Abstract
In the present study, we examined the ability of post-training injections of cocaine to facilitate spatial memory performance using the Morris water maze (MWM). We also investigated the role that hippocampal protein kinase A (PKA) and extracellular signal-regulated kinase 1/2 (ERK) signaling may play in cocaine-mediated spatial memory consolidation processes. Male and female C57BL/6 mice were first trained in a MWM task (eight consecutive trials) then injected with cocaine (0, 1.25, 2.5, 5, or 20 mg/kg), and memory for the platform location was retested after a 24 hr delay. Cocaine had a dose-dependent effect on spatial memory performance because only the mice receiving 2.5 mg/kg cocaine displayed a significant reduction in latency to locate the platform. No sex differences in MWM performance were observed; however, females showed higher hippocampal levels of PKA when compared to males. A second experiment demonstrated that 2.5 mg/kg cocaine enhanced MWM performance only when administered within 2, but not 4 hr after spatial training. We also found that cocaine (2.5 mg/kg) increased ERK2 phosphorylation within the hippocampus and one of its downstream targets (ribosomal S6 kinase), a mechanism that may be responsible, at least in part, for the enhanced cocaine-mediated spatial memory performance. Overall, these data demonstrate that a low dose of cocaine (2.5 mg/kg) administered within 2-hr after training facilitates MWM spatial memory performance in C57BL/6 mice.
Keywords: Morris water maze, hippocampus, spatial memory, cocaine, protein kinase A, extracellular signal-regulated kinase, ribosomal s6 kinase
INTRODUCTION
Cocaine, a central nervous system stimulant, increases dopamine levels in the synaptic cleft by blocking presynaptic dopamine transporter reuptake in brain regions such as the nucleus accumbens and striatum (for review see Anderson and Pierce, 2005). This increase in dopamine in the nucleus accumbens and striatal regions is important for the addictive and locomotor stimulating properties of cocaine (Di Chiara et al., 2004; Koob and Nestler, 1997). Cocaine also increases synaptic dopamine and other monoamines in the hippocampus (Krasnova et al., 2008), a brain area important for spatial memory (Duva et al., 1997; Nadel, 1991). Given that increased synaptic levels of monoamines are known to enhance learning and memory performance (Brown et al., 2000; Luine et al., 1990), cocaine administration would be expected to also improve memory. This idea is supported by studies showing that cocaine administration increases memory-associated proteins in the hippocampus (Thompson et al., 2002). For example, cocaine increases protein kinase A (PKA) and extracellular signal-regulated kinase 1/2 (ERK) signaling (Freeman et al., 2001; Tropea et al., 2008; Valjent et al., 2004), which are important mediators for long-term-potentiation (LTP), a phenomena underlying synaptic plasticity (Frey and Morris, 1998). The second messenger cyclic adenosine monophosphate (cAMP) is presumed to be the mechanism through which LTP is induced after dopamine receptor stimulation (Gurden et al., 2000; Jay et al., 1998). Thus, when stimulated, dopamine receptors activate cAMP, which in turn activates PKA and ERK signaling (Ambrosini et al., 2000; Bertran-Gonzalez et al., 2008). ERK signaling, via the activation of other downstream kinases, such as ribosomal S6 kinase of 90 kd (90RSK), leads to gene transcription via cAMP-response-element-binding-protein (CREB), which is necessary for the growth of new synaptic connections associated with LTP (Bailey and Kandel, 1993; McGauran et al., 2008).
Since spatial memory is positively correlated with synaptic monoamine levels (Beatty and Rush, 1983; Luine et al., 1990; Packard and White, 1989) and post-training administration of dopamine agonists have been found to enhance memory consolidation in several memory systems (Bekinschtein et al., 2010; Brown et al., 2000; Castellano et al., 1996), cocaine may be a useful tool to assess whether increases in dopamine levels facilitate spatial memory consolidation in challenging hippocampal-dependent memory tasks (Martin and Clark, 2007). While the pattern of cocaine’s effects on memory consolidation processes across different memory systems is unclear, experimental evidence suggests that acute administration of cocaine (in low doses) after training may enhance memory performance in fear conditioning paradigms (White et al., 1995; Wood et al., 2007), while chronic-administration (or high dose treatments) may impair memory acquisition in spatial tasks (Quirk et al., 2001). Although most of the data on cocaine’s effect on memory comes from basic research, its effects on memory are evident in humans as well. For example, stimulants (with similar properties to cocaine) have been used as cognitive enhancers in both academic and military settings (Butcher, 2003; Caldwell et al., 1995; Grayson et al., 2004), where they have been found to promote mental arousal and/or wakefulness in low to moderate doses (Greely et al., 2008). In addition, high doses of cocaine in humans can lead to abuse and result in poor memory performance (Bolla et al., 1999; Simon et al., 2002). Therefore, the goal of this study was to assess whether a single post-training injection of a low dose of cocaine facilitates spatial memory in C57BL/6 mice after Morris water maze (MWM) training, and to determine whether PKA and ERK activity within the hippocampus correlate with MWM performance as a function of acute cocaine administration.
MATERIALS AND METHODS
Animals
Male and female C57BL/6 mice (7 weeks old at time of arrival) were purchased from Harlan Laboratories (Indianapolis, IN). Mice were housed 3-4 per cage and allowed to acclimate to the colony room for 9 days prior to handling and behavioral testing. Specifically, mice were housed in a room with a temperature of 22-23°C with a 12:12 hr light/dark cycle, and with food and water accessible ad libitum. All procedures were conducted according to the 1996 National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee at California State University San Bernardino.
Drug Treatment
Mice were randomly assigned to one of five conditions, and received a single intraperitoneal (i.p.) injection of saline (VEH) or cocaine hydrochloride (1.25, 2.5, 5.0, or 20.0 mg/kg; Sigma St. Louis, MO) dissolved in 0.9% NaCl, immediately, 1, 2, or 4 hr after the completion of spatial memory training as described below.
Apparatus
Morris Water Maze
The MWM was a white circular water tank 97 cm in diameter and 58 cm in height. The maze was filled with water to a depth of 18 cm. The water was made opaque with white nontoxic paint, and its temperature was maintained at 24±1°C using a standard heat-lamp. Around the perimeter of the water tank, four starting points (north, south, east, west) were equally positioned, thus dividing the maze into four equal quadrants. During spatial training, the escape platform (10 × 10 cm2) was submerged to a depth of 0.5 cm on the north-east quadrant. Extra-maze cues were placed throughout the walls of the testing room.
Experiment 1
Habituation
All mice were handled for five days (5 min each time) in order to habituate them to the experimenter. On the last day of handling (day 5), mice were also habituated to the testing room for 20 min. This procedure was followed to reduce levels of stress by handling and exposing the mice to the testing environment. Lastly, mice were also habituated to the water immersion process (as described by Gresack and Frick, 2006). Briefly, mice were given 4 shaping trials. On trial 1, each mouse was placed for 10 sec on the escape platform (visible above water). For the remaining trials, each mouse was placed at three distances progressively further from the platform and allowed to swim to the platform. If the mouse did not find the platform within 60 sec, it was led to the platform by the experimenter. No data were collected during habituation.
Spatial Training
Spatial water maze testing was performed as previously described (Gresack and Frick, 2006; Packard and Teather, 1997b). The mice received one training session of eight-trials (Training Day). Mice were placed in the water maze at one of the four starting points and allowed 60 sec to freely swim and find the submerged escape platform. Every starting point was used twice within the eight trials in a randomized fashion. If a mouse did not locate the hidden platform within the allotted 60 sec, the experimenter directed it to the escape platform. Once on the escape platform, each mouse was allowed 10 sec to view its surroundings (extra-maze cues). After every trial, each mouse was dried with a towel and placed in a holding cage for a 45 sec inter-trial. At the end of the eight trials, the mice were immediately injected with either VEH or cocaine (1.25, 2.5, 5.0, or 20.0 mg/kg), and placed back into its home cage.
Test Day
Twenty-four hr after the last training trial and drug injection, the mice were returned to the MWM for a single memory retention trial. All mice were released from the same starting point (north point). Latency (sec) and velocity (cm/sec) to find the escape platform were recorded via an automated computer tracking system (NOLDUS®). Lower swim latencies were interpreted as better memory (Leon et al., 2010), while swim velocity was used as a control for potential differences in swimming ability.
Tissue Preparation
Mice were killed by rapid decapitation immediately after behavioral testing and their hippocampi were extracted bilaterally on dry ice and stored at −80°C until assayed. Frozen tissue was placed in homogenization buffer [50 mM Tris (pH 7.4), 100 ng/ml aprotinin, and 5 mM EDTA] and homogenized using a hand-held Teflon homogenizer (Crawford et al., 2006). Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA) based on the Bradford method (Bradford, 1976), using bovine serum albumin (BSA) as a standard.
PKA Assay
PKA assays were performed as previously described (Crawford et al., 2004, 2006). Specifically, duplicate hippocampi homogenates containing approximately 4 μg of protein for each subject were incubated for 5 min at 30°C in phosphorylation buffer [50 mM Tris (pH 7.4), 10 mM MgCl2, and 0.25 mg/ml BSA], containing 50μg of kemptide and 100 μg [γ-32P]ATP (ICN, Costa Mesa, CA). In addition, the buffer contained either cAMP (10 μM) or protein kinase inhibitor (PKI, 6-22) amide (1 μM/reaction). Following incubation, the phosphorylation mixture was blotted on phosphocellulose filter paper. The filter paper was washed twice with 1% phosphoric acid for 5 min, followed by two 5 min washes with double-distilled water. Filters were then placed in scintillation fluid and quantified by liquid scintillation spectrometry. PKA activity was defined as the difference between PKA activity in the presence of 8-bromo-cAMP and that measured in the presence of PKI. PKA activity was measured in units of nmol/min/mg protein.
Experiment 2
Because no differences in spatial memory performance were observed between male and female mice in Experiment 1, we selected to use only male mice in this follow up experiment. Here, mice were habituated and trained on the MWM in a similar manner as in Experiment 1 (see above). Mice were randomly assigned to receive an acute injection of VEH or cocaine (2.5 mg/kg), immediately, 1, 2, or 4 hr after spatial training (i.e., after the completion of trial-8). Mice were returned to the water maze 24 hr later for a single retention trial (Test Day). Immediately after, mice were decapitated and bilateral hippocampi were extracted on dry ice and stored at −80°C until assayed. In addition, a separate group of naïve mice (no MWM spatial training) received an acute injection of either VEH or cocaine (2.5 mg/kg) and were killed 40 min later, in order to assess the effects of acute cocaine on ERK signaling. This approach was taken because acute stressors (i.e., swimming and/or temperature changes) increase the phosphorylation of ERK in various brain regions (Iñiguez et al., 2010a), including the hippocampus (Shen et al., 2004; Zheng et al., 2008).
Western Blot Analysis
Western blots were performed as described (Iñiguez et al., 2010a). Briefly, hippocampi from mice were sonicated in a standard lysis buffer and then centrifuged at 14,000 rpm for 15-min. Samples (20 μg; estimated through Bradford assay) were treated with β-mercaptoethanol and subsequently electrophoresed on precast 4%-20% gradient gels (Bio-Rad, Hercules, CA). Proteins were transferred to a polyvinylidene fluoride membrane, washed in 1× Tris-buffered saline with 0.1% Tween-20 (TBST), and blocked in milk dissolved in TBST (5% w/v) for 1 hr at 25°C. Blots were probed (overnight at 4°C) with antibodies against the phosphorylated forms of the protein [except glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] and then stripped (Restore; Thermo Fisher Scientific) and probed with antibodies against total protein of the same type. Antibodies were from Cell Signaling Technology, Beverly, MA [ERK1/2, 90RSK, and GAPDH, phospho (p)-protein and total (t)-protein] and were used according to the instructions of the manufacturer (in 5% milk dissolved in TBST). After further washes, membranes were incubated with peroxidase-labeled goat antirabbit IgG or horse antimouse IgG (1:40,000; Vector Laboratories, Burlingame, CA). Bands were visualized with SuperSignal West Dura substrate (Pierce Biotechnology, Rockford, IL) and quantified (normalized to GAPDH) using ImageJ (NIH).
Data Analysis
The behavioral data was analyzed using two- and one-way analysis of variance (ANOVA) for repeated measures with experimental group (sex and drug) and swim-trial (repeated measure) as sources of variance for spatial memory (similar to Choopani et al., 2008; Leon et al., 2010; Packard and Teather, 1997a; Packard and Teather, 1997b). Post hoc comparisons were analyzed using Tukey test. Hippocampal PKA activity was also analyzed using two- and one-way ANOVAs with drug and/or sex as sources of variance. Western immunoblots were analyzed using student’s t-test. Data are expressed as the mean ± SEM. In all cases, statistical significance was defined as p<0.05.
RESULTS
Experiment 1
Body Weights
The body weight of each mouse (females, n=55; males, n=52) was recorded before and after drug administration in order to evaluate whether cocaine treatment would influence weight-gain between the training and testing days (see Table 1). A two-way ANOVA indicated that body weight did not differ prior to drug administration (Training Day) between the groups as a function of group assignment (main effect, p>0.05), or a sex by group assignment interaction (p>0.05). Conversely, a significant sex main effect was observed (F1,97=124.99, p<0.0001), with females displaying lower weights when compared to males. Similar results were obtained on the Test Day (24 hr post drug administration), with females showing lower body weight when compared to males (F1,97=127.08, p<0.0001), but this effect was not dependent on drug administration (main effect, p>0.05), or their interaction (sex by drug; p>0.05). Importantly, this finding indicates that water maze performance was independent of cocaine’s effects on body weight between male and female mice.
Table 1.
Body Weights (g)
| Cocaine (mg/kg) |
|||||
|---|---|---|---|---|---|
| Females | 0 | 1.25 | 2.5 | 5 | 20 |
| Spatial Training |
19.39 (±0.44) |
19.90 (±0.61) |
19.31 (±0.50) |
19.14 (±0.32) |
18.98 (±0.30) |
| Test Day | 19.58 (±0.39) |
20.12 (±0.63) |
19.53 (±0.43) |
19.27 (±0.26) |
19.25 (±0.33) |
| Subjects | n=10 | n=12 | n=11 | n=11 | n=11 |
| Cocaine (mg/kg) |
|||||
|---|---|---|---|---|---|
| Males | 0 | 1.25 | 2.5 | 5 | 20 |
| Spatial Training |
22.62 (±0.51) |
22.90 (±0.62) |
22.40 (±0.60) |
23.85 (±0.61) |
23.50 (±0.54) |
| Test Day | 22.85 (±0.57) |
23.05 (±0.61) |
22.54 (±0.58) |
24.10 (±0.53) |
23.45 (±0.54) |
| Subjects | n=10 | n=10 | n=11 | n=10 | n=11 |
Numbers in parenthesis indicate standard error of the mean (SEM). Spatial Training, weight prior to 8 training trials on water maze. Test Day, weight 24 hr after drug administration.
Spatial Memory Training
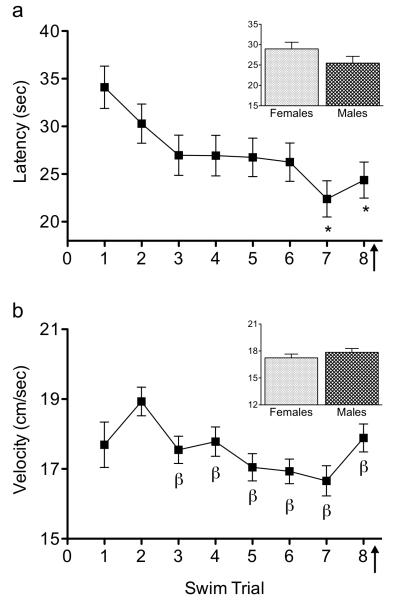
All mice (N=107) learned to locate the escape platform in a similar fashion during spatial training (swim trials 1-8) prior to drug administration (n=10-12/group). There were no statistical mean group differences in latency [time to locate the escape platform (sec)] prior to drug injection as a function of group assignment (main effect, p>0.05), sex (main effect, p>0.05; Fig. 1a inset), or group assignment by sex interaction (p>0.05). Similarly, there were no group differences in swim velocity (cm/sec) prior to cocaine exposure on the Training Day as a function of group assignment (main effect, p>0.05), sex (main effect, p>0.05, Fig. 1b inset), or group assignment by sex interaction (p>0.05). Importantly, during spatial training (trials 1-8), significant main effects of swim trial for latency (F7,679=4.08, p<0.0001; Fig. 1a) and velocity (F7,679=5.29, p<0.0001; Fig. 1b) in the absence of significant trial by group assignment (p>0.05), trial by sex (p>0.05), and trial by group assignment by sex (p>0.05) interactions, indicated that mice were taking less time (while swim velocity was variable) to locate the platform across the eight training trials. Specifically, Tukey post hoc comparisons indicated that for swim latency (sec), the last two trials (trails 7-8) where significantly lower when compared to swim trial 1 (p<0.05). In a somewhat similar pattern, the last six swim trials (trials 3-8) were significantly lower when compared to swim trial 2 (p<0.05), but not swim trial 1 (p>0.05), when swim velocity (cm/sec) was assessed. To ensure that no differences between the groups existed by the end of spatial training, and that all subjects performed similarly, additional one-way ANOVAs were conducted on trial 8 with group assignment (although no drug had been administered) as an independent variable for latency (p>0.05) and velocity (p>0.05). Also, a separate non-significant (p>0.05) one-way ANOVA, with sex as the independent variable, indicated that females and males performed similarly in trial 8 to locate the platform (latency) prior to drug injection, further demonstrating that female and male mice did not differ prior to drug exposure. Together, all behavioral data from the Training Day indicated that all mice performed similarly and did not differ as a function of group assignment or sex prior to cocaine administration. Because sex differences were not detected throughout spatial memory training (trials 1-8) all subsequent data of both male and female mice were collapsed for further analysis on Test Day.
Figure 1.
All mice exhibited similar acquisition performance during spatial training (eight training trials) of the Morris water maze task. (a) Mean latency (sec) and (b) swim velocity (cm/sec) decreased across training trials, indicating the acquisition of the spatial memory task by all mice. No differences between male and female C57BL/6 mice [latency to locate the escape platform (inset a) or swimming velocity (inset b)] were observed. Mice received an injection of cocaine (0, 1.25, 2.5, 5, or 20 mg/kg) immediately after trial 8 (arrow). *Indicates p<0.05 when compared to swim trial 1. βIndicates p<0.05 when compared to swim trial 2.
Test Day: MWM Performance 24 hr Post Cocaine Administration
The effects of post-training injections of cocaine (0, 1.25, 2.5, 5, and 20 mg/kg) on spatial memory retention are displayed in Figure 2. In contrast to trial 8 (last trial prior to drug administration), the groups statistically differed 24 hr post injection (Test Day) as a function of drug dose. More specifically, a one-way ANOVA with drug as source of variance for latency (F4,102=3.90, p<0.05), followed by Tukey post hoc tests, indicated that the mice receiving 2.5 mg/kg cocaine found the escape platform in significantly less time (sec) when compared to controls (see Figure 2a). Furthermore, the 2.5 mg/kg cocaine group differed from the groups administered with 1.25, 5.0, and 20.0 mg/kg cocaine (Tukeys, p<0.05, respectively). Importantly, swim velocity was not affected by the administration of cocaine on Test Day (drug main effect, p>0.05; Figure 2b). Together, these data indicate that MWM performance on Test Day was dependent on cocaine’s effect on spatial memory retention and not on individual or drug-induced effects on motor ability.
Figure 2.
Effects of a post-training injection of cocaine (0, 1.25, 2.5, 5 or 20 mg/kg) on (a) latency (sec) and (b) swim velocity (cm/sec) to locate the escape platform 24 hr after spatial training on the Morris water maze (Test Day). Mice receiving 2.5 mg/kg of cocaine performed significantly better than controls or those receiving 1.25, 5 or 20 mg/kg on Test Day. No differences in swim velocity were observed between any of the groups on the Test Day (b). Data is presented as a percent of VEH treated controls. *Indicates p<0.05 when compared to controls.
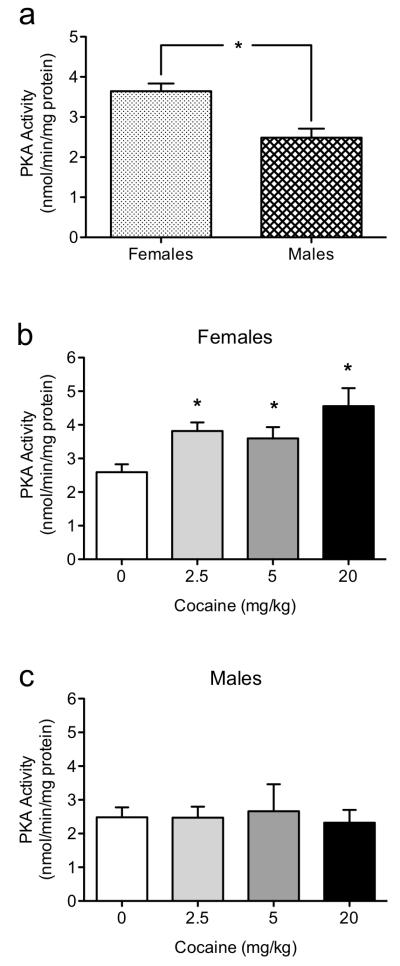
Hippocampal PKA Activity
On Test Day, there was a significant difference in hippocampal PKA activity between female (n=20) and male (n=20) mice (sex main effect: F1,32=9.37, p<0.05). As shown in Figure 3a, female mice exhibited higher levels of hippocampal PKA activity than male mice. Further analysis showed that PKA activity was dependent of drug administration within the female group (F3,16=11.36, p<0.05; Figure 3b). When compared to controls, the female mice (n=5/group) administered with 2.5 (p<0.05), 5.0 (p<0.05) and 20.0 (p<0.05) mg/kg of cocaine displayed higher hippocampal PKA activity. This cocaine-dependent PKA increase was not observed in male mice (p>0.05; Figure 3c; n=5/group).
Figure 3.
Mean hippocampal PKA activity (nmol/min/mg protein) of female and male C57BL/6 mice on Test Day, 24 hr after eight training trials on the Morris water maze and injected with cocaine (0, 2.5, 5, or 20 mg/kg). Female mice displayed overall higher PKA activity when compared to males 24 hr after spatial memory training and cocaine administration (a). Female mice showed a dose-dependent increase of hippocampal PKA as a function of cocaine administration (b). No differences in PKA activity as a function of cocaine were observed in male mice (c). *p<0.05 when compared to controls.
Experiment 2
Cocaine facilitates spatial memory in a time-dependent manner
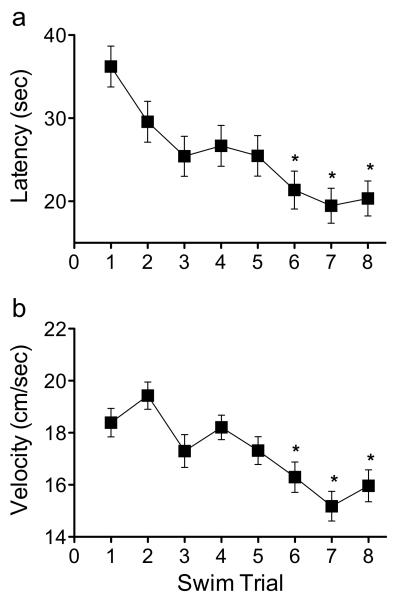
To assess whether the enhancement of spatial memory performance was dependent on time of cocaine administration, we trained a separate group of male C57BL/6 mice (N=80) on the MWM. Here, mice received an injection of cocaine (0 or 2.5 mg/kg) immediately, 1, 2, or 4 hr (n=9-11/group) after the last training trial (trial 8). Twenty-four hrs later (Test Day), mice returned to the MWM for a single retention swim trial in order to assess memory for the location of the escape platform. As observed in Experiment 1, the data from the Training Day in this second experiment indicated that all mice learned the location of the platform in a similar fashion (Fig. 4). This was evident by a non-significant group assignment main effect (p>0.05) and by a lack of swim trial by group assignment interaction (p>0.05) on the time (sec) it took the mice to locate the platform, without affecting swim velocity (cm/sec) as a function of group assignment (main effect, p>0.05) or by the absence of a trial by group assignment interaction (p>0.05). On the other hand, significant swim trial (within group variable) main effects on the time to locate the platform (latency: F7,546=7.85, p<0.0001) and swim velocity (F7,546=6.51, p<0.0001) were reached (see Fig. 4a-b, respectively). Thus indicating that mice, regardless of group assignment, learned the location of the platform in a similar fashion (decreasing time and velocity to locate the platform across trials) prior to drug injection. This was evident by significant swim trial differences between trial 1 and the last three swim trials (trials 6-8) in both the latency (Fig. 4a) and velocity (Fig. 4b) to reach the escape platform (p<0.05, respectively). On the Test Day (Fig. 5), a two-way ANOVA (drug by time-delay) indicated that mice treated with 2.5 mg/kg cocaine spent significantly less time (sec) to locate the escape platform when compared to mice treated with VEH (drug main effect: F1,72=14.37, p<0.0001). Furthermore, a significant time-delay main effect (F3,72=14.48, p<0.0001) indicated that the time to locate the escape platform was dependent on delay of injection after the last swim trial (Fig. 5a). That is, the mice receiving cocaine located the platform in shorter time when the drug was administered immediately (p<0.05), 1 (p<0.05), or 2 hr (p<0.05) after the last training trial when compared to controls. No differences in time to locate the escape platform (p>0.05) were observed between the groups administered with 2.5 mg/kg cocaine or VEH 4 hr after the last training trial. Importantly, no differences in swim velocity (cm/s) were observed between the groups as a function of drug treatment (main effect; p>0.05), time-delay (main effect; p>0.05), or their interaction (drug by time-delay; p>0.05), thus indicating that performance in the MWM was independent of any potential individual- or cocaine-induced locomotor effects (Fig. 5b).
Figure 4.
All male mice exhibited similar acquisition performance during spatial training (eight training trials) of the Morris water maze task. (a) Mean latency (sec) and (b) swim velocity (cm/sec) decreased across training trials, indicating the acquisition of the spatial memory task by all mice. Mice received an injection of cocaine (0 or 2.5 mg/kg) immediately, 1, 2, or 4 hr after trial 8. *Indicates p<0.05 when compared to swim trial 1.
Figure 5.
Effects of post-training injections of cocaine (2.5 mg/kg) after 0, 1, 2, or a 4 hr delay after the last spatial training trial. The moderate low dose of cocaine facilitated spatial memory performance on the Morris water maze only when administered within 2 hr of spatial memory training (a). Cocaine did not influence swim velocity (cm/sec) between any of the groups across the different time delays (b). Data is presented as a percent of VEH-treated controls. *Indicates p<0.05 when compared to controls.
Cocaine Increases Hippocampal ERK Signaling
Immunoblot analysis was used to examine the effects of cocaine (2.5 mg/kg) on ERK signaling (Fig. 6), as measured by phosphorylation levels of ERK1, ERK2, and one of its downstream targets, 90RSK, within the hippocampus of mice exposed to cocaine immediately after spatial training (n=6/group; all normalized to GAPDH). Figure 6a shows immunoblots of hippocampi homogenates representing averages of expression levels of pERK1, pERK2, p90RSK, and GAPDH of mice on Test Day. Cocaine increased the levels of p90RSK when compared to VEH-treated controls (t10=3.31, p<0.01). Also, cocaine appeared to increase the levels of pERK2, although this increase was not statistically significant (t10=2.01, p=0.07), without influencing pERK1 (p>0.05). Lastly, cocaine (2.5 mg/kg) did not affect the total levels of these proteins: tERK1, tERK2, and t90RSK (p>0.05, respectively; all normalized to GAPDH) when compared to VEH-treated controls (see Fig. 6b).
Figure 6.
Representative Western blot of the effects of acute cocaine (2.5 mg/kg) on expression of hippocampal ERK1/2 signaling in C57BL/6 male mice. On Test Day (a-b), 24 hr after spatial memory training and drug administration, cocaine (2.5 mg/kg) increased the levels of p90RSK, without affecting pERK1 (p>0.05), pERK2 (αp=0.07), or total (p>0.05) levels of protein when compared to controls (all normalized to GAPDH). Acute cocaine (2.5 mg/kg) in naive C57BL/6 mice (c-d) selectively increased hippocampal pERK2, without affecting pERK1, p90RSK, or total levels of protein (all normalized to GAPDH). VEH, saline-treated animals. *Indicates p<0.05 when compared to controls.
A separate group of naïve male mice (n=5/group) received an acute cocaine (0 or 2.5 mg/kg) injection and hippocampal tissue were extracted 40 min after to assess whether cocaine alone (without MWM training) influenced ERK signaling within this brain region (see Fig. 6c-d). We chose this approach because acute stress (i.e., swimming) increases hippocampal ERK activity (Shen et al., 2004; Zheng et al., 2008). In Figure 6c we show that acute cocaine administration increased the levels of pERK2 (t8=2.45, p<0.05), without changing pERK1 (p>0.05), p90RSK (p>0.05), or the total levels of these enzymes (p>0.05, respectively; Fig. 6d) when compared to VEH-treated controls.
DISCUSSION
The goal of the present study was to assess whether cocaine facilitates MWM spatial memory consolidation in C57BL/6 mice. This approach was taken because (1) previous work in rodents and humans suggest that stimulant use in low doses are beneficial, whereas high doses are detrimental, to performance on cognitive tasks (Wood et al., 2007), (2) stimulants increase dopamine, and dopamine modulates memory consolidation processes (i.e., LTP) by increasing intracellular levels of PKA and, along other signaling molecules, (3) converge on the ERK signaling cascade (Hinoi et al., 2002; Impey et al., 1998). Once activated, hippocampal ERK plays a role in gene expression by phosphorylating transcription factors and promoting chromatin modification, essential components for spatial long-term memory consolidation processes (Blum et al., 1999; Chwang et al., 2006; Levenson et al., 2004; Satoh et al., 2007). To this end, male and female C57BL/6 mice were trained on a single-day MWM spatial task, injected with VEH or cocaine and the memory for the escape platform was tested after a 24 hr delay. This behavioral protocol was adopted in order to avoid any possible confounding effects of pre-training drug administration on test performance given the motor enhancing properties of cocaine.
Our results show that both male and female mice performed similarly on the MWM, as there were no differences in the latency or swim velocity to locate the escape platform between the groups throughout spatial training. Although previous research suggests that males usually outperform females on spatial tasks (Gresack and Frick, 2003), gender differences are not always reported (Faraji et al., 2010; Healy et al., 1999), or they occur as a function of task difficulty (Berger-Sweeney et al., 1995; Coluccia and Louse, 2004), thus it is possible that our single-day protocol may not have been challenging enough to promote/unmask sex differences. Interestingly, the optimal dose of cocaine to facilitate spatial ability (in both male and female mice) was 2.5 mg/kg (see Fig. 2a), with an overall u-shaped function where both the lowest and higher doses did not affect MWM performance. These results are in agreement with previous reports demonstrating that 2.5 mg/kg cocaine facilitated memory performance on shock avoidance memory tasks in mice (Castellano et al., 1996; Cestari and Castellano, 1996). Importantly, our data further suggests that these cocaine-induced effects are time dependent, since enhanced spatial memory performance was observed only in animals receiving 2.5 mg/kg cocaine within 2 hr of MWM training (see Fig. 5a). These results are likely due to cocaine’s ability to facilitate spatial memory consolidation processes, since no differences in swim velocity or body weight as a function of cocaine treatment were observed between any of the groups (see Table 1, Figs. 2b and 5b). Interestingly, the highest cocaine dose (20 mg/kg) did not impair MWM performance, and although these mice took longer to locate the platform, they did not differ statistically from controls. Previous investigations have reported that high doses of cocaine (20-40 mg/kg) administered chronically prior to, or during training, impair memory consolidation on MWM-related tasks (Coluccia and Louse, 2004; Quirk et al., 2001). However, in the present study, exposure to 20 mg/kg cocaine after training did not impair MWM performance. These variable results can be attributed to differences in experimental design, as cocaine was administered acutely (one time only) after training in the present investigation. Alternatively, it is possible that 20 mg/kg was not high enough to induce memory impairment, as it was marginally close to those cocaine doses previously found to facilitate memory performance in rats tested in a Pavlovian appetitive approach test or a lever-release conditioned avoidance task (Taylor and Jentsch, 2001; White et al., 1995).
We further report here that female mice displayed higher levels of PKA activation as compared to males when assessing the role of hippocampal PKA in spatial memory consolidation processes after cocaine administration. In addition, we demonstrate that hippocampal PKA levels increased as a function of cocaine within the female groups. This finding is not surprising given that female rodents are generally more sensitive than males to drugs of abuse by demonstrating greater behavioral responses (Becker et al., 2001; Chin et al., 2002; Festa et al., 2004; Halladay et al., 2009). These results further parallel the work by others that have reported sex differences in PKA activity after acute cocaine administration within brain areas other than the hippocampus (Nazarian et al., 2009), or after drug pretreatment during the preweanling period, with females displaying higher PKA levels, as compared to males, when assessed in adulthood (Crawford et al., 2006). Interestingly, this cocaine dose-dependent increase of hippocampal PKA activity was not observed in the male mice (see Fig. 3c). This was surprising, since hippocampal PKA signaling is known for playing a critical role in associative memory formation (Kandel et al., 2000; Micheau and Riedel, 1999; Sunyer et al., 2009). Within this framework, it is conceivable that the lack of cocaine-induced PKA changes in the male groups indicates that hippocampal PKA-related signaling mechanisms underlying cocaine-facilitated spatial memory (in a single-day spatial MWM task) may differ between male and female mice in a task-dependent manner (Abel et al., 1997). This is likely the case as recent evidence suggests that other types of learning, such as contextual fear conditioning, exhibit sex-specific molecular differences within this brain region (Gresack et al., 2009).
Another signaling molecule that plays an important role in spatial memory is ERK (McGauran et al., 2008; Morozov et al., 2003; Peng et al., 2010). To assess the role of ERK in spatial memory consolidation processes after cocaine administration, we extracted hippocampal tissue of male C57BL/6 mice immediately after the MWM task in our second experiment. We found evidence that ERK signaling was increased in the animals showing better spatial performance (lower time to locate the platform), as shown by a significant increase in the phosphorylation of one of its downstream targets, namely 90RSK. Because acute stress also activates ERK signaling (Iñiguez et al., 2010a; Shen et al., 2004; Zheng et al., 2008), we also assessed the effects of acute 2.5 mg/kg cocaine alone on ERK signaling in a separate group of male naïve mice to rule out stress-induced effects on ERK phosphorylation. Indeed, we found that cocaine was sufficient to increase the phosphorylation of ERK2, without influencing 90RSK, indicating that ERK signaling was dependent on the spatial task (Cahill et al., 2003; Sandi et al., 1997) in addition to cocaine, thus likely contributing to facilitated spatial memory retention (McGauran et al., 2008).
Together, our findings suggest that ERK2 phosphorylation induced by cocaine (2.5 mg/kg) administration correlates with better spatial MWM performance, although not activated directly via PKA between males and females. This is possible because activation of the ERK pathway can be mediated via various signaling pathways that include PKA, protein kinase C, growth factors, tyrosine kinases, calcium, and a variety of guanine nucleotide binding proteins (G-proteins) through both Ras-dependent and Ras-independent signaling pathways (for review see, Peng et al., 2010; Yao and Seger, 2009). Interestingly, PKA has also been found to increase or decrease the phosphorylation of ERK via different isoforms of the upstream G-protein Raf (Cook and McCormick, 1993; Konig et al., 2001; Kwong and Chin, 2010). Thus, cocaine-induced facilitation of spatial memory may be mediated via different signaling molecules upstream of ERK, with females, but not males, showing a hippocampal PKA-dependent mechanism. Clearly, further molecular research is needed to better assess this hypothesis.
Although it is well established that the hippocampus is of critical importance for spatial memory performance (Duva et al., 1997; Nadel, 1991; Sekeres et al., 2010), we cannot rule out the possibility that other brain areas involved in cocaine-induced motivated behaviors, such as the ventral tegmental area (Iñiguez et al., 2008), the striatum (Velazquez-Sanchez et al., 2009), and the prefrontal cortex (Li et al., 2008), may contribute to the cocaine-induced facilitated spatial memory retention observed in our investigation (see Leon et al., 2010; Mizumori et al., 2009; Swanson, 1982). For example, in the ventral tegmental area of the midbrain, stress and cocaine influence the activity of ERK signaling (Bradberry and Roth, 1989; Iñiguez et al., 2010a; Iñiguez et al., 2010b) and the firing rate of dopaminergic neurons within this brain region (Marinelli et al., 2006), factors that have been linked to learning associations between contextual stimuli and motivational/rewarding events (Fields et al., 2007). The hippocampus receives dopaminergic inputs from the ventral tegmental area (Swanson, 1982), and disruption of its firing rate negatively influences spatial memory tasks (i.e., radial arm maze performance; Martig and Mizumori, 2010). Therefore, it is conceivable that the acute stress of swimming, in addition to the low dose of cocaine (2.5 mg/kg), may have resulted in additive effects by increasing the firing rate of ventral tegmental area neurons projecting to the hippocampus, which, in turn, may increase ERK2 signaling within this brain region. Within this framework, it is possible to conceive the notion that the facilitation of spatial memory consolidation by systemic administration of cocaine may be the result of a multidimensional and complex interaction between different brain regions, in addition to the hippocampus, that govern distinct types of dopamine-associated learning (Jay, 2003; Mizumori et al., 2009).
In summary, our results demonstrate that an acute low dose of cocaine (2.5 mg/kg) after spatial training can facilitate performance in the MWM task in C57BL/6 mice. This behavioral alteration is accompanied by increases of molecular markers involved in hippocampal dependent memory function, namely ERK2 signaling (Hebert and Dash, 2002; Martin and Clark, 2007). Our data further highlights the importance of drug dose as a critical factor in mediating enhanced spatial memory performance, and the hippocampal mechanisms that may contribute to the effects of stimulants on memory function.
Acknowledgments
This research was partially supported by an ASI research grant (CSUSB) to SDI. SDI was supported by a National Research Service Award (F31DA027300) from the National Institute on Drug Abuse, and a McKnight Fellowship from the Florida Education Fund.
Grant Sponsor: National Institute on Drug Abuse, Grant Numbers: 5SC1DA027683 (CAC); 1R01DA026854 (CABG); and 1F31DA027300 (SID).
REFERENCES
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88(5):615–26. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Ambrosini A, Tininini S, Barassi A, Racagni G, Sturani E, Zippel R. cAMP cascade leads to Ras activation in cortical neurons. Brain Res Mol Brain Res. 2000;75(1):54–60. doi: 10.1016/s0169-328x(99)00294-6. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106(3):389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Rush JR. Spatial working memory in rats: effects of monoaminergic antagonists. Pharmacol Biochem Behav. 1983;18(1):7–12. doi: 10.1016/0091-3057(83)90242-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk L, Gonzalez C, Dorman G, Cammarota M, Izquierdo I, Medina JH. Persistence of Long-Term Memory Storage: New Insights into its Molecular Signatures in the Hippocampus and Related Structures. Neurotox Res. 2010 doi: 10.1007/s12640-010-9155-5. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Arnold A, Gabeau D, Mills J. Sex differences in learning and memory in mice: effects of sequence of testing and cholinergic blockade. Behav Neurosci. 1995;109(5):859–73. doi: 10.1037//0735-7044.109.5.859. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28(22):5671–85. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19(9):3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11(3):361–9. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Roth RH. Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdialysis. Neurosci Lett. 1989;103(1):97–102. doi: 10.1016/0304-3940(89)90492-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown RW, Bardo MT, Mace DD, Phillips SB, Kraemer PJ. D-amphetamine facilitation of morris water task performance is blocked by eticlopride and correlated with increased dopamine synthesis in the prefrontal cortex. Behav Brain Res. 2000;114(1-2):135–43. doi: 10.1016/s0166-4328(00)00225-4. [DOI] [PubMed] [Google Scholar]
- Butcher J. Cognitive enhancement raises ethical concerns. Academics urge pre-emptive debate on neurotechnologies. Lancet. 2003;362(9378):132–3. doi: 10.1016/s0140-6736(03)13897-4. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003;10(4):270–4. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JA, Caldwell JL, Crowley JS, Jones HD. Sustaining helicopter pilot performance with Dexedrine during periods of sleep deprivation. Aviat Space Environ Med. 1995;66(10):930–7. [PubMed] [Google Scholar]
- Castellano C, Zocchi A, Cabib S, Puglisi-Allegra S. Strain-dependent effects of cocaine on memory storage improvement induced by post-training physostigmine. Psychopharmacology (Berl) 1996;123(4):340–5. doi: 10.1007/BF02246644. [DOI] [PubMed] [Google Scholar]
- Cestari V, Castellano C. Caffeine and cocaine interaction on memory consolidation in mice. Arch Int Pharmacodyn Ther. 1996;331(1):94–104. [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945(1):123–30. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Choopani S, Moosavi M, Naghdi N. Involvement of nitric oxide in insulin induced memory improvement. Peptides. 2008;29(6):898–903. doi: 10.1016/j.peptides.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13(3):322–8. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccia E, Louse G. Gender differences in spatial orientation: A review. Journal of Environmental Psychology. 2004;24(3):329–340. [Google Scholar]
- Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262(5136):1069–72. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Choi FY, Kohutek JL, Yoshida ST, McDougall SA. Changes in PKA activity and Gs alpha and Golf alpha levels after amphetamine- and cocaine-induced behavioral sensitization. Synapse. 2004;51(4):241–8. doi: 10.1002/syn.10301. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV. Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [35S]GTPgammaS binding in adult rats. Brain Res. 2006;1077(1):178–86. doi: 10.1016/j.brainres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–41. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JP, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behav Neurosci. 1997;111(6):1184–96. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- Faraji J, Metz GA, Sutherland RJ. Characterization of spatial performance in male and female Long-Evans rats by means of the Morris water task and the ziggurat task. Brain Res Bull. 2010;81(1):164–72. doi: 10.1016/j.brainresbull.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46(5):672–87. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Robertson DJ, Roberts DC, Vrana KE. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001;108(3):371–80. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21(5):181–8. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Grayson JK, Gibson RL, Shanklin SL, Neuhauser KM, McGhee C. Trends in positive drug tests, United States Air Force, fiscal years 1997-1999. Mil Med. 2004;169(7):499–504. doi: 10.7205/milmed.169.7.499. [DOI] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456(7223):702–5. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water-escape radial arm maze task. Brain Res. 2003;982(1):98–107. doi: 10.1016/s0006-8993(03)03000-2. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84(1):112–9. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Schafe GE, Orr PT, Frick KM. Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience. 2009;159(2):451–67. doi: 10.1016/j.neuroscience.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, Iñiguez SD, Furqan F, Previte MC, Chisum AM, Crawford CA. Methylphenidate potentiates morphine-induced antinociception, hyperthermia, and locomotor activity in young adult rats. Pharmacol Biochem Behav. 2009;92(1):190–6. doi: 10.1016/j.pbb.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SD, Braham SR, Braithwaite VA. Spatial working memory in rats: no differences between the sexes. Proc Biol Sci. 1999;266(1435):2303–8. doi: 10.1098/rspb.1999.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AE, Dash PK. Extracellular signal-regulated kinase activity in the entorhinal cortex is necessary for long-term spatial memory. Learn Mem. 2002;9(4):156–66. doi: 10.1101/lm.48502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi E, Balcar VJ, Kuramoto N, Nakamichi N, Yoneda Y. Nuclear transcription factors in the hippocampus. Prog Neurobiol. 2002;68(2):145–65. doi: 10.1016/s0301-0082(02)00078-3. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21(4):869–83. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010a;30(22):7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Neve RL, Nestler EJ, Russo SJ, Bolaños-Guzmán CA. Insulin receptor substrate-2 in the ventral tegmental area regulates behavioral responses to cocaine. Behav Neurosci. 2008;122(5):1172–7. doi: 10.1037/a0012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolaños-Guzmán CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res. 2010b;214(2):460–4. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69(6):375–90. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jay TM, Gurden H, Yamaguchi T. Rapid increase in PKA activity during long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in vivo. Eur J Neurosci. 1998;10(10):3302–6. doi: 10.1046/j.1460-9568.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Kandel E, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill Compaines; 2000. [Google Scholar]
- Konig S, Guibert B, Morice C, Vernier P, Barnier JV. Phosphorylation by PKA of a site unique to B-Raf kinase. C R Acad Sci III. 2001;324(8):673–81. doi: 10.1016/s0764-4469(01)01356-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9(3):482–97. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Li SM, Wood WH, McCoy MT, Prabhu VV, Becker KG, Katz JL, Cadet JL. Transcriptional responses to reinforcing effects of cocaine in the rat hippocampus and cortex. Genes Brain Behav. 2008;7(2):193–202. doi: 10.1111/j.1601-183X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- Kwong LN, Chin L. The brothers RAF. Cell. 2010;140(2):180–2. doi: 10.1016/j.cell.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn Mem. 2010;17(6):297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279(39):40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Li T, Yan CX, Hou Y, Cao W, Chen T, Zhu BF, Li SB. Cue-elicited drug craving represses ERK activation in mice prefrontal association cortex. Neurosci Lett. 2008;448(1):99–104. doi: 10.1016/j.neulet.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Luine V, Bowling D, Hearns M. Spatial memory deficits in aged rats: contributions of monoaminergic systems. Brain Res. 1990;537(1-2):271–8. doi: 10.1016/0006-8993(90)90368-l. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5(1):79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- Martig AK, Mizumori SJ. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus. 2010 doi: 10.1002/hipo.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Clark RE. The rodent hippocampus and spatial memory: from synapses to systems. Cell Mol Life Sci. 2007;64(4):401–31. doi: 10.1007/s00018-007-6336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGauran AM, Moore JB, Madsen D, Barry D, O’Dea S, Mahon BP, Commins S. A possible role for protein synthesis, extracellular signal-regulated kinase, and brain-derived neurotrophic factor in long-term spatial memory retention in the water maze. Behav Neurosci. 2008;122(4):805–15. doi: 10.1037/0735-7044.122.4.805. [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G. Protein kinases: which one is the memory molecule? Cell Mol Life Sci. 1999;55(4):534–48. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Puryear CB, Martig AK. Basal ganglia contributions to adaptive navigation. Behav Brain Res. 2009;199(1):32–42. doi: 10.1016/j.bbr.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin D, Winder DG, Adams JP, Sweatt JD, Kandel ER. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron. 2003;39(2):309–25. doi: 10.1016/s0896-6273(03)00404-5. [DOI] [PubMed] [Google Scholar]
- Nadel L. The hippocampus and space revisited. Hippocampus. 1991;1(3):221–9. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology (Berl) 2009;203(3):641–50. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Double dissociation of hippocampal and dorsal-striatal memory systems by posttraining intracerebral injections of 2-amino-5-phosphonopentanoic acid. Behav Neurosci. 1997a;111(3):543–51. doi: 10.1037//0735-7044.111.3.543. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997b;68(2):172–88. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Memory facilitation produced by dopamine agonists: role of receptor subtype and mnemonic requirements. Pharmacol Biochem Behav. 1989;33(3):511–8. doi: 10.1016/0091-3057(89)90378-x. [DOI] [PubMed] [Google Scholar]
- Peng S, Zhang Y, Zhang J, Wang H, Ren B. ERK in Learning and Memory: A Review of Recent Research. Int J Mol Sci. 2010;11(1):222–32. doi: 10.3390/ijms11010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk PL, Richards RW, Avery DD. Subchronic cocaine produces training paradigm-dependent learning deficits in laboratory rats. Pharmacol Biochem Behav. 2001;68(3):545–53. doi: 10.1016/s0091-3057(01)00462-2. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9(4):637–42. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;27(40):10765–76. doi: 10.1523/JNEUROSCI.0117-07.2007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Neve RL, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn Mem. 2010;17(6):280–3. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21(1):61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Sunyer B, Shim KS, An G, Hoger H, Lubec G. Hippocampal levels of phosphorylated protein kinase A (phosphor-S96) are linked to spatial memory enhancement by SGS742. Hippocampus. 2009;19(1):90–8. doi: 10.1002/hipo.20484. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1-6):321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4- methylenedioxymethamphetamine (“Ecstasy”) Biol Psychiatry. 2001;50(2):137–43. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology. 2002;42(8):1039–42. doi: 10.1016/s0028-3908(02)00059-x. [DOI] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem. 2008;106(4):1780–90. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19(7):1826–36. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Ferragud A, Hernandez-Rabaza V, Nacher A, Merino V, Carda M, Murga J, Canales JJ. The dopamine uptake inhibitor 3 alpha-[bis(4′-fluorophenyl)metoxy]-tropane reduces cocaine-induced early-gene expression, locomotor activity, and conditioned reward. Neuropsychopharmacology. 2009;34(12):2497–507. doi: 10.1038/npp.2009.78. [DOI] [PubMed] [Google Scholar]
- White IM, Christensen JR, Flory GS, Miller DW, Rebec GV. Amphetamine, cocaine, and dizocilpine enhance performance on a lever-release, conditioned avoidance response task in rats. Psychopharmacology (Berl) 1995;118(3):324–31. doi: 10.1007/BF02245962. [DOI] [PubMed] [Google Scholar]
- Wood SC, Fay J, Sage JR, Anagnostaras SG. Cocaine and Pavlovian fear conditioning: dose-effect analysis. Behav Brain Res. 2007;176(2):244–50. doi: 10.1016/j.bbr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Seger R. The ERK signaling cascade--views from different subcellular compartments. Biofactors. 2009;35(5):407–16. doi: 10.1002/biof.52. [DOI] [PubMed] [Google Scholar]
- Zheng G, Chen Y, Zhang X, Cai T, Liu M, Zhao F, Luo W, Chen J. Acute cold exposure and rewarming enhanced spatial memory and activated the MAPK cascades in the rat brain. Brain Res. 2008;1239:171–80. doi: 10.1016/j.brainres.2008.08.057. [DOI] [PubMed] [Google Scholar]