Abstract
The Acute Coagulopathy of Trauma (ACOT) has been described as a very early hypocoagulable state, but the mechanism remains controversial. One proposed mechanism is tissue hypoperfusion leading to protein C activation, with subsequent inhibition of Factors V and VIII. Variability in trauma has impeded the use of clinical data towards the elucidation of the mechanisms of ACOT, but thrombelastography (TEG) may provide insight by assessing hemostatic function from initial thrombin activation to fibrinolysis. We hypothesized that, in a controlled animal model of trauma/hemorrhagic shock, clotting factor dysfunction is the predominant mechanism in early ACOT.
Methods
Rats anesthetized by inhaled isoflurane (n=6) underwent laparotomy, and hemorrhage was induced to maintain a MAP of 35 mmHg for 30 minutes. Rats were then resuscitated with twice their shed blood volume in normal saline. TEG was performed at baseline, shock, and post-resuscitation periods. No heparin was given. Statistical analysis was performed by ANOVA with post-hoc Fisher’s test.
Results
Coagulation factor function was significantly impaired in the early stages of trauma/hemorrhagic shock. TEG R and SP-values were significantly increased from baseline to shock (p<0.001) and from shock to post-resuscitation periods (p<0.05). Delta (R-SP), a measure of thrombin generation, showed a significant increase (p<0.05) from baseline to shock. No significant changes were found in K, Angle, MA, and LY30 values.
Conclusion
Clotting factor derangement leading to impaired thrombin generation is the principle etiology of ACOT in this model and not the dynamics of clot formation, fibrin cross-linking, clot strength/platelet function, or fibrinolysis.
Keywords: Acute Coagulopathy of Trauma, Thrombelastography (TEG), Disseminated Intravascular Coagulation (DIC), Protein C, Trauma, Hemorrhagic Shock, Thrombin Generation
Introduction
The acute coagulopathy of trauma (ACOT) is recognized as a very early hypocoagulable state leading to increased transfusion requirements, and significant mortality. In spite of the first clinical descriptions of this phenomenon during the Korean and Vietnam wars1,2, the fundamental mechanism and management of this coagulopathy remain elusive. Since its discovery, the derangement seen in the ACOT has been presumed as disseminated intravascular coagulation (DIC)2,3. It was not until recently, however, that the thrombomodulin-protein C pathway has been implicated as the primary mechanism.4 Plasma-based coagulation studies have been limited in elucidating the basic components involved in post-injury coagulopathies. However, the science of hemostasis has evolved rapidly over the past decade and there is now strong evidence that thrombotic control mechanisms play an active role in hemostatic processes than originally believed.
A novel whole blood-based model of hemostasis, which encompasses both the cellular and the fluid phases of coagulation, has challenged the classical clotting cascade leading to new insights in the ACOT.5 Consequently, this has questioned the validity of plasma-based laboratory tests, such as the activated partial thromboplastin time (aPTT) and the international normalized ratio (INR), in identifying this phenomenon.6,7 In fact, these tests were originally designed to monitor hemophilia and anticoagulation therapy and do not reveal interactions between clotting factors, cells expressing tissue factor, and the surface of platelets. Therefore, whole blood viscoelastic hemostatic assays, such as thrombelastography (TEG), have been proposed as a more appropriate test to identify ACOT since it contains all the components of whole blood and assesses hemostatic function from initial thrombin activation to fibrinolysis.
Thrombelastography is re-emerging as a useful tool in the management of post-injury coagulopathy in order to obtain comprehensive assessments of coagulation. Developed by Hartert of Germany in 1948, TEG was brought to the United States by a fellow German, von Kaulla, who along with Swan at the University of Colorado in the 1950’s, used TEG to mange coagulation derangements during Swan’s ground-breaking clinical application of hypothermic arrest in cardiac surgery.8 A decade later, TEG was instrumental in guiding blood component therapy during the birth of Starzl’s liver transplantation program in Denver.9 Its use clinically, as a rapid point-of-care test in trauma, is quickly growing since results can be obtained in less than 15 min. Therefore, it is possible that with future studies, TEG can help predict which patients will develop the ACOT, employ earlier activations of rapid transfusion protocols, and guide resuscitation with appropriate products, ultimately leading to decreased mortality.
Several studies have exploited TEG in an effort to understand the ACOT, but due to the variation in the mechanism and severity of trauma, the results have been inconsistent.10 However, these studies have shown diametrically opposing correlation with coagulopathy depending on injury severity scores (ISS). Specifically, in patients with lower ISS, hypercoagulability predominates; whereas, patients with higher ISS tend to be hypocoaguable, and even hyperfibrinolytic in the most severe cases of trauma.10–13 Furthermore, hypocoagulable states have been associated with increased mortality, and the most common early coagulopathies were clotting factor derangements.14
Consequently, the purpose of this study was to determine the changes in the basic components of post-injury coagulation using TEG in a clinically relevant model of trauma/hemorrhagic shock.
Methods
Animals
Adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 350–450 g were housed under barrier-sustained conditions with 12 hr light/dark cycles and allowed free access to food and water for a minimum of one week before use. All animals were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, and this study was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee.
Materials
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO). Thrombelastography equipment and supplies were obtained from Haemonetics Corporation (Niles, IL); 0.9% injection grade normal saline (NS) was purchased from Baxter Healthcare (Deerfield, IL); iSTAT equipment and supplies were purchased from Abbott Laboratories (Abbott Point of Care, Princeton, NJ); Isoflurane inhalant was purchased from VET one (Meridian, ID); Polyethylene tubing was acquired from Fisher Scientific (Pittsburgh, PA).
Trauma/Hemorrhagic Shock Model
Animals (n=6) were anesthetized by inhaled isoflurane, and a cardiac puncture was performed to collect a blood sample for a baseline TEG. Local anesthesia was given by subcutaneous injection of 1% lidocaine. The femoral artery and vein were then cannulated with polyethylene (PE-50) tubing for continuous invasive pressure monitoring using a ProPaq device and to establish venous access. A tracheotomy was performed, at which point the animal was placed on 40% FiO2 using an air-oxygen mixer (Sechrist, Anaheim, CA) at a flow rate of 2 liters per minute. The animal’s body temperature was measured rectally and kept at euthermic with the use of a heat lamp. After a 45-minute observation period, a laparotomy was performed to simulate trauma, and hemorrhage was induced over a period of 10-minutes through the arterial catheter to maintain a MAP of 35 mmHg for 30 minutes. NS was infused at a rate of 0.4 ml/hr to maintain patency of the arterial line during shock. At the end of shock, animals were resuscitated with twice their shed blood volume in NS over a 30-minute period. Animals were then observed for 1 hour following resuscitation. TEG was performed at baseline, at the end of shock, and post-resuscitation periods. No heparin was given in this study.
Thrombelastography
Thrombelastography (TEG) was performed with blood collected from cardiac puncture at baseline, and through the femoral arterial catheter during shock and post-resuscitation periods. One ml of blood was anticoagulated with 100 µl of 3.2% sodium citrate and vortexed briefly to prevent platelet clumping. Kaolin was used as an activator, and 340 µl of the blood was added to 20 µl of 0.2M calcium chloride in a disposable plastic cup.
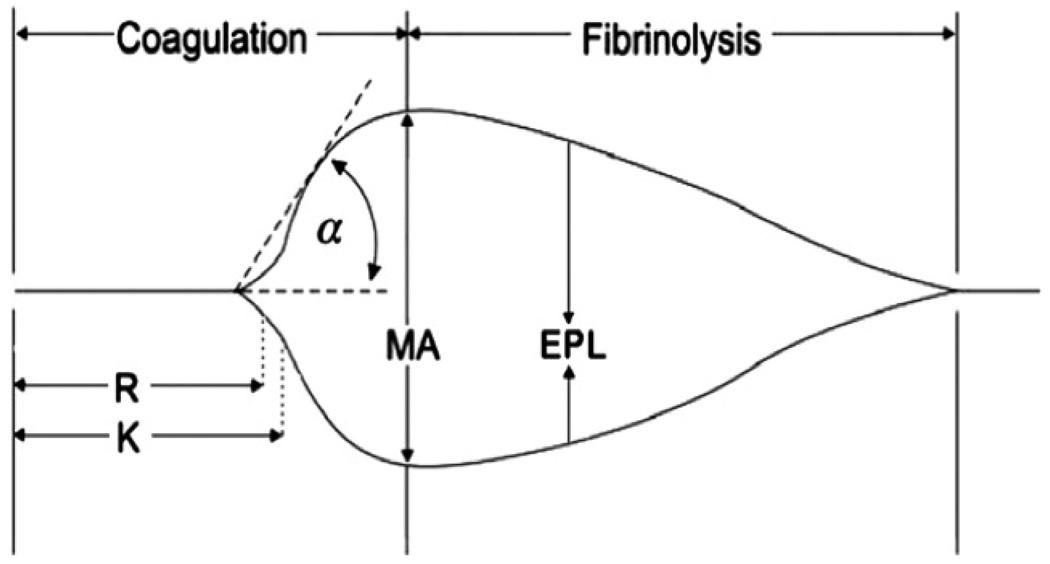
Thrombelastogram was performed at 37°C within 10 minutes of blood collection. All TEG parameters were recorded from standard tracings: split point (SP, minutes), reaction time (R, minutes), coagulation time (K, minutes), Delta (Δ, minutes), Angle (α, degrees), maximum amplitude (MA, mm), clot strength (G, dynes/cm2) and estimated percentage lysis (EPL, %). The various components of the TEG tracing are depicted in Fig 1. The SP time is a measure of the time to initial clot formation, interpreted from the earliest resistance detected by the TEG analyzer causing the tracing to split; this is the terminus of all other platelet-poor plasma clotting assays (e.g., aPTT and INR). The R value, the time elapsed from the start of the test until the developing clot provides enough resistance to produce a 2 mm amplitude reading on the TEG tracing, represents the initiation phase of enzymatic clotting factors. K measures the time from clotting factor initiation (R) until clot formation reaches amplitude of 20 mm. Delta (Δ) is calculated from the difference between R time and the SP of the TEG tracing (R – SP), representing the time interval of greatest clot growth secondary to peak thrombin generation.15 The angle (α) is formed by the slope of a tangent line traced from the R to the K time measured in degrees. K time and angle denote the rate at which the clot strengthens and is most representative of thrombin cleavage of fibrinogen into fibrin. The MA indicates the point at which clot strength reaches its maximum amplitude in millimeters on the TEG tracing, and reflects the end result of platelet-fibrin interaction via the GPIIb-IIIa receptors.16 G is a calculated measure of total clot strength derived from amplitude (A, mm): G=1/4(5000 X A)/(100 X A). Being derived from the progressive increase of amplitude, G is a more realistic representation of overall clot strength.17 Fibrinolysis, which begins the process of clot dissolution and a decrease in clot strength is measured by the percent lysis (LY30); the degree of fibrinolysis 30 minutes after MA is reached.
Figure I.
Standard TEG parameters. Reaction (R) time, clot formation (K) time, fibrin cross-linking (angle = α), clot strength (maximal amplitude [MA]), and estimated percent lysis (EPL).
Statistical analysis
Data are reported as the mean ± standard deviation and were compared by analysis of variance using the Fisher protected least significant difference test for post hoc comparisons. A p-value of <0.05 was considered statistically significant.
Results
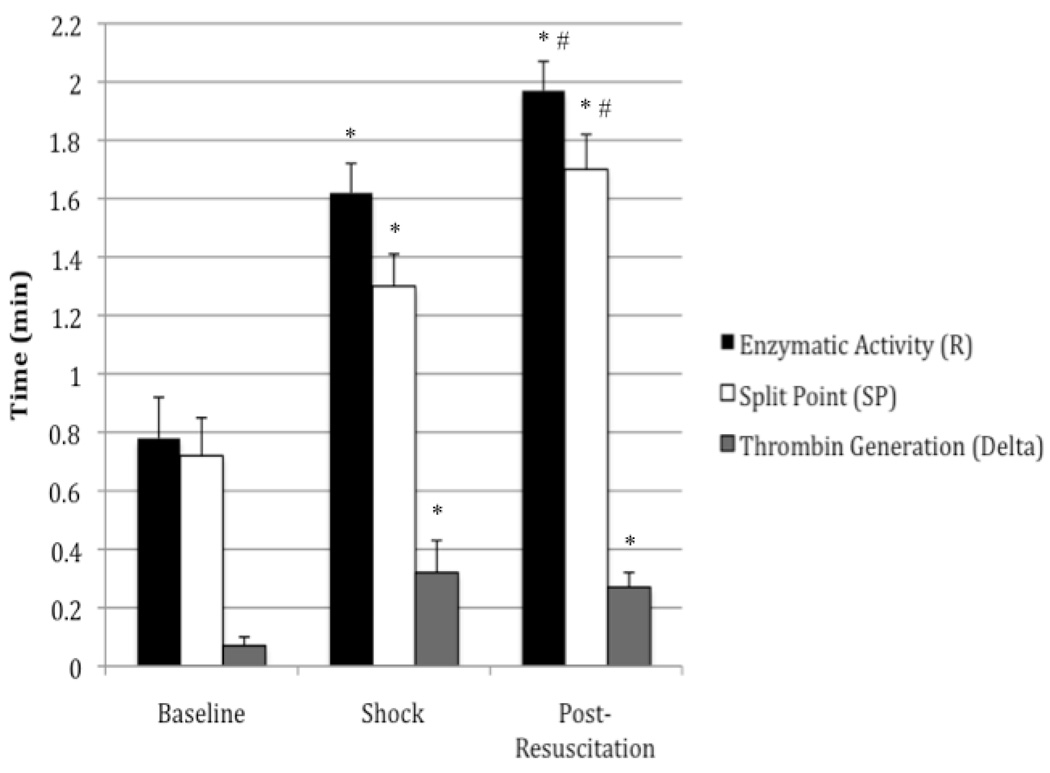
TEG values from the trauma/hemorrhagic shock experiments are summarized in Table I. Figure II shows the graphical relationship between R, SP, and Delta. To ensure a clinically relevant model of trauma/hemorrhagic shock, 40–60% of the animal’s total blood volume was removed and measurements of hemoglobin (Hb), hematocrit (Hct), and base deficit (BD) were obtained at baseline, shock, and post-resuscitation periods using an iSTAT device. Baseline Hb and Hct values decreased from 14.1±1.47 g/dL and 41.5±4.23 % to 7.42±0.65 g/dL and 22.0±1.92 % respectively post-resuscitation. At baseline, a base excess was measured at 4.75±1.26 mEq/L, and at the end of shock, a base deficit was obtained measuring 9.2±3.7 mEq/L.
Table I.
TEG parameters in the trauma/hemorrhagic shock experiment.
| Baseline | Shock | Post- Resuscitation |
|
|---|---|---|---|
| Enzymatic Activity (R) | 0.78 (±0.14) | 1.62 (±0.10)* | 1.97 (±0.10)*# |
| Split Point (SP) | 0.72 (±0.13) | 1.30 (±0.11)* | 1.70 (±0.12)*# |
| Thrombin Generation (Delta) | 0.07 (±0.03) | 0.32 (±0.11)* | 0.27 (±0.05)* |
| Clot Formation (K) | 0.87 (±0.07) | 0.80 (±0.00) | 0.83 (±0.03) |
| Fibrin Cross-linking (Angle) | 81.8 (±1.42) | 82.65 (±0.46) | 82.07 (±0.35) |
| Platelet-Fibrin Interaction (MA) | 70.67 (±2.91) | 73.93 (±0.81) | 70.63 (±0.75) |
| Clot Strength (G) | 12.75 (±3.48) | 13.68 (±1.59) | 12.22 (±1.06) |
| Percent Lysis (LY30) | 0.15 (±0.13) | 1.03 (±0.66) | 1.40 (±1.15) |
R (min), SP (min), Delta (min), K (min), Angle (degrees), MA (mm), G (dynes/cm2), and EPL (%). Data are reported as mean ± standard deviation. These data show that coagulation factor function was significantly impaired in the early stages of trauma/hemorrhagic shock. TEG R, SP, and delta values were significantly increased from baseline to shock. No significant changes were found in K, angle, MA, G, and LY30 values. Significant data measured from baseline are labeled (*) with p-values <0.0001. Significant data measured from shock are labeled (#) with p-values <0.05.
Figure II.
Coagulation factor function was significantly impaired in the early stages of trauma/hemorrhagic shock. TEG R and SP-values were significantly increased from baseline to shock and from shock to post-resuscitation periods. The time for thrombin generation, showed a significant increase from baseline to shock. Significant data measured from baseline are labeled (*) with p-values <0.001. Significant data measured from shock are labeled (#) with p-values <0.05.
Enzymatic Activity
Enzymatic activity, as reflected by the reaction time (R) in thrombelastography, is ultimately a measure of protease (coagulation factor) activity in initial clot formation. The reaction time was significantly increased from 0.78 min at baseline to 1.62 min during shock (p<0.001), and to 1.97 min post-resuscitation (p<0.05) representing significantly impaired coagulation factor function.
Thrombin Generation
The time interval of greatest clot growth secondary to thrombin generation, as measured by delta (Δ), significantly increased from 0.07 min to 0.32 min (p<0.05), reflecting a decreased rate of thrombin generation. There was no significant difference between shock and post-resuscitation values.
Clot Formation
Clot formation, as measured by the angle, represents the cleavage of fibrinogen to fibrin by thrombin, and the cross-linking of fibrin to begin the strengthening of the clot. The value of the angle at baseline, shock, and post-resuscitation periods were 81.8, 82.65, and 82.07 degrees respectively. There was no significant difference between any periods.
Clot Strength
Clot strength, represented by G on the TEG tracing, corresponds to both platelet and enzymatic contributions to the clot. There were no significant changes between baseline, shock, and post-resuscitation values, which measured 12.75, 13.68, and 12.22 dynes/cm2 respectively.
Fibrinolysis
Fibrinolysis, as measured by LY30, represents the percentage of clot lysis 30 min after MA is reached. The baseline, shock, and post-resuscitation values were 0.15, 1.03, and 1.4 percent respectively. Although there was a trend toward increasing fibrinolysis in the shock and post-resuscitation periods, no statistical significance was obtained.
Discussion
The results of the present in vitro study suggest that clotting factor derangement leading to impaired thrombin generation is fundamental to the development of the ACOT rather than the dynamics of clot formation, fibrin cross-linking, clot strength/platelet function, or fibrinolysis. This animal model of ACOT employing TEG provides similar results as those recently reported in severely injured patients.14 In this model, significant tissue injury and hemorrhage of approximately 50% of the total blood volume was achieved to generate a base deficit with a mean difference of 13.95 mEq/L from baseline. In addition, resuscitation with twice the volume of shed blood with NS did cause hemodilution; however, care was maintained to avoid critical anemia (Hb < 7.0 g/dL), making this model clinically relevant.
As in all animal models, there are inherent limitations which do not entirely reflect the human condition, and as with this study, there are pronounced species differences in thrombelastography coagulation profiles between humans and rats.18 At baseline, rats form clots significantly faster and stronger, and have less lysis compared to humans. In spite of these differences, the highly conserved pathways of coagulation have analogous functions throughout species, and likely respond to trauma/hemorrhagic shock similarly. Thus, animal models utilizing TEG are still instrumental in identifying the basic components of post-injury coagulopathy. Currently, there are two primary mechanisms proposed for the ACOT, which continue to be debated. The first proposed mechanism suggests DIC with consumption of coagulation factors as the primary etiology.3,19 The current consensus of DIC is that it has multiple etiologies, but is defined by two stages.20 The first stage involves a widespread and unregulated generation of clotting factors and thrombin, which induces platelet activation and aggregation in addition to fibrin deposition in the microvasculature. In addition, there are increased levels of plasminogen activator inhibitor-1 (PAI-1), which prevents fibrinogen breakdown, leading to a hypercoagulable state. Consequently, this surge in thrombin allows excess substrate to bind to receptors on endothelial cells, which stimulates the release of TPA, as well as activation of thrombin-activatable fibrinolysis inhibitor, neutrophil elastase, and plasmin. These anti-thrombotics, along with the excessive activation and consumption of coagulation factors and platelets, lead to the second stage of secondary fibrinolysis. This is evident by decreased levels of circulating clotting factors, fibrinogen, and platelets.
The alternative mechanism involves the thrombomodulin-protein C pathway.4 In the setting of both tissue hypoperfusion and injury, thrombomodulin is expressed on the endothelium, and ultimately forms a complex with thrombin. This complex then activates protein C, which inhibits factors V and VIII as well as PAI-1. Consequently, less fibrin is formed and the fibrin already in existence is degraded by plasmin due to unopposed tissue plasminogen activator (TPA) activity.
This study is limited in discerning between these two proposed mechanisms since no direct measurements of clotting factors, platelets, d-dimer, or fibrinogen were obtained. However, the activity or presence of these factors can be quantified using thrombelastography. Furthermore, TEG tracings are pathognomonic for specific coagulopathies including coagulation factor dysfunction and DIC (Figure III). If the common pathway of DIC is excessive thrombin generation and increased PAI-1 activity leading to a hypercoagulable state, TEG should reveal shortened R and delta values along with a stable or increased LY30 value from baseline to shock. The opposite of this was seen in this study, in which R and delta were prolonged and the LY30 did not significantly change at the end of shock. Even following resuscitation, no hypercoagulable state was detected, making this hypothesis less likely.
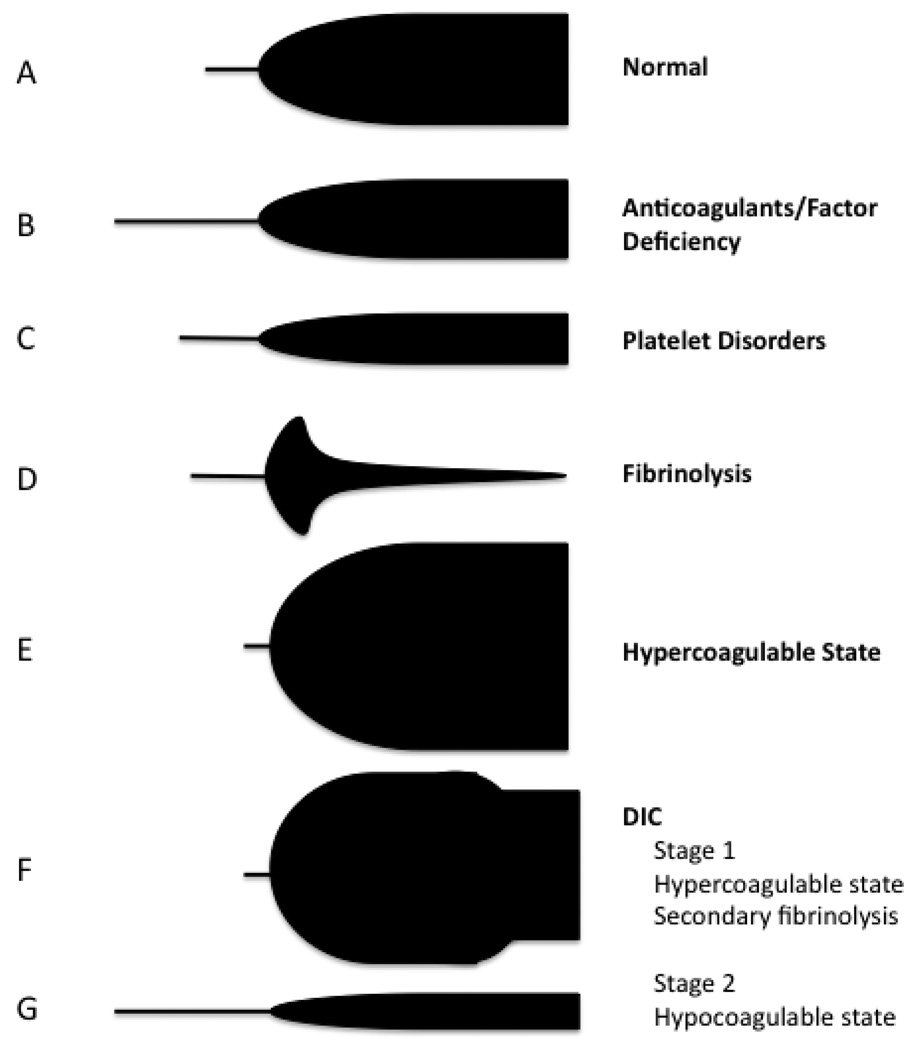
Figure III.
Characteristic tracings of a normal thrombelastogram and specific coagulopathies. A: Represents a normal thrombelastogram. B: Thrombelastogram reflecting impaired protease activity as represented by a prolonged R and K-time. F: Represents Stage I of DIC with an initial hypercoaguable state with secondary fibrinolysis. G: Represents Stage 2 of DIC with an overall hypocoaguable state as represented by a prolonged R and K-time, as well as a decreased MA secondary to clotting factor consumption.
These data show that even though thrombin generation was impaired, overall clot integrity was preserved, which favors the proposed mechanism of activated protein C inhibiting Factor V and VIII activity. Although, to fully support this mechanism, factor levels, as well as protein C levels, would need to be quantified in this model. A further limitation of this study is that only three time points were used to evaluate TEG parameters, and therefore, the course of this post-injury coagulopathy cannot be determined. One might speculate that an early hypercoagulable state is possible within the shock period, but other factors are implicated rather than DIC. The sympathetic response to injury, simulated by infusion of stress hormones in animal and clinical experiments, is sufficient to cause a hypercoagulable state.21–23 Even under adequate anesthesia, significant stress is likely following femoral vessel cannulation, tracheotomy, and laparotomy. For this reason, the baseline TEG blood sample was acquired through cardiac puncture. It is unlikely that an earlier, hypercoagulable state due to DIC is missed by these time points since hemostatic potential persists to the end of the experiment, suggesting no consumption of clotting factors.
Furthermore, additional time points following resuscitation were not measured. The proponents of the DIC hypothesis state that clinically, patients develop a hypercoagulable state (DIC with a prothrombotic phenotype) 3–5 days following the initial trauma secondary to increased levels of PAI-1.3 Extending time points over several days, to determine if a compensatory hypercoagulable state is evident, would require frequent blood draws from a small animal already anemic from hemorrhage, and thus, making this experiment prohibitive in this model.
It is also important to note that clinically, the mechanisms and severity of trauma vary greatly between blunt, penetrating, and crush injuries. This model focuses on one standardized type of injury (penetrating trauma associated with hemorrhagic shock) and cannot be generalized to all trauma patients. However, the purpose of this study was to evaluate the basic components of post-injury coagulopathy utilizing TEG in this specific model. Therefore, it is difficult to speculate how TEG parameters would change in different models of trauma, but this warrants further investigation. The proponents of DIC would likely hypothesize that tissue ischemia is all that is needed to incite DIC, and therefore, there would be no change in the TEG parameters. Alternatively, the proponents of the thrombomodulin-activated protein C pathway would propose that the mechanism of trauma matters since both tissue factor and tissue ischemia are required to develop the ACOT. Therefore, with worse tissue injury (increased tissue factor), a more pronounced ACOT would develop.
In summary, clotting factor derangement leading to impaired thrombin generation is fundamental to the development of the ACOT. Due to the limitations of this study, it is difficult to discern between the DIC or the thrombomodulin-protein C mechanism as the fundamental pathway of the ACOT. Although the thrombomodulin-protein C pathway appears likely, additional pathways must be considered due to the complexities of the coagulation, anti-thrombotic, and inflammatory systems. These data warrant further investigation to further elucidate a mechanism in this animal model.
Acknowledgements
I would like to acknowledge Joon Lee, MD for his assistance and guidance in the statistical analysis.
This study was supported by the National Institutes of Health (P50GM049222 T32GM008315 grants).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accepted for presentation at the Academic Surgical Congress on February 3, 2011.
References
- 1.Scott R. Changes in the coagulation mechanism following wounding and resuscitation with stored blood; a study of battle casualties in Korea. Blood. 1954;9:609–621. [PubMed] [Google Scholar]
- 2.Simmons R, Collins J, Heisterkamp C, et al. Coagulation disorders in combat casualties. I. Acute changes after wounding. II. Effects of massive transfusion. III. Post-resuscitative changes. Ann Surg. 1969;169:455–482. doi: 10.1097/00000658-196904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gando S. Disseminated intravascular coagulation in trauma patients. Semin Thromb Hemost. 2001;27:585–592. doi: 10.1055/s-2001-18864. [DOI] [PubMed] [Google Scholar]
- 4.Brohi D, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemeic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman M, Monroe D. Rethinking the coagulation cascade. Curr Hematol Rep. 2005;4:391–396. [PubMed] [Google Scholar]
- 6.Martini W, Cortez D, Dubick M, et al. Thrombelastography is better than PT, aPPT, and activated clotting time in detecting clinically relevant clotting abnormalities after hypothermia, hemorrhagic shock and resuscitation in pigs. J Trauma. 2008;65:535–543. doi: 10.1097/TA.0b013e31818379a6. [DOI] [PubMed] [Google Scholar]
- 7.Plotkin A, Wade C, Jenkins D, Smith K, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–S68. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 8.von Kaulla KN, Swan H. Clotting deviations in man associated with open-heart surgery during hypothermia. J Thorac Surg. 1958;36:857–868. [PubMed] [Google Scholar]
- 9.von Kaulla KN, Kaye H, von Kaulla E, Marchioro TL, Starzl TE. Changes in blood coagulation. Before and After Hepatectomy or Transplantation in Dogs and Man. Arch Surg. 1966;92:71–79. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson P, Stissing T, Bochsen L, Ostrowski S. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann C, Dwyer K, Crews J, Dols S, et al. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma. 1997;42:716–720. doi: 10.1097/00005373-199704000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber M. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11:590–597. doi: 10.1097/01.ccx.0000186374.49320.ab. [DOI] [PubMed] [Google Scholar]
- 13.Levrat A, Gros A, ruger L, Inaba K, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100:792–797. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 14.Carroll R, Craft R, Langdon R, Clanton C, et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl Res. 2009;154:34–39. doi: 10.1016/j.trsl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez E, Kashuk J, Moore EE, Silliman C. Differentiation of enzymatic from platelet hypercoagulability using the novel thrombelastography parameter delta. J Surg Res. 2010;163:96–101. doi: 10.1016/j.jss.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana S, Mattson JC, Westley S, et al. Monitoring platelet glycoprotein IIb/IIIa-fibrin interaction with tissue factoractivatedthromboelastography. J Lab Clin Med. 1997;130:401. doi: 10.1016/s0022-2143(97)90040-8. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen VG, Geary BT, Band MS. Evaluation of the contribution of platelets to clot strength by thrombelastography in rabbits: The role of tissue factor and cytochalasin D. Anesth Analg. 2000;91:35. doi: 10.1097/00000539-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Siller-Matula JM, Plasenzotti R, Spiel A, et al. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100:397–404. [PubMed] [Google Scholar]
- 19.Sawamura A, Hayakawa M, Gando S, et al. Disseminated intravascular coagulation with a fibrinolytic phyenotype at an early phase of trauma predicts mortality. Thrombosis Research. 2009;124:608–613. doi: 10.1016/j.thromres.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 20.Levi M. Disseminated intravascular coagulation: What’s new? Crit Care Clin. 2005;21:449–467. doi: 10.1016/j.ccc.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Cannon WB, Gray H. Factors affecting the coagulation time of blood II. The hastening or retarding of coagulation by adrenalin injections. Am J Physiol. 1914;34:232–242. [Google Scholar]
- 22.Hardaway RM, Neimes RE, Burns JW, et al. Role of norepinephrine in irreversible hemorrhagic shock. Ann Surg. 1962;156:57–60. doi: 10.1097/00000658-196207000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfeld BA, Faraday N, Campbell D, et al. Hemostatic effects of stress hormone infusion. Anesthesiology. 1996;84:485–486. doi: 10.1097/00000542-199411000-00005. [DOI] [PubMed] [Google Scholar]