8:00 am after overnight fasting as described 7. CSF (free from visible blood contamination) was obtained from the L4/L5 lumbar space using an atraumatic 22 Ga Sprotte spinal needle via gravity flow into a 50 ml polypropylene tube and put immediately on wet ice. Within 1 hour after collection, samples were gently inverted to avoid possible gradient effects, briefly centrifuged at low speed (2000g, 15 min, 4°C) to pellet any cellular debris and aliquoted (500μl) into polypropylene tubes prior to freezing at −80° C.
CSF biomarker assessment
CSF samples were analyzed for Aβ 1-42, total tau, and phospho-tau181 (p-tau181) by plate-based enzyme-linked immunosorbant assay (ELISA) (INNOTEST®, Innogenetics, Ghent, Belgium) by the Biomarker Core at WU-ADRC according to the manufacturer’s instructions, and by Luminex xMAP bead-based methods (INNO-BIA AlzBio3™, for research-use reagents, Innogenetics, Ghent, Belgium) by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Biomarker Core at the University of Pennsylvania, with performance characteristics and assay conditions as described 18. INNOTEST® reagents included monoclonal capture/detection antibodies 21F12/3D6 for Aβ1-42, AT120/HT7 and BT2 for total tau, and HT7/AT270 for p-tau181. INNO-BIA reagents included monoclonal capture/detection antibodies 4D7A3/3D6 for Aβ1-42, AT120/HT7 for total tau, and AT270/HT7 for p-tau181. Assays were performed by trained core technologists at each site who were blind to the clinical and PIB status of the participants. For all biomarker measures, samples were continuously kept on ice, and assays were performed in duplicate on sample aliquots after a single thaw following initial freezing.
In vivo amyloid imaging with Pittsburgh Compound B (PIB)
All participants underwent in vivo amyloid imaging via PET PIB within two years of LP as described 19. The cerebellum was chosen as a region with very low specific PIB binding for use as a reference region, and Logan graphical analyses were performed to calculate the mean cortical PIB distribution volume (MCBP) 20 for each participant. MCBP was defined by averaging the distribution volumes for the prefrontal cortex, precuneus, lateral temporal cortex and gyrus rectus. For certain analyses, amyloid-positivity was defined by a MCBP cut-off of 0.18 based on prior studies 19, 21.
Statistical analyses
Statistical analyses were carried out using SAS (SAS Institute, Inc., Cary, North Carolina). The relationships between CSF analyte values obtained with each assay platform and between MCBP and the various CSF measures were evaluated using the Spearman rho correlation coefficient (α=0.05). Because different correlations were estimated from the same sample of individuals, these estimates were correlated. Comparative analyses of correlations were based on standard normal tests after applying Fisher’s z-transformation to the correlated correlations 22. Receiver Operating Characteristic (ROC) and Area Under the Curve (AUC) analyses were used to assess the sensitivity and specificity of the various CSF measures to discriminate individuals with cortical amyloid deposition (PIB+) from those without (PIB-) using an MCBP cut-off value of 0.18 19, 21. Because the distributions of biomarker values were skewed, a logarithmic transformation was first used to approximate normality. Sensitivity was then estimated when specificity was fixed at 80% across biomarkers. Confidence intervals for AUCs were based on standard normal distributions 23. Since AUCs were estimated from the same set of individuals, comparative analysis of AUCs across biomarkers took into account the correlation between estimated AUCs and was based on standard normal tests 23–24.
Results
Study participants meeting the LP and PIB interval inclusion criteria included 89 who were cognitively normal (CDR 0), 11 who were very mildly demented (CDR 0.5), one who was mildly demented (CDR 1) and two who were moderately demented (CDR 2) (Table 1). Participants spanned a wide age range (46–89 years), with a mean ± SD of 67.8 ± 9.9 years. Sixty-eight percent were female, and 44% had at least one APOE ε4 allele. The median interval between LP and PIB scan was 97 days (3.2 months). The shortest interval was zero days and the longest was 719 days (1.97 years). The vast majority (80%) of participants had LP prior to PIB, including the 12 with the longest LP-PIB test intervals (ranging from 401 to 719 days).
According to data provided by the kit manufacturer, the intra-assay variability should be <6% with the INNOTEST® ELISAs and <4% with the INNO-BIA platform 25. In the present study, we observed a mean (± SD) coefficient of variation (% CV) of 4.2 ± 3.8%, 4.5 ± 4.8% and 1.7 ± 1.7% for Aβ1-42, total tau and p-tau181, respectively, using INNOTEST® (Table 2). Similar CVs were observed for INNO-BIA, with 3.7 ± 2.8%, 3.6 ± 3.1% and 3.9 ± 3.1% for Aβ1-42, total tau and p-tau181, respectively (Table 2).
Table 2.
Within-assay performance (% coefficient of variability)
| Analyte | Range | Mean (SD) | Median (25th, 75th percentile) | |
|---|---|---|---|---|
| INNOTEST® | Aβ1-42 | 0–16.1 | 4.2 (3.8) | 3.7 (1.45, 5.05) |
| Total tau | 0–20.2 | 4.5 (4.8) | 2.5 (0.9, 6.25) | |
| P-tau181 | 0–10.7 | 1.7 (1.7) | 1.2 (0.7, 2.35) | |
| INNO-BIA | Aβ1-42 | 0.01–11.7 | 3.7 (2.8) | 3.0 (1.5, 5.3) |
| Total tau | 0–15.3 | 3.6 (3.1) | 2.7 (1.3, 5.1) | |
| P-tau181 | 0–15.0 | 3.9 (3.1) | 3.6 (1.2, 6.2) |
Abbreviations: Aβ1-42, amyloid-β1-42; P-tau181, tau phosphorylated at tyrosine 181
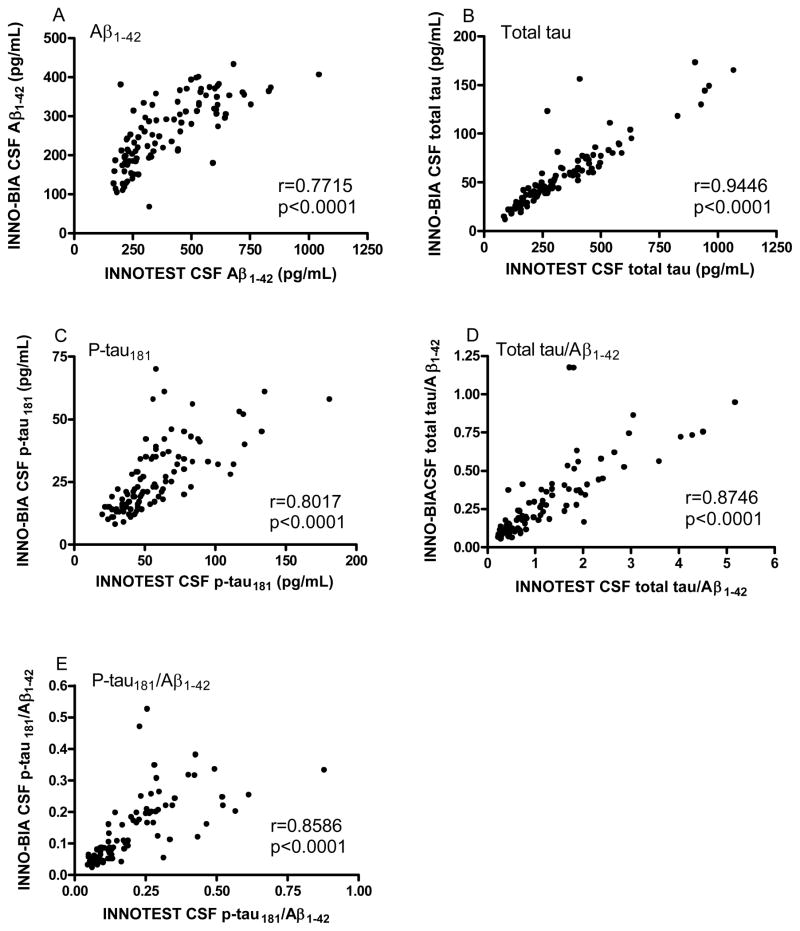
We first assessed the relationship between the analyte values obtained with the two platforms. Although the absolute values for Aβ1-42, total tau and p-tau181 obtained with the two platforms were different, the values correlated well with each other (Aβ1-42, r = 0.7715, p<0.0001; total tau, r = 0.9446, p<0.0001; p-tau181, r = 0.8017, p<0.0001) (Figure 1A–C), as did the ratios of total tau/Aβ1-42 (r = 0.8746, p<0.0001) and p-tau181/Aβ1-42 (r = 0.8586, p<0.0001) (Figure 1D, E).
Figure 1.
Relationship between the assay platforms for the various CSF analytes. Analytes include A) Aβ1-42; B) total tau; C) P-tau181; D) total tau/Aβ1-42; and E) p-tau181/Aβ1-42. r, Spearman rho correlation coefficient.
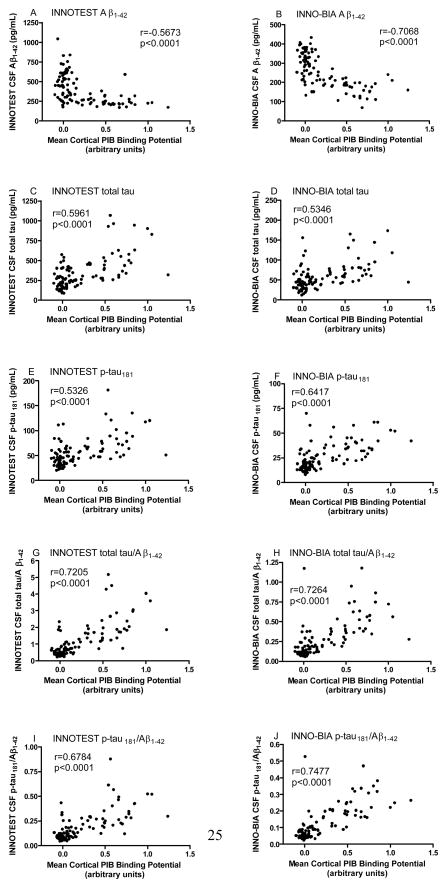
Given that a low level of CSF Aβ1-42 has been shown by several groups to be a marker of brain amyloid (as assessed by PET PIB), we next compared head-to-head the amount of cortical PIB binding (MCBP) with CSF Aβ1-42 as determined by the two platforms. Overall, increased MCBP, indicating the presence of cortical amyloid, was associated with low levels of CSF Aβ1-42 as determined by both assays (Figure 2A, B). MCBP was positively correlated with levels of CSF total tau and p-tau181 (Figure 2C–F), as well as the tau/Aβ1-42 and p-tau181/Aβ1-42 ratios (Figure 2G–I). When comparing PIB-CSF correlation coefficients between the two assay platforms, the correlation between MCBP and INNO-BIA Aβ1-42 (r=−0.7068) was stronger than that for MCBP and INNOTEST® Aβ1-42 (r=−0.5673, p=0.00006); INNOTEST® total tau (r=0.5961) was slightly stronger than that for INNO-BIA total tau (r=0.5346, p=0.0045); INNO- BIA p-tau181 (r=0.6417) was stronger than that for INNOTEST® p-tau181 (r=0.5326, p=0.0053); INNO-BIA p-tau181/Aβ1-42 (r=0.7477) was stronger than that for INNOTEST® p-tau181/Aβ1-42 (r=0.6784, p<0.00001); whereas the correlation between MCBP and the ratio of total tau/Aβ1-42 did not differ between the two platforms (INNOTEST® r=0.7205, INNO-BIA r=0.7264, p=0.4674). These relationships were not driven by different distributions between the CDR 0 and CDR>0 groups; similar correlative relationships were observed within the individual clinical groups (data not shown).
Figure 2.
Relationship between CSF analytes and cortical amyloid load. Analytes include A, B) Aβ1-42; C, D) total tau; E, F) P-tau181; G, H) total tau/Aβ1-42; and I, J) p-tau181/Aβ1-42. Left panels, INNOTEST®. Right panels, INNO-BIA. r, Spearman rho correlation coefficient.
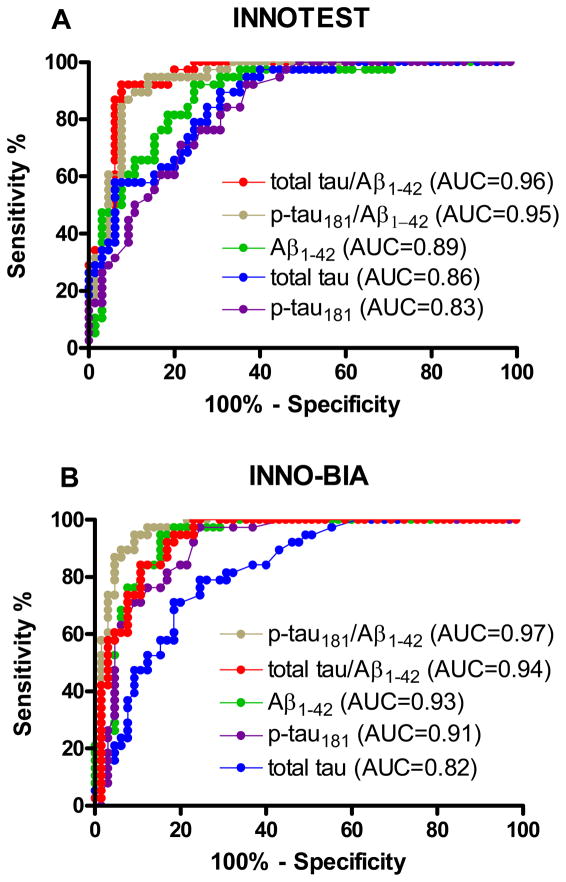
We next employed ROC analyses to determine the sensitivity and specificity of the CSF measures for discriminating amyloid+ from amyloid− individuals using a dichotomous PIB cutoff (MCBP ≥ 0.18) 19, 21. All analytes, regardless of assay platform, performed well in discriminating between the two amyloid groups (n=38 amyloid+, n=65 amyloid−), with AUCs ranging from 0.82 (95% confidence intervals [CI]: 0.73, 0.90) to 0.97 (CI: 0.95, 0.99) (Table 3). However, the tau(s)/Aβ1-42 ratios performed the best, even better than Aβ1-42 alone, with sensitivities of 92% (INNO-BIA) and 95% (INNOTEST®) for total tau/Aβ1-42 and 94% (INNOTEST®) and 98% (INNO-BIA) for p-tau181/Aβ1-42 (compared to 85% [INNOTEST®] and 89% [INNO-BIA ] for Aβ1-42), all at 80% specificity (Table 3 and Figure 3). Whereas the AUCs of Aβ1-42 and the tau(s)/Aβ1-42 ratios did not differ statistically as a function of assay platform, the INNOTEST® total tau values yielded better PIB group discrimination than the INNO-BIA total tau (76% vs 65% sensitivity, p=0.0067), and the INNO-BIA p-tau181 values performed better than the INNOTEST® p-tau181 (86% vs 69% sensitivity, p=0.016) (Table 3). The results were the same when using a MCBP cut-off of 0.20 since the 0.18 and 0.20 dichotomized cohorts were identical in this study.
Table 3.
Comparison of the accuracy of the various CSF analytes to discriminate amyloid-positive from amyloid-negative participants (MCBP cut-off = 0.18)
| Analyte | Assay | AUC (95% CI) | P value (comparing AUCs) | Sensitivity at 80% specificity |
|---|---|---|---|---|
| Aβ1-42 | INNOTEST® | 0.89 (0.84, 0.95) | 0.2573 | 85% |
| INNO-BIA | 0.93 (0.88, 0.98) | 89% | ||
| Total tau | INNOTEST® | 0.86 (0.79, 0.93) | 0.0067* | 76% |
| INNO-BIA | 0.82 (0.73, 0.90) | 65% | ||
| P-tau181 | INNOTEST® | 0.83 (0.75, 0.91) | 0.016* | 69% |
| INNO-BIA | 0.91 (0.85, 0.96) | 86% | ||
| Total tau/Aβ1-42 | INNOTEST® | 0.96 (0.92, 0.99) | 0.2644 | 95% |
| INNO-BIA | 0.94 (0.90, 0.98) | 92% | ||
| P-tau181/Aβ1-42 | INNOTEST® | 0.95 (0.91, 0.99) | 0.1025 | 94% |
| INNO-BIA | 0.97 (0.95, 0.99) | 98% |
Statistically different
Abbreviations: Aβ1-42, amyloid-β1-42; AUC, Area Under the Curve; CI, confidence interval; MCBP, mean cortical PIB binding potential; P-tau181, tau phosphorylated at tyrosine 181
Figure 3.
Receiver operating characteristic (ROC) curves and area under the curve (AUC) describing CSF biomarker sensitivity and specificity for discriminating amyloid-positive (MCBP≥0.18) from amyloid-negative (MCBP<0.18) participants. CSF values were obtained with A) the INNOTEST® or B) the INNO-BIA assay.
Discussion
Given the expanding literature supporting the diagnostic and prognostic potential of AD biomarkers 26, inclusion of biomarker data for use in disease diagnosis for research and/or clinical purposes is currently being proposed 27. However, biomarker validation and standardization must be fully demonstrated before such measures will be considered acceptable for individual patient diagnosis in the clinical care setting. To this end, we compared the performances of the two most commonly used commercial assays for CSF biomarkers of AD in identifying PIB+ amyloid plaque pathology in living individuals. Each of the assays exhibited high levels of intra-assay reliability, with mean coefficients of variability <5% for INNOTEST® and <4% for INNO-BIA. The two methods yielded different (~2–6-fold) absolute values for the various analytes, but the relative values were highly correlated (r values from 0.77 to 0.94), in agreement with previous reports 28–29. Also consistent with previous studies using individual assays 7–12, 30, the various CSF analytes were found to correlate with cortical PIB binding, albeit to differing degrees. The ratios of tau(s)/Aβ1-42 obtained with both assay platforms outperformed each single analyte (including Aβ1-42) in discriminating PIB+ from PIB- individuals, reaching high levels of sensitivity. Together these data demonstrate that the two assay platforms both performed well in identifying individuals with underlying amyloid plaque pathology, especially the tau(s)/Aβ1-42 ratios, and furthermore, support a strong relationship between amyloid and tau pathologies in AD.
Despite differences in the general assay platform, the specific Aβ antibodies employed, and the different absolute values generated by the two assays, the two assays performed equally well in discriminating amyloid+ from amyloid− individuals (AUC=0.89 and 0.93, respectively, p=0.2573). This was the case in the full cohort (of mixed CDRs) as well as in the sub-cohort of CDR 0 cases (data not shown), providing further support that CSF Aβ1-42 measures are able to identify amyloid-positive cases even in the earliest (preclinical) stage of the disease 9. However, the fact that the two assay platforms generated different absolute values (although positively correlated) poses a challenge for biomarker standardization efforts in so far as being able to define universal cut-off values to identify groups with or without underlying disease pathology. The similarity in performance of the two Aβ1-42 assays precludes endorsement of one assay platform over the other. Other factors, such as test sample volume requirements, reproducibility between laboratories and overall assay cost, will likely have to be considered when making protocol standardization recommendations.
Similar to the findings observed for CSF Aβ1-42, CSF total tau and p-tau181 measures obtained by both assays correlated significantly with the amount of cortical PIB binding, consistent with previous studies utilizing one assay platform 9–12, 30. Although the strengths of the correlations between amyloid load and the various CSF analytes differed between the two assay platforms, these differences were not consistent; certain analyte/platform combinations correlated better with cortical amyloid load than other combinations. The ratios of tau(s)/Aβ1-42 obtained with both platforms exhibited the strongest correlation with PIB binding compared to the single analytes. This was also reflected in the greatest AUCs obtained for the ratios (0.92–0.98 for the ratios compared with 0.65–0.89 for the single analytes) in identifying amyloid+ vs amyloid-groups. The CSF tau(s)/Aβ1-42 ratios have also been shown to predict future cognitive decline in cognitively normal individuals 31–33 and those with mild cognitive impairment or very mild dementia 34–36. Together, these data suggest a relationship between the development of amyloid plaque and tangle pathologies and neurodegeneration in AD that eventually leads to dementia. Although the pattern of elevated CSF tau only in the presence of substantial amyloid load suggests that Aβ1-42 aggregation is driving subsequent tangle formation and/or neurodegeneration, analysis of within-subject longitudinal clinical, CSF and amyloid (and eventually neurofibrillary tangle) imaging results will be required to determine the precise timing of the development of these pathologies during the course of the disease.
Establishment of a link between fluid biomarker profiles and underlying disease pathology is a necessary requisite for proposing their usefulness for disease diagnosis and eventually, therapeutic intervention. All published AD therapeutic trials to date have utilized clinical criteria (i.e., mild cognitive impairment or mild-moderate AD dementia) for patient enrollment despite the fact that by the time any clinical symptoms are apparent, significant AD pathology has already developed, including substantial synaptic and neuronal loss that will likely be unable to be reversed. Therefore, it is perhaps not surprising that no proposed disease modifying treatment has resulted in a positive clinical outcome. Given that various CSF measures are reflective of underlying amyloid pathology and neurodegeneration, even when cognitive symptoms are absent, these biomarkers may be useful in the design and evaluation of more appropriate “prevention” trials. Such markers could be used for patient selection (e.g., high CSF tau/Aβ 1-42 ratio for identifying individuals with cortical amyloid and neurodegeneration), thus helping to reduce enrollment requirements and trial duration. These markers could also be used to monitor response to therapy, especially for therapies designed to directly influence disease pathology itself. The ultimate goal, of course, will be to utilize biomarkers to assist in making treatment decisions once such disease-modifying treatments for AD become available.
Acknowledgments
Funding Sources: Supported by NIH grants: P50 AG05681 (J.C.M.), P01 AG03991 (J.C.M.), P01 AG026276 (J.C.M.), P30 NS057105 (D.M.H.), P30 NS048056 (M.A.M.), UL1 RR024992, U01 AG024904, 1RC AG036535, P30 AG10124 (J.Q.T.); the Dana Foundation (M.A.M); and the Charles and Joanne Knight Alzheimer Research Initiative (J.C.M.).
We thank the WU-ADRC Biomarker, Clinical, Genetics and Imaging Cores and the ADNI Biomarker Core. The funding organizations did not have any role in the design or conduct of the study, the collection, management, analysis or interpretation of the data, or the preparation, review or approval of the manuscript. Dr. Fagan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Footnotes
Dr. Fagan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Financial Disclosure Information:
Dr. Fagan reports no disclosures.
Dr. Shaw reports consulting for Innogenetics but no conflict of interest exists.
Dr. Xiong reports no disclosures.
Dr. Vanderstichele is an employee of Innogenetics, the manufacturer of the CSF assays in the present study. He has no further financial involvement in the company, nor does he receive benefits related to the sales of the assays or publication of manuscripts. No conflict of interest exists.
Dr. Mintun is currently Chief Medical Officer at Avid Radiopharmaceuticals (a subsidiary of Eli Lilly) which is developing a non-PIB radiopharmaceutical to image amyloid plaques
Dr. Trojanowski reports no disclosures.
Dr. Coart is an employee of Innogenetics, the manufacturer of the CSF assays in the present study. He has no further financial involvement in the company, nor does he receive benefits related to the sales of the assays or publication of manuscripts. No conflict of interest exists.
Dr. Morris reports no disclosures.
Dr. Holtzman reports consulting for Innogenetics and is on the scientific advisory board of En Vivo, Satori, and C2N Diagnostics. No conflict of interest exists.
Author Contributions:
Study concept and design: Fagan, Holtzman, Vanderstichele Acquisition of data:Fagan, Shaw, Morris, Mintun
Analysis and in terpretation of data: Fagan, Xiong, Holtzman, Vanderstichele, Shaw, Coart
Drafting of the manuscript: Fagan
Critical revision of the manuscript for important intellectual content: Xiong, Holtzman, Vanderstichele, Shaw, Coart, Trojanowski, Morris, Mintun
Statistical analysis: Xiong, Coart
Obtained funding: Fagan, Shaw, Trojanowski, Mintun, Morris, Holtzman
Administrative, technical, and material support: Fagan, Vanderstichele, Shaw, Trojanowski, Morris, Mintun
Study supervision: Fagan, Holtzman, Trojanowski
References
- 1.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Price J, McKeel D, Buckles V, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 5.Knopman D, DeKosky S, Cummings J, et al. Practice parameter: Diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 6.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fagan A, Mintun M, Mach R, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 8.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Fagan A, Mintun M, Shah A, et al. Cerebrospinal fluid tau and ptau181 increase with cortical amyloid deposition in cognitively normal individuals: Implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolboom N, VanDerFlier W, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 11.Grimmer T, Riemenschneider M, Förstl H, et al. Beta amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagust W, Landau S, Shaw L, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapiola T, Alafuzoff I, Herukka S, et al. Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 14.Klunk W, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 15.Morris J, McKeel D, Fulling K, Torack R, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 16.Berg L, McKeel DW, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease - Relation of histologic markers to dementia severity, age, sex, and apoE genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR). Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Shaw L, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mintun M, LaRossa G, Sheline Y, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 20.Logan J, Fowler J, Volkow N, Wang G, Ding Y, Alexoff D. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Morris J, Roe C, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- 23.McClish D. Analyzing a portion of the ROC curve. Med Decis Making. 1989;9:190–195. doi: 10.1177/0272989X8900900307. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Obuchowski N, McClish D. Statistical Methods in Diagnostic Medicine. New York: Wiley-Interscience; 2002. [Google Scholar]
- 25.Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 26.Perrin R, Fagan A, Holtzman D. Multi-modal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois B, Feldman H, Jacova C, et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 28.Reijn T, Rikkert M, Van Geel W, De Jong D, Verbeek M. Diagnostic accuracy of ELISA and xMAP technology for analysis of amyloid β42 and tau proteins. Clin Chem. 2007;53:859–865. doi: 10.1373/clinchem.2006.081679. [DOI] [PubMed] [Google Scholar]
- 29.Lewczuk P, Kornhuber J, Vanderstichele H, et al. Multiplexed quantification of dementia biomarkers in the CSF of patients with early dementias and MCI: a multicenter study. Neurobiol Aging. 2008;29:812–818. doi: 10.1016/j.neurobiolaging.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Forsberg A, Almkvist O, Engler H, Wall A, Långström B, Nordberg A. High PIB retention in Alzheimer’s disease is an early event with complex relationship with CSF biomarkers and functional parameters. Curr Alzheimer Res. 2010;(7):56–66. doi: 10.2174/156720510790274446. [DOI] [PubMed] [Google Scholar]
- 31.Fagan A, Roe C, Xiong C, Mintun M, Morris J, Holtzman D. Cerebrospinal fluid tau/Aβ42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Sokal I, Quinn J, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 33.Craig-Schapiro R, Perrin R, Roe C, et al. YKL-40: A novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 35.Snider B, Fagan A, Roe C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]