Abstract
Cognitively normal (NL) individuals with a maternal history of late-onset Alzheimer’s disease (MH) show reduced brain glucose metabolism on FDG-PET as compared to those with a paternal history (PH) and those with negative family history (NH) of Alzheimer’s disease (AD). This FDG-PET study investigates whether metabolic deficits in NL MH are associated with advancing maternal age at birth. Ninety-six NL individuals with FDG-PET were examined, including 36 MH, 24 PH, and 36 NH. Regional-to-whole brain gray matter standardized FDG uptake value ratios were examined for associations with parental age across groups using automated regions-of-interest and statistical parametric mapping. Groups were comparable for clinical and neuropsychological measures. Brain metabolism in AD-vulnerable regions was lower in MH compared to NH and PH, and negatively correlated with maternal age at birth only in MH. There were no associations between paternal age and metabolism in any group. Evidence for a maternally inherited, maternal age-related mechanism provides further insight on risk factors and genetic transmission in late-onset AD.
Keywords: Alzheimer’s disease, family history, PET imaging, glucose metabolism, age at birth, early detection
1. Introduction
After advanced age, having a first-degree family history of late onset Alzheimer’s disease (LOAD) is a major risk factor for developing the disease among cognitively normal (NL) individuals. While the rare early-onset forms of AD have autosomal dominant genetic inheritance, the genetics of LOAD remain elusive. Evidence for genetic transmission in LOAD comes from the familial aggregation of many cases. Risk is 4 to 10 times increased when a parent is affected (Farrer et al., 1989; Green et al., 2002).
Among adult children of parents with LOAD, those with a maternal history (MH, i.e., only the mother is affected) present with progressive reductions of brain glucose metabolism on 2-[18F]fluoro-2-Deoxy-D-glucose Positron Emission Tomography (FDG-PET) compared to those with a paternal history (PH, i.e., only the father is affected) and to those with negative family history (NH, i.e., neither parent is affected) (Mosconi et al., 2007, 2009). Moreover, NL MH showed increased fibrillar amyloid-beta (Aβ) depositions on N-methyl-[11C]2-(4’-methylaminophenyl)-6-hydroxybenzothiazole (PiB)-PET, a major hallmark of Alzheimer’s disease pathology, compared to PH and NH (Mosconi et al., 2010b). These data indicate that as yet unidentified, maternally transmitted factors influence brain pathophysiology in the offspring of LOAD-mothers, leading to altered glucose and Aβ metabolism years before symptoms might arise (Mosconi et al., 2010a).
Among several possible mechanisms involved in maternal transmission of LOAD (Mosconi, et al., 2010a), advanced maternal age at birth has been investigated as a possible risk factor. In humans, as in other mammals, female fertility and oocyte quality diminish with age (Navot et al., 1991). Advanced maternal age at birth has been associated with a variety of conditions, ranging from increased risk for stillbirths and congenital malformations to dyslexia, as well as with some neurological disorders (Heffner, 2004). There is a well-established increase in aneuploidy with increasing maternal age (Heffner, 2004), a typical example of which is Down’s syndrome (DS). DS is caused by trisomy of Chromosome 21 which is most frequently due to chromosomal non-disjunction during maternal meiosis with advanced maternal age (Yoon et al., 1996). In the 1980’s, clinical and pathological similarities were recognized between DS and AD (Masters et al., 1985), as both diseases are associated with increased accumulation of brain Aβ followed by dementia (Masters et al., 1985). In DS, this is due to the fact that Chromosome 21 contains the amyloid precursor protein (APP) gene, and as such individuals carrying 3 copies of the chromosome show increased brain Aβ accumulation over time (Masters et al., 1985). According to a popular theoretical model in AD, the “amyloid cascade hypothesis”, Aß dysmetabolism is a primary event in the pathogenesis of AD (Hardy and Selkoe, 2002). Despite genetic differences between AD and DS, the clinico-pathologic similarities have led investigators to examine late maternal age as a risk factor for AD, especially for the late-onset forms of AD which are likely due to a complex interaction of genetic and non-genetic factors (Hardy and Selkoe, 2002).
Some epidemiological studies in AD patients provided evidence for an association between maternal age and risk for AD (Cohen et al., 1982; Amaducci et al., 1986; Urakami et al., 1989; Whalley et al., 1982), while others did not (Farrer et al., 1991; Hofman et al., 1990; Bertram et al., 1998). A re-analysis of existing data sets showed that, across all studies, late maternal age at birth increases risk of developing AD, particularly for late-onset AD patients (Rocca et al., 1991). However, these studies did not take into account the patients’ family history and did not examine parental age at birth as a risk factor for NL individuals. Moreover, there are no studies that examined the relationship between parental age and biological markers of AD.
This FDG-PET study examined the associations between maternal and paternal age at birth and brain glucose metabolism in NL individuals as a function of the subjects’ family history of LOAD.
2. Methods
This study retrospectively examined clinically and cognitively NL individuals who received an FDG-PET scan and thorough family history evaluations at New York University School of Medicine. Subjects came from multiple community sources, including individuals interested in research participation and risk consultation, as well as spouses, family members, and caregivers of impaired patients. Written informed consent was obtained from all subjects after a complete description of this Institutional Review Board-approved study.
All subjects received a standardized diagnostic evaluation that included medical, psychiatric, neuropsychological, and clinical MRI examinations completed within a three-month window. Individuals with medical conditions or history of conditions that may affect brain structure or function (i.e. stroke, diabetes, head trauma, any neurodegenerative diseases, depression), showing MRI evidence of hydrocephalus, intracranial mass, and infarcts including lacunes, and those using psychoactive medications were excluded.
Subjects were 25–85 years of age, had education ≥12 years, Clinical Dementia Rating=0, Global Deterioration Scale ≤2, Modified Hachinski Ischemia Scale scores<4, MMSE ≥28, and normal cognitive test performance on a testing battery including evaluation of verbal and non verbal memory, attention/psychomotor speed, language and vocabulary tests (De Santi et al., 2008). ApoE genotype was determined using standard Polymerase Chain Reaction procedures. Subjects with one or two copies of the ApoE ε4 allele were grouped as ε4 carriers.
A family history of LOAD that included at least one 1st degree relative whose dementia onset was after age 60 was elicited by using a standardized questionnaire (Mosconi et al 2009, 2010b). Participants were asked to fill in names, dates of birth, age at death, cause of death, and clinical information of all affected family members over 3 generations. The information was confirmed with other family members in the clinical interview with the study physician. We only included subjects whose parents’ diagnosis was certified by an expert clinician according to established criteria for AD, and whose parents had lived to a minimum age of 60. A total of 114 NL with complete family history and FDG-PET exams were available for analysis. Of these, 18 subjects were excluded, as 5 had both parents affected, 3 had only siblings affected, 5 had only 2nd degree relatives affected, and 5 had a family history of an unspecified dementia. The remaining 96 subjects included 36 MH, 24 PH and 36 NH. The affected parents of 5 subjects (3 MH and 2 PH) received the post-mortem diagnosis of AD.
2.1 Image Acquisition and Analysis
Subjects received a PET scan using FDG as the tracer after at least 8 hours fasting, on either an ECAT EXACT HR+ (Siemens, Knoxville, TN; field of view=15.5 cm, full width at half maximum=4.3 mm) or an LS Discovery scanner (G.E. Medical Systems, Milwaukee, WI; field of view =20 cm, full width at half maximum =5.4 mm) using a standardized protocol (Mosconi et al., 2008b). Subjects received 5–10 mCi of FDG intravenously while lying supine in a dimly lit room. Scans started 40 min after injection and lasted 20 min. All images were corrected for photon attenuation, scatter, and radioactive decay, and reconstructed into a 128x128 matrix spaced every 4.25 mm.
Image processing and data analyses were performed blind to clinical information using MIDAS 1.9 (Mosconi et al., 2005; Li et al., 2008). Summed PET images corresponding to the 40–60 min of FDG data were created, and parametric standardized uptake value ratio (SUVR) images generated by dividing each voxel by the whole-brain gray matter to normalize for individual variability in global FDG uptake (Mosconi et al., 2008b). FDG SUVR images were processed using automated regions-of-interest (ROI) (Mosconi et al., 2005; Li et al., 2008) and Statistical Parametric Mapping (SPM’5) (Friston et al., 1995). Briefly, each FDG scan was spatially normalized to a standardized FDG brain template image, which approximates the Talairach and Tournoux space, using high-order polynomial transformations. The reverse of this transformation was then applied to the template ROI to map them back to the original FDG-PET image (Li et al. 2008). We focused on pre-selected, AD-vulnerable, bilateral ROI, including: inferior parietal lobe (IPL), hippocampus (HIP), lateral temporal lobe (LTL), posterior cingulate cortex (PCC) and prefrontal cortex (PFC) (Mosconi et al.,2007, 2009). An AD-mask was also created by combining these ROI. The thalamus (THAL) and occipital cortex (OCC) were examined as metabolically non-affected regions (Mosconi et al., 2008b). Spatially normalized SUVR images were then smoothed with a 12 mm FWHM gaussian filter and examined for correlations between metabolism and parental age at birth on a voxel-wise basis using SPM’5 (Friston et al., 1995). As a result of the normalization of FDG data into SUVR, no proportional scaling or grand mean scaling was performed. The gray matter threshold was set at 0.8 and only clusters exceeding an extent threshold of 30 voxels were considered significant.
2.2 Statistical Analysis
Statistical analyses were done with SPSS 12.0 (SPSS inc., Chicago, IL) and SPM’5. Differences in clinical and demographical measures between groups were examined with χ2 tests, Fisher’s exact test, and the general linear model (GLM), as appropriate.
FDG-PET group differences
For ROI analysis, a multivariate GLM with follow-up univariate post-hoc comparisons performed using F statistics and least-square difference tests was used to test for metabolic differences across groups. For SPM’5 analysis, the GLM was used to test for regional differences in FDG SUVR images across groups using post-hoc t-contrasts. All analyses were repeated controlling for age, education, gender, and ApoE genotype.
Associations between FDG-PET and parental age at birth
The associations of parental age with metabolism were investigated with multiple linear regressions. Three different models were evaluated across all subjects and for each family history group, which included the following predictors of brain metabolism: maternal or paternal age at birth and subject’s age at PET [Model I]; maternal or paternal age at birth, subject’s age, sex and education [Model II]; maternal or paternal age at birth, subject’s age, sex, education and ApoE genotype [Model III]. All analyses of maternal age were performed controlling for paternal age, and vice-versa. An interaction term was included in each model to test for slope differences across groups.
Size-matched groups
Given the smaller number of PH subjects (n=24), we created 3 size-matched groups of 24 subjects each, demographically balanced based on age (within 3 yrs), gender, and education (within 2 yrs). All analyses were repeated for this group, with and without controlling for ApoE.
Results were considered significant at P<0.05 for ROI, and at P<0.001, uncorrected, for SPM’5. Anatomical location of brain regions showing significant effects was described using Talairach and Tournoux coordinates [http://www.talairach.org], after coordinates conversion to the Talairach space using linear transformations [http://www.mrc-cbu.cam.ac.uk/Imaging].
3. Results
There were no group differences for age, gender, education, and neuropsychological data (Table 1). The proportion of ApoE ε4 carriers was lower in NH than in subjects with a family history (P=0.02), and was comparable between MH and PH (Table 1). There were no differences for maternal and paternal age across groups (Table 1).
Table 1.
Subject characteristics and FDG-PET regions-of-interest measures by family history group
| NH | PH | MH | |
|---|---|---|---|
| N | 36 | 24 | 36 |
| Age (yrs) | 63(12) | 58(9) | 60(9) |
| Gender (males/females) | 11/25 | 13/11 | 10/26 |
| Education (yrs) | 16(2) | 17(2) | 16(2) |
| Apolipoprotein E ε4 genotype (ε4-/ε4+) | 28/8 | 11/13* | 19/17* |
| Maternal age at subjects’ birth (yrs) | 29(4) | 28(5) | 28(5) |
| Paternal age at subjects’ birth (yrs) | 32(5) | 34(7) | 32(6) |
| Affected parent’s age at onset (yrs) | - | 77(10) | 69(8) |
| Mini Mental State Examination | 29.4(1.2) | 29.7(0.8) | 29.7(0.5) |
| Designs | 6.2(2.2) | 7.4(2.5) | 6.9(1.9) |
| Digit symbol substitution test | 58.0(9.5) | 62.0(9.3) | 56.5(8.6) |
| Object naming | 56.2(4.5) | 52.1(8.1) | 53.9(5.7) |
| Paired Associates delayed recall | 9.9(4.0) | 10.2(2.3) | 8.9(2.9) |
| Paragraph delayed recall | 6.1(2.1) | 6.2(3.1) | 5.9(2.2) |
| Visual recognition test | 13.3(14.7) | 12.5(14.3) | 18.6(11.9) |
| WAIS vocabulary | 67.9(7.8) | 70.6(8.7) | 66.7(9.2) |
Values are means (SD)
Different from NH, P<0.05
FDG-PET group differences
The MH group had lower metabolism compared to the other two groups in all AD-vulnerable ROIs (P’s≤0.05), and not in THAL and OCC (Table 2). Results remained significant controlling for age, gender, education, and ApoE (Table 2). Consistent with ROI, SPM analysis showed reduced metabolism in parietal, temporal, frontal, anterior and posterior cingulate cortices, angular gyrus and precuneus of MH compared to PH and NH (P’ s<0.001, uncorrected) (Supplemental Figure 1). There were no regions showing differences between PH and NH.
Table 2.
Regional FDG-PET measures by family history group
| NH | PH | MH | |
|---|---|---|---|
| AD-mask | 1.28(0.20) | 1.31(0.22) | 1.19(0.18)*† |
| 1.28(0.22) | 1.30(0.20) | 1.19(0.14)*† | |
| Hippocampus | |||
| Left | 0.81(0.11) | 0.80(0.11) | 0.74(0.11)* |
| 0.81(0.13) | 0.79 (0.13) | 0.74(0.12)* | |
| Right | 0.84(0.10) | 0.83(0.11) | 0.78(0.10)*† |
| 0.83(0.10) | 0.83(0.12) | 0.79(0.11)*† | |
| Inferior parietal lobule | |||
| Left | 1.18(0.11) | 1.18(0.12) | 1.16(0.11)* |
| 1.18(0.10) | 1.17(0.16) | 1.15(0.10)* | |
| Right | 1.17(0.11) | 1.17(0.11) | 1.16(0.11) |
| 1.17(0.10) | 1.16(0.14) | 1.15(0.09)* | |
| Lateral temporal lobe | |||
| Left | 1.03(0.11) | 1.04(0.11) | 1.01(0.12)† |
| 1.02(0.12) | 1.03(0.15) | 1.01(0.10) | |
| Right | 1.05(0.10) | 1.06(0.10) | 1.03(0.10)*† |
| 1.05(0.11) | 1.05(0.13) | 1.03(0.09)*† | |
| Posterior cingulate cortex | |||
| Left | 1.38(0.15) | 1.35(0.15) | 1.30(0.15)*† |
| 1.37(0.17) | 1.34(0.19) | 1.30(0.12)*† | |
| Right | 1.31(0.16) | 1.27(0.16) | 1.22(0.17)*† |
| 1.32(0.20) | 1.30 (0.21) | 1.22(0.13)*† | |
| Prefrontal cortex | |||
| Left | 1.10(0.12) | 1.17(0.12) | 1.09(0.13)† |
| 1.12(0.14) | 1.16(0.14) | 1.09(0.13)† | |
| Right | 1.05(0.13) | 1.11(0.12) | 1.05(0.12)† |
| 1.11(0.13) | 1.11(0.13) | 1.04(0.12)*† | |
| Occipital cortex | |||
| Left | 1.28(0.12) | 1.28(0.18) | 1.27(0.13) |
| 1.28(0.12) | 1.25(0.17) | 1.28(0.13) | |
| Right | 1.25(0.13) | 1.25(0.18) | 1.25(0.14) |
| 1.25(0.12) | 1.24(0.18) | 1.25(0.13) | |
| Thalamus | |||
| Left | 1.08(0.28) | 1.07(0.33) | 1.10(0.28) |
| 1.17(0.28) | 1.17 (0.32) | 1.16(0.26) | |
| Right | 1.25(0.15) | 1.25(0.20) | 1.22(0.15) |
| 1.25(0.14) | 1.27(0.19) | 1.23(0.14) |
Values are means (SD). FDG measures are regional-to-whole brain gray matter standardized uptake value ratios. Age, gender, education, and ApoE-adjusted measures are in italics.
Different from NH,
Different from PH; P<0.05
Associations between FDG-PET and parental age at birth
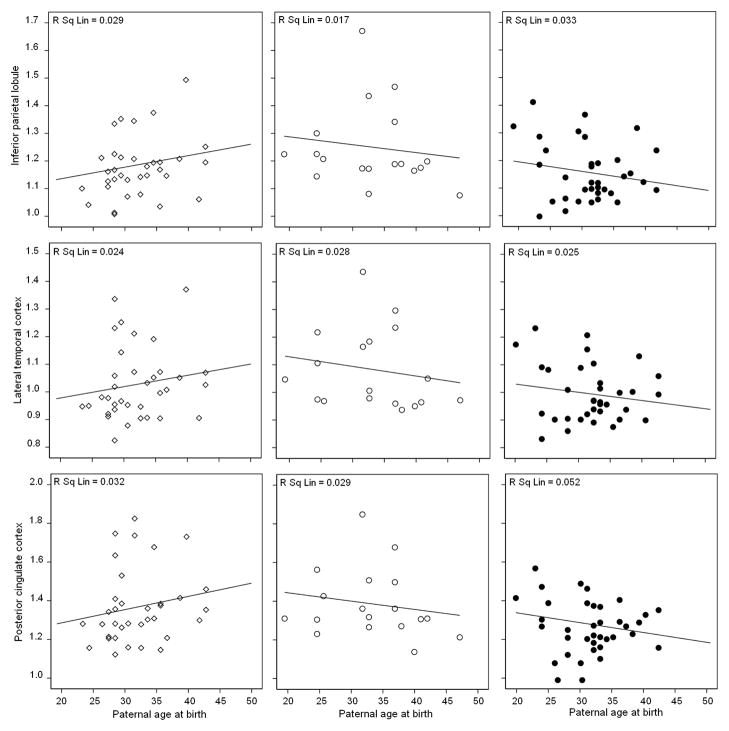
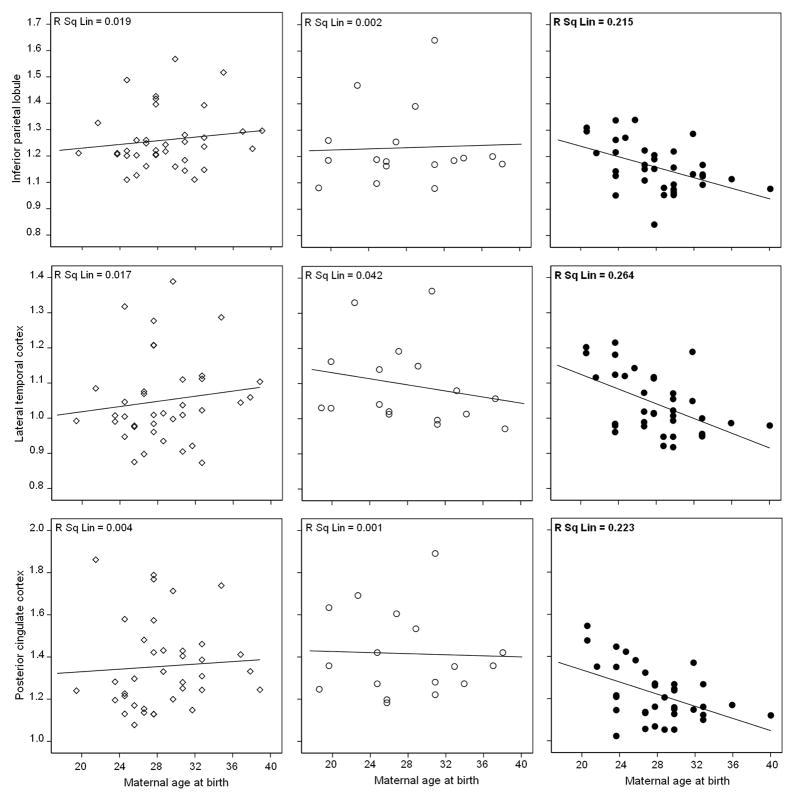
With or without controlling for subject’s age, there were no significant correlations between paternal age and metabolism in any regions of any group (Models I-III, Figure 1a and Supplemental figure 2a). With or without correcting for subjects’ age, significant associations between maternal age and metabolism were found only for MH subjects, which included IPL, LTL, PCC and AD-mask (R2 range: 0.20 in IPL to 0.42 in PCC, P’s<0.03, Figure 1b and Supplemental figure 2b). Unstandardized beta coefficients in these regions ranged between −0.009 in IPL to −0.016 in PCC. The higher maternal age, the lower metabolism in these regions, with FDG SUVR decreasing by an average of −0.012 units for every year increase in maternal age of MH subjects. These associations remained significant controlling for paternal age (P’s≤0.04, Model I). In these regions, there was a significant interaction between maternal age and family history, as MH subjects showed steeper regression slopes of metabolism on maternal age than PH and NH (Pinteraction≤0.02; Figure 1b and Supplemental figure 2b). Results were not modified by further adjusting for education, gender, and ApoE genotype (Model II–III, Supplemental Figure 2; P’s≤0.04). After controlling for these covariates, the MH group showed significant associations between maternal age and metabolism in IPL, LTL, PCC and AD mask (Model III; R2 range: 0.18 in LTL to 0.26 in AD-mask, P’s<0.02, Supplemental figure 2b), with unstandardized beta coefficients ranging between −0.01 in LTL to −0.016 in AD-mask. There were no significant associations between maternal age and metabolism in THAL and OCC (Model I-III). There were no significant correlations between maternal age and metabolism for PH and NH in any regions (Model I-III, Supplemental Figure 2b).
Figure 1. Associations between parental age and brain glucose metabolism in AD-vulnerable regions of NL MH (black circles), NL PH (white circles) and NL NH (white diamonds), without including covariates.
(a) Lack of significant associations between paternal age at birth (years) and metabolism (SUVR, unitless).
(b) Associations between maternal age at birth (years) and metabolism (SUVR, unitless). R2 (R Sq Linear) values are displayed in the top right corner of each panel. Significant R2 values are in bold.
Associations between metabolism and parental age at birth after adjusting for age, gender, education and ApoE (Model III) are shown in Supplemental Figure 2.
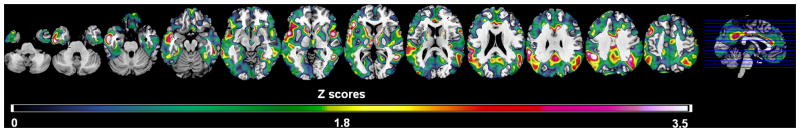
On SPM analysis, negative correlations between maternal age and metabolism were observed only for the MH group, involving the temporal, parietal, frontal, anterior and posterior cingulate cortices (Model I, P’s<0.001, Table 3 and Figure 2). Results remained unchanged by adjusting for education, gender, and ApoE (Model II-III, P’s<0.001). There were no significant correlations between maternal age and metabolism in any regions of PH and NH, or between paternal age and metabolism in any group.
Table 3.
Brain regions showing significant negative correlations between brain glucose metabolism and maternal age at birth in NL MH.
| Cluster extent | Coordinates* | Z† | Functional area | Brodmann Area |
|---|---|---|---|---|
| 793 | −63, −37, 2 | 3.90 | Inferior parietal gyrus | 40 |
| −63, −41, 24 | 2.95 | Inferior parietal gyrus | 40 | |
| −59, −45, 35 | 2.88 | Inferior parietal gyrus | 40 | |
| 615 | −61, −9, −16 | 3.60 | Inferior temporal gyrus | 21 |
| −52, 9, −1 | 3.33 | Superior temporal gyrus | 22 | |
| 332 | 61, −48, 16 | 3.14 | Superior temporal gyrus | 22 |
| 312 | −2, −20, 35 | 3.91 | Cingulate cortex | 24 |
| 481 | 4, −37, 40 | 3.42 | Posterior cingulate gyrus | 31 |
| −3, −44, 42 | 3.24 | Posterior cingulate gyrus | 31 | |
| 278 | −5, 24, 24 | 3.61 | Anterior cingulate cortex | 24 |
| 244 | 57, −53, 35 | 2.88 | Inferior parietal lobule | 40 |
| 174 | −59, 8, 14 | 2.82 | Inferior frontal gyrus | 44 |
| 121 | 55, 9, −1 | 2.93 | Superior temporal gyrus | 22 |
Coordinates (x, y, z) from the atlas of Talairach and Tournoux: x is the distance in mm to the right (+) or left (−) of midline; y is the distance anterior (+) or posterior (−) to the anterior commissure, and z is the distance superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures.
Z values at the peak of maximum statistical significance at P<0.001.
Figure 2.
Statistical parametric maps (SPMs) showing significant negative correlations between maternal age at birth and brain metabolism in NL MH, correcting for subjects’ age (Model I). Areas of maternal age-related hypometabolism are represented on color-coded scale reflecting Z scores between 0 and 3.5 (P<0.001, uncorrected). SPMs are displayed onto the axial views of a standard, spatially normalized MRI.
Size-matched groups
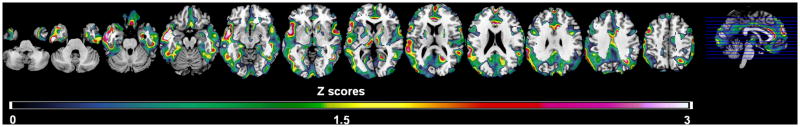
As with the entire group, MH had reduced metabolism in AD-regions compared to PH and NH, which remained significant after adjusting for ApoE genotype (P’s<0.05; Supplemental Table 1). With or without correcting for ApoE, significant associations between maternal age and metabolism were restricted to MH group (R2 range: 0.22 in IPL to 0.32 in LTL, P’s≤0.02), with an average of −0.011 units for every year increase in maternal age. A similar regional pattern of negative correlations restricted to the MH group was observed on SPM analysis (P≤0.001, Figure 3). Results were not modified by including ApoE as a covariate (Ps<0.001). There were no significant associations between paternal age and metabolism in any regions.
Figure 3.
Statistical parametric maps (SPMs) showing significant negative correlations between maternal age at birth and brain metabolism in NL MH from analysis of size-matched, demographically balanced groups (n=24 subjects/group), correcting for subjects’ age. Areas of maternal age-related hypometabolism are represented on color-coded scale reflecting Z scores between 0 and 3 (P<0.001, uncorrected). SPMs are displayed onto the axial views of a standard, spatially normalized MRI.
4. Discussion
This FDG-PET study shows that reductions in brain glucose metabolism in NL MH are significantly associated with advanced maternal age at birth. Hypometabolism involved the same brain regions that are typically affected at the prodromal stages of AD (de Leon et al., 2001; Mosconi et al., 2008a; Drzezga et al., 2003), potentially suggesting an increased risk for AD in NL MH. In contrast, brain metabolism in PH was comparable to controls and was not influenced by parental age. Advanced paternal age was not significantly associated with metabolism in any group.
These results confirm our previous findings of selectively reduced metabolism in NL MH using a sample twice as large (Mosconi et al., 2007, 2009), and are consistent with imaging evidence for increased Aβ, atrophy and altered white matter integrity in MH compared to PH and NH (Mosconi et al., 2010b; Honea et al., 2009; Andrawis et al., 2010; Bendlin et al., 2010), as well as with findings of increased levels of Aβ pathology and oxidative stress in cerebrospinal fluid of NL MH (Mosconi et al., 2010c). Present resent results also add biological evidence to epidemiological findings showing that having a maternal history of LOAD is a major risk factor, especially in offspring of older mothers. While there is evidence for both maternal and paternal transmission in LOAD families (Edland et al., 1996; Ehrenkrantz et al., 1999), maternal transmission is more frequent than paternal transmission, and is associated with higher risk of AD, poorer cognitive performance and a more predictable age at onset in the offspring (Edland et al., 1996; Silverman et al., 2005; Gomez-Tortosa et al., 2007; Debette et al., 2009).
Among neurological disorders, advanced maternal age has been associated with risk of DS (Yoon et al., 1996) and mental retardation (Croen et al., 2001), and paternal age with risk of schizophrenia (Malaspina et al., 2001) and autism (Reichenberg et al., 2006). In the late 1980’s and early 1990’s, epidemiological studies investigated the relationship between parental age at birth and risk for AD, providing results of either an association with maternal age (Cohen et al., 1982; Amaducci et al., 1986; Urakami et al., 1989; Whalley et al., 1982; Rocca et al., 1991), or with paternal age (Farrer et al., 1991; Bertram et al.,1998), or no associations (Hofman et al., 1990; White et al., 1986). Discrepant results may be due to the fact that previous studies examined AD patients’ without taking into account their parents diagnoses. Our study shows that maternal age influences risk for AD in individuals whose mothers were affected, but not in those with paternal or negative family history.
While not discounting the influence of possible age-related psychosocial factors that may confer risk for Alzheimer’s to the offspring of older mothers, or the effects of diminished female fertility with age (Navot et al., 1991), such effects would have led to hypometabolism in the children irrespective of their family history. Instead, maternal age had an impact only on the offspring of women who developed AD, and who were cognitively normal when they gave birth, suggesting a genetic component to the metabolic deficits. Previous studies on maternal age capitalized on reports of genetic and pathological links between DS and AD (Masters et al., 1985). While trisomy of Chromosome 21 is not present in AD, other studies are needed to examine whether advanced maternal age affects APP metabolism in NL MH through molecular pathways other than chromosomal aneuploidy. Previous studies showed that NL MH harbor increased brain Aβ deposition compared to PH and NH (Mosconi et al., 2010b), although the biological mechanisms accounting for this effect are unknown. Future studies are warranted to examine the relationship between maternal age at birth and Aβ pathology as measured by amyloid-PET scans or in cerebrospinal fluid of NL with LOAD parents.
Reproductive behavior and physiology are extremely complex, and as such all possible permutations could not be taken into account in the present study. That being said, one possible mechanism for the maternal age effect is through mitochondrial genes. Mitochondrial DNA (mtDNA) is entirely maternally inherited in humans, mitochondria are tightly involved with cellular glucose metabolism (Lin and Beal, 2006), and mtDNA mutations in human oocytes are associated with advanced maternal age (Barritt et al., 2000). Oocyte quality is determined primarily by the level of metabolism, and therefore by mitochondrial physiology (Wilding et al., 2005). In general, a physiologically normal level of free radical production, when extended over a 30–40 year period, could damage the oocyte mtDNA sufficiently to reduce the oocytes’ capacity for oxidative phosphorylation, explaining the maternal age effect’ (Wilding et al., 2005). Specifically for adult children of LOAD-mothers, if maternal transmission of disease involves mtDNA, affected mothers would have defective oocyte mitochondria to start with, and those who gave birth at an advanced age would have more impaired oocytes, exacerbating metabolic deficits in the offspring. Another possible mechanism is genomic imprinting, a genetic process that results in monoallelic gene expression in a parent-of-origin specific manner without altering the genetic sequence (Constancia et al., 2004). The majority of imprinted genes in mammals have roles in the control of embryonic growth and post-natal development, including development of the placenta, and roles affecting suckling and metabolism (Constancia et al., 2004). Metabolic imprinting is thought to have long term consequences for health (Preece and Moore, 2000).
Steps were taken to ensure that the diagnosis in the subjects’ parents was accurate by including only subjects whose parents’ AD diagnosis was clinician certified. However, our cohort may have included subjects whose parents did not have AD but another dementia, which would have conservatively reduced power in detecting group differences. We caution that there may also be phenotypic differences in NL PH that were not detected because of the relatively small samples, conservative image analysis and statistical procedures.
While present results were independent of ApoE genotype, a relatively high proportion of subjects were ε4 carriers. This is likely due to the fact that ApoE ε4 genotype is over-represented in LOAD families, and individuals with affected parents tend to worry about their cognitive status and seek clinical attention. Therefore, the frequency of ApoE ε4 carriers is often higher in the worried-well’ subjects that self-refer to memory clinics such as ours. Other studies with larger samples are needed to examine ApoE status as an effect modifier and its interactions with maternal history of AD on metabolism.
The majority of subjects in our study were born before year 1950, and their mothers were on average 28 years old when they gave birth, with 39% of mothers over the age of 30. At that time, parturition after 30 would have been considered advanced maternal age. However, with improving nutritional and health practices, as well as changing social pressures, maternal and paternal ages have increased over the years, and at present it is not uncommon for women to have healthy pregnancies after age 35. It remains to be explored what effects such changes will have on future risk for AD in current generations.
Finally, while altered brain metabolism in MH suggest increased risk for AD compared to PH and controls, only longitudinal studies and continued follow-up examinations of our subjects can establish with certainty whether the observed biomarker abnormalities are predictive of future AD or rather represent a steady-state feature associated with MH.
In conclusion, our FDG-PET study shows that adult children of mothers who gave birth at an advanced age and developed LOAD may be at increased risk for developing the disease. These findings may be helpful in designing clinical and prevention trials in AD, and in clinical practice particularly in the areas of genetic counseling, and possible future gene therapy.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute on Aging AG13616, AG032554, AG035137 and AG022374, the National Institute of Health-National Center for Research Resources M01RR0096, and Alzheimer’s Association IIRG-09-132030.
Footnotes
Disclosures
Drs. Mosconi, Tsui and de Leon have a patent on a technology that was licensed to Abiant Imaging Inc. by NYU and, as such, have a financial interest in this license agreement and hold stock and stock options on the company. Drs. Mosconi, Li and de Leon have received compensation for consulting services from Abiant Imaging. Dr. Glodzik is PI on an investigator initiated clinical trial supported by Forrest Labs. Dr. de Leon has received honoraria from the French Alzheimer Foundation, and is PI on an investigator initiated clinical trial supported by Neuroptix. Dr. De Santi is an employee at Bayer Healthcare Pharmaceuticals. Drs. Murray, McHugh, Pirraglia, Williams and Vallabhajosula report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amaducci LA, Fratiglioni L, Rocca WA, Fieschi C, Livrea P, Pedone D, Bracco L, Lippi A, Gandolfo C, Bino G. Risk factors for clinically diagnosed Alzheimer's disease. Neurology. 1986;36:922–931. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- Andrawis JP, Hwang KS, Green AE, Kotlerman J, Elashoff D, Morra JH, Cummings JL, Toga AW, Thompson PM, Apostolova LG. Effects of ApoE4 and maternal history of dementia on hippocampal atrophy. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.07.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt JA, Cohen J, Brenner CA. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod Biomed Online. 2000;1:96–100. doi: 10.1016/s1472-6483(10)61946-3. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Canu E, Sodhi A, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer's disease. Alzheimers Dement. 2010;6:394–403. doi: 10.1016/j.jalz.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Busch R, Spiegl M, Lautenschlager NT, Muller U, Kurz A. Paternal age is a risk factor for Alzheimer disease in the absence of a major gene. Neurogenetics. 1998;1:277–280. doi: 10.1007/s100480050041. [DOI] [PubMed] [Google Scholar]
- Cohen D, Eisdorfer C, Leverenz J. Alzheimer's disease and maternal age. J Am Geriatr Soc. 1982;30:656–659. doi: 10.1111/j.1532-5415.1982.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Constancia M, Kelsey G, Relk W. Resourceful imprinting. Nature. 2004;432:53–57. doi: 10.1038/432053a. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Selvin S. The epidemiology of mental retardation of unknown cause. Pediatrics. 2001;107:86. doi: 10.1542/peds.107.6.e86. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schyler D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, Pirraglia E, Barr WB, Babb J, Williams S, Rogers K, Glodzik L, Brys M, Mosconi L, Reisberg B, Ferris S, de Leon MJ. Robust and conventional neuropsychological norms: Diagnosis and prediction of age-related cognitive decline. Neuropsychology. 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Wolf PA, Beiser A, Au R, Himali JJ, Pikula A, Auerbach S, Decarli C, Seshardi S. Association of parental dementia with cognitive and brain MRI measures in middle-aged adults. Neurology. 2009;73:2071–2078. doi: 10.1212/WNL.0b013e3181c67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer's disease cases: evidence for maternal inheritance. Neurology. 1996;47:254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- Ehrenkrantz D, Silverman JM, Smith CJ, Birstein S, Marin D, Mohs RC, Davis KL. Genetic epidemiological study of maternal and paternal transmission of Alzheimer's disease. Am J Med Genet. 1999;88:378–382. doi: 10.1002/(sici)1096-8628(19990820)88:4<378::aid-ajmg15>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Connor L, Wolf PA, Growdon JH. Association of decreased paternal age and late-onset Alzheimer's disease. An example of genetic imprinting? Arch Neurol. 1991;48:599–604. doi: 10.1001/archneur.1991.00530180051017. [DOI] [PubMed] [Google Scholar]
- Farrer LA, O'Sullivan DM, Cupples AC, Growdon JH, Myers RH. Assessment of genetic risk for Alzheimer's disease among first-degree relatives. Ann Neurol. 1989;25:485–493. doi: 10.1002/ana.410250511. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gomez-Tortosa E, Barquero MS, Baron M, Sainz MJ, Manzano S, Payno M, Ros R, Almaraz C, Gómez-Garré P, Jiménez-Escrig A. Variability of Age at Onset in Siblings with Familial Alzheimer Disease. Arch Neurol. 2007;64:1743–1748. doi: 10.1001/archneur.64.12.1743. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, Williams M, Hipps Y, Graff-Radford N, Bachman D, Farrer LA. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The Amyloid Hypothesis of Alzheimer's disease: Progress and Problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Heffner LH. Advanced maternal age- how old is too old? N Eng J Med. 2004;351:1927–1929. doi: 10.1056/NEJMp048087. [DOI] [PubMed] [Google Scholar]
- Hofman A, van Duijn CM, Schulte W, Tanja TA, Haaxma R, Lameris AJ, Saan RJ. Is parental age related to the risk of Alzheimer's disease? Br J Psychiatry. 1990;157:273–275. doi: 10.1192/bjp.157.2.273. [DOI] [PubMed] [Google Scholar]
- Honea RA, Swerdlow RH, Vidoni E, Goodwin J, Burns JM. Reduced Gray Matter Volume in Normal Adults with a Maternal Family History of Alzheimer Disease. Neurology. 2009;74:113–120. doi: 10.1212/WNL.0b013e3181c918cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, De Santi S, Kemppainen N, Någren K, Kim BC, Tsui W, de Leon MJ. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment and Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008;35:2169–2181. doi: 10.1007/s00259-008-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald B, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Swerdlow RH, Mistur R, Pupi A, Duara R, de Leon MJ. Maternal transmission of Alzheimer's disease: Prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010a;4:170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci USA. 2007;104:19067–19072. doi: 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008a;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Glodzik L, Mistur R, McHugh P, Rich KE, Javier E, Williams S, Pirraglia E, De Santi S, Mehta PD, Zinkowski R, Blennow K, Pratico D, de Leon MJ. Oxidative stress and amyloid-beta pathology in normal individuals with a maternal history of Alzheimer’s. Biological Psychiatry. 2010c;68:913–21. doi: 10.1016/j.biopsych.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Glodzik L, Switalski R, Brys M, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer's. Neurology. 2009;72:513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Rinne JO, Tsui W, Berti V, Li Y, Wang H, Murray J, Scheinin N, Någren K, Williams S, Glodzik L, De Santi S, Vallabhajosula S, de Leon MJ. Increased fibrillar amyloid-β burden in normal individuals with a family history of late-onset Alzheimer's disease. Proc Natl Acad Sci USA. 2010b;107:5949–54. doi: 10.1073/pnas.0914141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, De Santi S, Li J, Rusinek H, Convit A, Li Y, Boppana M, de Leon MJ. Reduced hippocampal metabolism in mild cognitive impairment and Alzheimer's disease: automated FDG-PET image analysis. Neurology. 2005;64:1860–1867. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman EM, Holthoff V, Kalbe E, Sorbi S, Diehl-Schmid J, Perneczky R, Clerici F, Caselli R, Beuthien-Baumann B, Kurz A, Minoshima S, de Leon MJ. Multi-center standardized FDG-PET diagnosis of Mild Cognitive Impairment, Alzheimer's disease and other dementias. J Nucl Med. 2008b;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, Grunfeld L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337:1375–1377. doi: 10.1016/0140-6736(91)93060-m. [DOI] [PubMed] [Google Scholar]
- Preece MA, Moore GE. Genomic Imprinting, Uniparental Disomy and Foetal Growth. Trends Endocrinol Metab. 2000;11:270–275. doi: 10.1016/s1043-2760(00)00277-0. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, Susser E. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63:1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Van Duijn CM, Clayton D, Chandra V, Fratiglioni L, Graves AB, Heyman A, Form AF, Kokmen E, Kondo K, Mortimer JA, Shalat SL, Soininen H, Hofman A the EURODEM Risk Factors Research group. Maternal Age and Alzheimer’s disease: A collaborative Re-analysis of Case-control studies. Int J Epidemiol. 1991;20(Suppl 2):S21–27. doi: 10.1093/ije/20.supplement_2.s21. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Ciresi G, Smith CJ, Marin DB, Schnaider-Beeri M. Variability of familial risk of Alzheimer disease across the late life span. Arch Gen Psychiat. 2005;62:565–573. doi: 10.1001/archpsyc.62.5.565. [DOI] [PubMed] [Google Scholar]
- Urakami K, Adachi Y, Takahashi K. A community-based study of parental age at the birth of patients with dementia of the Alzheimer type. Arch Neurol. 1989;46:38–39. doi: 10.1001/archneur.1989.00520370040016. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Carothers AD, Collyer S, De Mey R, Frackiewicz A. A study of familial factors in Alzheimer's disease. Br J Psychiatry. 1982;140:249–256. doi: 10.1192/bjp.140.3.249. [DOI] [PubMed] [Google Scholar]
- White J, McGue M, Heston L. Fertility and parental age in Alzheimer's disease. J Gerontology. 1986;41:40–43. doi: 10.1093/geronj/41.1.40. [DOI] [PubMed] [Google Scholar]
- Wilding M, Di Matteo L, Dale B. The maternal age effect: a hypothesis based on oxidative phosphorylation. Zygote. 2005;13:317–323. doi: 10.1017/S0967199405003382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.