Abstract
Objectives.
Although research indicates that depressive symptoms and memory performance are related in older adults, the temporal associations between these variables remain unclear. This study examined whether depressive symptoms predicted later memory change and whether memory predicted later change in depressive symptoms.
Methods.
The sample consisted of more than 14,000 adults from the Health and Retirement Study, a biannual longitudinal study of health and retirement in Americans older than age 50 years. Measures of delayed recall and depressive symptoms served as the main study variables. We included age, sex, education, and history of vascular diseases as covariates.
Results.
Using dynamic change models with latent difference scores, we found that memory performance predicted change in depressive symptoms 2 years later. Depressive symptoms did not predict later change in memory. The inclusion of vascular health variables diminished the size of the observed relationship, suggesting that biological processes may partially explain the effect of memory on depressive symptoms.
Implications.
Future research should explore both biological and psychological processes that may explain the association between worse memory performance and subsequent increases in depressive symptoms.
Keywords: Cardiovascular disease, Depression, Health and Retirement Study, Longitudinal change, Memory
LONGITUDINAL research indicates that episodic memory, or memory of specific events, and depressive symptoms worsen with age (McArdle, Fisher, & Kadlec, 2007; Mirowsky & Ross, 1992; Rönnlund, Nyberg, Bäckman, & Nilsson, 2005; Yang, 2007).Moreover, a sizeable percentage of older adults exhibit comorbid memory decline and depression (for reviews, see Burt, Zembar, & Niederehe, 1995; Kindermann & Brown, 1997; Steffens & Potter, 2008).Specifically, a population-based study found that 20% of individuals with mild cognitive impairment and 32% of participants with dementia had depressive symptoms compared with 7% of the general population (Lyketsos et al., 2002). Moreover, the comorbidity of depressive symptoms and memory problems in older adults is more common than that of depressive symptoms with other cognitive problems (Lockwood, Alexopoulos, Kakuma, & Van Gorp, 2000). Thus, the nature of the association between depressive symptoms and memory deficits seems particularly important to understand.
Many case–control and epidemiological prospective studies have found that a prior history of depressive symptoms increase the risk of a dementia diagnosis and cognitive decline (Chodosh, Kado, Seeman, & Karlamagla, 2007; Dotson, Resnick, & Zonderman, 2008; Jorm, 2001; Sachs-Ericsson, Joiner, Plant, & Blazer, 2005). A popular explanation for these results is that depressive symptoms are an independent risk factor for later memory problems and dementia. For instance, depressive symptoms are associated with reduced functioning in the hippocampus and structural and functional declines in the dorsolateral prefrontal cortex (Hickie et al., 2005; Taylor et al., 2004), all of which also affect memory functioning.
On the other hand, some studies have found that memory impairment (Ritchie, Gilham, Ledesert, Touchoun, & Kotzki, 1999; Vinkers, Gussekloo, Stek, Westendorp, & van der Mast, 2004) and recently diagnosed dementia (Chen, Ganguli, Mulsant, & DeKosky, 1999) predict the development of depressive symptoms. This may reflect the presence of underlying brain diseases such as cerebral atrophy, limbic atrophy, and white matter lesions that are often seen in both dementia and late onset depression (O’Brien, Ames, & Schwietzer, 1996; Schweitzer, Tuckwell, O’Brien, & Ames, 2002). Imbalances in the functioning of serotonin, dopamine, and norepinephrine are also seen in both depression and dementia and may account for their association (Schweitzer et al., 2002). Alternatively, cognitive decline may cause depressive symptoms if individuals have negative emotional reactions or experience functional impairment because of memory problems (Vinkers et al., 2004). For example, older adults with cognitive impairment who also reported a recent decrease in their ability to perform everyday tasks were more likely to develop depressive symptoms than were individuals who remained functionally intact (Ritchie et al., 1999).
In sum, there is evidence that depressive symptoms predict memory decline and that memory problems predict future depressive symptoms. These results need not be mutually exclusive: Depressive symptoms may be an independent risk factor for memory decline, and memory impairment may contribute to depressive symptoms through biological and/or psychological mechanisms. One way that researchers can test both these hypotheses at the same time is to examine the bidirectionality of the association between memory functioning and depressive symptoms. Despite the large number of prospective studies in this field, the majority only examined one hypothesized direction. We know of only two studies that tested for bidirectionality (Chen et al., 1999; Vinkers et al., 2004), both of which found that memory impairment predicted depressive symptoms but not the reverse. However, these studies used relatively small community-based samples and did not model change over time for depressive symptoms or memory.
The current study aimed to explore the directionality of the association between depressive symptoms and episodic memory performance in older adults. We used data from the Health and Retirement Study (HRS), a longitudinal study of health, retirement, and aging in Americans older than age 50. Using this same data set, Gonzalez, Bowen, and Fisher (2008) found that higher depressive symptoms at one time point were related to decreased memory up to that same time point. Unfortunately, the authors did not model changes in depressive symptoms over time; neither did they report on whether depressive symptoms predicted later memory decline. In this study, we used a bivariate dynamic model with latent difference scores (McArdle, 2001) to model the rates of change of both depressive symptoms and memory and their interdependence over time. The development of dynamic models using latent difference scores allows for the combination of time-series analysis with latent growth curve modeling. Using a set of these models, we compared (1) no dynamic coupling, (2) memory as a leading indicator of depressive symptoms, (3) depressive symptoms as a leading indicator of memory, and (4) dynamic coupling between both variables (bidirectionality). By leading indicator, we mean that within-person changes in that variable will occur in time before changes in the other (lagging) variable. These methods have been used with older adults to examine lead–lag relationships between perceptual speed and knowledge changes, perceptual speed and social participation, and physical activity and cognitive decline (McArdle, 2009). As with other longitudinal regression models, causation is implied but not directly tested.
METHOD
Participants and Procedure
Participants came from the HRS, a biannual survey of health and retirement measures given to Americans older than age 50 (Juster & Suzman, 1995). Currently, the HRS includes more than 30,000 participants. Participants and their spouses were interviewed in person the first time and subsequently over the telephone (see Heeringa & Connor, 1995 for HRS design and method).
We included all participants who were older than 50 years of age and had cognitive testing information or depressive symptom scores from at least one wave of data collection. Because the HRS became demographically representative of the U.S. population older than 50 in 1998, we only included waves starting in or after 1998 (the HRS has data starting from 1992). One participant was randomly selected from each household. This resulted in a sample size of 14,789 with a maximum of five waves of data for each person for a total of 52,169 observations. The HRS adds new cohorts every 6 years (here in 1998 and 2004) to maintain representativeness to the U.S. population. Because of the open design, not all participants included have been interviewed all five times (M = 3.69, SD = 1.54). The Ns for the different waves are 11,770 in 1998, 10,515 in 2000, 9,546 in 2002, 10,787 in 2004, and 10,001 in 2006. The HRS has sample weights to compensate for unequal selection probabilities in geographical areas and ethnic groups (see Heeringa & Connor, 1995). We used sampling weights from the last wave for each individual, so the sample statistics and results are expected to characterize people in the Unites States older than 50 years old.
Measures
Memory.—
The cognition items were adapted from the Telelphone Interview for Cognitive Status (Brandt, Spencer, & Folstein, 1988). The memory measures included immediate and delayed free recall of a list of 10 common nouns. Assessors read the 10 nouns out loud (one word per second). Participants were asked to recall as many words as they could in any order. Recall was assessed immediately and after 3 min of interference tasks, with a possible score of 0–10 on each measure. Chen and colleagues (2000) found that delayed but not immediate recall discriminated between individuals who were presymptomatic for dementia and those who would remain nondemented at follow-up. Thus, we examined only the delayed verbal memory scores.
Depressive symptoms.—
Depressive symptoms were measured using a self-report eight-item subset of the Center for Epidemiological Studies-Depression scale (CES-D; Radloff, 1977). The brief version taps the same symptom dimensions in older adults as the original 20-item CES-D (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993). Participants indicated (yes or no) whether they had experienced each symptom in the past week. We summed the scores (0 or 1) across all items. Internal consistency was good (Cronbach’s α = .77–.83 in different waves).
Vascular disease and risk factors.—
Participants were asked at each wave if a doctor had ever told them that they had (1) heart problems, (2) stroke, (3) diabetes, and (4) hypertension. We created time-varying dichotomous variables that were coded as 0 if the participant endorsed not having these problems until that time point; otherwise, health variables were coded as 1.
Analytic Strategy
We used a structural equation modeling (SEM) approach with latent difference scores (McArdle, 2001). We first built univariate growth and change models separately for delayed recall and depressive symptoms based on latent difference scores. Model building consisted of the following steps:
(1) The measured score for each individual at each time [t] of measurement was modeled as a latent true score plus a residual error term. A person-level intercept provided the initial or starting point to the true score at Wave 1.
(2) Change from [t] to [t + 1] was modeled as a latent change variable (Δ). We had five waves and four latent change variables with identical assumptions and estimates. Latent true scores after Wave 1 were influenced by the previous wave true score plus the intervening latent change variables.
(3) The first source of individual variation in the change variables was a person-level slope representing linear stable change over time.
(4) The second source of variation in change was a proportional change over time component (β). This dynamic component indicates the degree to which latent change depends on the previous wave true score.
(5) Time-invariant covariates (sex, age at the first wave, and years of education) were added to explain the variance in the individual’s intercept and slope. Because significant positive practice effects have been reported with the HRS memory items (e.g., McArdle et al., 2007), we included the number of times participants had been given the memory test before 1998 as a time-invariant covariate for memory level and slope.
(6) We added time-varying covariates of vascular health to examine their dynamic impact on the latent curve parameters for memory and depressive symptoms.
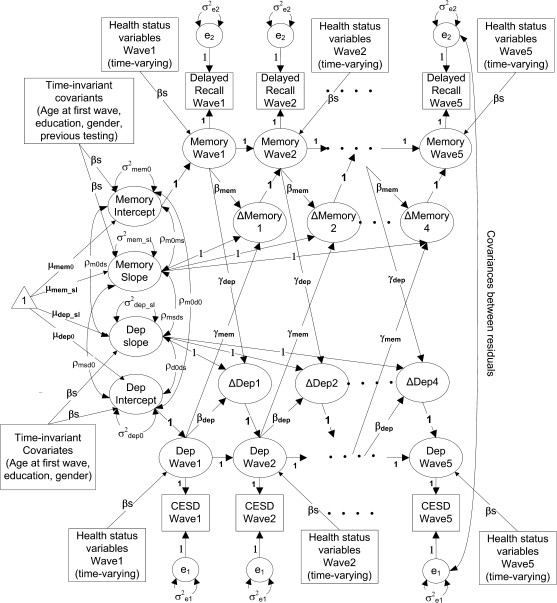
(7) We combined the individual change models for memory and depressive symptoms to form a bivariate change model (Figure 1). Also included are cross-variable paths from memory at [t] to change in depressive symptoms at [t + 1] (γdep), and from depressive symptoms at [t] to change in memory at [t + 1] (γmem). These paths capture the within-person longitudinal relationship between memory and depressive symptoms. The intercepts and slopes for memory and depressive symptoms were allowed to correlate to model between-person cross-sectional associations between these variables (see McArdle, 2001 for details).
Figure 1.
Longitudinal dynamic latent change score model for memory and depressive symptoms. Rectangles represent measured variables, ovals are latent constructs, and the triangle represents means and intercepts.
Because of the large sample size, we selected a significance level of .0001. We conducted all analyses using Mplus version 4 (Muthén & Muthén, 1998–2006).
RESULTS
Descriptive Statistics
Table 1 lists the characteristics for the weighted sample. Memory recall was normally distributed, but depressive symptoms were positively skewed. Transformations of the depressive symptoms variable did not result in improved skewness. Moreover, SEM estimation procedures require residual terms to be randomly distributed not the outcome variables themselves. Thus, we used the untransformed depressive symptoms variable in our analyses. The plots of the estimated values from the SEM models indicated a good approximation to the observed distribution of the depression variable.
Table 1.
Descriptive Statistics for Participants at First Testing and Over All Occasions
| At first testing |
Over all occasions |
|||||||
| Variable | M (SD)/% | Range | Skewness | Kurtosis | M (SD)/% | Range | Skewness | Kurtosis |
| Age | 62.32 (10.7) | 50–105 | .794 | −.412 | 65.92 (10.5) | 50–109 | .536 | −.605 |
| Female | 53.49 | — | — | — | 54.51 | — | — | — |
| Caucasian | 83.53 | — | — | — | 84.88 | — | — | — |
| Education (years) | 12.67 (3.45) | 0–20 | −.556 | 1.216 | 12.66 (3.43) | 0–20 | −.518 | 1.254 |
| Hypertension | 43.96 | — | — | — | 50.96 | — | — | — |
| Diabetes | 13.21 | — | — | — | 16.39 | — | — | — |
| Heart disease | 17.21 | — | — | — | 22.36 | — | — | — |
| Stroke | 5.49 | — | — | — | 6.42 | — | — | — |
| Memory | 4.59 (2.1) | 0–10 | −.215 | −.064 | 4.49 (2.1) | 0–10 | −.178 | −.071 |
| CES-D | 1.63 (2.0) | 0–8 | 1.377 | 1.161 | 1.57 (2.0) | 0–8 | 1.416 | 1.275 |
Notes: N = 14,789. Number of data points for all occasions = 52,169. All statistics are weighted with respondent level sampling weights to adjust to a U.S. national norm. Memory = score on delayed memory recall task. CES-D = Center for Epidemiological Studies-Depression.
Attrition Analyses
Even though the HRS has an excellent follow-up rate (93%–96% in different years; HRS staff, 2008), we have significant missing data because of inability or refusal to complete measures and due to death of participants (13.9% died during follow-up). Excluding missing data due to death, 72.0% participants had CES-D data at all possible time points, 13.8% were missing data on one time point, 5.7% on two, 4.6% on three, and 3.9% on four time points. The corresponding percentages for memory were 69.3%, 14.8%, 6.5%, 4.8%, and 4.2%. Fifty-six participants were missing memory scores during all five waves. We used regression analyses to identify characteristics of participants with any missing data. Less than 5% of the variance was accounted for by previously measured variables (age at last wave, sex, education, history of vascular disease, CES-D, and recall at the last time of testing). Lower memory scores, less education, history of stroke, and male sex were predictive of participants with missing data on both measures, but all coefficients were small (standardized βs < .1). We also repeated our SEM analyses after excluding the 13.9% (n = 2,053) participants who died and found results to be almost identical to when they were included.
For all analyses described subsequently, we expected nonrandom attrition and used methods that accounted for this. All available information was used to build maximum likelihood estimates with missing data points included as latent variables. The missing at random (MAR) assumption in Mplus assumes that the pattern of missing data is either random or can be fully accounted for by already-measured variables (Little & Rubin, 1987). If these assumptions are met, then the estimates for the parameters are as if everyone had continued to participate. In all analyses, participant-level weights were used with maximum likelihood estimation with robust standard errors. Demographic covariates were centered with a mean of zero (precentering means for age = 62.16 years, education = 12.68 years, sex = 0.53 [female = 1, male = 0], retest = 1.12 tests before 1998).
Univariate Dual Change Score Models
Memory recall.—
We first built a growth and change score model for memory by using delayed recall scores at each wave (top half of Figure 1). Recall scores were available at two-year intervals, so a two-year period was used as the time lag interval. The latent true score on the recall task in 1998 represented the initial level of memory for each participant. Initial memory was modeled as a mixed effect with fixed and random effects. Initial memory was regressed on demographic covariates (age at first wave, sex, and education) and number of recall tests answered before 1998 by the participant. The first model was a no-change over time model against which to compare other models. Subsequent models represented explicit theories of change. Respondent level weights were used in all models to ensure representativeness to the U.S. norm. The no-change model fit was good: χ2 (33) = 1096.4, Comparative Fit Index (CFI) = 0.928, and Root Mean Square Error of Approximation (RMSEA) = 0.047. An RMSEA of 0.05 or less indicates a close fit in relation to the degrees of freedom (Browne & Cudeck, 1993) and a CFI of greater than 0.90 is commonly accepted as a good fit.
Next, change in memory was modeled as an individual-specific mixed effect of slope (stable linear change). There was considerable between-person variation in the initial level (M = 4.62, SD = 1.65) and slope (M = −0.145, SD = 0.21). The random components of initial level and slope were allowed to covary (r = −.331, p < .0001). Including linear slope resulted in a much better fit than the no-change model: χ2 (26) = 226.4, CFI = 0.986, and RMSEA = 0.023. The Satorra–Bentler–scaled chi-squared difference test was used to evaluate the significance level (Muthén & Muthén, 1999): Δχ2 (7) = 928.2, p < .0001.
Next, we added the component of proportional change (βmem), so that how much people changed on memory depended on their memory at the previous testing point. This parameter (βmem = −.153, SE = 0.042) resulted in some improved fit but was not significant at the .0001 level: χ2 (25) = 214.8, CFI = 0.987, RMSEA = 0.023; Δχ2 (1) = 12.01, p = .0005. This suggests that the previous linear change model may be the better model.
We then added the four vascular variables as time-varying covariates to the linear change model. The latent true score for memory at each wave was regressed on these health variables. See Table 2 for estimates. The average participant recalled 4.67 words (SD = 1.62). Over time, memory declined by an average of 0.15 words in 2 years (SD = 0.21). Age at the first wave affected initial memory, with every decade resulting in 0.70 fewer words recalled. Age at first wave also negatively predicted change in memory. Memory decline accelerated over time: an additional loss of 0.46 words every 2 years for every decade of age. Participants recalled 0.17 more words for every year of education. Women recalled 0.67 more words than men but declined slightly faster. Practice effects resulted in an advantage of 0.22 words for each previous occasion of testing. Those who had no previous testing declined slightly less on average. Covariates accounted for 38.1% of the variance in initial memory and 16.2% of the slope. Some vascular variables had a dynamic cumulative effect on memory. Participants with stroke history became progressively worse with an average of 0.13 fewer words in each wave. Diabetes and hypertension also affected memory, although their effect was smaller. Heart disease did not have a significant effect on memory.
Table 2.
Univariate Latent Change Models for Memory and Depressive Symptoms
| Parameter | Memory constant change model |
Depressive symptoms dual change model |
||||||
| Initial level |
Slope |
Initial level |
Slope |
|||||
| Est (SE) | Std | Est (SE) | Std | Est (SE) | Std | Est (SE) | Std | |
| Mean (μ) | 4.668* (.020) | — | −.152* (.006) | — | 1.663* (.021) | — | .870* (.074) | — |
| Variance (σ) | 2.619* (.078) | 1 | .045* (.007) | 1 | 2.008* (.079) | 1 | .689* (.104) | 1 |
| Regression paths to initial level and slope | ||||||||
| Education (in years) | .175* (.005) | .370 | −.002 (.002) | −.031 | −.123* (.006) | −.297 | −.067* (.006) | −.276 |
| Sex (female) | .673* (.036) | .207 | −.047 (.012) | −.109 | .325* (.039) | .114 | .218* (.027) | .131 |
| Age (in decades) | −.704* (.017) | −.462 | −.050* (.006) | −.249 | −.075 (.019) | −.056 | −.066* (.011) | −.084 |
| Number of tests before 1998 | .225* (.013) | .179 | −.033* (.004) | −.197 | — | — | ||
| Regression estimates for time-varying covariates and self-feedback paths to memory and depressive symptoms | ||||||||
| Heart disease | −.011 (.010) | −.002 | .289* (.029) | .077 | ||||
| Stroke | −.131* (.020) | −.019 | .352* (.045) | .057 | ||||
| Diabetes | −.058* (.011) | −.012 | .149* (.027) | .035 | ||||
| Hypertension | −.031 (.008) | −.009 | .138* (.019) | .048 | ||||
| β | “=0” | −.531* (.046) | ||||||
Notes: N = 14,789. Est = nonstandardized estimate, SE = standard error, Std = standardized estimate. “=0” = parameter constrained to zero. Models fit with sampling weights and the missing at random assumption.
*p < 10−-6.
Depressive symptoms.—
An analogous procedure of model building was adopted for depressive symptoms (bottom half of Figure 1), except that amount of previous testing was not included. The fit of the no-change model was good: χ2 (29) = 229.2, CFI = 0.980, and RMSEA = 0.022. The linear stable change model with slope resulted in significant improvement in fit: χ2 (23) = 57.3, CFI = 0.997, RMSEA = 0.010; Δχ2 (6) = 182.8, p < .0001. Addition of the proportional change path (βdep) resulted in further improvement in fit: χ2 (22) = 34.5 (22), CFI = 0.999, RMSEA = 0.006; Δχ2 (1) = 17.4, p < .0001.
Finally, we added vascular health status variables as time-varying covariates. See Table 2 for estimates. The average participant reported 1.66 symptoms (SD = 1.42). The slope indicated an increase in depressive symptoms over time, (M = 0.87, SD = 0.83). However, the proportional change paths were negative (βdep = −.531, SE = 0.05), indicating that individuals who were initially high on depressive symptoms did not increase as rapidly or had reduced symptoms over time. Participants endorsed 0.12 fewer symptoms for every extra year of education. Those with more education also had a less positive slope. Age at first wave had a small effect on initial level: Every decade of age resulted in 0.075 fewer symptoms. There were also some nonlinear effects of age with less rapid increases in depressive symptoms in older adults. Women endorsed 0.325 more symptoms than men and also increased more in symptoms over time (0.22 symptoms every 2 years). Covariates accounted for 10.1% of the variance in initial depressive symptoms and 9.3% of the linear change over time. All four vascular health variables were associated with cumulatively more depressive symptoms over time: Stroke, heart disease, diabetes, and hypertension increased depressive symptoms every 2 years by 0.35, 0.29, 0.15, and 0.14, respectively.
Dynamic Change Models of Memory and Depressive Symptoms
The final best-fitting univariate models for memory and depressive symptoms were combined into a bivariate model (Figure 1). Age, education, sex, and retest were included as covariates as before. The models were made increasingly complex to test the across-time paths between memory and depressive symptoms. In the first model, memory and depressive symptoms did not interact over time (no γs in Figure 1), although passive correlations between memory and depressive symptoms initial levels and slopes were allowed. This initial simple model fit the data very well: χ2 (73) = 330.7, CFI = 0.990, and RMSEA = 0.015.
In a second model, we added the paths from memory at [t] to change in depressive symptoms at [t + 1] (γdep in Figure 1; memory as the leading variable). The addition of these paths to the model resulted in a significantly improved fit: χ2 (72) = 290.0, CFI = 0.992, RMSEA = 0.014; Δχ2 (1) = 52.5, p < .0001.
In a third model, we added the paths from depressive symptoms at [t] to change in memory at [t + 1] (γmem in Figure 1; depressive symptoms as the leading variable). The paths from memory at [t] to change in CES-D at [t + 1] from Model 2 were set to zero (γdep = 0) . This model did not fit better than Model 1: χ2 (72) = 332.9, CFI = 0.990, RMSEA = 0.016; Δχ2 (1) = 0.08, p = .78.
The fourth model tested the bidirectionality hypothesis by freely estimating both coupling parameters (γdep and γmem). Although better than Model 1, it did not result in improvement in fit over Model 2: χ2 (71) = 291.5, CFI = 0.992, RMSEA = 0.014; Δχ2 (1) from Model 2 = 0.01, p = .90.
Thus, although all the models had very good fit, the second model (with memory as the leading variable) was best in terms of relative fit. This suggests that memory predicted change in depressive symptoms, but depressive symptoms did not reliably predict change in memory two years later.
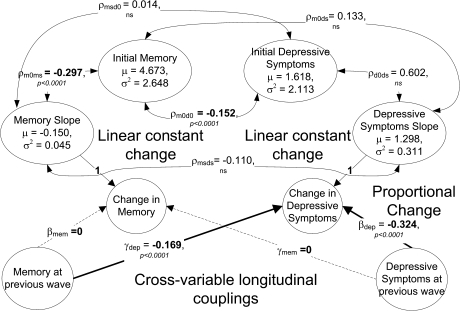
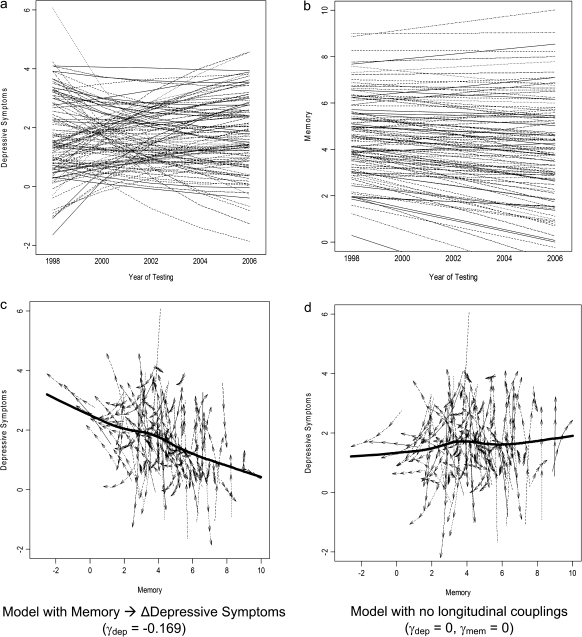
Figure 2 shows the different factors contributing to longitudinal changes in memory and depressive symptoms in this best-fitting dynamic model (Model 2). The only factor contributing to longitudinal changes in memory scores was the memory slope (M = −0.150, SD = 0.212). This model accounted for a majority of the total variance in individual delayed recall scores (ranging from 56.2% to 59.1% in the different waves). There were three sources of change in depressive symptoms. (1) Symptoms were influenced by the within-person, linear slope (M = 1.298, SD = 0.557). (2) The proportional change component (βdep = −.324) also significantly affected change in depressive symptoms, such that more symptoms at [t] were associated with a greater decrease in symptoms at [t + 1] two years later. (3) Finally, previous memory affected change in depressive symptoms (γdep = −.169), indicating that better memory at [t] was associated with greater decrease in depressive symptoms at [t + 1] two years later. In Figure 3a–c, we have plotted the longitudinal trajectories for depressive symptoms and memory over time and as a function of each other. Figure 3d shows trajectories for depressive symptoms and memory for the no-coupling case (the first bivariate model described previously). We can see the effect of the longitudinal coupling parameter γdep by contrasting Figures 3c and d. There is a strong negative trend in 3c with better memory serving as a protective factor against rising depressive symptoms.
Figure 2.
Parameter estimates for best-fitting dynamic latent change score bivariate model for memory and depressive symptoms.
Figure 3.
Expected trajectories for memory and depressive symptoms from bivariate model for hundred simulated participants (a and b). Trajectories over time from model in Figure 2 for depressive symptoms and memory (c and d). Plots of directional vectors for depressive symptoms and memory from two separate models. The solid line represents the mean relationship between memory and depressive symptoms. Trajectories plotted in (c) are from the model in Figure 2 with memory as a leading indicator of negative change in depressive symptoms (γdep = −.169, γmem = 0) and in (d) from a model without longitudinal couplings (γdep = 0, γmem = 0).
Overall, this model accounted for a majority of the total variance in observed depressive symptoms (ranging from 55.9% to 61.6% in the different waves). Between-person cross-sectional relationships also existed between these variables (see Figure 2): ρm0d0 = −.152, participants who had better memory performance endorsed somewhat fewer depressive symptoms and vice versa; ρm0ms = −.297, better initial memory was associated with less memory decline. Other correlations were not significant.
Finally, we included vascular health variables as time-varying covariates to examine whether these variables account for the longitudinal relationship between memory and depressive symptoms. This model fit the data well: χ2 (264) = 749.4, CFI = 0.984, and RMSEA = 0.011.The longitudinal relationship between memory and depressive symptoms diminished but still remained significant (γdep = −.120, SE = 0.027, p < .0001). This represented a 29% change in the value of this parameter from the previous model without vascular health variables.
DISCUSSION
This longitudinal study examined the complex association between memory performance and depressive symptoms in a demographically representative sample of more than 14,000 older Americans. We first modeled how both depressive symptoms and memory change over time. We then created bivariate dynamic models to examine whether depressive symptoms predict later memory change and whether memory predicts later change in depressive symptoms. Several important findings emerged.
Consistent with previous research (McArdle et al., 2007; Nilsson, Bäckman, Erngrund, & Nyberg, 1997), memory performance declined over time for the entire sample. Moreover, older individuals evidenced worse memory performance at their initial testing as well as greater memory decline over time. Thus, our results add support to findings (Rönnlund et al., 2005) that decline in episodic memory accelerates with age in older adults.
Depressive symptoms on average increased over time, although there was significant variability in this trend and factors other than age accounted for the increase. Specifically, as the proportion of women and health problems increased over time, so did depressive symptoms. After accounting for these factors, older individuals actually tended to report fewer initial depressive symptoms and a less rapid increase (or a decrease) of symptoms over time. These results are in accord with previous findings that levels of depressive symptoms are lower in older than younger adults (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000).
Unlike many other studies (see Ownby, Crocco, Acevedo, John, & Loewenstein, 2006; Steffens & Potter, 2008), depressive symptoms at one time point did not predict later memory decline. Instead, memory performance predicted later depressive symptoms across the entire sample. Specifically, higher memory scores were associated with less increase in depressive symptoms two years later.
The association between memory and future depressive symptoms could reflect either biological or psychological processes. As discussed earlier, depressive symptoms appearing after memory loss may reflect the same brain pathology that caused the memory impairment. In addition, memory functioning may indicate health-related problems that also influence the development of depressive symptoms. In particular, vascular diseases like diabetes, hypertension, stroke, and heart disease increase the risk for both depressive symptoms and memory impairment (Luchsinger et al., 2005; Mast, Neufeld, MacNeill, & Lichtenberg, 2004). In our data, vascular health variables were significant covariates of changes in both memory and depressive symptoms. Moreover, including these variables in our bivariate models diminished the longitudinal relationship between memory and depressive symptoms. We should note that the association was still significant, indicating that the memory–depression link reflects more than the presence of vascular factors. Future studies might explore whether other chronic health conditions such as rheumatoid arthritis, multiple sclerosis, and sleep problems account for additional shared variance.
Psychologically, memory deficits may make individuals worried and distressed, contributing to depressive symptoms. Relatedly, poor memory may preclude the use of effective coping and self-regulation skills. Previous literature indicates that memory functioning is associated in particular with social functioning (Tan, Hultsch, & Strauss, 2009). Although the reasons for this are unknown, we speculate that adults who show memory impairment may either lack the cognitive skills for appropriate social functioning and/or may avoid social interactions in order to prevent people from noticing their decline. A decrease in social engagement will likely lead to increased depressive symptoms (Glass, De Leon, Bassuk, & Berkman, 2006).
The fact that depressive symptoms did not predict memory decline in our sample conflicts with previous findings that depressive symptoms increase the risk of dementia and cognitive decline (Jorm, 2001; Ownby et al., 2006). However, this association is stronger for longer intervals between depressive symptoms and dementia (Ownby et al., 2006). Because the HRS did not assess first onset of depressive symptoms, we could not test whether earlier depression history predicted memory functioning. Moreover, the association between depressive symptoms and subsequent memory decline has not appeared in several large prospective studies (Cervilla, Prince, Joels, & Mann, 2000; Chen et al., 1999; Comijs, Jonker, Beekman, & Deeg, 2001; Henderson, Korten, Jacomb, & Mackinnon, 1997; Vinkers et al., 2004).Differences in participant characteristics (e.g., age of onset and level of initial depressive symptoms) and methods used for assessing depressive symptoms and memory (e.g., interviews vs. self-report vs. medical records) may explain some of the discrepant findings. It is also possible that the association we found reflects a process in individuals with a normative range of depressive symptoms, whereas individuals with stronger clinical histories might experience a different process
The measures administered in the HRS were brief. These procedures are often necessary for large-scale studies. However, memory in particular is a complex construct that should ideally be measured in multiple ways (Lezak, Howieson, Loring, Hannay, & Fischer, 2004). Our use of a single indicator of memory performance limits the scope of our conclusions. Both memory functioning and depressive symptoms are also influenced by other cognitive domains like attention, processing speed, and executive functioning (Steffens & Potter, 2008). Because the HRS did not assess a full range of cognitive domains (McArdle et al., 2007), we cannot examine whether other cognitive processes account for our results. Furthermore, because the memory measure was a single indicator and the HRS does not have short-term test–retest data available, we could not assess the reliability of the memory measure. It is possible that lower reliability in the memory measure may have reduced our power to find significant effects in this direction. Given these limitations, researchers should continue to explore the bidirectionality of this relationship in both clinical and nonclinical samples.
Severely cognitively impaired or depressed individuals are likely underrepresented in this sample. Because the HRS did not include participants who were institutionalized at first assessment (Heeringa & Connor, 1995), individuals with serious memory deficits and/or depressive symptoms were less likely to be in the data set. Seriously impaired individuals may have also been less likely to participate in subsequent assessments. Our use of all available data, with the MAR assumption, circumvents this problem somewhat. Nevertheless, our results may underestimate memory problems and/or depressive symptoms in older adults. Because a smaller variance would diminish the likelihood of finding significant paths, these data may in fact underestimate true effects.
We would also like to add a note about a limitation of the method itself. The models used here depend on the assumption that between- and within-person results converge. Because of our large sample, we have a lot of between-person variance but not as much within-person variance. Thus, the longitudinal results reported here rely on both between-person differences as well as within-person changes in memory and depressive symptoms. As the HRS continues following participants over the years, the amount of within-person information collected on these variables will grow. Future replications of these analyses with more waves of data may help diminish the over reliance on between-person factors.
In conclusion, our finding that memory performance predicted future change in depressive symptoms has potential clinical implications. Specifically, good memory functioning may be protective against depressive symptoms. Better vascular health may be responsible for some of this. With the many potential stressors that adults face at this stage in life, identifying mechanisms that may protect against depressed mood is important. Older adults who maintain strong memory may be better able to cope with major life changes and challenges. Researchers have suggested interventions that may enhance memory functioning, such as healthy diets, physical exercise, mnemonics, and stimulating mental activities (Hoyer & Verhaeghen, 2006). We suggest that older adults should engage in these memory-enhancing activities to not only maintain their memory functioning but also protect against depressed mood.
FUNDING
This work was supported by National Institute on Aging Grant AG-007137 to John J. McArdle and by National Institute on Aging Grant AG-027010, which provided additional support.
Acknowledgments
We are thankful to John J. McArdle and Kevin Grimm for helpful suggestions during the analytic phase of the study. We would also like to thank Jennifer Dave for useful leads for the research background and Kelly Kadlec for help with HRS data.
References
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1988;1:111–117. [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: A meta-analysis of the association, its pattern and specificity. Psychological Bulletin. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. doi:10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Carstensen L, Pasupathi M, Mayr U, Nesselroade J. Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology. 2000;79:644–655. doi:10.1037/0022-3514.79.4.644. [PubMed] [Google Scholar]
- Cervilla J, Prince M, Joels S, Mann A. Does depression predict cognitive outcome 9 to 12 years later? Evidence from a prospective study of elderly hypertensives. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2000;30:1017–1023. doi: 10.1017/s0033291799002779. doi:10.1017/S0033291799002779. [DOI] [PubMed] [Google Scholar]
- Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia: A community-based prospective study. Archives of General Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. doi:10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle S, Cauley J, DeKosky S, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Kado D, Seeman T, Karlamagla A. Depressive symptoms as a predictor of cognitive decline: MacArthur studies of successful aging. American Journal of Geriatric Psychiatry. 2007;15:406–415. doi: 10.1097/01.JGP.0b013e31802c0c63. doi:10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Jonker C, Beekman AF, Deeg DH. The association between depressive symptoms and cognitive decline in community-dwelling elderly persons. International Journal of Geriatric Psychiatry. 2001;16:361–367. doi: 10.1002/gps.343. doi:10.1002/gps.343. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, Zonderman AB. Differential association on concurrent baseline, and average depressive symptoms with cognitive decline in older adults. American Journal of Geriatric Psychiatry. 2008;16:318–330. doi: 10.1097/JGP.0b013e3181662a9c. doi:10.1097/JGP.0b013e3181662a9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass T, DeLeon C, Bassuk S, Berkman L. Social engagement and depressive symptoms in late life: Longitudinal findings. Journal of Aging and Health. 2006;18:604–628. doi: 10.1177/0898264306291017. doi:10.1177/0898264306291017. [DOI] [PubMed] [Google Scholar]
- Gonzalez HM, Bowen ME, Fisher GG. Memory decline and depressive symptoms in a nationally representative sample of older adults: The health and retirement study (1998–2004) Dementia and Geriatric Cognitive Disorders. 2008;25:266–271. doi: 10.1159/000115976. doi:10.1159/000115976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa SG, Connor JH. Technical description of the Health and Retirement Study sample design. 1995. (Institute for Social Research Pub. DR-002). Ann Arbor: University of Michigan. [Google Scholar]
- Henderson A, Korten A, Jacomb P, Mackinnon A. The course of depression in the elderly: A longitudinal community-based study in Australia. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 1997;27:119–129. doi: 10.1017/s0033291796004199. doi:10.1017/S0033291796004199. [DOI] [PubMed] [Google Scholar]
- Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Parker G. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. British Journal of Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. doi:10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- Hoyer WJ, Verhaeghen P. Memory aging. In: Birren JE, Schaire K, Birren JE, Schaire K, editors. Handbook of the psychology of aging. 6th ed. Amsterdam, The Netherlands: Elsevier; 2006. pp. 209–232. [Google Scholar]
- HRS Staff. Health and Retirement Study: Sample sizes and response rates (2002 and beyond) 2008. Retrieved from http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf. [Google Scholar]
- Jorm A. History of depression as a risk factor for dementia: An updated review. Australian and New Zealand Journal of Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. doi:10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Juster FT, Suzman R. An overview of the Health and Retirement Study. Journal of Human Resources. 1995;30:S7–S56. doi:10.2307/146277. [Google Scholar]
- Kindermann SS, Brown GG. Depression and memory in the elderly: A meta-analysis. Journal of Clinical and Experimental Neuropsychology. 1997;19:625–642. doi: 10.1080/01688639708403749. doi:10.1080/01688639708403749. [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. Journal of Aging and Health. 1993;5:179–193. doi: 10.1177/089826439300500202. doi:10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D, Hannay H, Fischer J. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Little R, Rubin D. Statistical analysis with missing data. New York: John Wiley & Sons; 1987. [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG. Subtypes of cognitive impairment in depressed older adults. American Journal of Geriatric Psychiatry. 2000;8:201–208. doi:10.1176/appi.ajgp.8.3.201. [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. doi:10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. Journal of the American Medical Association. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. doi:10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Mast BT, Neufeld S, MacNeill SE, Lichtenberg PA. Longitudinal support for the relationship between vascular risk factors and late-life depressive symptoms. American Journal of Geriatric Psychiatry. 2004;12:93–101. doi:10.1176/appi.ajgp.12.1.93. [PubMed] [Google Scholar]
- McArdle JJ. A latent difference score approach to longitudinal dynamic structural analyses. In: Cudeck R, du Toit S, Sorbom D, editors. Structural equation modeling: Present and future. Lincolnwood, IL: Scientific Software International; 2001. pp. 342–380. [Google Scholar]
- McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. doi:10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Fisher GG, Kadlec KM. Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992–2004. Psychology and Aging. 2007;22:525–545. doi: 10.1037/0882-7974.22.3.525. doi:10.1037/0882-7974.22.3.525. [DOI] [PubMed] [Google Scholar]
- Mirowsky J, Ross CE. Age and depression. Journal of Health and Social Behavior. 1992;33:187–205. doi:10.2307/2137349. [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide (May 2006, version 4.1 ed.) Los Angeles, CA: Author; 1998–2006. [Google Scholar]
- Muthén LK, Muthén BO. Chi-square difference testing using the Satorra-Bentler scaled chi-square. 1999. Retrieved from http://www.statmodel.com/chidiff.shtml. [Google Scholar]
- Nilsson L, Bäckman L, Erngrund K, Nyberg L. The Betula prospective cohort study: Memory, health, and aging. Aging, Neuropsychology, and Cognition. 1997;4:1–32. doi:10.1080/13825589708256633. [Google Scholar]
- O’Brien J, Ames D, Schwietzer I. White matter changes in depression and Alzheimer's disease: A review of magnetic resonance imaging studies. International Journal of Geriatric Psychiatry. 1996;11:681–694. doi:10.1002/(SICI)1099-1166(199608)11:8<681::AID-GPS426>3.0.CO;2-U. [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. doi:10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Ritchie K, Gilham C, Ledesert B, Touchoun J, Kotzki P. Depressive illness, depressive symptomotology and regional cerebral blood flow in elderly people with subclinical cognitive impairment. Age & Ageing. 1999;28:385–391. doi: 10.1093/ageing/28.4.385. doi:10.1093/ageing/28.4.385. [DOI] [PubMed] [Google Scholar]
- Rönnlund M, Nyberg L, Bäckman L, Nilsson L. Stability, growth, and decline in adult life span development of declarative memory: Cross-sectional and longitudinal data from a population-based study. Psychology and Aging. 2005;20:3–18. doi: 10.1037/0882-7974.20.1.3. doi:10.1037/0882-7974.20.1.3. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Joiner T, Plant E, Blazer D. The influence of depression on cognitive decline in community-dwelling elderly persons. American Journal of Geriatric Psychiatry. 2005;13:402–408. doi: 10.1176/appi.ajgp.13.5.402. doi:10.1176/appi.ajgp.13.5.402. [DOI] [PubMed] [Google Scholar]
- Schweitzer I, Tuckwell V, O’Brien J, Ames D. Is late onset depression a prodrome to dementia? International Journal of Geriatric Psychiatry. 2002;17:997–1005. doi: 10.1002/gps.525. doi:10.1002/gps.525. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Potter GG. Geriatric depression and cognitive impairment. Psychological Medicine. 2008;38:163–175. doi: 10.1017/S003329170700102X. doi:10.1017/S003329170700102X. [DOI] [PubMed] [Google Scholar]
- Tan J, Hultsch D, Strauss E. Cognitive abilities and functional capacity in older adults: Results from the modified scales of independent behavior—Revised. The Clinical Neuropsychologist. 2009;23:479–500. doi: 10.1080/13854040802368684. doi:10.1080/13854040802368684. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan K. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. The American Journal of Psychiatry. 2004;161(7):1293–1296. doi: 10.1176/appi.ajp.161.7.1293. doi:10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Vinkers DJ, Gussekloo J, Stek ML, Westendorp RJ, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: Prospective population based study. British Medical Journal. 2004;329:881–884. doi: 10.1136/bmj.38216.604664.DE. doi:10.1136/bmj.38216.604664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Is old age depressing? Growth trajectories and cohort variations in late-life depression. Journal of Health and Social Behavior. 2007;48:16–32. doi: 10.1177/002214650704800102. doi:10.1177/002214650704800102. [DOI] [PubMed] [Google Scholar]