Abstract
Objective
We sought to determine if maternal depression, anxiety, and/or treatment with selective serotonin reuptake inhibitors (SSRIs) affect placental human serotonin transporter (SLC6A4), norepinephrine transporter (SLC6A2), and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) gene expression.
Methods
Relative mRNA expression was compared among placental samples (n=164) from healthy women, women with untreated depression and/or anxiety symptoms during pregnancy, and women who used SSRIs.
Results
SLC6A4 expression was significantly increased in placentas from women with untreated mood disorders and from women treated with SSRIs, compared to controls. SLC6A2 and 11β-HSD2 expression was increased in non-control groups, though the differences were not significant. SLC6A4, SLC6A2, and 11β-HSD2 expression levels were positively correlated.
Conclusions
The finding that maternal depression/anxiety affects gene expression of placental SLC6A4 suggests a possible mechanism for the effect(s) of maternal mood on fetal neurodevelopmental programming. SSRI treatment does not further alter the elevated SLC6A4 expression levels observed with exposure to maternal depression or anxiety.
Keywords: serotonin transporter, norepinephrine transporter, 11beta-hydroxysteroid dehydrogenase, depression, anxiety, placenta, fetal programming
INTRODUCTION
Knowledge of the effects of maternal mood and SSRI exposure on neonatal outcome impacts large numbers of pregnant women and their offspring, given that approximately 7–13% of infants born in the United States each year are exposed to maternal major depressive disorder during gestation (Bennett, Einarson, Taddio, Koren, & Einarson, 2004) and that an estimated 7.6% of all pregnant women take an antidepressant (Andrade et al., 2008). SSRIs are a current first-line choice for pharmacologic treatment during pregnancy because of their low side-effect profiles and relatively low risk to the fetus (Kalra, Born, Sarkar, & Einarson, 2005). Depression exhibits significant co-morbidity with anxiety disorders, for which SSRIs are also a first-line pharmacologic treatment. SSRI use during pregnancy has increased from 1.5% in 1996 to 6.2% of all pregnant women in 2005 (Andrade et al., 2008).
Children of depressed parents are at increased risk for depression, and both genetic and environmental factors are implicated in this association (Sullivan, Neale, & Kendler, 2000; Silberg, Maes, & Eaves, 2010). Exposure to maternal depression in utero, independent of postnatal exposure, has been linked to adverse neurobehavioral development and temperament (Davis et al., 2007; Lundy et al., 1999). Adverse effects of in utero exposure to maternal depression are further supported by the finding of altered fetal neurobehavior in the setting of prenatal depression exposure (Monk et al., 2004). In addition, prenatal exposure to stress has been linked to disturbances in behavioral and emotional regulation in animal studies, independent of postnatal conditions (Coe & Lubach, 2005). The physiological correlates of elevated maternal cortisol levels late in pregnancy have been associated with dysregulated infant behavior and temperament (Davis et al., 2007; de Weerth, van Hees, & Buitelaar, 2003). Such findings suggest prenatal conditions may have a long-term impact on stress regulation and the risk for later development of mood disorders in the offspring.
Despite the associations between prenatal exposure to anxiety and major depressive disorder with long-term emotional, behavioral, and social problems in offspring (Field et al., 2010; Salisbury et al., 2007; Burt & Quezada, 2009; Marcus, 2009), little is known about the mechanisms of these disturbances such as dysregulation of key neurotransmitter systems in utero. The serotonin transporter (SLC6A4), norepinephrine transporter (SLC6A2), and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) genes have been implicated in perturbations of the hypothalamic pituitary adrenocortical (HPA) axis and in the development and treatment of mood disorders. This includes major depressive disorder, generalized anxiety disorder, and panic disorder (Strug et al., 2010; Gotlib, Joormann, Minor, & Hallmayer, 2008; Holmes et al., 2006; Welberg, Seckl, & Holmes, 2000; Ressler & Nemeroff, 2000). Depression is associated with elevated circulating cortisol, elevated norepinephrine, and decreased serotonin (Lundy et al., 1999; Field et al., 2004; Gillespie & Nemeroff, 2005). Neonatal levels of cortisol, norepinephrine, and serotonin reflect maternal biochemistry (Field et al., 2004). Accordingly, urinary cortisol is elevated and the 5-HIAA serotonin metabolite is decreased in depressed mothers compared to non-depressed mothers, and also in neonates of those depressed mothers compared to neonates of healthy mothers (Field et al., 2004). Thus alterations in maternal neurotransmitters are reflected in the fetus.
The placenta is the key regulatory organ maintaining fetal homeostasis. It is thus a critical site to examine alterations in neurotransmitter expression and potential dysregulation of the intrauterine environment (Coe & Lubach, 2005). The SLC6A4, SLC6A2, and 11β-HSD2 genes mediate placental uptake of serotonin, norepinephrine, and cortisol. Although previous studies have shown dysregulation of placental gene expression in response to maternal drug exposure, there are no known investigations of altered placental gene expression in response to maternal mood disorders and antidepressant use. SLC6A4 is downregulated in the placenta in response to cocaine or amphetamine exposure (Prasad, Leibach, Mahesh, & Ganapathy, 1994; Ramamoorthy, Ramamoorthy, Leibach, & Ganapathy, 1995a). Because of their ability to cross the placenta (Kim et al., 2006), antidepressants in the class of Selective Serotonin Reuptake Inhibitors (SSRIs) have the potential to alter SLC6A4 regulation in the placenta, however this has not been previously investigated, however this has not yet been investigated. SSRIs bind to SLC6A4 and induce internalization of the transporter from the neuronal surface, thereby blocking serotonin reuptake from the extracellular space into the pre-synaptic neuron and subsequently enhancing synaptic serotonin levels (Kittler, Lau, & Schloss, 2010). Chronic intrauterine stress, including exposure to cocaine, amphetamine, intrauterine growth retardation and maternal preeclampsia, are associated with downregulated human placental SLC6A2 expression (Ramamoorthy et al., 1995a; Bzoskie et al., 1997; Bottalico et al., 2004) and this downregulation is associated with an elevation in umbilical arterial plasma norepinephrine concentrations (Bzoskie et al., 1997). The 11β-HSD2 enzyme oxidizes cortisol to its inactive cortisone form. The abundant expression of this gene in the placenta creates a barrier from circulating maternal cortisol levels, protecting the fetus (Holmes et al., 2006; Welberg et al., 2000; McTernan et al., 2001). 11β-HSD2 is thus a key regulator of intrauterine growth. Cortisol exposure increases 11β-HSD2 mRNA levels in human placental trophoblast cells (van Beek, Guan, Julan, &Yang, 2004). In contrast, norepinephrine inhibits placental 11β-HSD2 expression (Sarkar et al., 2001), increasing fetal exposure to maternal cortisol.
Prenatal SSRI exposure is linked to both fetal (Salisbury, Ponder, Padbury, & Lester, 2009) and newborn (Moses-Kolko et al., 2005; Levinson-Castiel, Merlob, Linder, Sirota, & Klinger, 2006; Zeskind & Stephens, 2004) neurobehavioral changes, including up to thirty percent of exposed neonates who have symptoms of a “neonatal abstinence syndrome,” resembling that seen in infants withdrawing from (mostly opioid) drugs (Moses-Kolko et al., 2005; Levinson-Castiel, et al., 2006; Zeskind & Stephens, 2004). SSRI-exposed infants have decreased basal cortisol levels in the early evening compared with non-exposed infants at 3 months of age, controlling for maternal depression symptoms, and this effect was not related to prenatal or current SSRI exposure level measured in infant plasma (Oberlander, 2008a). Most studies of SSRI exposure outcomes to date have searched for teratogenic effects (Rahimi, Nikfar, & Abdollahi, 2006,Chambers et al., 2006; Louik, Lin, Werler, Hernández-Díaz, & Mitchell, 2007), however long-term consequences of prenatal SSRI exposure on developmental outcomes and risk for future mood disorders remains unclear.
Because it is not known whether intrauterine exposure to maternal mood disorders (depression/anxiety) and/or antidepressant treatment alters placental gene expression, the aim of this study was to determine if maternal depression/anxiety or treatment with an SSRI affects expression of placental genes that are implicated in mood disorders and stress, including human placental SLC6A4, SLC6A2, and 11β-HSD2 genes. Understanding the effects of maternal mood and antidepressant use on the expression of human placental genes would suggest roles for these factors in fetal programming mechanisms and contribute to the risk-benefit discussion of untreated maternal depression versus antidepressant medication exposure effects on neonates.
METHODS
Study Population and Sample Collection
Samples (n = 164) were collected from patients delivering at Women & Infants Hospital of Rhode Island. All research protocols were approved by the hospital’s institutional review board (IRB). Samples obtained for the hospital cohort were exempt from informed consent because the placenta is considered residual tissue and personal identifiers were not collected. Study investigators determined the presence of maternal mood disorders, medication use, and whether samples conformed to inclusion and exclusion criteria through chart review of prenatal and delivery records at the time of delivery. Samples were categorized in the “depression/anxiety” (”DEP/ANX”) group if prenatal and/or delivery records indicated a diagnosis of depression or anxiety with endorsement of symptoms present at any time during the pregnancy (self-reported by the patient or elicited by the medical provider then endorsed by the patient) and no pharmacological treatment. Women with a history of depression or anxiety but no symptoms and no SSRI use during the current pregnancy were excluded from the study. Samples from women who were treated with an SSRI antidepressant for depression and/or anxiety during at least the third trimester were categorized in the “SSRI” group. Samples were excluded from the study if SSRIs were used in only the first and/or second trimesters. Therefore samples in the SSRI group were exposed to between one and three trimesters of SSRI use, including the third trimester. The “control” group consisted of women with no history and no current symptoms or diagnosis of a mood disorder. The majority of participants received prenatal care through the study site hospital and therefore prenatal records were available in addition to the delivery record. If participants received their prenatal care at another facility and prenatal records were unavailable, study investigators reviewed the delivery record to determine if a subject met the study’s inclusion criteria (i.e. via a reference in the subject’s pregnancy history or medications section. If medication use during the third trimester or presence of symptoms during pregnancy was not clearly indicated, these samples were not included in the study). Seventy women met criteria for the control group, 62 women met criteria for the DEP/ANX group, and 32 women met criteria for the SSRI group.
Validation of the group categorization obtained by chart review was obtained in a portion of the samples collected (n = 32) as these participants were enrolled in a concurrent prospective cohort study of maternal depression during pregnancy in which participant depression and anxiety diagnoses and symptom severity scores were measured during the second and third trimesters of pregnancy. Trained staff members provided the sub-study participants with an IRB-approved informed consent form to read; study procedures, risks and benefits were then verbally explained prior to the participant signing the consent form. These women were interviewed between 24 and 28 weeks of gestation for psychiatric diagnoses using the Structured Clinical Interview for the DSM-IV-R (SCID, Williams et al., 1992). Mood and anxiety symptom severity was obtained at 28 and 34 weeks of gestation using the Inventory of Depressive Symptomatology (IDS-C), (Trivedi et al., 2004), and the Hamilton Anxiety Rating Scale (HAM-A, Vaccarino, Evans, Sills, & Kalali, 2008). A study investigator, not involved in the structured interviews, conducted a chart review at the time of delivery/placental sample collection for this subset of women in the same manner that the other participant information was collected. The resulting diagnosis and a detailed medication history obtained by structured interview were used to corroborate the diagnostic and medication categorization obtained by chart review in these 32 participants. To further minimize confounding effects, we excluded women with gestational age <35 weeks, maternal age <18 or >42, other psychotropic medications, maternal smoking, illicit drug use, alcohol use during pregnancy, or diabetes (gestational, type 1, or type 2).
RNA Extraction and Reverse Transcription
Preliminary studies showed that placental RNA levels were unchanged up to three hours following delivery (data not shown). Therefore, all samples were collected within three hours of delivery and then stored in RNAlater® (Ambion, Inc., Austin, TX). Preliminary studies showed that this approach was equal to or superior to immediate immersion in liquid nitrogen for prevention of RNA degradation (data not shown).
Samples were taken from the placental parenchyma with care to avoid the maternal decidua and areas of hemorrhage or calcification. After overnight storage in RNAlater®, the samples were stored at −80°C until later extraction. For RNA extraction, tissue samples were homogenized in TRIzol Reagent® and the RNA was extracted according to the manufacturer’s instructions with the addition of an extra precipitation step for optimized RNA quality. RNA concentration and quality were determined spectrophotometrically using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Witec, and Littau, Switzerland). Total RNA was then dissolved in sterile, deionized, diethylpyrocarbonate (DEPC) treated water and stored at −80°C. The SuperScript® III First Strand Synthesis System Supermix was used to synthesize cDNA from 3µg of RNA from each sample and a pooled internal control sample using random hexamer primers.
Real-Time PCR
Real-time quantitative PCR was carried out using the cDNA from each placental sample to determine RNA expression levels. Samples were analyzed using an Eppendorf Mastercylcer Ep Realplex 2.0 (Eppendorf North America Inc., Westbury, NY) sequence detector or an Applied Biosystems 7500 Real-Time PCR System (Applied Bio-systems, California, USA). Gene Expression Assays from Applied Biosystems (Assay number Hs00169010_m1 (SLC6A4), Hs00388669_m1 (11β-HSD2), and Hs00426573_m1 (SLC6A2)) were used. Each sample was run in triplicate for each of the three genes of interest (SLC6A4, SLC6A2, 11β-HSD2). The mean CT of each sample for each gene was determined. Values were excluded if the CT was undetectable or greater than two standard deviations from the overall mean. mRNA expression was determined relative to the multiplexed 18S housekeeping gene, which has stable expression levels in placental tissue regardless of gestational age and delivery method (Patel, Boyd, Johnston, & Williamson, 2002) and also relative to a pooled internal control sample, using the method controlling for PCR amplification efficiency described by Pfaffl (2001).
Statistical Analysis
Power analysis indicated that a sample size of 159 would be needed to detect an effect size of 0.50 with α =.05 with 80% power (Faul, Erdfelder, Lang, & Buchner, 2007). Statistical analyses were conducted using SPSS statistical software (Version 17.0; SPSS Inc, Chicago, Illinois). One-way analysis of variance (ANOVA) was conducted for each gene of interest using maternal exposure group as the independent variable and either SLC6A4, SLC6A2 or 11β-HSD2 as outcome variables. Significant main effects were further analyzed using planned comparisons, Tukey HSD and Sidak tests, where appropriate. Pearson correlations were carried out to evaluate the relationship between the dependent variables and potential confounding variables; significant (p < .05) variables were included as covariates in further analyses.
RESULTS
Maternal and Neonatal Characteristics
Placental samples (n = 164) were collected from 70 controls, 62 women with a diagnosis of depression and/or anxiety symptoms during the current pregnancy (DEP/ANX group), and 32 women who used SSRIs in at least the third trimester of the current pregnancy (SSRI group). Diagnostic classification of a depressive or anxiety disorder obtained from the medical record abstraction for the sub-study cohort (n = 32) matched the diagnostic classification based on the SCID interview at 36 weeks gestational age in 87.5% (n = 28) of the cohort. Discrepancies were noted in only three women (9.4%) diagnosed with Major Depressive Disorder in the cohort group who did not have an indication of MDD in their medical chart during review and were re-categorized based on this knowledge. In addition, one woman who described SSRI use throughout pregnancy (3.1%) in the cohort group had no indication of this medication in her medical chart and she was re-categorized based on this knowledge.
Prenatal Maternal Depression and Anxiety Symptom Severity
Thirty-one participants completed all questions of the Inventory of Depressive Symptomatology and 26 completed all questions of the Hamilton Anxiety Rating Scale. There was a significant group main effect for prenatal maternal depression (F(2,28) = 3.39, p < .05) and anxiety symptom severity scores (F(2,23) = 5.26, p < .05; Figure 1A–B). Post hoc comparisons revealed significantly higher depression scores in the SSRI group compared to controls (p < .05). Depression scores were higher in the DEP/ANX group compared to controls, but not at a significant level. Women in both the DEP/ANX (p < .05) and the SSRI group (p < .05) did not differ in their anxiety symptom scores, but exhibited significantly higher anxiety symptom scores compared to controls.
Figure 1.
Maternal depression and anxiety symptom scores in the cohort sample. (A) Mean depression score during pregnancy (averaged from 28 and 34 weeks gestational age) measured by the Inventory of Depression Symptomatology according to maternal category. (B) Mean anxiety symptom score during pregnancy (averaged from 28 and 34 weeks gestational age) measured by the Hamilton Anxiety Scale; *p < .05, compared to controls.
Maternal and neonatal characteristics for the control, DEP/ANX, and SSRI maternal exposure groups were compared. Maternal age, hypertension status, race, and ethnicity were significantly different across maternal exposure groups (Table 1). Women taking SSRIs were significantly older than controls (F(2,161) = 4.05, p < .05) and had a higher frequency of hypertension (pre-existing or pregnancy-induced hypertension or preeclampsia) (F(2,161) = 4.46, p < .05). Women taking SSRIs were also more likely to be of non-Hispanic ethnicity than women in either the control group (F(2,161) = 5.99, p < .01) or the DEP/ANX group (p < .05) and were more likely than controls(F(2,161) = 7.23, p < .01) and women in the DEP/ANX group (p < .05) to be Caucasian. The SSRI’s used by women in this study included sertraline (n=26, average daily dose = 70 milligrams (mg), range 25–150mg), fluoxetine (n=3, average dose 30mg/day), citalopram (n=1, 10mg/day), escitalopram (n=1, dose unknown), and both sertraline and citalopram (n=1, citalopram dose unknown).
Table 1.
Maternal & Neonatal Characteristics
| Control (n=70) |
Dep ± Anx (n=62) |
SSRI (n=32) |
Sig. (ANOVA or Chi Sq) |
|
|---|---|---|---|---|
| Birthweight (g) | 3402 ± 463 | 3410 ± 411 | 3403 ± 407 | p = 0.99 |
| Gestational Age (days) | 275.5 ± 12.0 | 276.4 ± 8.8 | 274.3 ± 9.4 | p = 0.62 |
| C-Section (%) | 16 (22.9) | 15 (24.2) | 5 (15.6) | p = 0.62 |
| Neonate Sex (% Male) | 35 (50.0) | 32 (51.6) | 16 (50.0) | p = 0.98 |
| Maternal Hypertension (%) | 1 (1.4) | 3 (4.8) | 5 (15.6) | p < .05* |
| Maternal Age (years) | 26.5 ± 6.7 | 27.7 ± 6.0 | 30.2 ± 5.0 | p < .05* |
| Pre-gestational BMI (kg/m2) | 25.6 ± 5.9 | 27.2 ± 6.1 | 27.2 ± 6.7 | p = 0.25 |
| Nulliparous (%) | 32 (45.7) | 28 (45.2) | 18 (56.3) | p = 0.55 |
| Private Insurance (%) | 28 (40.0) | 34 (54.8) | 20 (62.5) | p = 0.07 |
| Maternal Race (% Caucasian) | 32 (45.7) | 37 (59.7) | 27 (84.4) | p < .01** |
| Maternal Ethnicity (% Hispanic) | 24 (33.8) | 17 (27.4) | 1 (3.1) | p < .01** |
Values are mean ± SD or count (%)
SLC6A4 Gene Expression
There was a significant main effect of maternal exposure category on placental SLC6A4 gene expression (F(2,155) = 9.10; Figure 2). Post hoc analysis revealed significantly increased SLC6A4 expression in the DEP/ANX group (p < .01) and the SSRI exposed group (p < .01) compared to controls. There were no differences between the SSRI and DEP/ANX exposure groups (p = .99). Maternal ethnicity was the only demographic factor that was significantly correlated with SLC6A4 expression. Hispanic ethnicity (vs. non-Hispanic) was associated with decreased SLC6A4 expression (R2 = .028, p < .05) and was therefore analyzed as a covariate in the ANCOVA. The maternal exposure category effect remained significant after controlling for ethnicity (F(5,152) = 4.12, p < .01), therefore the unadjusted means are presented. Maternal age, pre-gestational BMI, parity, insurance status, infant sex, gestational age, delivery type (vaginal versus C-section), and birth weight were not related to SLC6A4 expression. SLC6A2 and 11β-HSD2 Gene Expression.
Figure 2.
The effect of maternal exposure category on relative placental SLC6A4 expression. Both the DEP/ANX exposed group and the SSRI exposed group exhibited significantly increased SLC6A4 mRNA expression compared to controls; F(2,155) = 9.10, partial Eta Squared = .105; **p < .01.
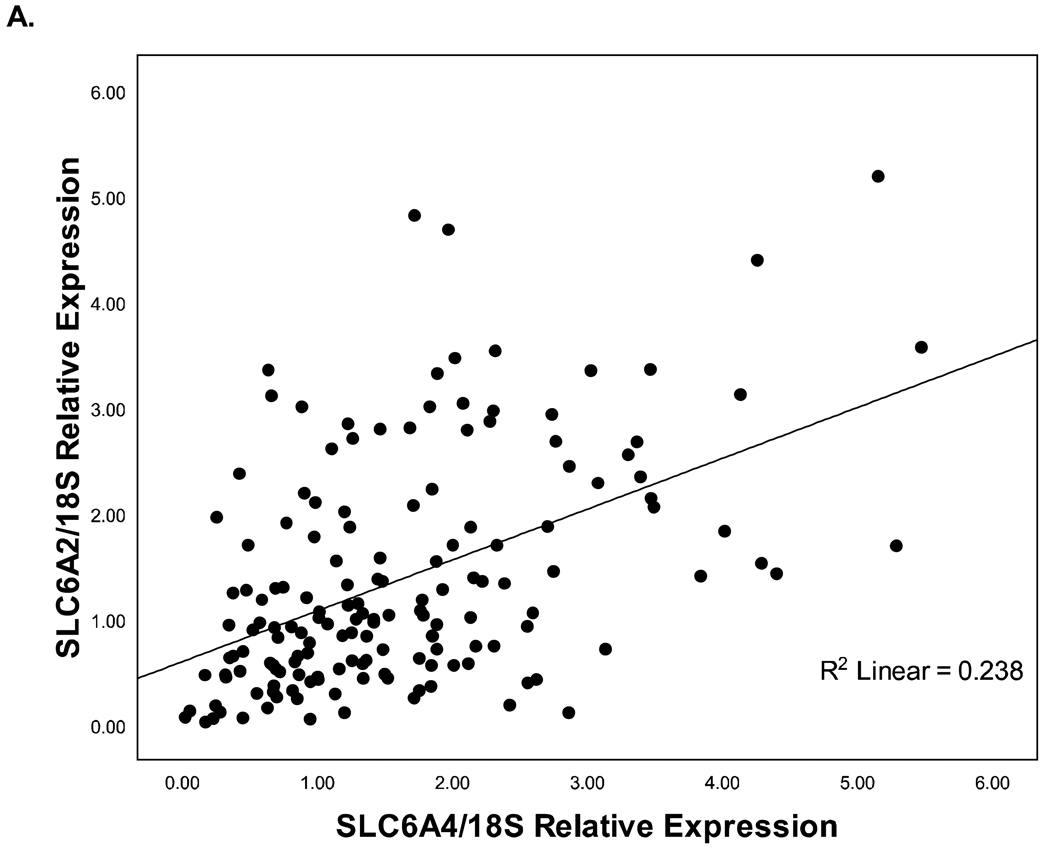
There were no significant main effects of maternal exposure category on SLC6A2 or 11β-HSD2 gene expression (Figures 3 & 4). Vaginal delivery (vs. C-section delivery) was associated with increased placental 11β-HSD2 expression (R2 = .028, p < .05). Maternal exposure category was not associated with changes in 11β-HSD2 expression when using delivery method as a covariate in the ANCOVA (F(5,150) = 1.67, p = .15). The other maternal and infant characteristics measured were not correlated with 11β-HSD2 expression or SLC6A2 expression. SLC6A4 was positively correlated with both SLC6A2 (Figure 5A; p < .001) and 11β-HSD2 (p < .01; Figure 5B) mRNA expression. SLC6A2 mRNA expression was also positively correlated with 11β-HSD2 (p < .001; Figure 5C).
Figure 3.
The effect of maternal exposure category on relative placental SLC6A2 expression; F(2,161) = 1.67, partial Eta Squared = .02, p = .191.
Figure 4.
The effect of maternal exposure category on relative placental 11β-HSD2 expression; F(2,153) = 1.53, partial Eta Squared = .02, p = .219.
Figure 5.
Inter-gene correlations. A)SLC6A4 vs. SLC6A2 (p < .001); B)SLC6A4 vs. 11β-HSD2 (p < .01); C)SLC6A2 vs. 11β-HSD2 (p < .001).
DISCUSSION
Our results demonstrate that maternal depression and/or anxiety disorders are associated with altered mRNA expression of SLC6A4 in the placenta. Women with depression and/or anxiety during pregnancy had a significant increase in placental SLC6A4 mRNA expression. Concomitant use of SSRIs did not further alter SLC6A4 expression. We did not observe significant alterations in placental SLC6A2 or 11β-HSD2 expression. The increased placental SLC6A4 expression in placenta from women with maternal mood disorders compared to controls is consistent with the previously reported association between maternal depression and decreased circulating serotonin in both mothers and newborns (Field et al., 2004). Investigations of SLC6A4 gene expression in the human placenta are necessary in order to understand the underlying developmental and behavioral changes observed in neonates with exposure to maternal depression or SSRI use. It is known that alterations in serotonin regulation through prenatal SSRI exposure has effects on both fetal (Salisbury, Ponder, Padbury, & Lester, 2009) and newborn (Moses-Kolko et al., 2005; Levinson-Castiel, Merlob, Linder, Sirota, & Klinger, 2006; Zeskind & Stephens, 2004) neurobehavior. The finding of altered SLC6A4 mRNA expression in association with maternal depression or SSRI use in the human placenta is a remarkable finding that has not been previously investigated, and has important implications for neonatal behavior. Altered mRNA expression has been associated with long term behavioral changes in previous studies, such as Hansen & Mikkelsen, in which a decrease in brain SLC6A4 mRNA expression was found in adult rats with a history of chronic SSRI exposure as neonates compared to controls, which also corresponded to greater depression-like behaviors (1998). Our studies complement those observations. The placenta serves as the interface between the maternal and fetal environments, and plays a key role in the regulation of the intrauterine environment. Altered human placental SLC6A4 expression in the setting of prenatal maternal depression/anxiety exposure suggests a biochemical mechanism for placental regulation of neurodevelopmental programming and the increased risk of depression in offspring of depressed mothers (Sullivan et al., 2000; Silberg, et al., 2010).
Alterations in placental gene expression and neurotransmitter activity due to maternal exposures have the potential to produce long-term effects on the neonate. Originally conceptualized as “Fetal Programming,” these effects are now known as the Developmental Origins of Health and Disease (Gillman, 2005; Gillman et al., 2007) and involve the neuroendocrine systems investigated in this study. Previous studies have shown that maternal stress and fetal growth restriction can have lasting effects on later health and disease including an increased risk of cardiovascular and metabolic disorders (Davis et al., 2007; Barker, 2004; Seckl, 2004). Fetal abnormalities in 11β-HSD2 activity, demonstrated in both animals with genetic disruption and/or using prenatal pharmacological inhibition of the enzyme, have been linked to “programming effects” resulting in increased anxiety in adult animals (Holmes et al., 2006; Welberg et al., 2000). Long-term studies are currently being conducted to determine if the altered SLC6A4 expression we observed could contribute to altered offspring outcomes. The finding that SSRI exposure was not associated with additional alteration in placental gene expression beyond the effects associated with maternal mood disorder is reassuring from a fetal programming standpoint.
In contrast to our findings, studies of SLC6A4 regulation in the brain show that chronic SSRI administration is typically associated with decreased SLC6A4 protein and variable mRNA expression, although the use of normal animal models in these studies could also contribute to the differences observed (Benmansour et al., 1999; Lesch et al., 1993; Benmansour, Owens, Cecchi, Morilak, & Frazer, 2002; Abumaria et al., 2007; Mirza, Nielsen, & Troelsen, 2007; Kugaya et al., 2003). The finding of increased placental SLC6A4 mRNA expression with exposure to maternal mood disorders and SSRI treatment compared to controls suggests SLC6A4 is differentially regulated in the placenta versus the brain. Previous studies have shown that SSRI regulation of SLC6A4 is tissue-dependent. There are variable effects in platelets (Little, Zhang, & Cook, 2006), peripheral blood lymphocytes (Lima & Urbina, 2002), kidney cell lines (Horschitz, Hummerich, & Schloss, 2001), and brain (Benmansour et al., 1999; Lesch et al., 1993; Benmansour et al., 2002; Abumaria et al., 2007). The differences observed between placental and brain SLC6A4 regulation could reflect temporal changes in regulation, as evidenced by one animal study demonstrating increased SLC6A4 mRNA expression in the brains of neonate rats in response to chronic SSRI exposure but significantly decreased SLC6A4 mRNA expression and depression like behaviors in the adult rats with a history of chronic SSRI exposure as neonates compared to controls (Hansen & Mikkelsen, 1998). This suggests altered long term SSRI regulatory effects on SLC6A4 after discontinuation, though the long term consequences of prenatal SSRI exposure on risk for future mood disorders remain unclear. The aforementioned findings support the hypothesis that SSRI exposure in neonates could increase serotonin levels acutely, but result in altered serotonin system development and overall activity later in life, increasing risk for depression (Oberlander, Gingrich, & Ansorge, 2009). An alternate hypothesis is that SSRI exposure and increased serotonin levels in neonates could counteract the deleterious effects of low serotonin levels seen in depression (Oberlander et al., 2009). This idea of a “buffering” effect of SSRI exposure on preventing or normalizing HPA axis dysregulation is supported by human and animal studies (Oberlander et al., 2008a; Ishiwata, Shiga, & Okado, 2005; Barden, Reul, & Holsboer, 1995). The consequences of prenatal SSRI exposure on risk for future mood disorders in humans should be explored further in long-term studies.
Maternal mood and SSRI use had less of an effect on placental SLC6A2. Expression was not downregulated as with other forms of intrauterine stress. We and others have demonstrated decreased placental SLC6A2 expression in association with adverse pregnancies due to drug exposure, fetal growth restriction and maternal hypertensive disorders (Bzoskie et al., 1997; Bottalico et al., 2004). This finding does not obviate a fetal origin or programming explanation for the dysregulated SLC6A2 expression seen in adults with depression (Klimek et al., 1997). The significant alteration of SLC6A4 but not SLC6A2 expression with exposure to maternal mood disorders reflects the complex and still unclear etiology of depression, which appears to go beyond a simple alteration in one or two monoamine levels. This complexity is illustrated in studies where elevated serotonin levels are necessary to sustain the antidepressant effects of an SSRI (Miller et al., 1996; Salomon, Miller, Delgado, & Charney, 1993), but where serotonin deficiency alone does not cause depression symptoms in previously healthy subjects (Miller et al., 1996; Zhao, Zhang, Bootzin, Millan, & O'Donnell, 2008). Current theories suggest that neurotransmitter dysregulation may not be the primary cause of depression, but may rather be a marker of disruptions, such as impaired synaptic plasticity or neurogenesis, elsewhere in the brain (Ressler & Nemeroff, 2000; Duman, Malberg, & Thome, 1999; Warner-Schmidt & Duman, 2006; Holderbach, Clark, Moreau, Bischofberger, & Normann, 2007). We did not observe changes in SLC6A2 expression with SSRI exposure, supporting findings of a lack of functional association between SLC6A2 and SSRI treatment (Benmansour et al., 1999; Horschitz et al., 2001; Benmansour et al., 2004). Future studies should nonetheless investigate effects of the SNRI class of antidepressants on placental SLC6A2 expression.
We observed a modest elevation in 11β-HSD2 mRNA expression in DEP/ANX and SSRI exposed placental samples compared to controls, but not at the level of significance. Increased 11β-HSD2 with SSRI exposure was expected given that prenatal SSRI exposure has regulatory effects on infant cortisol reactivity (Oberlander et al., 2008a) and that 11β-HSD2 is upregulated in placental cells in response to cortisol (van Beek et al., 2004). The enzyme 11β-HSD2 converts cortisol to its inactive form, creating a barrier to passage of maternal cortisol to the fetus. Elevated placental 11β-HSD2 in the adverse maternal exposure groups could represent a protective response to increased maternal cortisol and stress, as seen with depression.
The genes of interest in this study likely share regulatory mechanisms. The positive correlation between SLC6A2 and 11β-HSD2 is consistent with previous studies revealing inhibition of both SLC6A2 (Bzoskie et al., 1997) and 11β-HSD2 (Sarkar et al., 2001) placental mRNA expression by increased circulating catecholamines, especially norepinephrine. The positive correlation between SLC6A4 and 11β-HSD2 expression is the first report of a serotonin-glucocorticoid relationship in placental tissue in vivo. This is consistent with previous studies’ demonstration of glucocorticoid stimulation of SLC6A4 expression in blood and B-lymphoblastoid cells and stimulation of serotonin synthesis in the central nervous system (Glatz, Mossner, Heils, & Lesch, 2003; Tafet, Toister-Achituv, & Shinitzky, 2001). Conversely, serotonin stimulates 11β-HSD2 activity in renal cell lines (Zallocchi, Damasco, Calvo, Lantos, & Matkovic, 2006).
The limitation of chart review information to verify duration of SSRI use and patient compliance is a recognized shortcoming. We endeavored to include only women with SSRI use in at least the third trimester. Women with early SSRI use who ceased treatment at the beginning of pregnancy were not included in the SSRI exposure group; however, it is possible that this early SSRI exposure could have long term effects on placental gene expression. Given estimates of undiagnosed depression (Andersson et al., 2003), it is possible that control participants may have also had anxiety or depression symptoms. We attempted to control for potential long-term effects of depression or undiagnosed depression in the control group by excluding women with a history of depression or anxiety who were not currently experiencing symptoms or who were not taking antidepressants. In this study, categorization based on chart review was accurate (87.5%) compared to categorization based on structured interviews in the smaller cohort of 32 consented participants, providing confidence in our method for determining maternal exposure category.
This study illustrates the inherent difficulties in isolating mood disorder effects from SSRI effects. Women taking SSRIs may still be experiencing depression/anxiety symptoms, as indicated by symptom measures of the substudy participants. Nonetheless, third-trimester maternal mood, independent of SSRI exposure, is related to increased infant hypothalamic-pituitary-adrenal reactivity (Oberlander et al., 2008b). Interestingly this effect is mediated by increased methylation of the human glucocorticoid receptor gene (Oberlander et al., 2008b), an example of epigenetic regulation.
Epigenetic changes, including DNA methylation, are an important potential mechanism for fetal programming which could further modulate the alterations in mRNA level we observed and may explain why SLC6A2 and 11β-HSD2 expression were not significantly affected by maternal mood. Although we did not examine SLC6A4 protein levels, SLC6A4 protein and mRNA expression are highly correlated in the placenta (Ramamoorthy et al., 1993; Ramamoorthy et al., 1995b).
Our future studies will determine if the SLC6A4 expression changes we observed could be modulated by gene-environment interactions (Alexander et al., 2009; Caspi, Sugden, & Moffitt, 2003). Neonatal sex (Ren-Patterson et al., 2006; Murphy et al., 2003; Mueller & Bale, 2008) and SLC6A4 genotype (Little et al., 2006; Greenberg et al., 1999; Lesch et al., 1996; Wendlend, Martin, Kruse, Lesch, & Murphy, 2006) are associated with altered expression of other placental and non-placental genes. SLC6A4 genotype did not affect the significant associations between SLC6A4 brain expression, cortisol reactivity, and mood in a previous investigation (Reimold et al., 2010). Our finding that ethnicity was significantly associated with SLC6A4 expression level is consistent with findings of population stratification of a SLC6A4 polymorphism across ethnic groups (Gerlernter, Cubells, Kidd, Pakstis, & Kidd, 1999), although maternal mood and SSRI use still exhibited significant effects after controlling for ethnicity in our study.
CONCLUSION
Our observations suggest that a possible mechanism for the effects of maternal mood disorders on fetal/neonatal neurodevelopmental programming is via altered placental serotonin transporter expression. Future studies are needed to determine if changes in protein expression or methylation patterns also exist. Nonetheless, the identification of altered SLC6A4 mRNA expression in the human placenta in the setting of maternal depression and anxiety or SSRI exposure is a remarkable finding that has not been previously investigated and illustrates the need for further exploration of placental gene regulation. Given the known adverse effects of stress, anxiety, and depression on fetal outcomes (Davis et al., 2007; Lundy et al., 1999; Monk et al., 2004; Coe & Lubach, 2005; de Weerth et al., 2003; Field et al., 2010; Salisbury et al., 2007; Burt & Quezada, 2009; Marcus, 2009) and that nearly 70% of women who cease antidepressants during pregnancy will relapse (Cohen et al., 2006), the risk-benefit analysis of not treating maternal depression versus administration of antidepressant medication during pregnancy creates a dilemma for patients and clinicians. Our study demonstrated alterations of placental SLC6A4 in the setting of maternal mood disorders. Importantly, there was no apparent additional effect of concomitant SSRI use. Long-term follow up studies are needed to determine how differential gene expression in this setting affects offspring outcomes. The continued effort to determine safety or potential benefits of SSRI use during pregnancy will help to achieve optimal neonatal outcomes.
Acknowledgements
This project was supported in part by the Braufman Fund at Brown University and grants from the NIH R01 MH078033 and NCRR P20 RR018728.
Glossary
- DEP/ANX
Depression and/or anxiety
- HPA
Hypothalamic pituitary adrenocortical
- SLC6A2
Norepinephrine transporter (gene)
- SLC6A4
Serotonin transporter (gene)
- SSRI
Selective Serotonin Reuptake Inhibitor
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2 (gene)
Footnotes
Financial Disclosures: None
Contributor Information
Kathryn L. Ponder, Email: KPonder@gmail.com.
Amy Salisbury, Email: ASalisbury@wihri.org.
Bethany McGonnigal, Email: bethanymcg@comcast.net.
Alyse Laliberte, Email: aml2085@gmail.com.
Barry Lester, Email: BLester@wihri.org.
REFERENCES
- Abumaria N, Rygula R, Hiemke C, Fuchs E, Havemann-Reinecke U, Rüther E, et al. Effect of chronic citalopram on serotonin-related and stress-regulated genes in the dorsal raphe nucleus of the rat. European Neuropsychopharmacology. 2007;17(6–7):417–429. doi: 10.1016/j.euroneuro.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: Implications for the etiology of depression. Psychoneuroendocrinology. 2009;34(9):1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Andersson L, Sundström-Poromaa I, Bixo M, Wulff M, Bondestam K, Åström M. Point prevalence of psychiatric disorders during the second trimester of pregnancy: a population-based study. Am J Obstet Gynecol. 2003;189(1):148–154. doi: 10.1067/mob.2003.336. [DOI] [PubMed] [Google Scholar]
- Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1–194.e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic–pituitary–adrenocortical system? Trends Neurosci. 1995;18:6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]
- Barker DJP. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Altamirano AV, Jones DJ, Sanchez TA, Gould GG, Pardon MC, et al. Regulation of the norepinephrine transporter by chronic administration of antidepressants. Biol Psychiatry. 2004;55:313–316. doi: 10.1016/s0006-3223(03)00676-0. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999;19(23):10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmansour S, Owens WA, Cecchi M, Morilak DA, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. American College of Obstetrics and Gynecologists. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Bottalico B, Larsson I, Brodszki J, Hernandez-Andrade E, Casslén B, Marsál K, et al. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004;25(6):518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Burt VK, Quezada V. Mood disorders in women: focus on reproductive psychiatry in the 21st century – Motherisk Update 2008. Can J Clin Pharmacol. 2009;16(1):e6–e14. [PubMed] [Google Scholar]
- Bzoskie L, Yen J, Tseng YT, Blount L, Kashiwai K, Padbury JF. Human placental norepinephrine transporter mRNA: expression and correlation with fetal condition at birth. Placenta. 1997;18:205–210. doi: 10.1016/s0143-4004(97)90094-1. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE. Influence of life stress on depression: moderation by a polymorphism in the serotoninT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, et al. Selective serotonin reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Prenatal origins of individual variation in behavior and immunity. Neuroscience & Biobehavioral Reviews. 2005;29(1):39–49. doi: 10.1016/j.neubiorev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74(2):139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Figueiredo B, Deeds O, Ascencio A, et al. Comorbid depression and anxiety effects on pregnancy and neonatal outcome. Infant Behavior and Development. 2010;33(1):23–29. doi: 10.1016/j.infbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, et al. Prenatal maternal biochemistry predicts neonatal biochemistry. International Journal of Neuroscience. 2004;114(8):933–945. doi: 10.1080/00207450490461305. [DOI] [PubMed] [Google Scholar]
- Gerlernter J, Cubells JF, Kidd JR, Pakstisx AJ, Kidd KK. Population studies of the polymorphisms of the serotonin transporter gene. American Journal of Medical Genetics. 1999;88:61–66. [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosomatic Medicine. 2005;67:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353(17):1802–1809. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, et al. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD) Pediatr Res. 2007;61(5):625–629. doi: 10.1203/pdr.0b013e3180459fcd. [DOI] [PubMed] [Google Scholar]
- Glatz K, Mossner R, Heils A, Lesch KP. Glucocorticoid-regulated human serotonin transporter (serotoninT) expression is modulated by the serotoninT gene-promotorlinked polymorphic region. J Neurochem. 2003;86:1072–1078. doi: 10.1046/j.1471-4159.2003.01944.x. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among serotoninTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BD, Tolliver TJ, Huang SJ, Li Q, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. American Journal of Medical Genetics. 1999;88:83–87. [PubMed] [Google Scholar]
- Hansen HH, Mikkelsen JD. Long-term effects on serotonin transporter mRNA expression of chronic neonatal exposure to a serotonin reuptake inhibitor. European Journal of Pharmacology. 1998;352(2–3):307–315. doi: 10.1016/s0014-2999(98)00349-5. [DOI] [PubMed] [Google Scholar]
- Holderbach R, Clark K, Moreau J-L, Bischofberger J, Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiatry. 2007;62:92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Abrahamsen CT, French KL, Paterson JM, Mullins JJ, Seckl JR. The mother or the fetus? 11beta-hydroxysteroid dehydrogenase type 2 null mice provide evidence for direct fetal programming of behavior by endogenous glucocorticoids. J Neurosci. 2006;26(14):3840–3844. doi: 10.1523/JNEUROSCI.4464-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horschitz S, Hummerich R, Schloss P. Structure, function and regulation of the 5-hydroxytryptamine (serotonin) transporter. Biochem Soc Trans. 2001;29:728–732. doi: 10.1042/0300-5127:0290728. [DOI] [PubMed] [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Kalra S, Born L, Sarkar M, Einarson A. The safety of antidepressant use in pregnancy. Expert Opin Drug Saf. 2005;4(2):273–284. doi: 10.1517/14740338.4.2.273. [DOI] [PubMed] [Google Scholar]
- Kim J, Riggs KW, Misri S, Kent N, Oberlander TF, Grunau RE, et al. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol. 2006;61:155–163. doi: 10.1111/j.1365-2125.2005.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler K, Lau T, Schloss P. Antagonists and substrates differentially regulate serotonin transporter cell surface expression in serotonergic neurons. Eur J Pharmacol. 2010;629(1–3):63–67. doi: 10.1016/j.ejphar.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Seneca NM, Snyder PJ, Williams SA, Malison RT, Baldwin RM, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology. 2003;28(2):413–420. doi: 10.1038/sj.npp.1300036. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Aulakh CS, Wolozin BL, Tolliver TJ, Hill JL, Murphy DL. Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Molecular Brain Research. 1993;17:31–35. doi: 10.1016/0169-328x(93)90069-2. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160(2):173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- Lima L, Urbina M. Serotonin transporter modulation in blood lymphocytes from patients with major depression. Cell Mol Neurobiol. 2002;22(5–6):797–804. doi: 10.1023/A:1021869310702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Zhang L, Cook E. Fluoxetine-induced alterations in human platelet serotonin transporter expression: serotonin transporter polymorphism effects. J Psychiatry Neurosci. 2006;31:333–339. [PMC free article] [PubMed] [Google Scholar]
- Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- Lundy BL, Jones NA, Field T, Nearing G, Davalos M, Pietro PA, et al. Prenatal depression effects on neonates. Infant Behavior and Development. 1999;22(1):119–129. [Google Scholar]
- Marcus SM. Depression during pregnancy: rates, risks and consequences. Can J Clin Pharmacol. 2009;16(1):e15–e22. [PubMed] [Google Scholar]
- McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, et al. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86(10):4979–4983. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, Heninger GR, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch Gen Psychiatry. 1996;53:117–128. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Nielsen EØ, Troelsen KB. Serotonin transporter density and anxiolytic-like effects of antidepressants in mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(4):858–866. doi: 10.1016/j.pnpbp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, et al. Fetal heart rate reactivity differs by women's psychiatric status: an early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43(3):283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. 2005;293(19):2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, et al. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86(6):672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, et al. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008a;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008b;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Patel P, Boyd CA, Johnston DG, Williamson C. Analysis of GAPDH as a standard for gene expression quantification in human placenta. Placenta. 2002;23:697–698. doi: 10.1053/plac.2002.0859. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nuc Acid Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PD, Leibach FH, Mahesh VB, Ganapathy V. Human placenta as a target organ for cocaine action: interaction of cocaine with the placental serotonin transporter. Placenta. 1994;15(3):267–278. doi: 10.1016/0143-4004(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;4:571–575. doi: 10.1016/j.reprotox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy JD, Ramamoorthy S, Leibach FH, Ganapathy V. Human placental monoamine transporters as targets for amphetamines. Am J Obstet Gynecol. 1995a;173(6):1782–1787. doi: 10.1016/0002-9378(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy JD, Ramamoorthy S, Papapetropoulous A, Catravas JD, Leibach FH, Ganapathy V. Cyclic AMP-independent up-regulation of the human serotonin transporter by staurosporine in choriocarcinoma cells. Journal of Biological Chemistry. 1995b;270:17189–17195. doi: 10.1074/jbc.270.29.17189. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Cool DR, Mahesh VB, Leibach FH, Melikian HE, Blakely RD, et al. Regulation of the human serotonin transporter. Cholera toxin-induced stimulation of serotonin uptake in human placental choriocarcinoma cells is accompanied by increased serotonin transporter mRNA levels and serotonin transporter-specific ligand binding. J Biol Chem. 1993;268(29):21626–21631. [PubMed] [Google Scholar]
- Reimold M, Knobel A, Rapp MA, Batra A, Wiedemann K, Ströhle A, et al. Central serotonin transporter levels are associated with stress hormone response and anxiety. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1903-y. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Lesch KP, Lu B, Murphy DL. Sex dependent modulation of brain monoamines and anxiety like behaviors in mice with genetic serotonin transporter and BDNF deficiencies. Cellular and Molecular Neurobiology. 2006;26:755–780. doi: 10.1007/s10571-006-9048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12 Supp.1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Salisbury AL, Lester BM, Seifer R, Lagasse L, Bauer CR, Shankaran S. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29(3):331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clinics in Perinatology. 2009;36(3):595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RM, Miller HL, Delgado PL, Charney D. The use of tryptophan depletion to evaluate central serotonin function in depression and other neuropsychiatric disorders. Int Clin Psychopharmacol. 1993;8 Suppl. 2:41–46. doi: 10.1097/00004850-199311002-00006. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Tsai SW, Nguyen TT, Plevyak M, Padbury JF, Rubin LP. Inhibition of placental 11beta-hydroxysteroid dehydrogenase type 2 by catecholamines via alpha-adrenergic signaling. Am J Regulatory Integrative Comp Physiol. 2001;281:R1966–R1974. doi: 10.1152/ajpregu.2001.281.6.R1966. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151 Suppl 3:U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Maes H, Eaves LJ. Genetic and environmental influences on the transmission of parental depression to children's depression and conduct disturbance: an extended Children of Twins study. J Child Psychol Psychiatry. 2010;51(6):734–744. doi: 10.1111/j.1469-7610.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strug LJ, Suresh R, Fyer AJ, Talati A, Adams PB, Li W, et al. Panic disorder is associated with the serotonin transporter gene (SLC6A4) but not the promoter region (5-HTTLPR) Mol Psychiatry. 2010;15(2):166–176. doi: 10.1038/mp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Toister-Achituv M, Shinitzky M. Enhancement of serotonin uptake by cortisol: A possible link between stress and depression. Cognitive, Affective, and Behavioral Neuroscience. 2001;1(1):96–104. doi: 10.3758/cabn.1.1.96. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- Vaccarino AL, Evans KR, Sills TL, Kalali AH. Symptoms of anxiety in depression: assessment of item performance of the Hamilton Anxiety Rating Scale in patients with depression. Depress Anxiety. 2008;25(12):1006–1013. doi: 10.1002/da.20435. [DOI] [PubMed] [Google Scholar]
- van Beek JP, Guan H, Julan L, Yang K. Glucocorticoids stimulate the expression of 11beta-hydroxysteroid dehydrogenase type 2 in cultured human placental trophoblast cells. J Clin End Met. 2004;89:5614–5621. doi: 10.1210/jc.2004-0113. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12(3):1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Wendlend JR, Martin BJ, Kruse MR, Lesch K-P, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of serotoninTLPR and rs25531. Molecular Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch Gen Psychiatry. 1992;49(8):630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Zallocchi ML, Damasco MC, Calvo JC, Lantos CP, Matkovic LB. MDCK cells express serotonin-regulable 11beta-hydroxysteroid dehydrogenase type 2. Biocell. 2006;30:469–477. [PubMed] [Google Scholar]
- Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Obstet Gynecol Surv. 2004;59(8):564–566. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Zhang H-T, Bootzin E, Millan MJ, O'Donnell JM. Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs. Neuropsychopharmacology. 2008;34(6):1467–1481. doi: 10.1038/npp.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]