Abstract
Background
The ability to understand how Parkinson’s disease (PD) neurodegeneration leads to cortical dysfunction will be critical for developing therapeutic advances in PD dementia (PD-D). The overall purpose of this project was to study the small amplitude cortical myoclonus in PD as an in vivo model of focal cortical dysfunction secondary to PD neurodegeneration. The objectives were to test the hypothesis that cortical myoclonus in PD is linked to abnormal levels of α-synuclein in primary motor cortex and to define its relationship to various biochemical, clinical, and pathological measures.
Methods
Primary motor cortex was evaluated for 11 PD subjects with (PD+Myoclonus group) and 8 without (PD group) electrophysiologically confirmed cortical myoclonus who had premortem movement and cognitive testing. Similarly assessed 9 controls were used for comparison. Measurements for α-synuclein, Aβ-42 peptide, and other biochemical measures were made in primary motor cortex.
Results
A 36% increase in α-synuclein was found in the motor cortex of PD+Myoclonus cases when compared to PD without myoclonus. This occurred without significant differences in insoluble α-synuclein, phosphorylated to total α-synuclein ratio, or Aβ-42 peptide levels. Higher total motor cortex α-synuclein levels significantly correlated with the presence of cortical myoclonus but did not correlate with multiple clinical or pathological findings.
Conclusions
These results suggest an association between elevated α-synuclein and the dysfunctional physiology arising from the motor cortex in PD+Myoclonus cases. Alzheimer’s disease pathology was not associated with cortical myoclonus in PD. Cortical myoclonus arising from motor cortex is a model to study cortical dysfunction in PD.
INTRODUCTION
A prerequisite for developing effective treatments of Parkinson’s disease (PD) dementia (PD-D) is to determine the mechanism(s) through which PD neurodegeneration causes cerebral cortical dysfunction in humans (1–9). Braak and others have associated PD-D with pathology spread to neocortical areas (3–9). This highlights the need for biomarker models to study cortical dysfunction in PD patients as well as to complement and validate findings from animal models.
The small amplitude cortical myoclonus of PD indicates in vivo dysfunction of the primary motor cortex in PD patients (10). We have characterized this myoclonus in our laboratory, both clinically and electrophysiologically (10). The mechanism for neuronal dysfunction caused by PD in the primary motor cortex of PD patients with myoclonus should have strong similarity to the pathophysiology in other neocortical areas. Instead of associating a type of diffuse dysfunction (i.e. dementia) with focal tissue samples from cortex regions with unknown local functional integrity, this biomarker allows specific correlation of primary motor cortex biochemistry with the known presence or absence of abnormal physiology (i.e. cortical myoclonus) in the primary motor cortex per se.
In this study, we tested the hypothesis that the small amplitude cortical myoclonus in PD is linked to abnormal levels of α-synuclein in primary motor cortex. The presence of cortical myoclonus in PD was correlated with biochemical, clinical, and pathological measures.
METHODS
Cases Studied
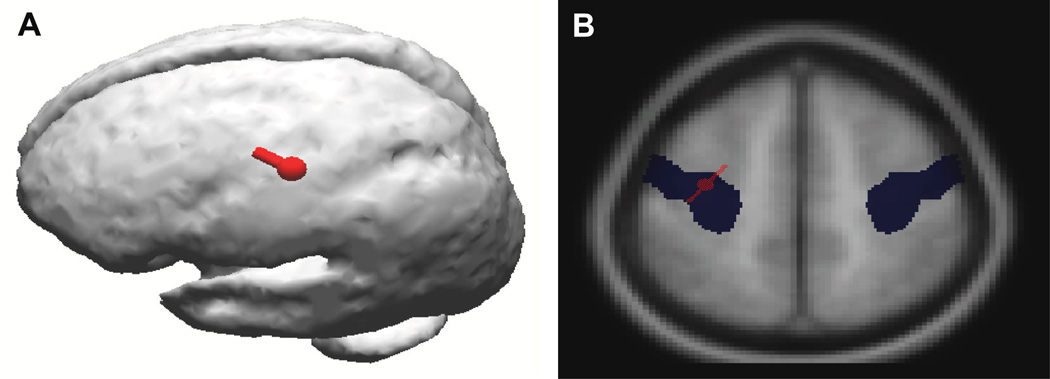
Biochemical studies were performed on 11 PD cases with small amplitude cortical myoclonus (PD+Myoclonus group) and 8 PD cases without myoclonus (PD group). For comparison, 9 Control cases were also studied. All subjects studied were from the Banner Sun Health Research Institute (SHRI) Brain and Body Donation Program (4,10–12). All subjects signed informed consent; were followed antemortem with standardized medical history; received movement, cognitive, electrophysiological assessments; were autopsied within 4 hours of death; and received a final diagnosis based on clinicopathologic correlation as per our previous reports (4,10–12). The neuropsychological battery included Folstein MMSE, long-term memory score on the Auditory-Verbal Learning Test (AVLT-LTM), Controlled Oral Word Association Test (COWAT), Stroop Interference, Trails B, WAIS-III Digit Span, and Judgment of Line Orientation test (JLO). DSM-IV criteria for dementia were used: abnormalities in memory and one other domain of cognition, functional decline related to cognitive deficit(s), and preservation of consciousness. Abnormal performance in any cognitive domain was determined by a consistent pattern of impaired performance on neuropsychological measures that load on that cognitive domain. Additionally, these subjects did not meet clinical criteria for dementia with Lewy bodies (13). Those in the PD+Myoclonus group had the clinical and electrophysiologic demonstration of bilateral small amplitude cortical myoclonus as previously described, within 2 years of death, including documentation of a back-averaged pre-myoclonus electroencephalographic (EEG) transient (10). Using CURRY software (Neuroscan, Charlotte, NC, USA), all PD+Myoclonus cases were confirmed to have a primary motor cortex myoclonus source as demonstrated in Figure 1 with EEG dipole source localization and mapping on the averaged MRI within the CURRY program. PD group cases were confirmed not to have myoclonus within 2 years of death. Controls were defined as having the absence of pre-mortem clinical or post-mortem pathological evidence of dementia or movement disorder secondary to neurodegenerative diseases. Control subjects did not have clinical myoclonus and did not received electrophysiological investigation.
Figure 1.
A-(left) shows dipole localization (red) of the abnormal electrical activity (represented by a dipole) from the cortical myoclonus generation in a PD+Myoclonus subject from the right hand/wrist. This shows that the physiologic abnormality that produces the cortical myoclonus is a highly focal neocortical location. B-(right) shows that the same cortical myoclonus electrical activity (red) also overlaps the Talairach coordinates (blue) of the precentral gyrus (primary motor cortex) on MRI. These data provide evidence that the primary motor cortex is the location of the pathology that produces the cortical myoclonus in PD.
Pathology Methods
Standardized neuropathology methods and diagnostic criteria for the SHRI Brain Bank were performed and have been described (11,12,). In brief, dissected brain tissue was frozen as coronal sections (right hemisphere) on dry ice and stored at −80°C. The opposite hemisphere was fixed for 48 hours in 4% paraformaldehyde and sectioned for histological studies. Each brain received a full neuropathological diagnosis by standard criteria for PD and Alzheimer’s disease (AD) (4). These procedures included assignment of a Lewy-related pathology (LRP) staging score (1 through 4) using immunohistochemical staining of anti-alpha synuclein (antibody LB509, Invitrogen, Carlsbad, CA) for each defined region as described in the consensus publication on dementia with Lewy bodies (13). As a measure of global Lewy related pathology, a total staging score was calculated of all regions and also summed separately for the three neocortical regions of frontal, temporal, and parietal. For primary motor cortex, sections were taken from dorsolateral arm area within precentral gyrus. LRP staging, senile plaques and neurofibrillary tangles were also scored in the primary motor cortex (dorsolateral precentral gyrus) with the same staining and scoring methods. We also assign a Unified Staging system score for LB stage which is along the lines of Braak (4).
Protein Studies
Alpha-synuclein and Aβ-42 peptide ELISA
To measure total levels of total α-synuclein and Aβ-42 peptide, samples of primary motor cortex gray matter were extracted in 4 volumes of 5 M guanidine hydrochloride (GHCL) (Thermo Scientific, Pierce) buffered in 50 mM Tris-HCl (pH7.4) to solubilize α-synuclein or Aβ (14). To measure concentrations of total α-synuclein and Aβ-42 peptide, commercial ELISA kits specific for human α-synuclein and Aβ-42 (Invitrogen) were utilized. ELISA was performed according to manufacturer’s instructions; results were calculated as pg/mg protein (Aβ-42) or ng/mg protein (α-synuclein).
ELISA for parvalbumin, synaptophysin, and glial fibrillary acidic protein (GFAP)
We developed in our laboratory ELISAs for measuring concentrations of parvalbumin (ng/mg), synaptophysin (ng/mg), and GFAP (µg/mg). For these assays, ELISA plates were coated with the optimal concentration of capture antibody; for synaptophysin, SY17 (IgM monoclonal, Covance) was used; for parvalbumin, affinity purified polyclonal (R&D Systems) was used; for GFAP, a cocktail of 3 monoclonal antibodies (BD Biosciences) was used. Purified proteins for each were available for use in preparation of standard curves (synaptophysin; ABNOVA, Taiwan; parvalbumin – AbCAM, MA; GFAP; Calbiochem, NJ). Samples were prepared by extraction of primary motor cortex gray matter with RIPA buffer. Bound proteins were detected with the appropriate detection antibody (Synaptophysin – rabbit polyclonal, Chemicon; parvalbumin – rabbit polyclonal – AbCAM; GFAP – Calibiochem) and followed by the appropriate horseradish peroxidase labeled secondary antibody. Bound proteins (sample and standards) were detected using tetramethylbenzidine (TMB) peroxidase substrate (R & D Systems). Results were measured using a plate reader (Absorbance, 570 nm) and calculated against the generated standard curves.
Western blots
Western blot analysis of the insoluble fraction of α-synuclein in primary motor cortex gray matter extracts was performed in 6 M urea/2% SDS extracted samples. To measure concentrations of phosphorylated α-synuclein, aliquots of RIPA extracts were dissolved in 4× LDS gel sample buffer, and separated by electrophoresis on 4–12% NuPAGE Bis Tris gels (Invitrogen). The blots of brain samples were probed with an antibody to phosphorylated α-synuclein (p-ser 129), the most abundant form of p-synuclein (15,16). Blots were reprobed with an antibody to unmodified α-synuclein (BD Biosciences). This analysis yielded values from western blot for both phosphorylated (pSyn) and total α-synuclein (tSyn) expressed as a ratio (pSyn:tSyn).
Data Analysis and Statistical methods
The mean and prevalence of characteristics for the PD+Myoclonus, PD, and Control groups were compared. Our hypotheses were related to differences between the PD+Myoclonus and PD groups, where the only difference was the presence of small amplitude cortical myoclonus in the PD+Myoclonus group. The Control group had different exclusions, especially the presence of dementia and any evidence of neurodegenerative disease, etc. Thus, we designated PD+Myoclonus versus PD group as the primary analysis and all other comparisons secondary, such as PD+Myoclonus versus Control group. Statistical significance was calculated by using the two-sample t-test or Fisher exact test. Distribution assumptions were verified by using the permutation test. The effect of characteristics that are observable in living subjects (sex, age, postmortem interval (PMI)), PD duration, Unified Parkinson’s Disease Rating Scale (UPDRS) part III (motor score) and Hoehn and Yahr (H&Y) at last clinical assessment, Levodopa (LD) dose equivalents, dementia, neuropsychological test battery variables, and ApoE4 gene status on the relationship between total α-synuclein and the occurrence of cortical myoclonus was assessed by using a general linear model with terms for cortical myoclonus and the covariate. The relationships of different measures with total α-synuclein were quantified by using the Pearson correlation. Significance was set at P<0.05.
RESULTS
a) Demographic, clinical and pathological comparisons of disease groups
The clinical and pathological features are presented in Tables 1 and 2. Two subjects did not have a full UPDRS motor score or H&Y staging completed before death. Unavailable samples from certain areas caused N values to be <19 for “Neocortical” LRP Classification (N=18), LRP Stage Total (N=17), and Neocortical LRP Stage Total (N=17). There were no demographic or clinical differences between the groups for gender, age, PD duration, UPDRS motor score, H&Y stages, LD dose equivalents, presence of dementia, and time since electrophysiological study (Table 1). Pathology findings (Table 2), including multiple Lewy-related and AD pathology measures and postmortem interval did not differ between groups. There was little Lewy-related pathology as the highest primary motor cortex stage was only grade 1 (sparse Lewy bodies or Lewy neurites) for any PD+Myoclonus or PD case, with many cases receiving a grade 0 (no Lewy bodies nor Lewy neurites).
Table 1.
Demographic and clinical findingsa. Total UPDRS and H&Y scores were not assessed for two PD+Myoclonus subjects.
| PD+Myoclonus | PD | Control | |
|---|---|---|---|
| Female; n/N (%) | 3/11 (27%) | 3/8 (38%) | 3/9 (33%) |
| Age (y); mean (SD) | 82.6 (6.5) | 78.6 (9.0) | 85.1 (6.8) |
| Duration of PD (y); mean (SD) | 13.6 (5.1) | 10.0 (4.6) | N/A |
| UPDRS III; mean (SD) | 33 (14), N=9 | 22 (13) | N/A |
| H&Y; mean (SD) | 3.2 (1.1), N=9 | 2.75 (0.71) | N/A |
| Levodopa Dose Equivalents (mg); mean (SD) | 690 (370) | 560 (290) | N/A |
| Dementia; n/N (%) | 6/11 (55%) | 5/8 (62%) | 0/9 |
| Time since EP (y); mean (SD) | 1.48 (0.85) | 1.31 (0.47) | 1.4 (1.2) |
| Post-Mortem Interval (hr); mean (SD) | 3.4 (2.4) | 4.9 (5.6) | 2.55 (0.46) |
No values were significantly different for PD+Myoclonus versus PD or versus Control for Female predominance, Age, Time since EP, and Post-Mortem Interval; two-sample t-test or Fisher exact test. H&Y=Hoehn and Yahr score; EP=electrophysiological study
Table 2.
Pathology findingsa. Unavailable samples from certain areas caused N values to be <19 for “Neocortical” LRP Classification (N=18), LRP Stage Total (N=17), and Neocortical LRP Stage Total (N=17).
| PD+Myoclonus | PD | |
|---|---|---|
| Primary Motor Cortex LRP non-zero stage; n/N (%) | 7/11 (64%) | 4/8 (50%) |
| “Neocortical” LRP Classification; n/N (%) | 5/10 (50%) | 1/8 (12%) |
| LRP Stage Total (all areas); mean (SD) | 23.4 (5.0) | 22.1 (8.4) |
| Unified LB Stage; mean (SD) | 3.00 (0.94) | 3.00 (0.76) |
| Neocortical LRP Stage Total; mean (SD) | 3.7 (1.5) | 3.6 (3.6) |
| AD Pathology Criteria; n/N (%) | 4/11 (36%) | 2/8 (25%) |
| Braak Stage; mean (SD) | 3.33 (0.71) | 2.71 (0.76) |
| Primary Motor Cortex Senile Plaques Score; mean (SD) | 1.7 (1.5) | 1.0 (1.2) |
| Primary Motor Cortex Neurofibrillary Tangle Count | 0 | 0 |
No values were significantly different for PD+Myoclonus versus PD; two-sample t-test or Fisher exact test.
LRP=Lewy Related Pathology Stage as defined by McKeith et al. (DLB-III) (13). Neocortical LRP Classification refers to the assignment of “Neocortical” for the Lewy Related Pathology classification as per McKeith et al. (DLB-III) (13). Neocortical LRP Stage Total=Frontal+Temporal+Parietal Stages. AD=Alzheimer’s disease. AD pathology refers to the presence of pathological criteria for AD (4).
b) Protein measurements (Table 3)
Table 3.
Biochemical results
| PD+Myoclonus Mean (SD) |
PD Group Mean (SD) |
Pa | Control Mean (SD) |
Pb | |
|---|---|---|---|---|---|
| Total α-Synuclein; ng/mg | 212 (58) | 156 (23) | .02 | 162 (24) | .02 |
| Parvalbumin; ng/mg | 360 (120) | 390 (110) | .65 | 373 (78) | .86 |
| Synaptophysin; ng/mg | 390 (170) | 340 (120) | .55 | 330 (170) | .53 |
| GFAP; µg/mg | 54 (17) | 67 (30) | .24 | 41 (12) | .16 |
| Aβ42; pg/mg | 16 (19) | 10 (17) | .50 | 0.7 (1.7) | .03 |
| pSyn:tSyn Ratio | 0.155 (0.081) | 0.155 (0.093) | >.99 | 0.071 (0.039) | .02 |
| ApoE ε4 Carrier; n/N (%) | 4/11 (36%) | 2/8 (25%) | >.99 | 1/9 (11%) | .09 |
Pa is for PD+Myoclonus Group versus PD Group,
Pb is for PD+Myoclonus Group versus Control Group; two-sample t-test.
There was a 36% increase in total α-synuclein in the PD+Myoclonus group primary motor cortex compared to the PD group (P=0.02), and was also increased compared to the Control group (P=0.02), suggesting that increased α-synuclein may play an important role in the neocortical neuronal dysfunction in primary motor cortex that produces PD+Myoclonus. Based on the relative exposure time for α-synuclein western blots between soluble and insoluble forms, the levels of insoluble α-synuclein in these samples were very low (data not shown). The parvalbumin, synaptophysin, and GFAP values did not differ between groups. There was significantly less Aβ-42 between the Control and PD and PD+Myoclonus groups. Despite a higher nonsignificant mean value for the Aβ-42 peptide in the PD+Myoclonus group, the high variability and very low values in some PD+Myoclonus cases suggests that Aβ-42 and PD+Myoclonus are not consistently linked. There was no difference between the PD and PD+Myoclonus groups for the relative proportion of phosphorylated to total α-synuclein as expressed in a ratio.
Table 4 shows the correlation of total primary motor cortex α-synuclein values with demographic, clinical, pathological, and Apo-E4 findings. Cortical myoclonus had the strongest and the only significant correlation with total α-synuclein. None of the clinical characteristics found during life affected the relationship between cortical myoclonus and total α-synuclein by more than 20%.
Table 4.
Correlation for combined PD+Myoclonus and PD Groups of primary motor cortex total α-synuclein with demographic, clinical, pathological, and Apo-ε4 findings. Total UPDRS and H&Y scores were not assessed for two PD+Myoclonus subjects. Unavailable samples from certain areas caused N values to be <19 for “Neocortical” LRP Classification (N=18), LRP Stage Total (N=17), and Neocortical LRP Stage Total (N=17). The correlations are listed in the order of increasing P value for the correlation.
| Total α-Synuclein versus | N | r | 95% CI | P |
|---|---|---|---|---|
| Cortical Myoclonus presence | 19 | .53 | .10 to .79 | .02 |
| Duration of PD | 19 | .40 | −.07 to .72 | .09 |
| “Neocortical” LRP Classification | 18 | .33 | −.16 to .69 | .19 |
| Female | 19 | .26 | −22 to .64 | .28 |
| Primary Motor Cortex LRP Stage | 19 | −.25 | −.63 to .23 | .30 |
| AD Pathology Criteria | 19 | −.25 | −.63 to .23 | .31 |
| MMSE | 19 | .27 | −.26 to .68 | .32 |
| LRP Stage Total (all areas) | 17 | .23 | −.30 to .65 | .38 |
| AVLT-LTM | 19 | .24 | −.31 to .67 | .38 |
| Dementia | 19 | −.19 | −.59 to .29 | .43 |
| UPDRS III | 17 | .22 | −.38 to .69 | .47 |
| Neocortical LRP Stage Total | 17 | .18 | −.33 to .61 | .48 |
| ApoE ε4 | 19 | .17 | −.31 to .58 | .48 |
| Trails B | 19 | .21 | −.39 to .68 | .49 |
| JLO | 19 | .26 | −.49 to .79 | .50 |
| Wais-III Digit Span | 19 | −.18 | −.65 to .39 | .54 |
| Hoehn & Yahr Stage | 17 | .10 | −.42 to .57 | .71 |
| COWAT | 19 | .09 | −.44 to .58 | .74 |
| Stroop Interference | 19 | .08 | −.45 to .57 | .78 |
| Post-Mortem Interval | 19 | −.05 | −.49 to .41 | .85 |
| Age | 19 | −.03 | −.48 to .43 | .91 |
| Braak Score | 19 | .02 | −.48 to .51 | .95 |
| Unified LB Stage | 18 | .01 | −.46 to .47 | .98 |
| Primary Motor Cortex Senile Plaques score | 19 | .00 | −.45 to .45 | .98 |
| Levodopa Dose Equivalents | 19 | .00 | −.45 to .45 | .99 |
DISCUSSION
Our findings indicate that the mean α-synuclein level measured by ELISA is increased 36% in the primary motor cortex of PD+Myoclonus cases when compared to PD without myoclonus subjects. This measure represents the total pool of extractable α-synuclein. It is remarkable that this did not occur with group differences, or with much presence in either group of insoluble α-synuclein, as assessed by western blot analysis and by Lewy body/neurite presence. As the primary motor cortex is the myoclonus source in our PD+Myoclonus cases, our results suggest a possible association between elevated α-synuclein and the dysfunctional physiology arising from this localized area of the cerebral cortex. The association of cortical myoclonus with higher α-synuclein levels is consistent with the concept that abnormal accumulation of α-synuclein (even when not insoluble) is pathogenic in PD. Moreover, higher total α-synuclein levels significantly correlated with PD+Myoclonus but did not correlate with multiple clinical or pathological findings (see table 4).
Other biochemical findings did not differ between the PD+Myoclonus and PD groups. In particular, the loss of important inhibitory influences in the primary motor cortex is a plausible explanation for the increased excitability of primary motor cortex neuronal circuits causing myoclonus, but no group differences in parvalbumin protein suggests that a loss of major inhibitory GABA neurons in the primary motor cortex is not responsible for cortical myoclonus generation in PD+Myoclonus cases. Both basket cells and chandelier cells can be identified with immunocytochemical stains for parvalbumin in mammalian (including primate) motor cortex (DeFelipe, 1998; Porter, 2000). However, parvalbumin staining neurons are known to be more powerful in inhibiting target neurons than calbindin- and calretinin-staining neurons (Thomson, 1997, DeFelipe, 1999).
We suggest that small amplitude cortical myoclonus is appropriate for study as a model of localized PD cortical dysfunction in the primary motor cortex. Autopsy studies of PD-D have shown that the relative proportions of pathological involvement between different cortical areas is not always consistent across all cases (3,4,6–9). For biochemical studies of PD neocortex, the state of “dementia” is commonly used as the independent variable. However, when focal tissue samples are obtained from PD neocortex (e.g. cingulate gyrus, etc.) in demented patients, it is difficult to know whether that focal tissue location is experiencing the same pathological dysfunction that truly represents all cortical areas, especially those affecting the clinical cognitive state. Likewise, it is tricky to assume that “non-dementia” tissue samples are free from localized neocortical dysfunction in PD without having a measure of function for that focal cortical area from which the sample is taken. In this study, the primary motor cortex elevated α-synuclein correlation was significant for the presence of cortical myoclonus but not for the more global measure of clinical dementia presence. Measurement of localized neocortical dysfunction through electrophysiological detection of abnormal physiology (e.g. PD+Myoclonus) may provide a more accurate assessment of disease-affected tissue samples than the simple presence or absence of generalized clinical dysfunction (dementia) or global neocortical Lewy-related pathology staging systems.
This study provides verification of small amplitude cortical myoclonus in 11 more cases of pathologically proven idiopathic Lewy body PD (10). Parkinsonism severity and medication doses were not different between the PD and PD+Myoclonus groups, and these results are consistent with our previous report on the clinical characteristics of small amplitude cortical myoclonus in PD (10). Cortical myoclonus is also seen in other disorders that have cortical Lewy bodies, such as dementia with Lewy bodies (DLB) and hereditary Lewy body disease due to α-synuclein triplication (21–23). We have previously demonstrated that PD+Myoclonus in these cortical Lewy body disorders show identical electrophysiological localization and characteristics to the small amplitude cortical myoclonus in PD (21–23). It is not known whether our present results may be generalized to cases with other cortical Lewy body disorders but this remains an interesting possibility.
There are several findings that suggest cortical myoclonus in PD is not associated with AD pathological changes. Myoclonus is common in AD and has been associated with severe primary motor cortex pathology of AD patients (24,25). Since AD pathology changes in the primary motor cortex seemed a possible explanation for the cortical myoclonus in PD, several variables were examined in the primary motor cortex of our PD groups for this purpose. First, Aβ-42 peptide mean levels did not show a significant increase or difference in PD+Myoclonus subjects. These levels were more variable from case to case in both PD subject groups than the α-synuclein levels. The Control group had less Aβ-42 peptide than either the PD+Myoclonus or PD groups. Second, the presence of senile plaques in the primary motor cortex did not differ between the groups. Third, no neurofibrillary tangles were present in primary motor cortex of any subject. Fourth, synaptophysin which has been found to be reproducibly decreased in AD was not different for protein level in PD+Myoclonus cases. Lastly, most PD+Myoclonus cases did not have histological criteria for AD; nor did they differ in Braak staging for AD. These data therefore suggest that AD pathology is not necessary to produce the physiologic dysfunction of PD+Myoclonus, but given the high Aβ-42 variability found in both PD groups, a possible role for AD pathology in some cases can not be ruled out.
There are limitations of this study. Higher numbers of autopsied subjects may have found differences between PD+Myoclonus and PD groups for more measures. In particular, there were possible trends for the PD+Myoclonus group to be older, have longer duration and more severe PD requiring more medication. More subjects studied may have found these trends to be significant. Such a possible trend may possibly suggest that PD+Myoclonus cases correlate with more severe disease. The monoamines serotonin and dopamine have been implicated in human myoclonus and animal models. Although not examined in this study, markers for these monoamines, and calbindinand calretinin GABA neuron markers, are worthy of study in this PD+Myoclonus model (26,27).
The mechanism by which higher α-synuclein levels could directly cause or be associated with primary motor cortex neuron toxicity is currently unknown. Several mechanisms have been proposed for α-synuclein neuronal toxicity and include membrane disruption; interference with signaling pathways; altering vesicle trafficking; post-translational modification; and others (28–31). Phosphorylation is an example of post-translational modification and has been proposed to be associated with toxicity and α-synuclein aggregation. However, the ratio of phosphorylated to total α-synuclein was not altered in our PD+Myoclonus cases, and other investigators have evidence that phosphorylation is not directly responsible for α-synuclein toxicity (31–33). Membrane disruption of α-synuclein can alter the electrical properties of neurons, causing abnormal neuron firing (21, 34–38). This phenomenon in pyramidal neurons could potentially cause the abnormal paroxysmal discharges associated with cortical myoclonus. More research is needed to determine how α-synuclein may be toxic to cortical neuron function.
In summary, small amplitude cortical myoclonus in PD may serve as a useful model of in vivo PD cortical dysfunction in humans. This cortical dysfunction is associated with higher total α-synuclein levels rather than measures of insoluble α-synuclein. The mechanism for this dysfunction should have relevance for other cortical areas in PD. Such a model may provide a more specific correlation for studying focal brain tissue sample abnormalities than generalized dementia. Finally, the clinical biomarker significance of small amplitude cortical myoclonus in PD deserves further study.
ACKNOWLEDGEMENTS
This work was funded by the gift of Beth and Larry Johnson, Mayo Clinic Foundation for Medical Research, Michael J Fox Foundation for Parkinson’s Research (Prescott Family Initiative), Arizona Disease Control Research Commission (contracts 04-800, 4001, 05-901), Arizona Biomedical Research Commission (contracts 0011, 1001), and Federal Grant P30 AG019610.
Footnotes
AUTHOR ROLES
Study Concept and Design: Caviness, Lue, Walker.
Acquisition of Data: Caviness, Lue, Beach, Sue, Adler, Sue, Sadeghi, Driver-Dunckley, Evidente, Sabbagh, Shill, Walker.
Analysis and Interpretation of Data: Caviness, Lue, Hentz, Walker.
Drafting of the manuscript: Caviness, Lue, Hentz, Walker.
Critical revision of the manuscript for important intellectual content: Caviness, Lue, Beach, Sue, Adler, Sue, Sadeghi, Driver-Dunckley, Evidente, Sabbagh, Shill, Walker.
Statistical Analysis: Hentz.
Obtained funding: Caviness, Lue, Beach, Adler, Walker.
Administrative, technical, and material support: Caviness.
FINANCIAL DISCLOSURES
| Stock Ownership in medically fields--None | Intellectual Property Rights--None |
| Consultancies--None | Expert Testimony--None |
| Advisory Boards--None | Employment--None |
| Partnerships--None | Contracts--None |
| Honoraria--None | Royalties--None |
| Grants--Michael J. Fox Foundation, Parkinson’s study group, Arizona Biomedical Research Foundation | Other--None |
| Stock Ownership in medically-related fields--None | Intellectual Property Rights—None |
| Consultancies--None | Expert Testimony—None |
| Advisory Boards--None | Employment—None |
| Partnerships--None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--NIH Grant P30 AG019610 | Other—None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships--None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--NIH Grant P30 AG019610. | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria—None | Royalties—None |
| Grants--Michael J. Fox Foundation, Arizona Biomedical Research Foundation | Other--None |
| Stock Ownership in medically-related fields—None. | Intellectual Property Rights—None. |
| Consultancies--Allergan, Ipsen, Merck Serono. | Expert Testimony—None. |
| Advisory Boards-- Bachmann Strauss Dystonia Foundation | Employment—None. |
| Partnerships—None. | Contracts—None. |
| Honoraria—None. | Royalties—None. |
| Grants—Michael J. Fox Foundation, Parkinson’s study group, Arizona Biomedical Research Foundation | Other—None. |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--NIH Grant P30 AG019610. | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria—None | Royalties—None |
| Grants-- NIH Grant P30 AG019610. | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--Michael J. Fox Foundation, Parkinson’s study group, Arizona Biomedical Research Foundation | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--Michael J. Fox Foundation, Parkinson’s study group, Arizona Biomedical Research Foundation | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--NIH Grant P30 AG019610 | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights—None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--Michael J. Fox Foundation, Parkinson’s study group, Arizona Biomedical Research Foundation | Other--None |
| Stock Ownership in medically-related fields—None | Intellectual Property Rights--None |
| Consultancies—None | Expert Testimony—None |
| Advisory Boards—None | Employment—None |
| Partnerships—None | Contracts—None |
| Honoraria--None | Royalties—None |
| Grants--NIH Grant P30 AG019610 | Other--None |
REFERENCES
- 1.Levy G, Tang M-X, Louis ED, Cote LJ, Alfaro MA, Mejia H, Stern Y, Marder K. The association of incident dementia with mortality in PD. Neurology. 2002;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 2.Maguire-Zeiss KA. α-Synuclein: A therapeutic target for Parkinson’s disease? Pharmacological Research. 2008;58:271–280. doi: 10.1016/j.phrs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Rub U, Steur ENHJ, Del Tredici KD, de Vos RAI. Cognitive status correlates with neuropathologic stage in Parkinson’s disease. Neurology. 2005;64:1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 4.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson’s disease. J Neural Transm. 2002;109:329–339. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- 6.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch. Neurol. 2002;59:102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 7.Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM-Y, Clark CM, Gosser G, Stern MD, Golllomp SM, Arnold SE. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 8.Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, Peterson LK, Caviness JN, Shill HA, Sue LI, Ziabreva I, Perry E, Ballard CG, Aarsland D, Walker DG, Beach TG. Parkinson’s disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer’s disease and other disorders. 2009;23(3):295–297. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. α-Synuclein Pathology Does Not Predict Extrapyramidal Symptoms or Dementia. Annals of Neurology. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 10.Caviness JN, Adler CH, Beach TG, Wetjen KJ, Caselli RJ. Small Amplitude Cortical Myoclonus in Parkinson’s Disease: Physiology and Clinical Observations. Movement Disorders. 2002;17:657–662. doi: 10.1002/mds.10177. [DOI] [PubMed] [Google Scholar]
- 11.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler CH, Hentz JG, Joyce JN, Beach T, Caviness JN. Motor Impairment in Normal Aging, Clinically Possible Parkinson’s Disease, and Clinically Probable Parkinson’s Disease: Longitudinal Evaluation of a Cohort of Prospective Brain Donors. Parkinsonism and Related Disorders. 2002;9:103–110. doi: 10.1016/s1353-8020(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 13.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C for the Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report on the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 14.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and A beta42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1997;l94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp.Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr.Protein Pept.Sci. 2008;9:507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 17.DeFelipe J, del Carmen Gonzalez-Albo M. Chandelier cell axons asre immunoreactive for GAT-1 in the human neocortex. NeuroReport. 1998;9:467–470. doi: 10.1097/00001756-199802160-00020. [DOI] [PubMed] [Google Scholar]
- 18.Porter LL, Matin D, Keller A. Characteristics of GABAergic neurons and their synaptic relationships with intrinsic axons in the cat motor cortex. Somatosensory & Motor Research. 2000;17(1):67–80. doi: 10.1080/08990220070319. [DOI] [PubMed] [Google Scholar]
- 19.Thomson AM, Deuchars J. Synaptic interactions in Neocortical local circuits: Dual intracellular recordings in Vitro. Cerebral Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- 20.DeFelipe J. Chandelier cells and epilepsy. Brain. 1999;122:1807–1822. doi: 10.1093/brain/122.10.1807. [DOI] [PubMed] [Google Scholar]
- 21.Caviness JN, Adler CH, Beach T, Wetjen K, Caselli RJ. Myoclonus in Lewy Body Disorders. In: Fahn S, et al., editors. Myoclonus and Paroxysmal Dyskinesias. Vol. 89. New York: Lippincott Williams & Wilkins, New York; 2002. pp. 23–30. Advances in Neurology. [PubMed] [Google Scholar]
- 22.Caviness JN. Clinical Neurophysiology of Myoclonus. Movement Disorders. In: Hallett M, editor. Handbook of Clinical Neurophysiology. Vol. 1. 2003. pp. 521–548. Ch. 32. [Google Scholar]
- 23.Caviness JN, Gwinn-Hardy KA, Adler CH, Muenter MD. Electrophysiological Observations in Hereditary Parkinsonism-Dementia with Lewy Body Pathology. Movement Disorders. 2000;15:140–145. doi: 10.1002/1531-8257(200001)15:1<140::aid-mds1022>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Golaz J, Bouras C, Hof PR. Motor cortex involvement in presenile dementia: Report of a case. J Geriatr Psychiatry Neurol. 1992;5:85–92. doi: 10.1177/002383099200500205. [DOI] [PubMed] [Google Scholar]
- 25.Horoupian DS, Wasserstein PH. Alzheimer’s disease pathology in motor cortex in dementia with Lewy bodies clinically mimicking corticobasal degeneration. Act Neuropathol. 1999;98:317–322. doi: 10.1007/s004010051087. [DOI] [PubMed] [Google Scholar]
- 26.Tai KK, Bhidayasiri R, Truong DD. Post-hypoxic animal model of myoclonus. Parkinsonism & Related Disorders. 2007;13(7):377–381. doi: 10.1016/j.parkreldis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Caviness JN. Pathophysiology and Treatment of Myoclonus. Neurologic Clinics. 2009;27(3):757–777. doi: 10.1016/j.ncl.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. Journal of Neurochemistry. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 29.Tofaris GK, Spillantini MG. Physiological and pathological properties of alpha-synuclein. Cell Mol. Life Sci. 2007;64:2194–2201. doi: 10.1007/s00018-007-7217-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soto C, Estrada L. Protein Misfolding and Neurodegeneration. Arch Neurol. 2008;65(2):184–189. doi: 10.1001/archneurol.2007.56. [DOI] [PubMed] [Google Scholar]
- 31.Azeredo da Silveira S, Schneider BL, Cifuentes-Diaz C, Sage D, Abbas-Terki T, Iwatsubo T, Unser Aevischer P. Phosphorylation does not prompt, nor prevent, the formation of alpha-synuclein toxic species in a rat model of Parkinson’s disease. Human Molecular Genetics. 2009;18(5):872–887. doi: 10.1093/hmg/ddn417. [DOI] [PubMed] [Google Scholar]
- 32.Libow LS, Frisina PG, Haroutunian V, Perl DP, Purohit DP. Parkinson’s disease dementia—A diminished role for the Lewy body. Parkinsonism and related disorders. 2009;15:572–575. doi: 10.1016/j.parkreldis.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalfo E, Portero-Otin M, Ayala V, Marinez A, Pamplona R, Ferrer I. Evidence of Oxidative Stress in the Neocortex in Incidental Lewy Body Disease. J Neuropathol Exp Neurol. 2005;64(9):816–830. doi: 10.1097/01.jnen.0000179050.54522.5a. [DOI] [PubMed] [Google Scholar]
- 34.Beyer K. Mechanistic aspects of Parkinosn’s disease: alpha-synuclein and the biomembrane. Cell Biochem Biophys. 2007;47:285–299. doi: 10.1007/s12013-007-0014-9. [DOI] [PubMed] [Google Scholar]
- 35.Volles MJ, Lansbury PT. Relationships between the sequence of alpha-synuclein and its membrane affinity, fibrillization propensity, and Yeast toxicity. J. Mol. Biol. 2007;366:1510–1522. doi: 10.1016/j.jmb.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukawa K, Matsuzaki-Kobayashi M, Hasegawa T, Kikuchi A, Sugeno N, Itoyama Y, Wang Y, Yao PJ, Bushlin I, Takeda A. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. Journal of Neurochemistry. 2006;97(4):1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 37.McLean PJ, Kawamata H, Ribieh S, Hyman BT. Membrane Association and Protein Conformation of α-Synuclein in Intact Neurons. The Journal of Biological Chemistry. 2000;275(12):8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 38.Pandey AP, Haque F, Rochet J-C, Hovis JS. Clustering of α-Synuclein on Supported Lipid Bilaters: Role of Anionic Lipid, Protein, and Divalent Ion Concentration. Biophysical Journal. 2009;96:540–551. doi: 10.1016/j.bpj.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]