Abstract
Cardiovascular disease in children is common and results in significant morbidity and mortality. The sickest children with cardiovascular disease may require support with extracorporeal membrane oxygenation (ECMO) which provides life-saving assistance for children with refractory cardiorespiratory failure. Many classes of cardiovascular drugs are used in children, but very few of these agents have been well studied in children. The knowledge gap is even more pronounced in children supported with ECMO. Pharmacokinetic (PK) data collected to date (primarily from antibiotics and sedatives) suggest that the ECMO circuit has the potential to significantly alter the pharmacokinetics of drugs including changes in clearance and volume of distribution. Of all cardiovascular drugs administered to children supported by ECMO, only 11 have been partially studied and reported in the medical literature. Esmolol, amiodarone, nesiritide, bumetanide, sildenafil, and prostaglandin E1 appear to require dosing modifications in children supported by ECMO, while it appears that hydralazine, nicardipine, furosemide, epinephrine, and dopamine can be dosed similarly to children not supported by ECMO. However, trials evaluating the PK of these drugs in patients supported by ECMO are extremely limited (i.e. case reports) and therefore definitive dosing recommendations are not plausible. Research efforts should focus on evaluating the PK of drugs in patients on ECMO in order to avoid therapeutic failures or unnecessary toxicities.
Keywords: cardiovascular agents, extracorporeal membrane oxygenation, pharmacology, pharmacokinetics
Introduction
Cardiovascular disease in children is common and results in significant morbidity and mortality. The most common cardiovascular conditions presenting in childhood are congenital heart disease (CHD), hypertension, and arrhythmias.1 In the U.S., it is estimated that 9 children per 1000 live births have some form of CHD and 2.3 per 1000 will either die or require invasive intervention within the first year of life.2 In children undergoing surgical repair for CHD, mortality is estimated at 4.8%, although this varies significantly by the type cardiac lesion3; atrial septum defects have the lowest attributed mortality (0.4%) whereas 26–36% of infants with hypoplastic left heart syndrome die or need a heart transplantation by 1 year of age.3,4
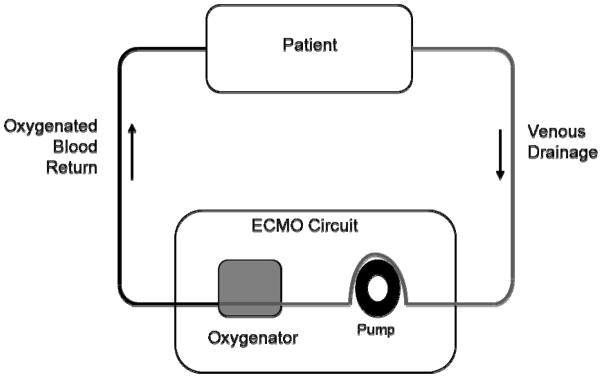
The sickest children with cardiovascular disease may require support with extracorporeal membrane oxygenation (ECMO) which provides life-saving assistance for children with refractory cardiorespiratory failure. ECMO is a form of cardiopulmonary bypass that has been adapted from the operating room to be used for prolonged periods of time in the intensive care unit (ICU). Pulmonary gas exchange and circulatory support are accomplished through the ECMO circuit which includes a pump, an oxygenator, tubing, and a heater. Mechanically, blood is drained from the venous system, pumped through the oxygenator where oxygen is added and carbon dioxide removed, and then, depending on the configuration of the circuit, returned to either the venous or arterial circulation (see figure 1). ECMO has been used for over three decades5 and has shown a survival benefit in both children and adults.6,7 Veno-arterial ECMO has been used extensively for pediatric cardiovascular disease either for failure to wean from cardiopulmonary bypass following cardiac surgery or as salvage from hemodynamic instability, with survival dependent on age and disease state (Table 1). CHD accounts for the majority of ECMO runs (73.6%) but has some of the lowest survival rates. The highest survival rates are observed among neonates with cardiomyopathy and infants and children with myocarditis. Therapeutic classes of cardiovascular drugs used in children with cardiovascular disease include inotropes and chronotropes, vasoconstrictors, vasodilators, and antiarrhythmic agents. These drugs work by affecting cardiac output through the interaction of preload, afterload, contractility, and heart rate. Unfortunately, very few of these agents have been well studied in children and medical providers are frequently forced to extrapolate dosing and efficacy data from that obtained in adults.8,9 The knowledge gap is even more pronounced in children supported with ECMO who are frequently treated with cardiovascular agents. Most studies evaluating the pharmacokinetic (PK) data of drugs in patients supported with ECMO are limited to small PK trials and case reports with most of the emphasis on antimicrobials and sedatives.10
Figure 1.
ECMO Circuit
Table 1. Cumulative ECMO runs for cardiovascular disease (1990–July, 2010)73.
| Diagnosis | Neonatal (0–30d) | Infant (30d–<1yr) | Pediatric (1yr–<16yr) | |||
|---|---|---|---|---|---|---|
| Total runs | Survival % | Total runs | Survival % | Total runs | Survival % | |
| Congenital defect | 3742 | 37 | 2159 | 44 | 1089 | 44 |
| Cardiomyopathy | 105 | 62 | 135 | 56 | 368 | 59 |
| Cardiac arrest | 61 | 23 | 68 | 46 | 87 | 40 |
| Cardiogenic shock | 57 | 39 | 33 | 36 | 71 | 46 |
| Myocarditis | 51 | 49 | 65 | 72 | 179 | 65 |
| Other cardiac failure | 351 | 42 | 340 | 46 | 530 | 51 |
PK data collected to date suggest that the ECMO circuit has the potential to significantly alter the PK of drugs including changes in clearance (CL) and volume of distribution (Vd). Because children supported with ECMO require a large volume of exogenous blood to prime the ECMO circuit as well as blood transfusions needed to maintain stable hemoglobin levels,10 it is assumed that the Vd of most drugs in this population is increased. This has been demonstrated with drugs such as gentamicin and vancomycin.11–18 In addition, the ECMO circuit itself also has the potential to increase Vd through the adsorption of drug as demonstrated by multiple in vitro and ex vivo studies.19–23 Investigators have shown that drugs with high lipophilicity (i.e. fentanyl and midazolam) are highly adsorbed by the circuit, presumably due to easier binding by the polyvinylchloride tubing and oxygenator materials.22,24,25
Changes in drug CL in patients supported by ECMO have also been observed. Studies of gentamicin,10–15 vancomycin,16,18,26 and bumetanide27 have all shown decreased CL in children supported with ECMO.. Decreased CL for renally excreted drugs is expected in children on ECMO as renal insufficiency is common in these patients.10 It is reasonable to assume in this critically ill population, often with multi-organ failure, that the CL of metabolized drugs might also be affected, but to our knowledge this has not been reported in the literature.
The purpose of this review is to describe the available evidence for the dosing of cardiovascular drugs in children with a specific focus in children supported with ECMO. Using the U.S. National Library of Medicine’s PubMed web search, we conducted a search using the following search terms: 1) extracorporeal membrane oxygenation AND ((pharmacology) OR (pharmacokinetics)), 2) extracorporeal membrane oxygenation AND cardiovascular agents, and 3) name of drug for each cardiovascular agent used in children8 AND extracorporeal with no limitations on age or time period. In addition we searched the abstracts for the previous five years (2006–2010) from the Pediatric Academic Society meeting. Finally, we accessed two theses published on the Rotterdam group’s experience with ECMO PK.23,28 For the purposes of this review, we will focus on the dosing of medications with direct cardiovascular effects.
Pediatric Drug Dosing in Critically Ill Children
The most commonly used cardiovascular drug classes in children include inotropes, vasoconstrictors, vasodilators, diuretics, and antiarrhythmics.8 Of these approximately 50 medications, only 11 have been reported or studied in children on ECMO support (Table 2), and the most extensively studied are furosemide, prostaglandin E1, and sildenafil. In addition, almost 40 cardiovascular medications commonly used in the pediatric ICU have no data to guide dosing on ECMO. Many of these medications have a narrow therapeutic index (e.g. most antiarrhythmics), which increases the risk of therapeutic failure or toxicity. This paper will review drugs for which literature exists in pediatric ECMO.
Table 2. Dosing of Cardiovascular Drugs on ECMO.
Unless otherwise cited, all dosing obtained from LexiComp Pediatric Dosage Handbook47
| Drug | Route | Routine Dose | Dose on ECMO | FDA Label in Children |
|---|---|---|---|---|
| Antiarrhythmics | ||||
|
| ||||
| Amiodarone | IV | Load 5mg/kg 5–15mcg/kg/min |
Multiple 5mg/kg loads 10–20mcg/kg/min40 |
N |
| PO | Load 5–15mg/kg 5mg/kg/day |
|||
|
| ||||
| Esmolol | IV | Load 100–500mcg/kg 50–1000mcg/kg/min |
Load 700mcg/kg/min 50–700mcg/kg/min31 |
N |
|
| ||||
| Vasodilators | ||||
|
| ||||
| Hydralazine | IV | 0.1–0.2mg/kg/dose q4-6h up to 1.7–3.5mg/kg/day div q4-6h) | 0.15mg/kg41 | Y |
| PO | Initial 0.75–1mg/kg/day div q12-24h (max 25mg/dose) Titrate over 3–4wks to max 5mg/kg/d in infants, 7.5mg/kg/day in children, max 200mg/day |
|||
|
| ||||
| Nesiritide | IV | Load 1–2mcg/kg48–52 0.005–0.05 mcg/kg/min48–52 |
0.01–0.09mcg/kg/min54 | N |
|
| ||||
| Nicardipine | IV | Infants: 0.5–2 mcg/kg/min43,44,57 Children: 0.5–5mcg/kg/min55,56 |
0.5–5mcg/kg/min43,58 | N |
|
| ||||
| Diuretics | ||||
|
| ||||
| Bumetanide | IV | Neonates: 0.01–0.05 mg/kg/dose q24-48h Infants and children: 0.015–0.1mg/kg/dose q6-24h (max 10mg/day)66 |
0.1mg/kg was inadequate28 | N |
|
| ||||
| Furosemide | IV | Neonates: 1–2mg/kg/dose q12-24h Infants and children: 1–2mg/kg/dose q6-12h Infusion 0.05–0.4 mg/kg/hr |
Load 1mg/kg Infusion 0.2mg/kg/hr65 |
Y |
| PO | Neonates: 1–4mg/kg/dose q12-24h Infants and children: 1–6mg/kg/dose q6-24h |
|||
|
| ||||
| Inotropes and Vasopressors | ||||
|
| ||||
| Dopamine | IV | Neonates 1–20mcg/kg/min Infants and children 1–20mcg/kg/min (max 50mcg/kg/min) |
No change21 | N |
|
| ||||
| Epinephrine | IV | Infants and children: 0.01–1mcg/kg/min | No change21 | Y |
|
| ||||
| Pulmonary medications | ||||
|
| ||||
| Sildenafil | PO | Neonates: 0.25–1mg/kg q6-12h74 Infants and children: 0.25–0.5mg/kg/dose q4-8h (max 2mg/kg/dose75–77) |
5–7mg/kg/day; decreased to 3–5mg/kg/day after decannulation28 | N |
|
| ||||
| Miscellaneous | ||||
|
| ||||
| Prostaglandin E1 | IV | Neonates: 0.05–0.1mcg/kg/min (max 0.4mcg/kg/min) | 0.8mcg/kg/min68 | Y |
IV –intravenous
PO – oral
Anti-arrhythmic Agents
Arrhythmias are common both as a primary diagnosis and as a complication of cardiovascular disease in children, especially in the post-operative period. However, experience with this drug class in children on ECMO has been reported for only two antiarrhythmic drugs.
Esmolol
Esmolol is an extremely short acting β1-selective β-blocker administered via continuous intravenous infusion for the control of hypertension and certain ventricular and supraventricular arrhythmias. It does not have FDA labeling for use in children. Recommended dosing in infants includes a loading dose of 500mcg/kg followed by an infusion of 25–200mcg/kg/min that can be titrated to effect.29 The maximum reported dose was 1000 mcg/kg/min which was well tolerated in children aged 2–16 years.30 There is one case report of esmolol use in a newborn infant with hypertrophic cardiomyopathy on venoarterial ECMO.31 The patient was loaded with 700 mcg/kg and an infusion started at 50 mcg/kg/min and titrated up to a maximum of 700 mcg/kg/min. No adverse events were noted, and the infant was able to be decannulated from ECMO 24h after initiation of the esmolol infusion. However, no information regarding pharmacologic effect was provided and no PK samples were collected.
Amiodarone
Amiodarone is a Class III antiarrhythmic commonly used for both ventricular and supraventricular tachycardias including junctional ectopic tachycardia (JET). Amiodarone is one of the few effective treatments for JET, however, it has the added side effect of myocardial depression which is problematic for children in the immediate post-operative period. Like esmolol, it does not carry FDA labeling for use in children. Amiodarone dosing for JET includes an intravenous loading dose of 5mg/kg followed by an intravenous infusion of 5–15mcg/kg/min32,33 aiming for a serum concentration of 0.8–2.8 mg/L.34–37 However, infusion rates as high as 25mcg/kg/min have been tolerated in infants in the post-operative period and those with incessant tachycardia.38,39
The data on amiodarone dosing while on ECMO are limited to a single case report of a 4 day old, full term infant who developed JET following tetralogy of Fallot repair.40 The patient was loaded with amiodarone 2.5mg/kg and started on an infusion of 10mcg/kg/min in the immediate post-operative period but required ECMO 5 hours post-operatively for persistent JET-associated hypotension. The patient remained in a JET rhythm for 5 days, ultimately requiring two additional boluses of amiodarone and an increase in the infusion rate to 20mcg/kg/min. Amiodarone concentrations measured during this period were within the therapeutic range (0.9mg/L and 2mg/L on days 2 and 4, respectively). In this case, higher amiodarone doses were required to reach therapeutic concentrations in a patient on ECMO. This could be due to amiodarone’s high lipophilicity and possible adherence to the ECMO circuit.
Vasodilators
Vasodilators are used frequently in children in the ICU for afterload reduction in the context of hypertension and decreased cardiac output related to increased systemic vascular resistance.41–45
Hydralazine
Hydralazine is a peripheral vasodilator with preference for arterioles that is thought to work by blocking calcium movement within smooth muscles.46 Hydralazine is labeled by the FDA for use in children with essential hypertension. Recommended dosing in the pediatric ICU is 0.1–0.6 mg/kg intravenously every 4 hours with a maximum dose of 1.7–3.5 mg/kg/day.47 In a study describing the impact of hydralazine on cardiac performance in infants supported by ECMO (n=23), infants treated with 0.15mg/kg did not show improvement in shortening fraction, cardiac output or cerebral blood flow index.41 Although there was a 9 mmHg decrease in mean arterial pressure after hydralazine dosing in infants who were initially hypertensive, the complex interactions between afterload and cardiac output in children on ECMO make it difficult to attribute this effect entirely to this low dose of hydralazine. So while a dose of 0.15mg/kg appeared well-tolerated more studies need to be performed to assess physiologic effect at different dosages.
Nesiritide
Nesiritide is a recombinant B-type natriuretic peptide administered as a continuous infusion that causes natriuresis, diuresis, and smooth muscle relaxation. It is FDA labeled for adults with congestive heart failure but is not labeled for use in children. Reports on pediatric dosing are scarce but based on several prospective and retrospective studies, the recommended dosage is 0.005–0.05 mcg/kg/min48–52 following a loading dose of 1–2 mcg/kg.48,52 Adult studies have demonstrated safety and efficacy with doses as high as 0.1 mcg/kg/min.53 A series of 2 neonates supported by ECMO (1 with congenital diaphragmatic hernia, 1 with hypoplastic left heart syndrome) reported the need of higher nesiritide maintenance doses (0.01–0.09 mcg/kg/min) in order to control mean arterial pressure.54 However, the safety of higher nesiritide doses in infants supported by ECMO has not been evaluated.
Nicardipine
Nicardipine is a calcium channel blocker that decreases SVR without negatively impacting myocardial inotropy, chronotropy, or dromotropy. In the ICU it is frequently used as an infusion due to its rapid onset of action and short half- life (40 mins). While it is not FDA labeled for use in children, Recommended dosing in the pediatric ICU is 0.5–5 mcg/kg/min by continuous infusion.43,44,55–57 Medications such as nicardipine with quick onset, short half-lives, and a readily measured effect (e.g. blood pressure change) are relatively easy to dose adjust in the setting of ECMO. A case report of a full term infant with congenital diaphragmatic hernia on venoarterial ECMO showed effective control of hypertension at nicardipine doses (0.5–1.5 mcg/kg/min) similar to those administered to patients not receiving ECMO support.43 Similarly, a 19 month old male on venoarterial ECMO after hydrocarbon ingestion was effectively managed by a nicardipine infusion starting at 5 mcg/kg/min and then titrated down to 1–3 mcg/kg/min.58 These limited data suggest that dosing changes are not necessary in patients supported with ECMO, but further studies are needed to confirm that observation.
Inotropes
Inotropes are used in infants and children in the ICU to support blood pressure by augmenting cardiac contractility and increasing systemic vascular resistance. Most infants and children are on some form of inotropic support prior to initiation of ECMO and some continue to require inotropic support while connected to the ECMO circuit.
Epinephrine and Dopamine
Numerous inotropic and vasopressor drugs are used in children on ECMO, but only epinephrine and dopamine have been studied in this patient population. An ex vivo study evaluated the extraction of dopamine and epinephrine by the ECMO circuit after administration of a single dose of dopamine (5 mg) and epinephrine (0.5 mg). Investigators measured epinephrine and dopamine concentrations at 30 min, 3h, and 24h post administration and calculated the extraction efficiency of the isolated ECMO circuit primed with blood. The authors found that the circuit by itself minimally impacts the serum concentration of these two drugs. To our knowledge there have been no in vivo studies looking specifically at dosing of inotropic medications in children on ECMO.
Diuretics
Infants and children with cardiovascular disease are often exquisitely sensitive to fluid balance and diuretics are a mainstay of fluid management. The ECMO circuit itself can trigger a significant inflammatory response which results in a combination of capillary leak syndrome, intravascular hypovolemia, and end-organ hypoperfusion. As such, most patients develop significant edema in the first few days of ECMO support and require diuretic therapy.
Furosemide
Furosemide is a loop diuretic and one of the best studied medications in children supported with ECMO. Dosing can be intermittent (0.5–2mg/kg IV up to every 6h) or continuous (up to 8 mg/kg/day). Several studies suggest continuous infusion may be preferable in critically ill children.59–63 A retrospective, single-center review of continuous infusion regimens in infants on ECMO suggested that such regimens are frequently used but that dosing varied widely, particularly with respect to the need for additional bolus doses.64 A subsequent prospective trial of a continuous furosemide infusion regimen in neonates supported with ECMO used PK modeling and found that a loading dose of 1 mg/kg followed by an infusion of 0.2 mg/kg/hr resulted in urine output in excess of 6 ml/kg/hr.65 This suggests that a lower furosemide dose may be required in this population supported by ECMO, however, due to the wide variability in urine output reported (0.8–8.4 ml/kg/hr) the most appropriate furosemide dose remains to be determined. While this was a well-designed study, it was limited by small sample size (n=7) and larger PK/PD studies would be helpful to determine optimal dosing of this drug.
Bumetanide
Bumetanide is another loop diuretic often used in infants and children with heart disease. Bumetanide does not carry a FDA-approved pediatric indication, but recommended dosing is 0.015–0.1mg/kg every 6–24 hours.66 A single-dose, PK trial of bumetanide in 11 full term neonates less than 1 month of age on ECMO support showed that using a single intravenous dose of 0.1mg/kg produced an initial doubling of UOP that remained above the baseline urine rate for approximately 3 hours.27 The Vd (0.44 +/− 0.1 L/kg) and CL (0.63 +/− 0.36 ml/min/kg) in these infants on ECMO27 was increased compared to critically ill infants not on ECMO (Vd 0.29 +/− 0.12 L/kg, CL 2.74 +/− 1.95 ml/min/kg).67 These results suggest that doses at the higher end of the recommended range but at decreased frequency are needed for patients supported by ECMO, however the infants included in these two studies had different chronological ages (ECMO 1-7d, non-ECMO 4-174d) which could also account for some of these differences in PK.27,67 More work needs to be done to better characterize the PK and PD of bumetanide in children supported with ECMO in order to provide appropriate dosing recommendations.
Pulmonary vasodilators
Pulmonary vasodilators are used to treat pulmonary hypertension and augment forward flow of blood across the pulmonary vasculature.
Sildenafil
Pulmonary hypertension is a common indication for ECMO in infants. The majority of these infants are treated with nitric oxide, however, in cases of refractory or poorly responsive pulmonary hypertension, oral sildenafil is often added off-label as adjunct therapy. Sildenafil is a potent phosphodiesterase (PDE-5) inhibitor dosed in infants and children at 2–8 mg/kg/day orally divided into four doses. A PK study of sildenafil in 23 infants (mean gestational age 38 weeks (37–42) with a mean post natal age of 9.6 days (1.4–1644)) supported by ECMO showed that doses of 5–7mg/kg/day were required to achieve adequate serum concentrations and that dosing adjustments down to 3–5 mg/kg/day were needed after decannulation.28 This was primarily due to increased Vd and suggests that when sildenafil is started in children supported by ECMO, the dose should be decreased after decannulation from ECMO. It is important to note that while there was a significant drop in the dose required to achieve adequate serum concentrations after decannulation, both the dose used on ECMO and after ECMO fell within the recommended dosing range.
Other
Prostaglandin
Prostaglandin E1 (PGE1) is used to maintain the patency of the patent ductus arteriosus in ductal-dependent congenital heart lesions prior to surgical repair. One case report of an infant with pulmonary valve stenosis, ductal-dependent pulmonary blood flow, and meconium aspiration showed that PGE1 doses of 0.8 mcg/kg/min (normal range 0.01–0.1 mcg/kg/min, max 0.4 mcg/kg/min) were required to maintain ductal patency68 As PGE1 remains primarily in the circulation with only minimal tissue distribution,69,70 by effectively tripling this infant’s blood volume with the exogenous blood needed to prime the ECMO circuit, the investigators were substantially altering the Vd and hence higher PGE1 doses were required.
Discussion
The data on drug dosing for infants and children supported with ECMO are limited in the number of drugs that have been studied and the size/design of these studies (e.g. case reports, small PK/PD trials, ex vivo studies). The results of these trials are mixed, with some drugs demonstrating markedly different PK (e.g. amiodarone, nesiritide, bumetanide, sildenafil) and others apparently exerting appropriate effects within the normal dosing range (e.g. nicardipine, epinephrine, dopamine). Several investigators have performed ex vivo and in vitro studies in an attempt to correlate drug characteristics with the likelihood that they will be affected by ECMO. Polyvinyl chloride tubing has been shown to adsorb lipophilic drugs,22–25 and early studies of highly lipophilic drugs such as fentanyl and midazolam showed almost complete disappearance of these drugs as opposed to less lipophilic drugs which were lost at rates of 10–35%.23 LogP, or partition coefficient, is a measure of lipophilicity and effort has been made to correlate LogP with altered PK/PD on ECMO. As noted above, Wildschut et al23 saw a significant association between a drug’s LogP and altered PK/PD. However, another study showed no significant association except for fentanyl.21 While the theory that high LogP is associated with higher adsorption by the ECMO circuit is biologically plausible, this conflicting data suggest that confounding characteristics of fentanyl and midazolam may be the causative factors.
Because ECMO is often associated with decreased renal function, it is also plausible that drugs which are primarily renally cleared might also show decreased CL. This has been shown with antibiotics such as vancomycin16–18,71,72 and gentamicin.11,12,14,15 Of the cardiovascular drugs, only bumetanide has had CL measured.27 CL was decreased in their study which is in line with its primary renal elimination (80%). The PD implications of these PK changes need to be further elucidated.
In summary, children on ECMO are among the most critically ill. Given the paucity of data and potentially important consequences of under- or over-dosing, efforts should be made to better understand drug dosing in this population. PK/PD trials in children are often limited by sparse sampling due to the desire to minimize blood draws. This is not a limitation with children supported by ECMO as they have frequent blood sampling that is clinically indicated, usually have indwelling catheters (e.g. peripheral arterial line) from which to obtain this blood, and receive frequent transfusions due to circuit-associated hemolysis. Additionally, children on ECMO are often on multiple drugs providing multiple targets of study. In conjunction with PK trials, efforts should be directed toward modeling the impact of various components of the ECMO circuit on drug disposition, identifying drug characteristics that make certain drugs more susceptible to circuit adsorption, and exploring the impact of organ injury on drug dosing. In addition, determining the PD of drugs in children supported by ECMO will be key to further determine the most appropriate dose required in this population. Research efforts should focus on the following drug classes:
Commonly used drugs in children on ECMO without PK information
Drugs with narrow therapeutic index
Drugs without a rapid measurable effect
Drugs with substantial renal elimination or hepatic metabolism
With an emphasis on PK/PD trials, modeling, and high-impact drug classes, a safer and evidence-based approach to pediatric dosing in children supported with ECMO can be achieved.
Acknowledgments
Funding Source: Dr. Watt receives support from the United States Government for his work in pediatric research (5T32HD043029-09)
Dr. Li received support from the United States Government (1U54RR023469-01) and from industry (research support from Sanofi Aventis, and Genzyme; honoraria from PTC Bio, and Daiichi Sankyo).
Dr. Benjamin receives support from the United States Government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01, and is the Principal Investigator of the Pediatric Trials Network, Government Contract HHSN275201000002I); the non profit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org); and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp).
Dr. Cohen-Wolkowiez receives support from NICHD for his work in pediatric and neonatal clinical pharmacology (1K23HD064814-01)
References
- 1.Pasquali SK, Hall M, Slonim AD, et al. Off-label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Circ Cardiovasc Qual Outcomes. 2008 Nov;1(2):74–83. doi: 10.1161/CIRCOUTCOMES.108.787176. [DOI] [PubMed] [Google Scholar]
- 2.Moller JH. Perspectives in pediatric cardiology: surgery of congenital heart disease: Pediatric Cardiac Care Consortium, 1984–1995. Vol. 6. Armonk, NY: Futura Publishing Company; 1998. [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010 May 27;362(21):1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 6.Group UCET. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trial Group. Lancet. 1996 Jul 13;348(9020):75–82. [PubMed] [Google Scholar]
- 7.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 Oct 17;374(9698):1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 8.Barnes S, Shields B, Bonney W, Hardin J, Abdulla R. The pediatric cardiology pharmacopoeia: 2004 update. Pediatr Cardiol. 2004 Nov-Dec;25(6):623–646. doi: 10.1007/s00246-003-0692-z. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Allen HD. Moss and Adams’ heart disease in infants, children, and adolescents : including the fetus and young adult. 7. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 10.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42(5):403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 11.Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1989 Jun;33(6):817–819. doi: 10.1128/aac.33.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen P, Collart L, Prober CG, Fischer AF, Blaschke TF. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J. 1990 Aug;9(8):562–566. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Dodge WF, Jelliffe RW, Zwischenberger JB, Bellanger RA, Hokanson JA, Snodgrass WR. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extracorporeal membrane oxygenation. Ther Drug Monit. 1994 Dec;16(6):552–559. [PubMed] [Google Scholar]
- 14.Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12(1):28–32. [PubMed] [Google Scholar]
- 15.Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans. 1991 Jan-Mar;37(1):16–18. doi: 10.1097/00002480-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hoie EB, Swigart SA, Leuschen MP, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990 Sep;9(9):711–715. [PubMed] [Google Scholar]
- 17.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996 May;40(5):1139–1142. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1998 Sep-Oct;18(5):1082–1086. [PubMed] [Google Scholar]
- 19.Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. 2005 Oct;20(6):309–315. doi: 10.1191/0267659105pf827oa. [DOI] [PubMed] [Google Scholar]
- 20.Dagan O, Klein J, Bohn D, Koren G. Effects of extracorporeal membrane oxygenation on morphine pharmacokinetics in infants. Crit Care Med. 1994 Jul;22(7):1099–1101. doi: 10.1097/00003246-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007 Jun;33(6):1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 22.Mulla H, Lawson G, von Anrep C, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000 Jan;15(1):21–26. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 23.Wildschut E, editor. Drug therapies in neonates and children during extracorporeal membrane oxygenation (ECMO); Keep your eyes open. Rotterdam, The Netherlands: Erasmus MC; 2010. [Google Scholar]
- 24.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993 Aug;15(4):263–266. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Rosen DA, Rosen KR, Silvasi DL. In vitro variability in fentanyl absorption by different membrane oxygenators. J Cardiothorac Anesth. 1990 Jun;4(3):332–335. doi: 10.1016/0888-6296(90)90041-d. [DOI] [PubMed] [Google Scholar]
- 26.Amaker RD, Bhatia J. ECMO and pharmacotherapy. Neonatal Netw. 1996 Dec;15(8):58. [PubMed] [Google Scholar]
- 27.Wells TG, Fasules JW, Taylor BJ, Kearns GL. Pharmacokinetics and pharmacodynamics of bumetanide in neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1992 Dec;121(6):974–980. doi: 10.1016/s0022-3476(05)80355-5. [DOI] [PubMed] [Google Scholar]
- 28.Ahsman MJ, editor. Determinants of pharmacokinetic variability during extracorporeal membrane oxygentation: A roadmap to rational pharmacotherapy in children. Rotterdam, The Netherlands: Erasmus MC; 2010. [Google Scholar]
- 29.Cuneo BF, Zales VR, Blahunka PC, Benson DW., Jr Pharmacodynamics and pharmacokinetics of esmolol, a short-acting beta-blocking agent, in children. Pediatr Cardiol. 1994 Nov-Dec;15(6):296–301. doi: 10.1007/BF00798123. [DOI] [PubMed] [Google Scholar]
- 30.Trippel DL, Wiest DB, Gillette PC. Cardiovascular and antiarrhythmic effects of esmolol in children. J Pediatr. 1991 Jul;119(1 Pt 1):142–147. doi: 10.1016/s0022-3476(05)81055-8. [DOI] [PubMed] [Google Scholar]
- 31.Robinson B, Eshaghpour E, Ewing S, Baumgart S. Hypertrophic obstructive cardiomyopathy in an infant of a diabetic mother: support by extracorporeal membrane oxygenation and treatment with beta-adrenergic blockade and increased intravenous fluid administration. ASAIO J. 1998 Nov-Dec;44(6):845–847. [PubMed] [Google Scholar]
- 32.Figa FH, Gow RM, Hamilton RM, Freedom RM. Clinical efficacy and safety of intravenous Amiodarone in infants and children. Am J Cardiol. 1994 Sep 15;74(6):573–577. doi: 10.1016/0002-9149(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 33.Perry JC, Fenrich AL, Hulse JE, Triedman JK, Friedman RA, Lamberti JJ. Pediatric use of intravenous amiodarone: efficacy and safety in critically ill patients from a multicenter protocol. J Am Coll Cardiol. 1996 Apr;27(5):1246–1250. doi: 10.1016/0735-1097(95)00591-9. [DOI] [PubMed] [Google Scholar]
- 34.Falik R, Flores BT, Shaw L, Gibson GA, Josephson ME, Marchlinski FE. Relationship of steady-state serum concentrations of amiodarone and desethylamiodarone to therapeutic efficacy and adverse effects. Am J Med. 1987 Jun;82(6):1102–1108. doi: 10.1016/0002-9343(87)90211-7. [DOI] [PubMed] [Google Scholar]
- 35.Haffajee CI, Love JC, Canada AT, Lesko LJ, Asdourian G, Alpert JS. Clinical pharmacokinetics and efficacy of amiodarone for refractory tachyarrhythmias. Circulation. 1983 Jun;67(6):1347–1355. doi: 10.1161/01.cir.67.6.1347. [DOI] [PubMed] [Google Scholar]
- 36.Latini R, Tognoni G, Kates RE. Clinical pharmacokinetics of amiodarone. Clin Pharmacokinet. 1984 Mar-Apr;9(2):136–156. doi: 10.2165/00003088-198409020-00002. [DOI] [PubMed] [Google Scholar]
- 37.Naccarelli GV, Rinkenberger RL, Dougherty AH, Giebel RA. Amiodarone: pharmacology and antiarrhythmic and adverse effects. Pharmacotherapy. 1985 Nov-Dec;5(6):298–313. doi: 10.1002/j.1875-9114.1985.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 38.Burri S, Hug MI, Bauersfeld U. Efficacy and safety of intravenous amiodarone for incessant tachycardias in infants. Eur J Pediatr. 2003 Dec;162(12):880–884. doi: 10.1007/s00431-003-1302-z. [DOI] [PubMed] [Google Scholar]
- 39.Plumpton K, Justo R, Haas N. Amiodarone for post-operative junctional ectopic tachycardia. Cardiol Young. 2005 Feb;15(1):13–18. doi: 10.1017/S1047951105000041. [DOI] [PubMed] [Google Scholar]
- 40.Kendrick JG, Macready JJ, Kissoon N. Amiodarone treatment of junctional ectopic tachycardia in a neonate receiving extracorporeal membrane oxygenation. Ann Pharmacother. 2006 Oct;40(10):1872–1875. doi: 10.1345/aph.1H148. [DOI] [PubMed] [Google Scholar]
- 41.Martin GR, Chauvin L, Short BL. Effects of hydralazine on cardiac performance in infants receiving extracorporeal membrane oxygenation. J Pediatr. 1991 Jun;118(6):944–948. doi: 10.1016/s0022-3476(05)82216-4. [DOI] [PubMed] [Google Scholar]
- 42.Sell LL, Cullen ML, Lerner GR, Whittlesey GC, Shanley CJ, Klein MD. Hypertension during extracorporeal membrane oxygenation: cause, effect, and management. Surgery. 1987 Oct;102(4):724–730. [PubMed] [Google Scholar]
- 43.McBride BF, White CM, Campbell M, Frey BM. Nicardipine to control neonatal hypertension during extracorporeal membrane oxygen support. Ann Pharmacother. 2003 May;37(5):667–670. doi: 10.1345/aph.1C366. [DOI] [PubMed] [Google Scholar]
- 44.Milou C, Debuche-Benouachkou V, Semama DS, Germain JF, Gouyon JB. Intravenous nicardipine as a first-line antihypertensive drug in neonates. Intensive Care Med. 2000 Jul;26(7):956–958. doi: 10.1007/s001340051287. [DOI] [PubMed] [Google Scholar]
- 45.Tobias JD. Nicardipine to control mean arterial pressure after cardiothoracic surgery in infants and children. Am J Ther. 2001 Jan-Feb;8(1):3–6. doi: 10.1097/00045391-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Mulrow JP, Crawford MH. Clinical pharmacokinetics and therapeutic use of hydralazine in congestive heart failure. Clin Pharmacokinet. 1989 Feb;16(2):86–89. doi: 10.2165/00003088-198916020-00003. [DOI] [PubMed] [Google Scholar]
- 47.Taketomo C, Hodding JH, Kraus DM. Pediatric Dosage Handbook. 16. Hudson, OH: Lexi-Comp; 2009. Lexicomp’s Drug Reference Handbooks. [Google Scholar]
- 48.Behera SK, Zuccaro JC, Wetzel GT, Alejos JC. Nesiritide improves hemodynamics in children with dilated cardiomyopathy: a pilot study. Pediatr Cardiol. 2009 Jan;30(1):26–34. doi: 10.1007/s00246-008-9272-6. [DOI] [PubMed] [Google Scholar]
- 49.Jefferies JL, Denfield SW, Price JF, et al. A prospective evaluation of nesiritide in the treatment of pediatric heart failure. Pediatr Cardiol. 2006 Jul-Aug;27(4):402–407. doi: 10.1007/s00246-005-1294-8. [DOI] [PubMed] [Google Scholar]
- 50.Jefferies JL, Price JF, Denfield SW, et al. Safety and efficacy of nesiritide in pediatric heart failure. J Card Fail. 2007 Sep;13(7):541–548. doi: 10.1016/j.cardfail.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Ryan A, Rosen DA, Tobias JD. Preliminary experience with nesiritide in pediatric patients less than 12 months of age. J Intensive Care Med. 2008 Sep-Oct;23(5):321–328. doi: 10.1177/0885066608320840. [DOI] [PubMed] [Google Scholar]
- 52.Simsic JM, Mahle WT, Cuadrado A, Kirshbom PM, Maher KO. Hemodynamic effects and safety of nesiritide in neonates with heart failure. J Intensive Care Med. 2008 Nov-Dec;23(6):389–395. doi: 10.1177/0885066608324296. [DOI] [PubMed] [Google Scholar]
- 53.Marcus LS, Hart D, Packer M, et al. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. A double-blind, placebo-controlled, randomized crossover trial. Circulation. 1996 Dec 15;94(12):3184–3189. doi: 10.1161/01.cir.94.12.3184. [DOI] [PubMed] [Google Scholar]
- 54.Smith T, Rosen DA, Russo P, et al. Nesiritide during extracorporeal membrane oxygenation. Paediatr Anaesth. 2005 Feb;15(2):152–157. doi: 10.1111/j.1460-9592.2004.01398.x. [DOI] [PubMed] [Google Scholar]
- 55.Flynn JT, Mottes TA, Brophy PD, Kershaw DB, Smoyer WE, Bunchman TE. Intravenous nicardipine for treatment of severe hypertension in children. J Pediatr. 2001 Jul;139(1):38–43. doi: 10.1067/mpd.2001.114030. [DOI] [PubMed] [Google Scholar]
- 56.Flynn JT, Pasko DA. Calcium channel blockers: pharmacology and place in therapy of pediatric hypertension. Pediatr Nephrol. 2000 Dec;15(3–4):302–316. doi: 10.1007/s004670000480. [DOI] [PubMed] [Google Scholar]
- 57.Gouyon JB, Geneste B, Semama DS, Francoise M, Germain JF. Intravenous nicardipine in hypertensive preterm infants. Arch Dis Child Fetal Neonatal Ed. 1997 Mar;76(2):F126–127. doi: 10.1136/fn.76.2.f126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tobias JD, Pietsch JB, Lynch A. Nicardipine to control mean arterial pressure during extracorporeal membrane oxygenation. Paediatr Anaesth. 1996;6(1):57–60. doi: 10.1111/j.1460-9592.1996.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 59.Klinge JM, Scharf J, Hofbeck M, Gerling S, Bonakdar S, Singer H. Intermittent administration of furosemide versus continuous infusion in the postoperative management of children following open heart surgery. Intensive Care Med. 1997 Jun;23(6):693–697. doi: 10.1007/s001340050395. [DOI] [PubMed] [Google Scholar]
- 60.Luciani GB, Nichani S, Chang AC, Wells WJ, Newth CJ, Starnes VA. Continuous versus intermittent furosemide infusion in critically ill infants after open heart operations. Ann Thorac Surg. 1997 Oct;64(4):1133–1139. doi: 10.1016/s0003-4975(97)00714-5. [DOI] [PubMed] [Google Scholar]
- 61.Martin SJ, Danziger LH. Continuous infusion of loop diuretics in the critically ill: a review of the literature. Crit Care Med. 1994 Aug;22(8):1323–1329. doi: 10.1097/00003246-199408000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Singh NC, Kissoon N, al Mofada S, Bennett M, Bohn DJ. Comparison of continuous versus intermittent furosemide administration in postoperative pediatric cardiac patients. Crit Care Med. 1992 Jan;20(1):17–21. doi: 10.1097/00003246-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 63.van der Vorst MM, Ruys-Dudok van Heel I, Kist-van Holthe JE, et al. Continuous intravenous furosemide in haemodynamically unstable children after cardiac surgery. Intensive Care Med. 2001 Apr;27(4):711–715. doi: 10.1007/s001340000819. [DOI] [PubMed] [Google Scholar]
- 64.van der Vorst MM, Wildschut E, Houmes RJ, et al. Evaluation of furosemide regimens in neonates treated with extracorporeal membrane oxygenation. Crit Care. 2006;10(6):R168. doi: 10.1186/cc5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Vorst MM, den Hartigh J, Wildschut E, Tibboel D, Burggraaf J. An exploratory study with an adaptive continuous intravenous furosemide regimen in neonates treated with extracorporeal membrane oxygenation. Crit Care. 2007;11(5):R111. doi: 10.1186/cc6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wells TG. The pharmacology and therapeutics of diuretics in the pediatric patient. Pediatr Clin North Am. 1990 Apr;37(2):463–504. doi: 10.1016/s0031-3955(16)36880-8. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan JE, Witte MK, Yamashita TS, Myers CM, Blumer JL. Pharmacokinetics of bumetanide in critically ill infants. Clin Pharmacol Ther. 1996 Oct;60(4):405–413. doi: 10.1016/S0009-9236(96)90197-6. [DOI] [PubMed] [Google Scholar]
- 68.Stone DM, Frattarelli DA, Karthikeyan S, Johnson YR, Chintala K. Altered prostaglandin E1 dosage during extracorporeal membrane oxygenation in a newborn with ductal-dependent congenital heart disease. Pediatr Cardiol. 2006 May-Jun;27(3):360–363. doi: 10.1007/s00246-005-1189-8. [DOI] [PubMed] [Google Scholar]
- 69.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem. 1971 Nov 25;246(22):6713–6721. [PubMed] [Google Scholar]
- 70.Rosenkranz B, Fischer C, Boeynaems JM, Frolich JC. Metabolic disposition of prostaglandin E1 in man. Biochim Biophys Acta. 1983 Feb 7;750(2):231–236. doi: 10.1016/0005-2760(83)90023-1. [DOI] [PubMed] [Google Scholar]
- 71.de Hoog M, Mouton JW, van den Anker JN. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet. 2004;43(7):417–440. doi: 10.2165/00003088-200443070-00001. [DOI] [PubMed] [Google Scholar]
- 72.Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005 Sep;60(3):265–275. doi: 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Extracorporeal Life Support Organization (ELSO) ECLS Registry Report: International Summary. 2010 July; [Google Scholar]
- 74.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117(4):1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 75.Humpl T, Reyes JT, Holtby H, et al. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation. 2005;111:3274–80. doi: 10.1161/CIRCULATIONAHA.104.473371. [DOI] [PubMed] [Google Scholar]
- 76.Karatza AA, Bush A, Magee AG. Safety and efficacy of Sildenafil therapy in children with pulmonary hypertension. Int J Cardiol. 2005;100:267–73. doi: 10.1016/j.ijcard.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Karatza AA, Narang I, Rosenthal M, et al. Treatment of primary pulmonary hypertension with oral sildenafil. Respiration. 2004;71:192–4. doi: 10.1159/000076684. [DOI] [PubMed] [Google Scholar]