Summary
Background
Formation of inhibitory antibodies is a frequent and serious complication of factor VIII (F.VIII) replacement therapy for the X-linked bleeding disorder hemophilia A. Similarly, hemophilia A mice develop high-titer inhibitors to recombinant human F.VIII after a few intravenous injections.
Objective
Using the murine model, the study sought to develop a short regimen capable of inducing tolerance to F.VIII.
Methods
A 1-month immunomodulatory protocol, consisting of F.VIII administration combined with oral delivery of rapamycin, was developed.
Results
The protocol effectively prevented inhibitor formation to F.VIII upon subsequent intravenous treatment (weekly for 3.5 months). Control mice formed high-titer inhibitors and had CD4+ T effector cell responses characterized by expression of IL-2, IL-4, and IL-6. Tolerized mice instead had a CD4+CD25+FoxP3+ T cell response to F.VIII that suppressed antibody formation upon adoptive transfer, indicating a shift from Th2 to Treg if F.VIII antigen was presented to T cells during inhibition with rapamycin. CD4+ T cells from tolerized mice also expressed TGF-β1 and CTLA4, but not IL-10. The presence of F.VIII antigen during the time of rapamycin administration was required for specific tolerance induction.
Conclusions
The study shows that a prophylactic immune tolerance protocol for F.VIII can be developed using rapamycin, a drug that is already widely in clinical application. Immune suppression with rapamycin was mild and highly transient, as the mice regained immune competence within a few weeks.
Keywords: rapamycin, tolerance, factor VIII, hemophilia, T helper cell, inhibitor
Introduction
Formation of inhibitory antibodies against coagulation factor VIII (F.VIII) is currently the most serious complication of replacement therapy in the X-linked bleeding disorder hemophilia A and occurs in 25–30% of treated patients. Inhibitors typically form within 20 days of exposure to clotting factor. Frequent high-dose administration of F.VIII, as performed during ITI (immune tolerance induction) protocols, can often eradicate the inhibitor but is very expensive and takes months to years to complete [1]. Once an inhibitor has formed, management of bleeding is complicated, and risks of morbitity and mortality are increased. At the same time, prediction of inhibitor formation in pediatric patients is steadily improving, taking into consideration the underlying mutation, family history, intensity of early treatment, and polymorphisms in promoters and other sequences of genes for cytokines and other immune functions (such as IL-10, TNFα, and CTLA-4) [2, 3]. There is also a concern that increased intensity of treatment during surgery could pose a heightened risk of inhibitor formation, including in adult patients [3, 4]. Ultimately, prophylactic tolerance induction in patients at high risk would be most desirable.
Several experimental drugs (such as anti-CD3 or blockers of co-stimulation) and genetic manipulations have been explored for tolerance induction in animal studies [5-11]. Rapamycin is an alternative moderate immunosuppressive agent widely used to prevent transplant rejection. It blocks IL-2 growth signaling to T cells by binding to the intracellular protein FKBP12, which in turn inhibits the protein kinase mammalian target of rapamycin (mTOR). The resulting block in cell cycle progression leads to activation-induced cell death (AICD). Importantly, antigen presentation in the presence of rapamycin selectively expands functional CD4+CD25+FoxP3+ Treg, which preferentially utilize Stat5 rather than mTOR signaling and which promote tolerance to coagulation factors [12-16]. In this study, we developed a rapamycin-based protocol that prevents inhibitor formation against full-length (FL) and B-domain deleted (BDD) F.VIII in hemophilia A mice.
Materials and Methods
Tolerance induction and treatment protocols for hemophilia A mice
Hemophilia A mice (C57BL/6/129 or BALB/c mice with targeted deletion of exon 16) were kindly provided by Drs. Kazazian and Lillicrap [17]. All mice were male and 6–8 weeks old at the start of experiments and housed in under specific pathogen free coditions. Ramamycin (LC Laboratories, Woburn, MA, USA) was administered 6 days per week (for 1 month) by oral gavage in 100 μl of sterile PBS at a dose of 4 mg/kg. Human F.VIII (BDD/Refacto, Wyeth Pharmaceuticals, Madison, NJ, now distributed as Xyntha by Pfizer; or full-length/Kogenate, Bayer Healthcare Pharmaceuticals, Berkeley, CA) was given by tail vein injection 3-times per week at 0.3 IU during tolerance induction and once per week at 1 IU for subsequent treatment. Blood samples were obtained by tail-bleed, and antibody titers against F.VIII were measured by Bethesda and immunocapture assays as published [18].
Viral immunization
In order to test for immune competence, mice were challenged with E1-deleted adenoviral vector 5 weeks after rapamycin administration was stopped. The virus was given subcutaneous at 1×1010 viral particles per mouse. Neutralizing antibodies (NAB) in serum were measured after 3 weeks. Two-fold serial dilutions of serum samples were incubated for 2 hrs at room temperature with ad-LacZ vector and were subsequently applied to HEK-293 cells in triplicate, which were stained for β-gal expression 18 hrs later. The highest dilution, at which less than 50% of β-gal expressing cells of control transduction (virus without animal serum, moi = 200 vp/cell) was achieved, was recorded as the NAB titer.
T cell assays
Isolated splenocytes were cultured in RPMI 1640 media (containing 50 μM β-mercaptoethanol, 100 mM insulin/transferrin/selenium, glutamine, and antibiotics) with or without BDD-F.VIII at 10 μg/ml for 48 hrs (37°C, 5% CO2). Transcript levels of cytokines were measured by quantitative RT-PCR using SA Biosciences arrays (Frederick, MD; RNA was extracted from 1×107 cells per spleen prior to cDNA synthesis) and a MyQ thermocycler (Biorad, Hercules, CA), and normalized based on GAPDH expression. Similarly, CD3+CD4+ and CD8+ cells were isolated from cultured cells by cell sorting (with an Aria cell sorter, BD Biosciences, San Jose, CA) and analyzed separately by RT-PCR. In addition, the percent of CD25+FoxP3+ cells of CD4+ cells was determined by flow cytometry following staining. Anti-CD3 was purchased from BD Biosciences. All other antibodies were from eBioscience (San Diego, CA).
Adoptive transfer studies
CD4+CD25+ splenocytes from hemophilia A BALB/c mice were purified using the Treg magnetic cell sorting kit from Stem Cell Technologies (Vancouver, Canada), pooled, and adoptively transferred to naïve mice of the same strain by tail vein injection (1×106 cells per mouse). Recipient mice were immunized by subcutaneous injection of 1 IU BDD-hF.VIII (in complete Freund’s adjuvant, CFA) 24 hrs later.
Comparison of STAT phosphorylation
Magnetically purified CD4+CD25+ splenocytes were stained with anti-CD4-Pacific Blue, anti-CD25-APC-Cy7, and PerCp-Cy5.5 anti-pY705-stat3 (or AlexaFlour 647 anti-pY694-stat5) and analyzed by flow cytometry.
Statistical analyses
Statistical differences between two experimental groups were analyzed by unpaired student’s T test using Prism (Irvine, CA) software. Significant differences are indicated in the figures as * for P<0.05, ** for P<0.01, *** for P<0.001, and so on.
Results
Prevention of inhibitor formation to BDD-F.VIII
Previously, we found that repeated intraperitoneal (IP) co-administration of a peptide (representing a dominant CD4+ T cell epitope), rapamycin, and cytokine IL-10 (3-times per week for 1 month) induced tolerance to factor IX (F.IX). However, using 1 IU of BDD-F.VIII (instead of a peptide) in this protocol failed to induce tolerance to F.VIII in hemophilia A mice (C57BL/6/129 background). Four subsequent weekly IV injections of 1 IU BDD-F.VIII resulted in high-titer inhibitors (39-58 BU/ml, data not shown). Therefore, we modified the protocol in several ways, keeping human treatment in mind. In humans, rapamycin is typically administered in oral form on a daily basis, while F.VIII is given intravenously (IV). Hence, rapamycin was given orally 6 days per week for 1 month. During this time, BDD-F.VIII was given IV 3-times per week at a reduced dose (0.3 IU/dose; high doses of therapeutic protein antigens often cause B cell activation before tolerance is established, unpublished observations). Although we previously found that IL-10 enhances the tolerogenic effects of rapamycin administration, this cytokine has only been an experimental drug in humans and was therefore not included in this modified protocol.
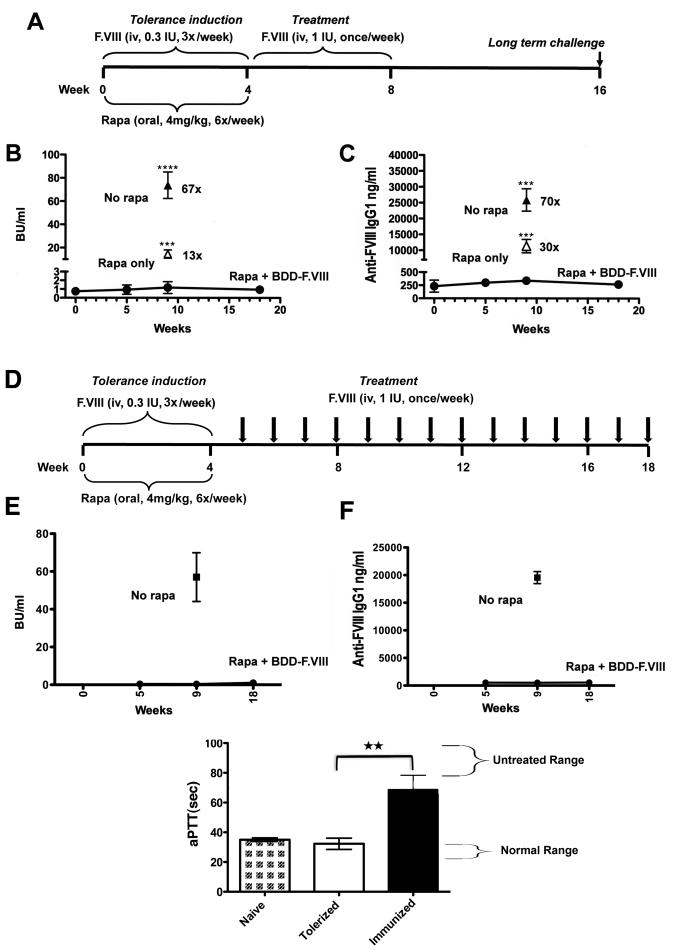
In control mice (no prior immune modulation), weekly IV injection of 1 IU BDD-F.VIII led to high-titer inhibitor formation (48 to 98 BU/ml) after the 4th week of treatment (Fig. 1A), corresponding to 16-32 μg IgG1/ml (Fig. 1B; no other IgG subclasses were detected, data not shown). Following identical treatment, immune modulated mice had ~70-fold lower antibody titers (0.5-2 BU/ml; 0.3-0.4 μg IgG1/ml), which were not above background (pre-treatment) values and did not increase after an additional challenge 2 months later (Fig. 1B, C). In order to exclude a general immune suppressed state as the reason for lack of inhibitor formation, another control group received the identical rapamycin regimen without F.VIII, followed by 1 month of weekly IV treatment. These mice still formed high-titer inhibitors (10-19 BU/ml; 9-13 μg IgG1/ml), indicating that the immune system had quickly recovered and that co-administration of rapamycin and the antigen was required for tolerance induction (Fig. 1B, C). Interestingly, while it took several months for lymphocyte frequencies in C3H/HeJ mice treated with the F.IX/rapamycin/IL-10 protocol to recover (published data) [12], frequencies of CD4+, CD8+, and B220+ peripheral lymphocytes in rapamycin-treated hemophilia A mice returned to normal within 5 weeks (data not shown).
Fig. 1.
Prophylaxis against inhibitor formation against BDD-F.VIII in hemophilia A mice (C57BL/6/129). A. Tolerance induction and treatment schedule. B. Inhibitor titers as a function of time in mice that received rapamycin (rapa) plus BDD-F.VIII (0.3 IU/mouse/dose) followed by treatment with BDD-F.VIII (circles, n=5). Open triangle: mice that received rapamycin without F.VIII followed by treatment with BDD-F.VIII (n=4). Solid triangle: mice that received no rapamycin followed by treatment with BDD-F.VIII (n=4). C. BDD-F.VIII-specific IgG1 titers in the same animals. Data are average±SD. Statistically significant differences for 5-week time point are indicated as described in Methods. Fold-differences between control groups and tolerized mice are also indicated. D. Schedule for long-term assessment of tolerance to BDD-F.VIII. E. Inhibitor titers and F. IgG1 titers after long-term treatment (n=8). Non-tolerized control mice formed high-titer antibodies (n=4). G. Correction of aPTT 10 min after IV administration of 2 IU BDD-F.VIII in i) naïve mice, ii) mice that had been tolerized and received long-term treatment, and iii) control mice with inhibitors (n=5 per group).
Longer-term follow-up of tolerance to BDD-F.VIII
In order to more rigorously test for maintenance of tolerance, hemophilia A mice received weekly IV injections of 1 IU BDD-F.VIII for 3.5 months following the rapamycin regimen (Fig. 1D). Here again, lack of antibody formation was sustained for the duration of the experiment (Fig. 1E, F). In the end, some of these mice received one additional IV injection of 2 IU BDD-F.VIII followed by blood collection 10 min later. The level of correction of the aPTT in these mice was similar to that in naïve mice, while inhibitor positive controls could not be corrected (Fig. 1G).
Prevention of inhibitor formation to FL-F.VIII
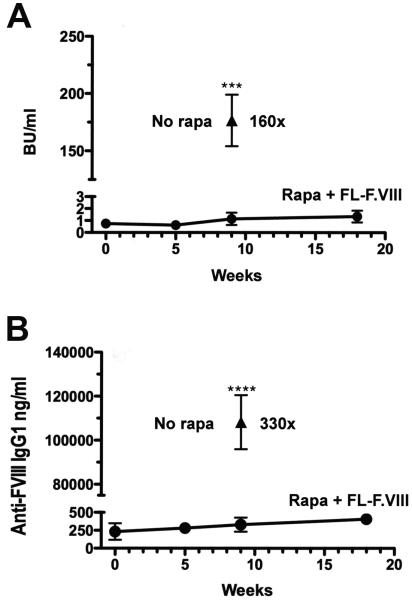
Next, effectiveness of the protocol was tested for FL-F.VIII, which represents a larger polypeptide sequence and contains the B-domain with potential additional epitopes. Control mice treated with weekly IV injections of 1 IU FL-F.VIII formed very high-titer inhibitors of 60-266 BU/ml (34-153 μg IgG1/ml, Fig. 2A, B). However, if mice were subjected to the tolerance regimen using FL-F.VIII, inhibitor titers were 160-fold lower (0.6-1.8 BU/ml, 0.2-0.4 μg IgG1/ml) and again did not increase after an additional challenge 2 months later (0.8-1.9 BU/ml). A minor increase in IgG1 titer was seen at the late time point (Fig. 2B).
Fig. 2.
Prophylaxis against inhibitor formation against FL-F.VIII in hemophilia A mice (C57BL/6/129). The protocol was identical as in Fig. 1A, except that FL-F.VIII was used instead of BDD-F.VIII. A. Inhibitor titers in mice that received rapamycin plus FL-F.VIII followed by treatment with FL-F.VIII (0.3 IU/mouse/dose; circles, n=5). Solid triangle: mice that received no rapamycin followed by treatment with full-length F.VIII (n=9). B. FL-F.VIII-specific IgG1 titers in the same animals. Data are average ±SD.
Consistent with earlier reports by Hoyer and colleagues, there was a good correlation between inhibitor and IgG titers in all treatment groups (data not shown) [19]. In contrast to our experience with F.IX protein therapy in hemophilia B C3H/HeJ mice [20], no allergic or anaphylactic reactions to F.VIII were observed.
Tolerized mice form NAB to viral particles
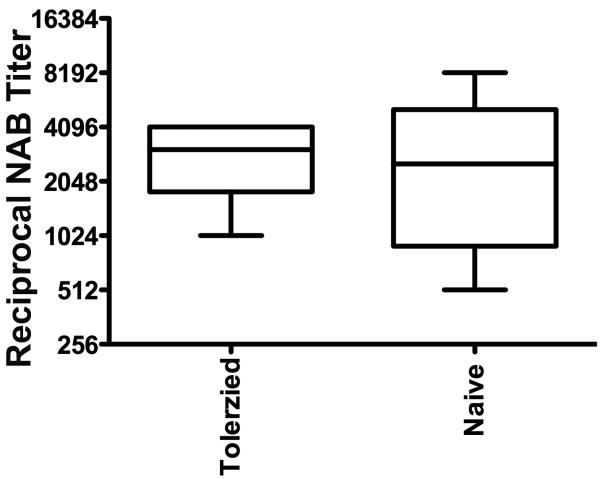
For clinical implementation, it will be important that transient immune suppression does not prevent subsequent responses to immunization or to pathogens. To address this point, rapamycin-treated mice were challenged 5 weeks later with adenovirus. As shown in Fig. 3, the mice formed similar titers of NAB as control mice.
Fig. 3.
Formation of NAB to adenovirus in sera of control mice or of mice that had been treated with rapamycin. Virus was administered 5 weeks after rapamycin had been discontinued, and NAB titers measured 3 weeks later. Shown are the ranges and means of NAB titers in a box-and-whisker plot (n=4 per group).
Induction of CD4+ T cell tolerance to F.VIII antigen
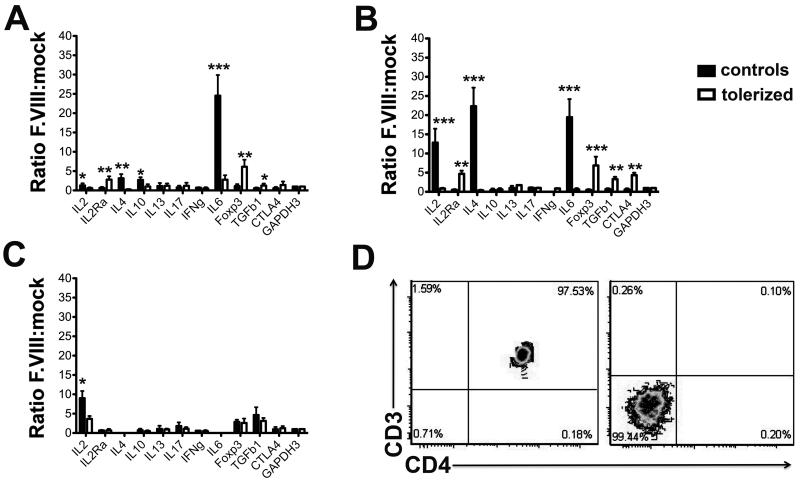
Inhibitor formation to F.VIII is known to be T helper cell-dependant [21]. Splenocytes from mice tolerized to and challenged with BDD-F.VIII were re-stimulated in vitro with this protein, and expression patterns of cytokines and other T cell markers were compared to responses in non-immune modulated mice (Fig. 4). Consistent with formation of IgG1, these control animals showed a response dominated by Th2 cytokines (including IL-4, IL-6, and IL-10), while no induction of transcripts of Treg markers (CD25, FoxP3, CTLA4, or TGF-β1) was detected (Fig. 4A). In addition, IL-2 was expressed. In contrast, the tolerance protocol completely prevented IL-2, IL-4, and IL-10 expression, and almost completely abolished IL-6 responses to F.VIII (Fig. 4A). At the same time, FoxP3, CD25, and TGF-β1 transcripts were significantly increased in the tolerized group (CTLA4 was also increased, although not reaching statistical significance). Neither tolerized nor control mice showed increases in transcripts of IFN-γ (a Th1 cytokine), IL-13 (another Th2 cytokine), or IL-17 after stimulation with F.VIII.
Fig. 4.
T cell responses to BDD-F.VIII. Relative levels of transcripts for cytokine and T cell marker genes in F.VIII- vs. mock-stimulated A. total splenocytes, B. splenic CD3+CD4+ T cells (purified from stimulated splenocyte cultures by flow sorting), and C. non-T cells (i.e. remaining cells after purification of T cells by sorting). Each data point is average for n=4 mice per group ±SD. Hemophilia A mice (C57BL/6/129) had been assayed individually, and all transcript levels were determined by quantitative RT-PCR and normalized for GAPDH expression. Open bars are mice tolerized to BDD-F.VIII followed by treatment with BDD-F.VIII. Black bars are for control mice treated with BDD-F.VIII. D. Purity of sorted CD3+CD4+ T cells and CD3− cells.
Because IL-6 is a cytokine that can also be produced by non-T cells, we wanted to further differentiate between CD4+ T cell and other cellular responses to F.VIII. The experiment was repeated, except that following in vitro stimulation with mock or F.VIII-containing media, CD3+CD4+ cells were purified by cell sorting. The resulting CD3+CD4+ cell population was >97% pure as determined by flow cytometric analysis (Fig. 5D). In control mice (treatment with BDD-F.VIII without prior tolerization), CD3+CD4+ T cells expressed high levels of IL-2, IL-4, and IL-6 transcripts in response to F.VIII (Fig. 4B). CD3+CD4+ T cells from tolerized mice completely lacked IL-2, IL-4, and IL-6 responses, and instead showed significant induction of transcripts for CD25 (IL2Ra), FoxP3, TGF-β1, and CTLA4 upon stimulation with hF.VIII (Fig. 4C). No induction of IL-10, IL-13, or IL-17 transcripts was detected in either experimental group in this experiment.
No induction of transcripts of any of the tested markers was found in CD8+ T cells of either group (data not shown). We also analyzed non-T cells (i.e. CD3− cells). These did not produce IL-6 or any of the other cytokines in response to stimulation with F.VIII with the exception of IL-2. However, IL-2 transcript levels were ~10-fold lower compared to levels in CD3+CD4+ T cells. These levels were further reduced in tolerized mice (Fig. 4D and data not shown).
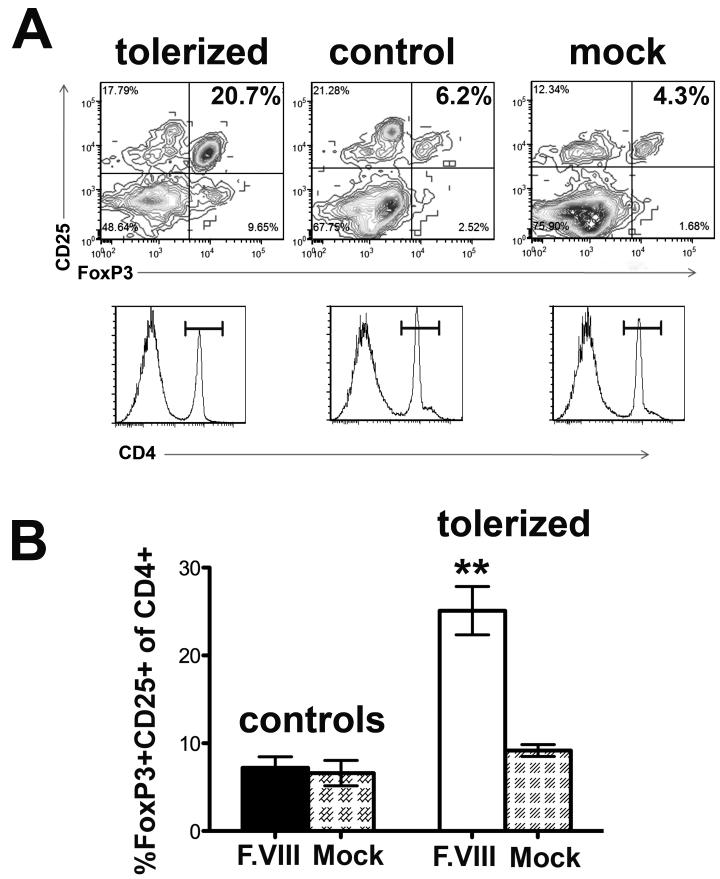
To confirm induction of CD4+CD25+FoxP3+ Treg in tolerized mice, cultured lymphocytes were analyzed by antibody stains followed by flow cytometry. Upon in vitro stimulation with hF.VIII, a significant increase in the frequency of CD4+CD25+FoxP3+ cells was seen for tolerized mice, while no Treg induction was found in control mice (Fig. 5A, B).
Fig. 5.
Frequencies of CD4+CD25+FoxP3+ T cells following in vitro stimulation of splenocytes with hF.VIII. A. Examples of results from tolerized and control hemophilia A mice stimulated with hF.VIII (first two panel; third panel: mock stimulated splenocytes from tolerized mouse). Control mice had been challenged with F.VIII without prior tolerance induction. Upper panels: CD25 and FoxP3 stains gated on CD4+ cells. Lower panels: corresponding histograms for CD4+ staining. B. Summary of results for n=4 mice per group. Data are average ±SD. **: statistically significant difference with P<0.01.
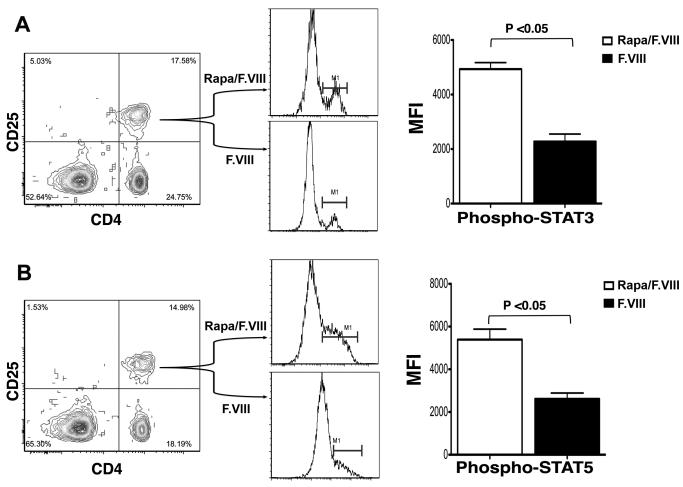
In contrast to Teff, Treg are less prone to AICD after blockage of mTOR (and thus able to proliferate) because of signaling through alternative pathways [12, 13]. This effect of rapamycin on Treg in our protocol was further studied using antibody stains for phosphorylated forms of Stat3 and Stat5. Flow cytometry revealed a significant 2.5-fold increase in phosphorylated forms for CD4+CD24+ splenocytes from rapamycin-treated compared to control mice (Fig. 6).
Fig. 6.
Enhanced phosphorylation of Stat3 (A) and Stat5 (B) one week after last F.VIII/rapamycin treatment of hemophilia A C57BL/6/129 mice compared to control mice (treated with identical dose of F.VIII without rapamycin). Following staining with antibodies specific for the phosphorylated forms, results are reported as mean geometric MFI (mean fluorescence intensity) ±SD (n=4 per group).
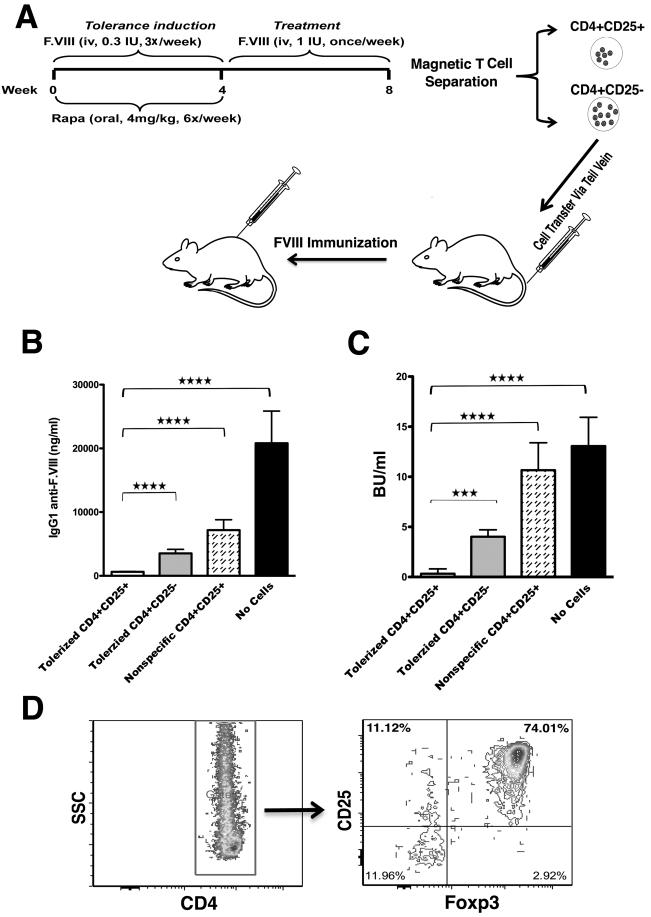
In order to address functionality of induced Treg, hemophilia A mice on a defined genetic background (BALB/c) were tolerized to BDD-F.VIII using the identical protocol followed by 1 month of therapy (Fig. 7A, D). Again, no anti-F.VIII was detected (data not shown). Adoptive transfer of CD4+CD25+ cells from tolerized mice to naïve hemophilia A BALB/c mice completely blocked antibody formation after subsequent immunization, whereas CD4+CD25− cells or non-specific CD4+CD25+ cells had only a partial effect (Fig. 7B, C).
Fig. 7.
Suppression of anti-BDD-F.VIII formation upon adoptive transfer of Treg from tolerized mice. A. Tolerance induction and treatment protocol for hemophilia A BALB/c mice (n=7) followed by adoptive transfer of Treg. B. Anti-F.VIII titers and C. inhibitor titers in hemophilia A BALB/c mice that were immunized with BDD-deleted F.VIII/CFA 24 hrs after receiving no cells or 1×106 control CD4+CD25+ Treg (from naïve donors), or CD4+CD25+ or CD4+CD25− splenocytes from tolerized mice (n=5/group). C. Magnetically isolated CD4+ splenocytes were >98% pure after negative selection, and subsequent positive selection for CD4+CD25+ cells resulted in >85% CD4+CD25+ cells (85-90% of which were also FoxP3+).
Discussion
Tolerance induction by co-administration of rapamycin and hF.VIII prevents Th2 and promotes Treg responses
Inhibitor formation in this murine hemophilia A model was driven by Th responses characterized by expression of cytokines IL-2, IL-4, and IL-6. IL-2 is generally important for T cell activation and generation of memory T cells and can also promote antibody production by B cells. While Th1 cells are known to constitutively express IL-2, lack of IFN-γ expression in response to F.VIII argues against a Th1 response here. IL-4 is the hallmark cytokine for Th2 cells and promotes IgG1 class-switch upon activation of B cells. Interestingly, we additionally found induction of IL-6 expression. This proinflammatory cytokine can be produced by different cell types, including non-T cells such as macrophages, and is often involved in innate immune responses. However, Th2 cells may also express IL-6. For example, during an inflammatory Th2 response, IL-6 can regulate neutrophil trafficking. IL-6 is also a potent B cell stimulatory factor that promotes immunoglobulin secretion and thus likely contributed to the antibody response to F.VIII.
Repeated administration of F.VIII to control mice did not induce Treg, and immune suppressive cytokines (TGF-β and IL-10) were also absent or, in the case of IL-10, inconsistently detected. Insufficient induction of Treg or of suppressive cytokine expression by activated Th2 cells likely caused an unregulated Th2 response, driving high-titer antibody formation. It is known that the combination of IL-6 and TGF-β can promote induction of Th17 cells, an inflammatory subset of CD4+ T cells. However, we found no evidence of F.VIII-specific Th17 responses in this model. Instead, co-administration of rapamycin and F.VIII suppressed Teff (in this case Th2) responses and induced CD4+CD25+FoxP3+ Treg.
Comparison to other tolerance protocols
Several immune tolerance protocols for coagulation factors have recently been reported based on studies in mice. Examples include hepatic gene transfer with adeno-associated viral, retroviral, or lentiviral vectors, ex vivo gene transfer to primary B cells, oral tolerance, non-Fc-binding anti-CD3 (for interference with T cell receptor signaling), and blockage of co-stimulatory pathways such as during administration of anti-ICOS, CTLA4-g combined with anti-CD40L, or Fc-GITR-L [5-11, 20, 22]. A prevalent mechanistic feature that has emerged is induction of CD4+CD25+FoxP3+ Treg. Induced Treg can further amplify the antigen-specific regulatory response by aiding in the conversion of naïve CD4+ T cells to Treg [23]. With the exception of the high-dose anti-CD3 protocol, these Treg were found to be required for tolerance [9]. Interestingly, antigen-specific Treg induced by retrovirally transduced B cells expressing an IgG fusion protein appeared more suppressive than pre-existing, endogenous Treg [24].
Somewhat different from the rapamycin approach, F.VIII administration combined with low-dose anti-CD3 not only induced Treg and prevented Th2 but also induced Th1 responses against F.VIII [10]. Nonetheless, the protocol successfully suppressed inhibitor formation. Th1 responses alone, i.e. in the absence of Th2, may not drive inhibitor formation [25].
Advantages of rapamycin
While the drugs and protocols described above have been largely confined to pre-clinical studies, anti-CD3 is at least in advanced clinical trials for treatment of type 1 (autoimmune) diabetes. However, rapamycin offers a distinct advantage in that there is already extensive clinical experience with this drug. Mechanistically, rapamycin may be ideal for the purpose of tolerance induction because it does not prevent signaling through the T cell receptor (signal 1), thereby allowing the T cell to undergo initial activation steps. Through inhibition of the mTOR pathway, subsequent signaling from growth factors is blocked, resulting in AICD. Utilizing alternative pathways, CD4+CD25+FoxP3+ Treg are able to expand and are required to maintain unresponsiveness of the tolerized CD4+ T cell population [12, 13]. The cytokine IL-10 further enhances the effects of rapamycin [12, 16] but is not absolutely necessary as shown here. Use of the entire coagulation factor protein as the tolerizing antigen (instead of small peptides that encode CD4+ T cell epitopes) required further optimization of dosing and schedule. Interestingly, rapamycin is known to increase levels of hemeoxygenase-1 (HO), a stress-inducible, anti-inflammatory enzyme [26]. A recent study found that HO activity promotes hyporesponsiveness to F.VIII [27]. Therefore, rapamycin may limit inflammatory signals in part by up-regulation of HO, thereby further facilitating the desired shift from Teff to Treg.
Implications for treatment of hemophilia
A short immune modulatory regimen using rapamycin could be considered for prophylactic tolerance induction in pediatric patients with high risk for inhibitor formation or prior to medical procedures that may increase this risk. Although the protocol was effective for both forms of F.VIII, lack of antibody formation was less complete for FL-F.VIII. If low-titer antibodies were still formed, subsequent F.VIII doses may have to be increased for effective treatment. Use of BDD-F.VIII completely prevented antibody formation. The rapamycin dose/kg was chosen based on prior studies in mice with hemophilia B or autoimmune lymphoproliferative syndrome [12, 28]. In humans, a lower dose is sufficient to maintain an effective trough level in the blood [29]. Undesired side effects include the risk of opportunistic infection. In addition, rapamycin affects lipid metabolism and can increase particular cholesterol levels. However, the regimen presented here is highly transient, and all data indicate that the animals quickly regained immune competence. Animals that were non-specifically suppressed with rapamycin still formed inhibitors during subsequent treatment. Lymphocyte frequencies recovered quickly, and, mimicking vaccination, the animals formed NAB to a virus (albeit that adenovirus provides strong inflammatory signals).
In summary, a short protocol of antigen administration in the presence of rapamycin effectively blocked inhibitor formation to F.VIII in subsequent factor replacement therapy by preventing Th2 immunity and establishing a Treg response. Given the wealth of clinical experience with this only moderately immune suppressive drug, this strategy may be translatable to treatment of humans with hemophilia A.
Acknowledgements
This work was supported by NIH grants R01 AI/HL51390 (to RWH), T32DK074367 (support for SN), and T32AI007110 (support for BKS); and by a Bayer Hemophilia Award to RWH.
Footnotes
Conflict of interest: None.
References
- 1.DiMichele DM. Immune tolerance: critical issues of factor dose, purity and treatment complications. Haemophilia. 2006;12(Suppl 6):81–5. doi: 10.1111/j.1365-2516.2006.01376.x. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh K, Shetty S. Immune Response to FVIII in Hemophilia A: An Overview of Risk Factors. Clin Rev Allergy Immunol. 2009;37:58–66. doi: 10.1007/s12016-009-8118-1. [DOI] [PubMed] [Google Scholar]
- 3.Coppola A, Santoro C, Tagliaferri A, Franchini M, G DIM. Understanding inhibitor development in haemophilia A: towards clinical prediction and prevention strategies. Haemophilia. 2010;16(Suppl 1):13–9. doi: 10.1111/j.1365-2516.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- 4.Peerlinck K, Jacquemin M. Mild haemophilia: a disease with many faces and many unexpected pitfalls. Haemophilia. 2010;16(Suppl 5):100–6. doi: 10.1111/j.1365-2516.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- 5.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–70. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C, Chuah M, VandenDriessche T, Miao CH, Hough C, Lillicrap D. A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells. Blood. 2009;114:677–85. doi: 10.1182/blood-2009-03-202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao CH, Ye P, Thompson AR, Rawlings DJ, Ochs HD. Immunomodulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice. Blood. 2006;108:19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng B, Ye P, Blazar BR, Freeman GJ, Rawlings DJ, Ochs HD, Miao CH. Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice. Blood. 2008;112:1662–72. doi: 10.1182/blood-2008-01-128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng B, Ye P, Rawlings DJ, Ochs HD, Miao CH. Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy. Blood. 2009;114:4373–82. doi: 10.1182/blood-2009-05-217315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters B, Qadura M, Burnett E, Chegeni R, Labelle A, Thompson P, Hough C, Lillicrap D. Anti-CD3 prevents factor VIII inhibitor development in hemophilia A mice by a regulatory CD4+CD25+-dependent mechanism and by shifting cytokine production to favor a Th1 response. Blood. 2009;113:193–203. doi: 10.1182/blood-2008-04-151597. [DOI] [PubMed] [Google Scholar]
- 11.Xu L, Mei M, Ma X, Ponder KP. High expression reduces an antibody response after neonatal gene therapy with B domain-deleted human factor VIII in mice. J Thromb Haemost. 2007;5:1805–12. doi: 10.1111/j.1538-7836.2007.02629.x. [DOI] [PubMed] [Google Scholar]
- 12.Nayak S, Cao O, Hoffman BE, Cooper M, Zhou S, Atkinson MA, Herzog RW. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J Thromb Haemost. 2009;7:1523–32. doi: 10.1111/j.1538-7836.2009.03548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao O, Loduca PA, Herzog RW. Role of regulatory T cells in tolerance to coagulation factors. J Thromb Haemost. 2009;7(Suppl 1):88–91. doi: 10.1111/j.1538-7836.2009.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James EA, Kwok WW, Ettinger RA, Thompson AR, Pratt KP. T-cell responses over time in a mild hemophilia A inhibitor subject: epitope identification and transient immunogenicity of the corresponding self-peptide. J Thromb Haemost. 2007;5:2399–407. doi: 10.1111/j.1538-7836.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 16.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 17.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 18.Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, Ertl HC, High KA, Herzog RW. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–42. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian J, Borovok M, Bi L, Kazazian HH, Jr., Hoyer LW. Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb Haemost. 1999;81:240–4. [PubMed] [Google Scholar]
- 20.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci U S A. 2010;107:7101–6. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian J, Collins M, Sharpe AH, Hoyer LW. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95:1324–9. [PubMed] [Google Scholar]
- 22.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, Herzog RW. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–40. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao CH, Harmeling BR, Ziegler SF, Yen BC, Torgerson T, Chen L, Yau RJ, Peng B, Thompson AR, Ochs HD, Rawlings DJ. CD4+FOXP3+ regulatory T cells confer long-term regulation of factor VIII-specific immune responses in plasmid-mediated gene therapy-treated hemophilia mice. Blood. 2009;114:4034–44. doi: 10.1182/blood-2009-06-228155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skupsky J, Zhang AH, Su Y, Scott DW. B-cell-delivered gene therapy induces functional T regulatory cells and leads to a loss of antigen-specific effector cells. Mol Ther. 2010;18:1527–35. doi: 10.1038/mt.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ettinger RA, James EA, Kwok WW, Thompson AR, Pratt KP. Lineages of human T-cell clones, including T helper 17/T helper 1 cells, isolated at different stages of anti-factor VIII immune responses. Blood. 2009;114:1423–8. doi: 10.1182/blood-2009-01-200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visner GA, Lu F, Zhou H, Liu J, Kazemfar K, Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–6. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 27.Dimitrov JD, Dasgupta S, Navarrete AM, Delignat S, Repesse Y, Meslier Y, Planchais C, Teyssandier M, Motterlini R, Bayry J, Kaveri SV, Lacroix-Desmazes S. Induction of heme oxygenase-1 in factor VIII-deficient mice reduces the immune response to therapeutic factor VIII. Blood. 2010;115:2682–5. doi: 10.1182/blood-2009-04-216408. [DOI] [PubMed] [Google Scholar]
- 28.Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, Hulitt J, Manno CS, Maris JM, Rhodin N, Sullivan KE, Brown VI, Grupp SA. Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS) Blood. 2006;108:1965–71. doi: 10.1182/blood-2006-01-010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, Manno C, Rappaport E, Schwabe D, Sheen C, Sullivan KE, Zhuang H, Wechsler DS, Grupp SA. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145:101–6. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]