Abstract
Findings on affective processing deficits in Huntington's disease (HD) have been inconsistent. It is still not clear whether HD patients are afflicted by specific deficits in emotion recognition and experience. We tested 28 symptomatic HD patients and presented them with pictures depicting facial expressions of emotions (Karolinska-Set) and with affective scenes (International Affective Picture System; IAPS). The faces were judged according to the displayed intensity of six basic emotions, whereas the scenes received intensity ratings for the elicited emotions in the viewer. Patients' responses were compared with those of 28 healthy controls. HD patients gave lower intensity ratings for facial expressions of anger, disgust and surprise than controls. Patients' recognition deficits were associated with reduced functional capacity, such as problems with social interactions. Moreover, their classification accuracy was reduced for angry, disgusted, sad and surprised faces. When judging affective scenes for the elicitation of happiness, disgust and fear, HD patients had a tendency to estimate them as more intense than controls. This finding points to a differential impairment in emotion recognition and emotion experience in HD. We found no significant correlations between emotion experience/recognition ratings and CAG repeats, symptom duration and UHDRS Motor Assessment in the patient group.
Keywords: Symptomatic Huntington's disease, Affective faces, Affective scenes, Intensity perception, Classification accuracy
1. Introduction
Huntington's disease (HD) is an autosomal dominant, neurodegenerative disorder. The disease is caused by an unstable expansion of the trinucleotide repeat cytosine-adenine-guanine (CAG) of the gene IT-15 on the short arm of chromosome 4 that codes for the protein huntingtin (Htt). CAG expansion beyond 35 repeats is associated with the expression of HD (Langbehn et al., 2004). Intranuclear inclusions of the aggregated mutant Htt lead to progressive cerebral degeneration starting in the caudate nucleus and in the putamen (Douaud et al., 2006; Bohanna et al., 2008). HD is characterised by increasing deterioration in motor function, psychiatric disturbance and cognitive impairment (Bohanna et al., 2008; Henley et al., 2009). There is a close relationship between the number of CAG repeats and age of disease onset as well as its progress (Langbehn et al., 2004). HD patients usually develop changes in personality and emotional functioning during the progress of disease, e.g., increase of hostility, impulsivity, depression and anxiety (Thompson et al., 2002; van Duijn et al., 2007).
One particular deficit in affective processing that has been identified in several studies relates to a selective disgust recognition deficit in preclinical gene-carriers (Gray et al., 1997; Hennenlotter et al., 2004; Sprengelmeyer et al., 2006). Such a deficit can be explained by neurobiological emotion models suggesting that basic feelings (e.g., disgust and fear) are mediated by specific central affect programs initiated in localized brain regions (Calder et al., 2001). Several studies led to the assumption that recognition of disgust stimuli relies on the basal ganglia (particularly the putamen) and the anterior insula (Phillips et al., 1997; Sprengelmeyer et al., 1998; Hennenlotter et al., 2004). Results by Kipps et al. (2007) showed a strong linear correlation between disgust recognition and insula volume in HD.
However, findings on symptomatic HD do not support the idea of a specific disgust processing deficit. Three studies on clinically manifest HD found impairments for various negative emotions but all investigated very small samples (Sprengelmeyer et al., 1996: n = 13; Wang et al., 2003: n = 6; Montagne et al., 2006: n = 8). Furthermore, Milders et al. (2003) revealed for 20 symptomatic HD patients that fear recognition was most severely impaired, but there were also problems in recognising facial expressions of anger, disgust and sadness. Snowden et al. (2008) found poorer performance in 10 symptomatic HD patients for the recognition of anger and to a lesser extent disgust. The manifest HD group in the study of Henley et al. (2008) was significantly worse than controls at recognising facial expressions of surprise, disgust, anger and fear. Impaired recognition of each emotion was associated with striatal volume loss. The results of Johnson et al. (2007), studying a large sample of 475 pre-symptomatic individuals, revealed that already gene-carriers performed worse than controls concerning all negative emotions.
In contrast to emotion identification the elicitation of feelings by the presentation of emotion-relevant scenes has hardly been studied in HD. The only study by Hayes et al. (2007) with 15 HD patients found that fewer scenes were classified as disgusting by symptomatic HD patients than by controls. Similar to disgust recognition it has been suggested that the experience of disgust feelings recruits the insula. In an fMRI investigation by Stark et al. (2007)participants' reported disgust feelings were correlated with activation of the insular cortex. However, other studies did not observe a specific involvement of a basal ganglia-insular circuit in the central representation of disgust experience (e.g. Schienle et al., 2005).
In summary, findings on affective processing dysfunctions in HD are inconsistent. One reason for this concerns small sample sizes in most studies, which limits the power to detect more subtle group differences and enhances the possibility of sample-dependent results. Furthermore, severity of symptoms, influenced by CAG length and disease duration, differed between studies. Unspecific symptoms can precede motor symptoms, which are commonly used as markers of clinical manifestation (Langbehn et al., 2004). Approximately 60% of patients start with mental and emotional dysfunctions (Di Maio et al., 1993), which are often not immediately attributed to HD. Specificity of emotion recognition deficits might vary with disease progression, starting with impaired disgust perception in asymptomatic gene-carriers, as revealed in two studies (Gray et al., 1997; Sprengelmeyer et al., 2006).
Adequate emotional experience and understanding other persons' emotional behaviour are important for social functioning and quality of life. HD results in a complex symptomatology especially during progressed stages, including impairment of voluntary motor functions, cognition, language and mental functions. HD is related to multiple psychosocial problems in the afflicted persons and their families. Abnormal emotional processing has negative implications for patients' daily lives, especially for social interactions. Findings of previous studies already demonstrated that the psychosocial well-being of patients with HD is strongly affected (e.g., Helder et al., 2001).
The primary objective of this study was to determine whether the impairment of facial disgust recognition in symptomatic HD extends to the experience of visually induced disgust. We asked the participants to rate facial expressions according to the displayed emotion intensities for all basic emotions (graded choice). This allowed the analysis of quantitative (intensity) as well as qualitative (classification accuracy) emotion processing deficits in HD. The same approach was applied for the analysis of emotions elicited by affective scenes. Further, we were interested whether HD patients show a disproportionately severe impairment in disgust recognition or a more general deficit that extends to other basic emotions.
2. Methods
2.1. Participants
We studied 28 genetically tested symptomatic HD patients, 17 men and 11 women (M = 48.4 years, S.D. = 9.4; age range = 30–64), who were inpatients at the University Hospital of Graz (Austria). Socio-economic status was based on the highest educational level completed. Mean years of education were 11.9 years (S.D. = 2.7). Twelve of the patients (42.9%) still pursued a profession. CAG repeat length varied between 41 and 54 (M = 45.1, S.D. = 2.5). Symptom onset had occurred 0.5 to 14.3 years ago (M = 4.8 years; S.D. = 3.5). Each patient was assessed using the Unified Huntington's Disease Rating Scale (UHDRS; Huntington Study Group, 1996), by the Total Functional Capacity Scale (TFC; Shoulson and Fahn, 1979) and by a clinical interview (Mini-DIPS; Margraf, 1994). Patients' motor score on the UHDRS ranged between 8 and 68 (M = 30.89, S.D. = 16.22), score on the UHDRS Functional Assessment Scale ranged between 11 and 25 (M = 20.71, S.D. = 4.38), and score on the UHDRS Independency Scale ranged between 55 and 100%. Shoulson's TFC scores ranged between 3 and 13 (M = 9.75, S.D. = 3.20). Handedness was taken as the hand used to write with. All patients with exception of one woman were right-handed. Fourteen of the patients (50%) received no pharmacologic treatment. Antidepressants or/and antipsychotics were applied to fourteen patients (antidepressants: 7 patients, antipsychotics: 2 patients, both: 5 patients). A conducted analysis of variance with the factors medication (medicated vs. non-medicated) and emotion (fear, anger, sadness, disgust, happiness, and surprise) revealed a non-significant medication effect for the ratings of affective faces and scenes and a non-significant medication × emotion interaction (all Fs < 1.36, all Ps > 0.260).
We further tested 28 mentally healthy subjects, 17 men and 11 women, matched for sex, age and socio-economic status. They had been recruited by advertisements in a local newspaper. The control group underwent the Mini-DIPS to exclude the presence of mental disorders. Age of controls ranged between 31 and 63 years, with a mean age of 47.2 years (S.D. = 7.5). Sixteen subjects of the control group (57.1%) were employed. Mean education level was 10.7 years (S.D. = 1.6). Controls were right-handed with the exception of one man and one woman. Groups did not differ in age (t(54) = 0.52, n. s.) and years of education (t(54) = 1.97, n. s.).
Exclusion criteria for patients and for controls were dementia (TFDD-Sore < 35; Ihl et al., 2000), substance abuse, and current use of sleep-inducing drugs or sedatives. The presence of neurological disorders for controls and of other neurological disorders besides HD for patients was also an exclusion criterion.
The project had been approved by the ethics committee of the University of Graz (vote number 18–138 ex 06/07). All participants gave informed written consent to the study.
2.2. Questionnaires
Cognitive performance was assessed using the TFDD (‘Test zur Früherkennung von Demenzen mit Depressionsabgrenzung’; Ihl et al., 2000). This scale ranges between 0 and 50 points and allows the detection of early signs of cognitive impairment. A score lower than 35 indicates a tentative dementia diagnosis (exclusion criterion). The Cronbach's alpha is 0.88.
The UHDRS (Huntington Study Group, 1996), only used for the patient group, is a standardized clinical rating scale for assessing motor, cognitive, behavioural, and functional capacity symptoms of Huntington's disease. The UHDRS Motor Assessment ranges from 0 to 124, with higher scores indicating higher motor impairment. The Functional Assessment Scale ranges up to a score of 25, and the Independence Scale up to 100%, with higher scores being indicative of higher functioning. The Cronbach's alpha of the UHDRS is 0.95.
The TFC (Shoulson and Fahn, 1979) is a standard measure of functional capacity consisting of five items assessing engagement in occupation, capacity to handle financial affairs, capacity to manage domestic responsibilities, capacity to perform activities of daily living and the type of residential care provided. Scores range from 0 to 13, with higher scores indicative of higher functioning and greater independence. The Cronbach's alpha of this scale is 0.95.
Habitual emotional reactivity was assessed by self-report inventories:
The Beck Depression Inventory (BDI; German version: Hautzinger et al., 1994) assesses depressive symptomatoloy. Cronbach's alpha is 0.88.
The Questionnaire for the Assessment of Disgust Proneness (QADS; Schienle et al., 2002a) measures disgust propensity and describes 37 situations, which have to be judged on 5-point scales with regard to the experienced disgust (0 = ‘not disgusting’; 4 = ‘very disgusting’; e.g., ‘You are just about to drink a glass of milk as you notice that it is spoiled’). The Cronbach's alpha of the total scale is 0.90.
The Trait scale of the State-Trait Anxiety Inventory (STAI; Laux et al., 1981) measures the frequency of anxious feelings on a 4-point scale. The Cronbach's alpha of the scale is 0.88.
The Trait scale of the State-Trait-Anger Inventory (STAXI; Schwenkmezger et al., 1992) assesses trait anger as well as anger expression. We only assessed trait anger. All items are rated on 4-point scales. Internal consistence of the STAXI is 0.90.
2.3. Stimuli for the picture perception tasks
All participants viewed emotional scenes and facial expressions on a computer screen (notebook, 15 in.). The participants sat at about 50 cm from the screen. Before starting the experiment participants were asked for their understanding of basic emotions by a short verbal description.
-
a)
Forty-two pictures with emotional facial expressions depicting happiness (6), fear (6), sadness (6), anger (6), disgust (6), surprise (6), and a neutral affective state (6) from the Karolinska-Set (Lundquist et al., 1998) were presented. Half of the posers were female, half were male.
-
b)
Twenty-four emotion-relevant scenes for the induction of happiness (6), fear (6), disgust (6), and an affectively neutral state (6) were presented. Most scenes were taken from the International Affective Picture System (IAPS; Lang et al., 2001). Disgust-inducing pictures were developed by Schienle et al. (2002b) and included scenes with animals (maggots, bluebottles, and slugs), a dirty toilet, carrion and an eczematous face. The fear-inducing pictures showed threatening situations either through attacks of animals (‘dog with its teeth bared’, IAPS 1300; ‘white shark’, developed by the authors) or human attacks (‘man threatening a woman with a knife’, IAPS 6350; ‘men with pistol’, IAPS 6230; ‘war scene’, IAPS 6940; ‘masked robber’, IAPS 6370). Happy pictures included animals (‘baby seal’, IAPS 1440; ‘young rabbits’, IAPS 1750; ‘playing dolphins’, IAPS 1920) and food (‘roast chicken’, IAPS 7230; ‘gateau’, IAPS 7282; ‘ice cream’, IAPS 7330). Neutral scenes consisted of office equipment (‘telephone’, ‘files’, and ‘mouse-pad’, developed by the authors), ‘buildings’ (IAPS 7491), ‘winter clothes’ (IAPS 7205) and a ‘funnel’ (IAPS 7185).
The stimulus material had been matched for item difficulty, complexity, brightness and colour. Since the IAPS does not include pictures which reliably induce anger, sadness and surprise these categories were omitted. It is known that IAPS scenes which should induce sadness or anger usually produce mixed emotions (e.g. 50% anger and 50% sadness). Affective neutral stimuli were included as a control (reference) condition.
Each picture was presented for a maximum of 15 s. Pre-tests had displayed that this time was reported as sufficient for identifying the pictures also by HD patients. The presentation could be terminated early by pressing a button on a three-button device, which had been developed for the experiment. Then, the subject was asked to rate the picture on a 9-point scale within 15 s. For the scenes, subjects rated how intense the six basic emotions were induced by a particular picture (e.g., ‘Please indicate how intense you experienced disgust while viewing the picture’: 1 = very little, 9 = very intense). For each facial expression subjects rated how intense the depicted person experienced the six basic emotions (e.g., ‘Please indicate how intense the depicted person experienced disgust’: 1 = very little; 9 = very intense). To avoid position effects, the order of the two picture perception tasks (recognition vs. experience), order of pictures, and order of basic emotions to rate were randomised.
2.4. Statistical analyses
All statistical analyses were carried out using PASW Statistics 18.0 for Windows. We computed mean emotion intensity ratings for affective scenes and facial expressions for each emotional condition and all six basic emotions. Repeated measures analyses of variance (2 × 6 ANOVAs) were carried out with the independent factors group (HD and Control) and sex (male and female) and the repeated measure factor emotion (fear, disgust, sadness, anger, happiness, and surprise). Alpha level significance was set at 0.05 for all statistical tests. To account for multiple comparisons we used the method by Shaffer (1986) with a family type I error of α = 0.05 for all exploratory analyses. Effect sizes were calculated by Cohen's d.
3. Results
3.1. Group comparisons for the questionnaires
Cognitive performance (TFDD) between HD patients and controls differed significantly (patients: M = 39.18, S.D. = 3.92; controls: M = 46.29, S.D. = 2.34; t(54) = − 8.22, P < 0.001), with patients showing lower scores. Groups did not differ in depressive symptoms (BDI; patients: M = 4.89, S.D. = 4.50; controls: M = 3.61, S.D. = 3.33; t(54) = 1.22, n. s.) and disgust propensity (QADS; patients: M = 2.13, S.D. = 0.93; controls: M = 2.01, S.D. = 0.70; t(54) = 0.56, n. s.). We also found no group effects for trait anxiety (STAI; patients: M = 34.66, S.D. = 8.16; controls: M = 32.50, S.D. = 7.43; t(54) = 0.94; n. s.) and trait anger (STAXI; patients: M = 15.19, S.D. = 4.08; controls: M = 16.62, S.D. = 3.89; t(54) = − 1.33; n. s.).
3.2. Group comparisons for the picture perception tasks (Table 1, Figs. 1 and 2)
Table 1.
Group comparison of the Mean scores (standard deviations) for intensity ratings in neutral stimuli for emotion perception tasks.
| HD | Controls | |
|---|---|---|
| N = 28 | N = 28 | |
| Neutral faces | ||
| Disgust | 2.38 (1.72) | 2.13 (1.26) |
| Anger | 3.24 (1.88) | 3.21 (1.60) |
| Fear | 2.85 (1.57) | 2.94 (1.78) |
| Sadness | 4.05 (1.94) | 4.17 (1.89) |
| Happiness | 3.78 (1.84) | 2.61 (1.42) |
| Surprise | 4.35 (2.17) | 2.81 (1.59) |
| Neutral scenes | ||
| Disgust | 1.60 (0.80) | 1.54 (0.94) |
| Anger | 1.80 (0.92) | 1.74 (1.01) |
| Fear | 1.65 (0.82) | 1.70 (0.96) |
| Sadness | 1.70 (1.00) | 1.64 (1.08) |
| Happiness | 4.74 (1.79) | 3.09 (1.66) |
| Surprise | 3.83 (2.15) | 2.95 (1.77) |
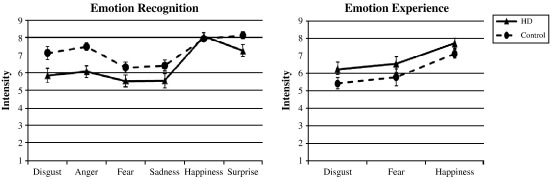
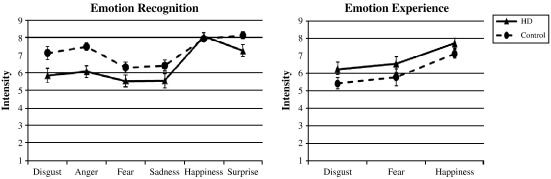
Fig. 1.
Group comparison of the mean scores (standard errors) for intensity ratings of target emotions. Left panel refers to affective facial expressions and right panel to affective scenes.
Fig. 2.
Group comparison of the mean scores (standard errors) for classification accuracy (difference between rated target emotion intensity and mean sum of non-target intensity ratings) for affective facial expressions. Higher scores mean better classification performance.
3.2.1. Facial expressions
-
a)
Intensities of target emotions: analyzing the intensity ratings of the six target emotions (anger, disgust, sadness, fear, happiness, and surprise; e.g., disgust intensity in faces depicting disgust) we revealed a significant main effect for emotion (F(5, 260) = 20.69, P < 0.001), for group (F(1, 54) = 8.55, P = 0.005), and a significant emotion × group interaction (F(5, 260) = 2.30, P = 0.046). Patients estimated target emotions in affective faces less intense than controls (mean difference (md) = 0.85), concerning anger (md = 1.40, P = 0.001; effect size: Cohen's d = 0.98), disgust (md = 1.27, P = 0.023; d = 0.63) and surprise (md = 0.88, P = 0.024; d = 0.63; see Fig. 1, left panel). Also, patients perceived more happiness than controls in angry, fearful and sad facial expressions (all mds > 0.73, all Ps < 0.037). We found no significant sex × group interaction for intensity estimations of affective faces.
-
b)
Classification accuracy of target emotions: for assessing participants' classification accuracy of facial expressions we calculated the difference between the rated target emotion intensity and the mean of all non-target intensity ratings (e.g., classification accuracy for a disgust expression = disgust intensity minus mean intensity (anger, fear, sadness, happiness, and surprise).
Analyses of classification accuracy of target emotions relative to non-target emotions revealed a significant main effect for emotion F(5, 260) = 57.13, P < 0.001) and for group F(1, 53) = 11.90, P = 0.001), and a significant emotion × group interaction (F(5, 260) = 2.89, P = 0.015). Patients showed lower classification accuracy concerning anger (md = 1.91, P < 0.001, d = 1.12), disgust (md = 1.72, P = 0.003, d = 0.85), surprise (md = 1.15, P = 0.028, d = 0.60), and sadness (md = 1.09, P = 0.050, d = 0.54; see Fig. 2) than controls.
-
c)
Perception of neutral faces: analyses of neutral faces' ratings revealed a main effect for the six basic emotions (F(5, 260) = 13.97, P < 0.001), a marginally significant group effect (F(1, 52) = 2.85, P = 0.098) and a significant emotion × group interaction (F(5, 260) = 4.21, P = 0.006). Patients reported marginally higher intensities for neutral faces than controls. Pairwise comparisons showed that patients perceived higher intensities of happiness (md = 1.17, P = 0.012; d = 0.70) and surprise (md = 1.54, P = .004; d = 0.81) in neutral faces than controls (see Table 1). Controls assessed sadness highest in neutral faces, differing significantly from all other emotions (all mds > 1.35, P < 0.010).
3.2.2. Affective scenes
-
a)
Intensity of target emotions: intensity analyses for the three target emotions (happiness, fear, and disgust) showed significant main effects for emotion (F(2, 104) = 18.35, P < 0.001) as well as a marginally significant main effect for group (F(1, 52) = 3.26, P = 0.077). Patients experienced emotions marginally more intense than controls. We found no significant sex × group interaction for intensity estimations of affective scenes (see Fig. 1, right panel).
-
b)
Experience of neutral scenes: ratings of the neutral scenes displayed a main effect for the six basic emotions estimated (F(5, 260) = 54.64, P < 0.001) and a significant emotion × group interaction (F(5, 260) = 6.60, P = 0.001). The main effect for group reached marginally significance (F(1, 52) = 3.82, P = 0.056). Patients tended to estimate neutral scenes as more intense than controls. Patients reported a higher intensity of happiness (md = 1.65, P = 0.001; d = 0.96) and marginally also for surprise (md = 0.89, P = 0.098; d = 0.45) than controls.
3.2.3. Correlative analyses
-
a)
Controlling for confounding variables: For both groups cognitive performance (TFDD) was not associated with emotion intensity ratings neither for recognition nor experience (all r < 0.32; n. s.). There were no significant correlations between recognition and experience of target emotions in patients and controls (all r < 0.21; n. s.).
-
b)
Habitual emotion reactivity: patients' fear experience and anxiety sensitivity were correlated (r = 0.46, P = 0.014). Their disgust experience (intensity) was correlated with anxiety sensitivity (r = 0.53, P = 0.004), trait anxiety (r = 0.42, P = 0.027) and trait anger (r = 0.48, P = 0.014). For controls correlations reached significance between fear experience and anxiety sensitivity (r = 0.60, P = 0.001).
-
c)
Measures of disease severity: the UHDRS Functional Assessment score correlated with patients' classification accuracy for angry (r = 0.52, P = 0.006), disgusted (r = 0.42, P = 0.032) and surprised (r = 0.42, P = 0.031) faces. Shoulson's TFC score was positively associated with happiness experience (r = 0.47, P = 0.012) and surprise recognition (r = 0.43, P = 0.025).
We found no significant correlations between emotion experience/recognition ratings and CAG repeats, symptom duration and UHDRS Motor Assessment in the patient group.
4. Discussion
In this study we investigated emotion recognition and emotion experience in symptomatic HD. The participants had been asked to judge the intensity of affective facial expressions as well as the intensity of emotional experiences elicited by affective scenes using graded choice over six basic emotions.
The analysis of the recognition task revealed lower intensity ratings of target emotions for angry, disgusted and surprised faces in HD patients compared to controls. This points to a quantitative recognition deficit in HD patients. Effect sizes were large for anger and medium for disgust and surprise. Besides this quantitative deficit, patients showed lower classification accuracy concerning facial expressions of anger and disgust (large effect sizes), and for sadness and surprise (medium effect sizes). The described deficits of our patients are in line with findings of Snowden et al. (2008) and Henley et al. (2008) who also identified the strongest impairment in HD for anger recognition. Fear recognition was comparably poor in patients as well as in healthy controls. This is in accordance with previous studies on healthy subjects where facial expressions of fear were typically recognised more poorly than other expressions (e.g., Ekman and Friesen, 1976).
The patients rated angry, fearful and sad faces as happier compared to controls. Also, they perceived neutral faces as happier and as more surprised than the healthy subjects. In contrast, the controls rated neutral faces as slightly sad, which is in line with earlier findings (e.g., Katsikitis, 1997; Lee et al., 2008). We hypothesize that this ‘positivity bias’ might be a consequence of patients' focusing on easy to decode emotions, such as happiness. Negative emotions are more difficult to decipher and the patients clearly displayed recognition deficits for affects with negative valence. Due to the reduced recognition of negative emotions, the sensitivity for signs of positive emotions might be enhanced.
Altogether, HD patients showed quantitative and qualitative deviations from healthy subjects as to emotion recognition. There were differences regarding the perceived intensity as well as the distinction of emotions with negative and inconclusive valence. We could also display that these deviations were not specifically linked with patients' poorer cognitive performance.
Our second main objective was to determine if the impairment in emotion recognition extends to experience. Interestingly, patients had a tendency to give higher intensity ratings for all experienced emotions (happiness, fear, and disgust) than controls. The group difference in experienced emotion intensity was only marginally significant and therefore should be interpreted with caution. However, our data clearly show that HD patients responded differently to affective scenes (increased intensity ratings) compared to affective faces (decreased intensity ratings). Perhaps HD patients start to focus more on their own emotions because the decoding of emotions in others becomes more and more difficult as the disease progresses. The diminished perception of emotions in others might lead to a (compensatory) enhancement of one's own feelings. Another explanation is also possible. Since HD patients experience strong emotions and are overwhelmed by them, this might interfere with the correct perception of emotional cues in others.
Moreover, patients and controls rated neutral scenes differently. They displayed a positivity bias (similar to the positivity bias for neutral faces) and experienced more happiness and surprise when viewing neutral scenes relative to the healthy participants. Future studies are needed in order to replicate this observation.
Hence our data show an HD-related differential impairment in emotion recognition and experience. Such a deficit seems possible as the two processes recognition and experience were independent from each other. The intensity ratings for emotional faces and scenes (for a given target emotion) were not correlated with each other, neither in patients nor in controls.
We also investigated whether heterogeneous findings on disgust recognition and experience in HD may be due to an influence of personality and gender-related factors. But we found no difference between patients and controls in habitual emotional reactivity concerning disgust, anxiety, anger and depression. This is in line with results of previous questionnaire assessments (Sprengelmeyer et al., 2006; Hayes et al., 2007; Johnson et al., 2007). We also found no gender-specific effects in HD. Associations between experience of visual emotional stimuli and habitual emotional reactivity concerned anxiety sensitivity, trait anxiety, disgust sensitivity, and trait anger being related to experienced disgust and fear. This corresponds with previous studies (e.g., Schienle et al., 2005).
Although no findings exist from previous studies on symptomatic HD we expected symptom severity to affect emotion processing. We found associations between functioning in everyday life and emotion recognition deficits. Huntington disease has a significant impact on the social life. Deficient comprehension of other persons' affect can lead to a breakdown in interpersonal relationships, especially when linguistic functionality becomes worse with progress of disease. Especially impaired perception of others' anger and fear may result in negative consequences for communication. Our results point to a relationship between these deficiencies and everyday functioning. Therefore, therapeutic approaches should address this aspect and supply emotional recognition trainings for symptomatic patients.
As a limitation of the present study it needs to be mentioned that the two applied emotional tasks differed in their difficulty. The emotion recognition task included six emotions, whereas the experience task only contained three conditions. This might have influenced the ratings. Furthermore, we plan to study a larger sample of HD patients in order to replicate the findings on enhanced emotion experience.
Acknowledgment
This research was supported by the research grant ‘Austrian Research Fund’, project number P20779-B02.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.psychres.2011.04.007.
Appendix A. Supplementary data
Supplementary application1: Disease severity in patient samples of previous studies.
Supplementary application 2: Clinical ratings of the patient sample.
References
- Bohanna I., Georgiou-Karistianis N., Hannan A.J., Egan G.F. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington's disease. Brain Research Reviews. 2008;58:209–225. doi: 10.1016/j.brainresrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Calder A.J., Lawrence A.D., Young A.W. Neuropsychology of fear and loathing. Nature Reviews. Neuroscience. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Di Maio L., Squitieri F., Napolitano G., Campanella G., Trofatter J.A., Conneally P.M. Onset symptoms in 510 patients with Huntington's disease. Journal of Medical Genetics. 1993;30:289–292. doi: 10.1136/jmg.30.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Gaura V., Ribeiro M.J., Lethimonnier F., Maroy R., Verny C. Distribution of grey matter atrophy in Huntington's disease patients: a combined ROI-based and voxel-based morphometric study. NeuroImage. 2006;32:1562–1575. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Consulting Psychological Press; Palo Alto, CA: 1976. Pictures of Facial Affect. [Google Scholar]
- Gray J.M., Young A.W., Barker W.A., Curtis A., Gibson D. Impaired recognition of disgust in Huntington's disease gene carriers. Brain. 1997;120:2029–2038. doi: 10.1093/brain/120.11.2029. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Worall H., Keller F. Bearbeitung der deutschen Ausgabe. Testhandbuch. Huber; Bern, Göttingen, Toronto, Seattle: 1994. Beck-Depressions-Inventar (BDI) [Google Scholar]
- Hayes C.J., Stevenson R.J., Coltheart M. Disgust and Huntington's disease. Neuropsychologia. 2007;45:1135–1151. doi: 10.1016/j.neuropsychologia.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Helder D.I., Kaptein A.A., van Kempen G.M., van Houwelingen J.C., Roos R.A. Impact of Huntington's disease on quality of life. Moving Disorders. 2001;16:325–330. doi: 10.1002/mds.1056. [DOI] [PubMed] [Google Scholar]
- Henley S.M., Wild E.J., Hobbs N.Z., Warren J.D., Frost C., Scahill R.I. Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia. 2008;46:2152–2160. doi: 10.1016/j.neuropsychologia.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Henley S.M., Wild E.J., Hobbs N.Z., Scahill R.I., Ridgway G.R., Macmanus D.G. Relationship between CAG repeat length and brain volume in premanifest and early Huntington's disease. Journal of Neurology. 2009;256:203–212. doi: 10.1007/s00415-009-0052-x. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A., Schroeder U., Erhard P., Haslinger B., Stahl R., Weindl A. Neural correlates associated with impaired disgust processing in pre-symptomatic Huntington's disease. Brain. 2004;127:1446–1453. doi: 10.1093/brain/awh165. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group Unified Huntington's Disease Rating Scale: reliability and consistency. Movement Disorders. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- Ihl R., Grass-Kapanke B., Lahrem P., Brinkmeyer J., Fischer S., Gaab N. Development and validation of a test for early diagnosis of dementia with differentiation from depression (TFDD) Fortschritte der Neurologie-Psychiatrie. 2000;68:413–422. doi: 10.1055/s-2000-11799. [DOI] [PubMed] [Google Scholar]
- Johnson S.A., Stout J.C., Solomon A.C., Langbehn D.R., Aylward E.H., Cruce C.B. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain. 2007;130:1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- Katsikitis M. The classification of facial expressions of emotion. A multidimensional scaling approach. Perception. 1997;26:613–626. doi: 10.1068/p260613. [DOI] [PubMed] [Google Scholar]
- Kipps C.M., Duggins A.J., McCusker E.A., Calder A.J. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington's disease. Journal of Cognitive Neuroscience. 2007;19:1206–1217. doi: 10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M., Cuthbert B. Center for Research in Psychophysiology. University of Florida; Gainsville, Florida: 2001. International affective picture system. [Google Scholar]
- Langbehn D.R., Brinkman R.R., Falush D., Paulsen J.S., Hayden M.R. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clinical Genetics. 2004;65:267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Laux L., Glanzmann P., Spielberger C.D. Beltz Testgesellschaft; Weinheim: 1981. State Trait Angstinventar (STAI) [Google Scholar]
- Lee E., Kang J.I., Park I.C.H., Kim J.J., An S.K. Is a neutral face really evaluated as being emotionally neutral? Psychiatry Research. 2008;157:77–85. doi: 10.1016/j.psychres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lundquist D., Flykt A., Öhman A. Department of Neurosciences Karolinska Hospital; Lawrence, Stockholm, Sweden: 1998. The Karolinska Directed Emotional Faces of Emotion and Attention. [Google Scholar]
- Margraf J. Verlag Springer; Berlin, Heidelberg, New York: 1994. Mini-DIPS — Diagnostisches Kurz-Interview bei psychischen Störungen. [Google Scholar]
- Milders M., Crawford J.R., Lamb A., Simpson S.A. Differential deficits in expression recognition in gene-carriers and patients with Huntington's disease. Neuropsychologia. 2003;41:1484–1492. doi: 10.1016/s0028-3932(03)00079-4. [DOI] [PubMed] [Google Scholar]
- Montagne B., Kessels R.P., Kammers M.P., Kingma E., de Haan E.H., Roos R.A. Perception of emotional facial expressions at different intensities in early-symptomatic Huntington's disease. European Neurology. 2006;55:151–154. doi: 10.1159/000093215. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Senior C., Brammer M., Andrew C., Calder A.J. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Schienle A., Walter B., Stark R., Vaitl D. A questionnaire for the assessment of disgust sensitivity. Zeitschrift für Klinische Psychologie und Psychotherapie. 2002;31:110–120. [Google Scholar]
- Schienle A., Stark R., Walter B., Blecker C., Ott U., Sammer G. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13:2023–2026. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Schienle A., Schäfer A., Walter B., Stark R., Vaitl D. Relationship between disgust sensitivity, trait anxiety and brain activity during disgust induction. Neuropsychobiology. 2005;51:86–92. doi: 10.1159/000084165. [DOI] [PubMed] [Google Scholar]
- Schwenkmezger P., Hodapp V., Spielberger C.D. Huber; Bern: 1992. State-Trait-Ärgerausdrucksinventar STAXI. [Google Scholar]
- Shaffer I.P. Modified sequentially rejective multiple test procedures. Journal of the American Statistical Association. 1986;81:826–831. [Google Scholar]
- Shoulson I., Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29:1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- Snowden J.S., Austin N.A., Sembi S., Thompson J.C., Craufurd D., Neary D. Emotion recognition in Huntington's disease and frontotemporal dementia. Neuropsychologia. 2008;46:2638–2649. doi: 10.1016/j.neuropsychologia.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R., Young A.W., Calder A.J., Karnat A., Lange H., Homberg V. Loss of disgust. Perception of faces and emotions in Huntington's disease. Brain. 1996;119:1647–1665. doi: 10.1093/brain/119.5.1647. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R., Rausch M., Eysel U.T., Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of Biological Sciences. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R., Schroeder U., Young A.W., Epplen J.T. Disgust in pre-clinical Huntington's disease: a longitudinal study. Neuropsychologia. 2006;44:518–533. doi: 10.1016/j.neuropsychologia.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Stark R., Zimmermann M., Kargerer S., Schienle A., Walter B., Weygand M., Vaitl D. Hemodynamic brain correlates of disgust and fear ratings. NeuroImage. 2007;37:663–673. doi: 10.1016/j.neuroimage.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Thompson J.C., Snowden J.S., Craufurd D., Neary D. Behavior in Huntington's disease: dissociating cognition-based and mood-based changes. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:37–43. doi: 10.1176/jnp.14.1.37. [DOI] [PubMed] [Google Scholar]
- van Duijn E., Kingma E.M., van der Mast R.C. Psychopathology in verified Huntington's disease gene carriers. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:441–448. doi: 10.1176/jnp.2007.19.4.441. [DOI] [PubMed] [Google Scholar]
- Wang K., Hoosain R., Yang R.M., Meng Y., Wang C.Q. Impairment of recognition of disgust in Chinese with Huntington's or Wilson's disease. Neuropsychologia. 2003;41:527–537. doi: 10.1016/s0028-3932(02)00171-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary application1: Disease severity in patient samples of previous studies.
Supplementary application 2: Clinical ratings of the patient sample.