Abstract
Objectives.
This study assessed the moderating role of marital quality in the effects of subjective and objective vision on functional limitations, social isolation, and depressive symptomatology.
Method.
Data from 738 married older adults drawn from a probability-based representative sample of elders residing in the United States were used. Assessments included subjective and objective vision, marital quality variables (relationship satisfaction, supportive spouse behaviors, and free time spent with one’s spouse), and three aspects of quality of life (functional limitations, social isolation, and depressive symptomatology).
Results.
Moderated regression analyses found that relationship satisfaction and supportive spouse behaviors moderated the effects of poor self-reported vision on functional limitations and depressive symptoms and the effects of poor visual acuity on functional limitations. As hypothesized, poorer vision was unrelated to functional limitations and depressive symptoms in more satisfying marriages but predicted higher levels of both outcomes in less satisfying marriages. Contrary to expectations, higher levels of supportive spouse behaviors were associated with more functional limitations in respondents who reported poorer subjective and objective vision.
Discussion.
A marriage that is highly satisfying can mitigate the adverse effects of poor vision on functional limitations and depressive symptomatology in late life. The moderating role of supportive spouse behaviors in the link between poor vision and quality of life is less intuitive, however. Whereas relationship satisfaction may operate as a traditional buffer in the context of poor vision, supportive spouse behaviors may increase in response to or be ineffective in this context.
Keywords: Marital quality, Moderation, Quality of life, Self-reported vision, Visual acuity
CONSIDERABLE evidence points to the enjoyment of better health and quality of life among married older adults relative to their non-married peers (e.g., Bookwala & Fekete, 2009; Kim & McKenry, 2002; Liu & Umberson, 2008; Pienta, Hayward, & Jenkins, 2000; Umberson, Hui, & Powers, 2009). These advantages in health and quality of life are likely to accrue at least partially from the benefits that marriage can bring in the form of supportive exchanges between spouses, ongoing companionship, and emotional attachment (e.g., Allen, Blieszner, & Roberto, 2000; Bradbury, Fincham, & Beach, 2000; Xu & Burleson, 2004). Past research has shown that being in a better quality marriage is directly related to superior quality of life in late adulthood (e.g., Antonucci, Lansford, & Akiyama, 2001; Bookwala, in press; Bookwala, 2005; Bookwala & Jacobs, 2004; Umberson, Williams, Powers, Liu, & Needham, 2006). More importantly, in the face of stressors, a good marriage can serve as perhaps the most important resource of support (Cutrona, 1996) by fostering responsiveness to a loved one’s needs and acts that communicate caring and facilitate adaptive coping with stress. Several studies accordingly have confirmed that a better quality marriage can mitigate the negative impact of late life stressors on quality of life (Bookwala & Franks, 2005; Choi & Marks, 2006; Tower & Kasl, 1995; Tower, Kasl, & Moritz, 1997).
The present study focuses on the impact of poor vision as one such age-related stressor. Specifically, it examines the role of a good marriage as a potential buffer in the adverse consequences of poor vision in late life. Using vision assessed both subjectively and objectively in a national probability-based sample of adults aged 57–85 years, it examines the extent to which different aspects of marital quality (relationship satisfaction, supportive spouse behaviors, and free time spent with one’s spouse) moderate the negative effects of poor vision on three specific indicators of quality of life: functional limitations, feelings of social isolation, and depressive symptomatology. These indices are in keeping with the World Health Organization’s (1998) multidimensional definition of quality of life that incorporates individuals’ physical and social functioning and psychological states as key components.
Impact of Poor Vision
Vision-related problems are widely prevalent during the late life years. Recent estimates obtained from the National Health Interview Survey indicate that 17% of individuals aged 65+ years reported having trouble with their vision even with corrective glasses and lenses (Federal Interagency Forum on Aging-Related Statistics, 2008). Vision-related problems can have demonstrable adverse effects on the lives of older adults (e.g., Crews, 1994; Federal Interagency Forum on Aging-Related Statistics, 2008; Horowitz, 2004). For example, studies have found a link between vision-related problems and global measures of subjective well-being and life satisfaction in a variety of samples. Wahl, Schilling, Oswald, and Heyl (1999) observed that greater visual impairment was related to lower subjective well-being in a sample of older adults recruited through physician practices. Bourque, Leger, Pushkar, and Beland (2007) reported that poor vision was related to lower life satisfaction in a representative sample of older Canadians, and Reinhardt (1996) found a similar relationship in a convenience sample of visually impaired older adults.
In terms of more specific aspects of quality of life, consistent with the World Health Organization’s (1998) definition of the construct, O’Donnell (2005) has described the challenges associated with poor vision in late life to be threefold involving functional limitations, losses in social interactions, and increases in depressive symptomatology. These challenges also are in keeping with the disablement process model (Verbrugge & Jette, 1994), according to which limitations in physical functioning and difficulty in performing day-to-day activities are likely consequences in the face of impairment in domains such as visual function. Moreover, the activity restriction model (Williamson & Christie, 2009; Williamson & Shaffer, 2000) also explains that limitations in functioning (both physical and social) and depressive symptomatology are likely adverse consequences older adults experience in the face of late life stressors, and this model is applicable to the context of poor vision (Bookwala & Lawson, 2011). Several studies accordingly have examined the effects of poor vision on functional levels, social relationships, and depressive symptoms.
Perhaps the most widely examined effect of poor vision is difficulty in the ability to carry out day-to-day physical activities, and studies consistently have found evidence for adverse effects (Crews, Jones, & Kim, 2006; Gunnel & Nordholm, 1999; Horowitz, 2004; LaForge, Spector, & Sternberg, 1992; Travis, Boerner, Reinhardt, & Horowitz, 2004). Gunnel and Nordholm (1999) found that reading, writing, and watching television were common functional problems associated with poor vision. In addition, mobility-related problems, such as difficulty with moving about outdoors, driving or using public transportation, and conducting bank-related business were commonly reported in their visually impaired elderly sample. Travis and colleagues (2004) found that older adults with impaired vision reported higher levels of difficulty with instrumental activities of daily living (e.g., selecting/locating and identifying clothing, food items, and money; using a telephone; writing checks; and taking medications) and that these individuals identified these visual problems rather than other health problems as the source of these difficulties. Crews and colleagues (2006) also found that older adults who reported visual impairment were more likely to report difficulty performing everyday tasks such as walking, climbing steps, and shopping relative to their age-matched peers. In addition to contributing to limitations in physical function, researchers propose that poor vision can have an adverse impact on a wide range of social functioning domains including social network size, relationship maintenance, and social integration (O’Donnell, 2005; Reinhardt, Boerner, & Benn, 2003; Wahl & Tesch-Romer, 2001). Accordingly, studies have found that poor vision is correlated with lower levels of social integration (Femia, Zarit, & Johansson, 2001), more difficulty engaging in social relationships (Crews et al., 2006), and higher levels of social isolation (Femia et al., 2001). Finally, greater depressed affect is commonly associated with poor vision. Lupsakko, Mantyjarvi, Kautiainen, and Sulkava (2002) found that higher levels of depressive symptomatology (but not clinical depression) occurred in a population-based sample of older adults relative to their non-impaired peers, and Crews and colleagues reported that mild or moderate levels of depressive symptoms are a common comorbid condition among elders who are visually impaired. Numerous other studies also have found that older adults who rated their vision to be poorer had higher levels of depressive symptomatology in convenience and probability-based samples (e.g., Brody et al., 2001; Casten, Rovner, & Edmonds, 2001; Chou, 2008; Femia et al., 2001; Reinhardt, 1996).
Buffering Role of Marital Relationships
Despite the link that has been reported widely in the literature between poor vision and quality of life outcomes, older adults are not uniform in their experience of adverse outcomes associated with poor vision. Social support theory (see Cohen, 2004) suggests that good quality relationships can offer a critical resource in adapting to life stressors; this is especially the case during the late life years (Fuller-Iglesias, Sellars, & Antonucci, 2008; Hatch, 2005; Ormel et al., 1997; Rowe & Kahn, 1997; Turner & Noh, 1988). According to social support theory, supportive and satisfying relationships can enhance quality of life both directly as well as via their buffering role in the face of personal challenges and stressors (Cohen, 2004). Specifically in the domain of adaptation to poor vision, several studies have documented that support from family members and friends can play a key role (e.g., Horowitz, 2004; McIlvane & Reinhardt, 2001; O’Donnell, 2005; Reinhardt, 1996; Thomas & Urbano, 1993). For example, Reinhardt (1996) found evidence for the direct contribution of support from both friends and family (siblings, children, and other close relatives) to lower depressive symptomatology and higher life satisfaction among visually impaired elders. In the same sample of visually impaired older adults, McIlvane and Reinhardt (2001) also found evidence for the buffering role of social support. Relative to visually impaired older adults who reported lower levels of social support from family and friends, those who enjoyed higher support from these sources experienced lower depressive symptomatology.
A particular type of personal relationship that has been largely overlooked, however, in terms of its potential capacity to mitigate the adverse impact of poor vision in late life is the resource of a close marital relationship. The spousal relationship has been identified as playing an especially important role in health and well-being during the late adulthood years (e.g., Antonucci et al., 2001; Bookwala, in press; Bookwala, 2005; Umberson et al., 2006) and can enhance broader social functioning by fostering larger social networks (Bookwala & Fekete, 2009). When compared with other types of relationships, a good quality marriage also shows a stronger link to better psychological well-being as assessed by negative affect, depressive symptomatology, life satisfaction, and self-esteem (Birditt & Antonucci, 2007; Walen & Lachman, 2000). Moreover, we know from previous studies that the spousal relationship can play an important role in modifying the impact of life stressors on specific quality of life indicators (Bookwala & Franks, 2005; Choi & Marks, 2006; Mancini & Bonnano, 2006; Tower & Kasl, 1995; Tower et al., 1997). The general pattern of findings shows that a good quality marriage buffers the stress-outcome association in mid- and late life. For example, Mancini and Bonnano (2006) found that greater marital closeness moderated the negative impact of physical disability on older adults’ depressive symptoms, anxiety levels, and self-esteem. Likewise, Tower and colleagues (1997) found that a close marriage buffers the stress-related outcomes of living with a spouse who is cognitively impaired or depressed. Bookwala and Franks also found that greater marital disagreement exacerbates the link between physical disability and depressive symptomatology.
The Current Study
The present study hypothesized that a marriage of good quality will buffer the negative impact of poor vision (assessed both subjectively and objectively) on quality of life among older adults. As described earlier, poorer vision has been found to be linked to more functional limitations, feelings of social isolation, and depressive symptomatology. The availability of a spouse with whom one shares a satisfying relationship, who engages in supportive behaviors, and with whom recreational time is spent can be expected to play a protective role for visually impaired elders. Thus, a marital relationship marked by these characteristics would buffer the negative effects of poor vision whereby, relative to their peers in lower quality marriages, older adults with poorer vision who were in better marriages would experience fewer functional limitations, feelings of social isolation, and symptoms of depression. Data to test this hypothesis were drawn from the National Social Life, Health, and Aging Project (NSHAP; O’Muircheartaigh, Eckman, & Smith, 2009; S. Smith et al., 2009), a survey-based study of a nationally representative sample of older adults residing in the United States.
METHOD
Sample
The sample consisted of older adults who participated in the NSHAP (O’Muircheartaigh et al., 2009; S. Smith et al., 2009). The NSHAP is a large-scale survey study that assessed components of health, social relationships, and well-being in older adults aged 57–85 years using face-to-face and self-administered questionnaires. The NSHAP data were collected in 2005–2006 for which eligible cases were identified as part of a larger national area probability sample of households (O’Muircheartaigh et al., 2009). The NSHAP sample was balanced on age and gender subgroups and oversampled African Americans and Latinos. The NSHAP study design involved randomly selecting 50% (N = 1,506) of its original sample (N = 3,005) to receive the objective vision assessment. For the present study, eligible cases included all NSHAP respondents whose vision was objectively assessed and were married or living with a partner at the time of data collection (N = 927). Of these eligible respondents, those who had no missing data on the study variables were included in the current analysis yielding a final sample of 738 older adults (79.6% of the 927 NSHAP respondents). On average, respondents were 68 years of age (SD = 7.5), and 40.4% of the sample was female (N = 298). The majority of the sample had at least a high school diploma or equivalent (82.4%, N = 608) with about one fourth of the sample holding a bachelor’s degree or higher (N = 188). Slightly more than three quarters of the sample described their ethnic background as White (78.3%, N = 578), 9.3% (N = 69) as Black, 9.6% (N = 71) as Hispanic non-Black, and 2.7% (N = 20) as other. All respondents were married (N = 715, 96.9%) or living with their partner (N = 23, 3.1%). (In the remainder of the document, the term spouse is used to refer collectively to the spouse and cohabiting partner.)
Measures
Vision.—
Self-reported vision (subjective vision) was assessed in the NSHAP using a single-item measure with a 5-point rating scale (Schumm et al., 2009). Self-report single-item measures of vision have been used successfully in other studies such as the Berlin Aging Study (J. Smith, Fleeson, Geiselmann, Settersten, & Kunzmann, 1999) and the Canadian Aging in the Community Study (see Bourque et al., 2007). NSHAP respondents were asked to rate their eyesight using the item “With your glasses or contact lenses if you wear them, is your eyesight poor, fair, good, very good, or excellent?” Responses were recoded such that higher scores represented poorer self-reported vision (M = 2.43, SD = 0.99). Slightly less than half the sample (N = 340, 46%) rated their own vision to be poor, fair, or good. The remaining respondents rated their vision to be very good or excellent (N = 398, 53.9%). Visual acuity (objective vision) was assessed in both eyes together at a distance of 3 m using a chart with Sloan optotypes manufactured by Precision Vision (catalog number 2104; Schumm et al., 2009). NSHAP interviewers were trained to follow a detailed protocol to ensure consistent distance from the chart (via use of a premeasured string laid out on the floor), line of sight (the interviewer seated the respondent and held the chart at the respondent’s eye level), and lighting (sufficient light for reading with low glare or strong backlighting). Respondents who normally wore glasses or contact lenses for driving or distance vision were instructed to wear them during the test and asked to begin reading the smallest discernible line. Success or not on this task resulted in the respondent being instructed to read the successive line directly below or above the just-read line, respectively, until the smallest discernible line successfully read was determined. Performance on the visual acuity test was coded on a continuum using standard guidelines (Schumm et al., 2009), whereby 20/20 vision or better was coded as “normal or better” vision, between 20/40 and 20/20 was coded as “good” vision, between approximately 20/60 and 20/40 was coded as “moderately decreased” vision, and worse than 20/60 was coded as “poor” vision. Respondents who were unable to read the largest line on the Sloan chart at 3 m represented visual acuity worse than 20/200 (N = 4); these individuals were coded to have a score of 20/200 or poor vision. Scores in the sample ranged from normal or better (0) to poor (3) (M = 1.02, SD = 0.70). Based on their performance on the visual acuity test, 18.8% (N = 139) had normal or better vision, almost two thirds had good vision (64.6%, N = 477), 12.1% (N = 89) had moderately decreased vision, and 4.5% (N = 33) scored in the poor vision range.
Marital quality.—
The present study used NSHAP assessments of three domains of marital quality: relationship satisfaction, supportive spouse behaviors, and free time spent with one’s spouse. Marital researchers have underscored the importance of measuring different domains of marital quality (e.g., Bradbury, 1995; Bradbury et al., 2000), and prior research shows that different aspects of marriage have differential relationships to physical and mental health indicators (e.g., Bookwala, 2005; Bookwala & Franks, 2005; Bookwala & Jacobs, 2004; Umberson et al., 2006). The three aspects of marital relationships were assessed via six items available in the NSHAP data. [The six marital quality items were factor analyzed using principal components analysis with varimax (orthogonal) rotation. A three-factor extraction was requested representing the three domains. The results of the orthogonal rotation yielded an interpretable three-factor solution that collectively explained 74.624% of the variance for the set of six variables (34.238% explained by Factor 1, 23.574% by Factor 2, and 16.812% by Factor 3) with the rotated factors obtaining eigenvalues ranging from 1.01 to 2.054. Three items loaded on Factor 1 that was labeled relationship satisfaction: items measuring relationship happiness (factor loading = .692); emotional satisfaction (factor loading = .855); and physical pleasure (factor loading = .864). Two items loaded on Factor 2, labeled supportive spouse behaviors: items measuring frequency of relying on the spouse (factor loading = .800) and opening up to the spouse (factor loading = .806). Factor 3, labeled free time spent with the spouse, had a single item load on that assessed preference for spending free time (factor loading = .984). All items loaded on a single factor and are described in detail below.] Relationship satisfaction was assessed by respondents rating (a) their relationship with their spouse “taking all things together” using a 7-point scale ranging from 1 = very unhappy to 7 = very happy, (b) how physically pleasurable they considered the relationship to be with the spouse (5-point scale ranging from 0 = not at all to 4 = extremely), and (c) how emotionally satisfying they found the relationship to be (5-point scale ranging from 0 = not at all to 4 = extremely). Mean responses were computed across the three items such that higher scores reflected greater relationship satisfaction (M = 4.12, SD = 0.82; Cronbach’s α = .75). Supportive spouse behaviors was measured using two items where respondents rated how often they could rely on their spouse and open up to their spouse. Responses were made using a 3-point scale ranging from 1 = hardly ever (or never) to 3 = often. Mean responses across the two items were computed such that higher scores represented more supportive spouse behaviors (M = 2.79, SD = 0.38). Free time spent with one’s spouse was assessed using a single item that asked if the respondent liked to spend free time doing things together with their spouse or doing things separately. Responses made on a 3-point scale (1 = together, 2 = some together and some different, and 3 = different/separate things) were dichotomized in keeping with NSHAP-based research that has used this variable (Brown & Kawamura, 2011); responses were dummy-coded into 0 (responses of some together and some different and different/separate things) or 1 (together). Almost 52% (51.8%, N = 382) indicated that they liked to spend free time doing things together with their spouse, whereas the remaining responded that they liked to do some things together and some different or that they liked to do different things (48.2%, N = 356).
Functional limitations was assessed using nine items on which respondents reported how much difficulty they had related to walking (e.g., walking a street block, walking across a room), self-care activities (e.g., dressing, eating, using the toilet, bathing or showering, getting in and out of bed), and driving (e.g., driving during the day, driving at night). Responses were made on a 4-point scale using codes ranging from 1 to 4 (no difficulty, some difficulty, much difficulty, and unable to do). Activities that had never been performed were scored as no difficulty; for the driving items, if respondents indicated that they no longer drove, the response was coded as unable to do. Responses to the nine items were summed such that higher scores represented greater difficulty in performing daily functions (or more functional limitations; M = 10.66, SD = 3.01). The 9-item scale was found to be internally consistent (Cronbach’s α = .80).
Feelings of social isolation were assessed using three items (how often do you feel isolated from others, that you lack companionship, and feel left out?) adapted from the UCLA Loneliness Scale (Russell, Peplau, & Cutrona, 1980). Similar items were used by Femia and colleagues (2001) in their study of outcomes associated with vision impairment to measure feelings of social isolation in older adults. Responses to these items were made using a 3-point scale ranging from 1 = hardly ever or never to 3 = often, and scores were summed such that higher scores represented more feelings of social isolation (M = 3.78, SD = 1.22; Cronbach’s α = .80).
Depressive symptomatology was measured using an 11-item version of the Center for Epidemiological Studies–Depression scale (Radloff, 1977) that assesses the severity of depressive symptoms over a 1-week recall period. Items (e.g., “I could not ‘get going’” and “I felt that everything I did was an effort”) were answered on a 4-point scale ranging from 0 = rarely/none of the time to 3 = most of the time. Item responses were summed with higher scores reflecting more symptoms of depression (M = 4.93, SD = 4.88); the scale was internally consistent (Cronbach’s α = .79).
Control variables.—
Sociodemographic variables were measured in the NSHAP using standard self-report items. These included age (in years), gender (1 = male, 2 = female), education (1 = less than high school, 2 = high school diploma or equivalent, 3 = some college/vocational education, and 4 = college education or higher), and ethnic background (coded as a dichotomous variable, 1 = White vs. 2 = non-White). These variables were used as covariates in the moderated regression models.
RESULTS
Zero-order correlations between major study variables are listed in Table 1. A small but statistically significant correlation was obtained between self-reported vision and performance on the visual acuity test (r = .24, p < .001). As expected, respondents who rated their vision to be poorer also reported greater functional limitations, feelings of social isolation, and depressive symptomatology. Those whose visual acuity was objectively assessed to be poorer also reported more functional limitations and symptoms of depression. The three marital quality domains (relationship satisfaction, supportive spouse behaviors, and free time spent with the spouse) were correlated with each other in the expected direction; these correlation coefficients ranged from .17 to .45, indicating that these factors assessed related but distinct aspects of marital quality (shared variance ranging from .030 to .203). In addition, consistent with existing research, poorer marital quality was related to more functional limitations, feelings of social isolation, and depressive symptoms. The quality of older adults’ marital relationship was uncorrelated with objectively assessed vision, whereas better self-rated vision was related to higher relationship satisfaction and more supportive spouse behaviors.
Table 1.
Bivariate Correlations for Major Study Variables (N = 738)
| (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Self-rated vision (1) | .24 | −.13 | −.10 | .06 | .26 | .17 | .31 |
| Visual acuity (2) | .03 | .03 | .06 | .22 | .01 | .12 | |
| Relationship satisfaction (3) | .45 | .22 | −.04 | −.37 | −.28 | ||
| Supportive spouse behaviors (4) | .17 | .01 | −.22 | −.17 | |||
| Free time spent with spouse (5) | .07 | −.09 | −.01 | ||||
| Functional limitations (6) | .11 | .35 | |||||
| Feelings of social isolation (7) | .45 | ||||||
| Depressive symptomatology (8) |
Notes: |r| ≥ .09 were significant at p < .05 or better. Higher scores represent poorer vision; higher relationship satisfaction, more supportive spouse behaviors, and preference for spending free time doings things together with the spouse; more functional limitations, feelings of social isolation, and depressive symptoms.
Baron and Kenny’s (1986) approach for testing moderator models was used to assess the protective role of marital quality in the link between poorer vision and the three indicators of quality of life, functional limitations, feelings of social isolation, and depressive symptomatology. Two separate moderated regression models were tested for each indicator of quality of life using the SPSS 17.0 statistical software package; one model tested the moderating role of marital quality in the effects of self-reported (subjective) vision controlling for visual acuity (objective vision) and the second tested the same in the effects of objective vision controlling for subjective vision. Each model introduced sociodemographic (control) variables on Step 1, self-reported vision, visual acuity, and marital quality factors on Step 2, and the interaction of each marital quality variable and either self-reported vision or objective vision on Step 3. To minimize multicollinearity, predictor variables were centered before they were multiplied (Judd & McClelland, 1989). A significant interaction term, indicating that marital quality moderates the effects of vision on the criterion variable (Baron & Kenny, 1986), was decomposed such that simple slopes were computed and tested for the relationship between vision and the quality of life indicator for respondents scoring 1 SD above and below the mean on the moderating marital quality variable (Aiken & West, 1991).
Table 2 contains the results of the moderated regression models. It presents the findings separately for the moderating role of marital quality in the effects of self-reported vision and visual acuity. The results for Step 1 (examining the role of sociodemographic variables) and Step 2 (examining the direct role of the vision and marital quality variables) were identical across the models focusing on self-reported vision and visual acuity; the results for Step 3 (the test of moderation) varied across these models. Step 2 indicates that vision assessments and marital quality variables as a set explained a significant proportion of variance in functional limitations, ΔF(5,728) = 9.13, p < .001, ΔR2 = .053, feelings of social isolation, ΔF(5,728) = 25.06, p < .001, ΔR2 = .142, and depressive symptomatology, ΔF(5,728) = 22.79, p < .001, ΔR2 = .127. After controlling for the role of sociodemographic variables, poorer self-reported vision was independently associated with more functional limitations, feelings of social isolation, and depressive symptomatology and poorer visual acuity predicted more functional limitations. Among the marital quality variables, lower relationship satisfaction predicted more feelings of social isolation and symptoms of depression. [The moderated regression models were retested using two health-related variables as additional covariates on Step 1, scores on a standard single item measuring self-rated health and a count of 17 health conditions for which the respondent had received physician diagnoses (e.g., arthritis, ulcers, emphysema). The addition of these health variables to the regression models did not significantly alter the results of the original models that are provided in Table 2, and thus, the results for the original models are presented. The improvement in model fit yielded by the test of the moderator model focusing on subjective and objective vision (Steps 3A and 3B) remained statistically significant. Likewise, all but one interaction term in Steps 3A and 3B remained statistically significant (the interaction of Objective Vision × Spouse Supportive Behaviors when predicting functional limitations dropped to marginal statistical significance at p < .08). These results can be obtained from the author upon request.]
Table 2.
Marital Quality as a Moderator in the Link From Vision to Functional Limitations, Feelings of Social Isolation, and Depressive Symptoms (N = 738)
| Functional limitations |
Feelings of social isolation |
Depressive symptomatology |
||||||||||
| B | β | SE | t | B | β | SE | t | B | β | SE | t | |
| Step 1 (sociodemographic variables) | ||||||||||||
| Gender | 0.86 | .14 | 0.22 | 3.96*** | 0.32 | .13 | 0.09 | 3.54*** | 1.59 | .16 | 0.36 | 4.44*** |
| Age | 0.06 | .16 | 0.01 | 4.42*** | −0.01 | −.06 | 0.01 | −1.70 | 0.05 | .08 | 0.02 | 2.31* |
| Education | −0.55 | −.19 | 0.11 | −5.22*** | −0.03 | −.02 | 0.04 | −0.56 | −0.72 | −.15 | 0.18 | −4.10*** |
| Race | 0.32 | .04 | 0.27 | 1.18 | 0.29 | .10 | 0.11 | 2.62** | −0.04 | −.00 | 0.44 | −0.09 |
| F(4,733) | 19.15*** | 6.30*** | 11.93*** | |||||||||
| R2 | .095 | .033 | .061 | |||||||||
| Step 2 (test of direct relationships) | ||||||||||||
| Gender | 0.74 | .12 | 0.22 | 3.42*** | 0.13 | .05 | 0.09 | 1.48 | 0.97 | .10 | 0.34 | 2.83** |
| Age | 0.04 | .11 | 0.02 | 2.97*** | −0.02 | −.10 | 0.01 | −2.70** | 0.02 | .04 | 0.02 | 0.99 |
| Education | −0.44 | −.15 | 0.11 | −4.13*** | −0.02 | −.02 | 0.04 | −0.52 | −0.56 | −.12 | 0.17 | −3.36*** |
| Race | 0.03 | .01 | 0.27 | 0.13 | 0.20 | .07 | 0.11 | 1.88 | −0.68 | −.06 | 0.42 | −1.62 |
| Self-reported vision | 0.56 | .19 | 0.11 | 5.00*** | 0.14 | .11 | 0.05 | 3.14** | 1.22 | .25 | 0.18 | 6.88*** |
| Visual acuity | 0.47 | .11 | 0.16 | 2.95** | 0.03 | .01 | 0.06 | 0.39 | 0.25 | .04 | 0.25 | 0.97 |
| Relationship satisfaction | −0.12 | −.03 | 0.15 | −0.78 | −0.47 | −.31 | 0.06 | −7.95*** | −1.32 | −.22 | 0.23 | −5.66*** |
| Supportive spouse behaviors | 0.44 | .06 | 0.31 | 1.43 | −0.22 | −.07 | 0.12 | −1.79 | −0.42 | −.03 | 0.48 | −0.86 |
| Time w/spouse | 0.19 | .03 | 0.21 | 0.90 | −0.05 | −.02 | 0.09 | −0.58 | 0.12 | .01 | 0.34 | 0.37 |
| ΔF(5,728) | 9.13*** | 25.06*** | 22.79*** | |||||||||
| ΔR2 | .053 | .142 | .127 | |||||||||
| Step 3A (moderator test for self-reported vision [subjective vision]) | ||||||||||||
| Gender | 0.74 | .12 | 0.22 | 3.44*** | 0.13 | .05 | 0.09 | 1.50 | 0.99 | .10 | 0.34 | 2.89** |
| Age | 0.05 | .12 | 0.01 | 3.27*** | −0.02 | −.09 | 0.01 | −2.61** | 0.03 | .04 | 0.02 | 1.23 |
| Education | −0.42 | −.15 | 0.11 | −4.03*** | −0.02 | −.02 | 0.04 | −0.41 | −0.52 | −.11 | 0.17 | −3.13** |
| Race | 0.02 | .00 | 0.26 | 0.07 | 0.20 | .07 | 0.11 | 1.84 | −0.72 | −.06 | 0.42 | −1.73 |
| Self-reported vision | 0.44 | .15 | 0.15 | 2.90** | 0.10 | .08 | 0.06 | 1.67 | 0.99 | .20 | 0.24 | 4.06*** |
| Visual acuity | 0.46 | .11 | 0.16 | 2.89** | 0.03 | .02 | 0.06 | 0.41 | 0.24 | .03 | 0.25 | 0.95 |
| Relationship satisfaction | −0.07 | −.02 | 0.15 | −0.51 | −0.46 | −.31 | 0.06 | −7.86*** | −1.28 | −.21 | 0.23 | −5.51*** |
| Supportive spouse behaviors | 0.23 | .03 | 0.31 | 0.74 | −0.21 | −.07 | 0.13 | −1.70 | −0.45 | −.04 | 0.50 | −0.90 |
| Time w/spouse | 0.16 | .03 | 0.21 | 0.77 | −0.05 | −.02 | 0.09 | −0.59 | 0.10 | .01 | 0.34 | 0.30 |
| Interaction of Self-reported Vision × | ||||||||||||
| Relationship Satisfaction | −0.51 | −.14 | 0.13 | −3.80*** | −0.06 | −.04 | 0.05 | −1.06 | −.71 | −.12 | 0.21 | −3.35*** |
| Supportive Spouse Behaviors | 1.00 | .11 | 0.35 | 2.86** | −0.03 | −.01 | 0.14 | −0.19 | 0.22 | .02 | 0.56 | 0.40 |
| Time w/Spouse | 0.16 | .04 | 0.22 | 0.75 | 0.08 | .05 | 0.09 | 0.94 | 0.49 | .07 | 0.34 | 1.42 |
| ΔF(3,725) | 5.78*** | 0.64 | 4.11** | |||||||||
| ΔR2 | .020 | .002 | .014 | |||||||||
| Step 3B (moderator test for visual acuity [objective vision]) | ||||||||||||
| Gender | 0.74 | .12 | 0.22 | 3.40*** | 0.13 | .05 | 0.09 | 1.50 | 0.94 | .10 | 0.34 | 2.74** |
| Age | 0.05 | .11 | 0.02 | 3.09** | −0.02 | −.10 | 0.01 | −2.71** | 0.03 | .04 | 0.02 | 1.16 |
| Education | −0.43 | −.15 | 0.11 | −4.07*** | −0.02 | −.02 | 0.04 | −0.53 | −0.55 | −.12 | 0.17 | −3.30*** |
| Race | 0.07 | .01 | 0.26 | 0.25 | 0.20 | .07 | 0.11 | 1.88 | −0.62 | −.05 | 0.42 | −1.49 |
| Self-reported vision | 0.56 | .18 | 0.11 | 4.95*** | 0.15 | .12 | 0.05 | 3.23*** | 1.21 | .25 | 0.18 | 6.81*** |
| Visual acuity | 0.31 | .07 | 0.24 | 1.29 | −0.05 | −.03 | 0.10 | −0.49 | 0.02 | .00 | 0.38 | 0.06 |
| Relationship satisfaction | −0.13 | −.04 | 0.15 | −0.91 | −0.46 | −.31 | 0.06 | −7.86*** | −1.34 | −.23 | 0.23 | −5.76*** |
| Supportive spouse behaviors | 0.48 | .06 | 0.31 | 1.56 | −0.22 | −.07 | 0.12 | −1.80 | −0.31 | −.02 | 0.48 | −0.64 |
| Time w/spouse | 0.19 | .03 | 0.21 | 0.88 | −0.05 | −.02 | 0.09 | −0.58 | 0.11 | .01 | 0.34 | 0.34 |
| Interaction of Visual Acuity × | ||||||||||||
| Relationship Satisfaction | −0.49 | −.09 | 0.21 | −2.40* | 0.04 | .02 | 0.08 | 0.43 | −0.58 | −.06 | 0.33 | −1.77 |
| Supportive Spouse Behaviors | 0.88 | .07 | 0.44 | 2.01* | −0.07 | −.01 | 0.18 | −0.38 | 1.79 | .09 | 0.70 | 2.57** |
| Time w/Spouse | 0.27 | .05 | 0.31 | 0.86 | 0.12 | .05 | 0.12 | 0.97 | 0.33 | .04 | 0.49 | 0.67 |
| ΔF(3,725) | 2.68* | 0.45 | 2.73* | |||||||||
| ΔR2 | .009 | .002 | .009 | |||||||||
Notes: Higher scores represent poorer vision; more relationship satisfaction, more supportive spouse behaviors, and preference for free time spent doing things with the spouse; more functional limitations, feelings of social isolation, and depressive symptoms. Results for Steps 1 and 2 are identical for the models testing moderation of marital quality for the effects of self-reported vision and visual acuity. On Step 1, being female was associated with more functional limitations, feelings of social isolation, and symptoms of depression; being older and being less educated were associated with more functional limitations and symptoms of depression, whereas being of non-Caucasian race/ethnicity was predictive of more feelings of social isolation. Time w/spouse = free time spent with one’s spouse.
*p < .05; **p ≤ .01; ***p ≤ .001.
Moderation of the Effects of Self-reported Vision (Subjective Vision)
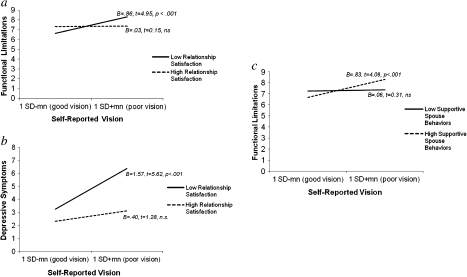
Step 3A presents the results for the test of marital quality as a moderator of the effects of poorer self-reported vision. Supporting the moderation hypothesis, the addition of the interaction terms between the marital quality variables and self-reported vision collectively resulted in a significant improvement in model fit when predicting functional limitations, ΔF(3,725) = 5.78, p < .001, ΔR2 = .020, and depressive symptoms, ΔF(3,725) = 4.11, p < .01, ΔR2 =.014; the test of the moderator model was not significant for feelings of social isolation. A significant interaction of Relationship Satisfaction × Self-reported Vision indicated that higher relationship satisfaction buffered the effects of self-reported vision on functional limitations (B = −0.51, β = −.14, t = −3.80, p < .001) and depressive symptomatology (B = −0.71, β = −.12, t = −3.35, p = .001). The simple slopes analysis showed that for those with low levels of relationship satisfaction (1 SD below the mean), poorer self-reported vision predicted significantly more functional limitations (B = 0.86, t = 4.95, p < .001). For those with high levels of relationship satisfaction (1 SD above the mean), however, self-reported vision was unrelated to functional limitations (B = 0.03, t = 0.15, p = n.s.). Likewise, the simple slopes analysis in the case of depressive symptoms showed that poorer self-reported vision predicted greater depressive symptomatology when relationship satisfaction was low (B = 1.57, t = 5.62, p < .001), but poor vision was unrelated to symptoms of depression for high relationship satisfaction (B = 0.40, t = 1.28, p = n.s.). Figure 1a and b graphically presents the simple slopes for predicting functional limitations and depressive symptoms, respectively, based on poor versus good self-reported vision (plotted at ± 1 SD around the mean) for those with low and high relationship satisfaction (computed at ± 1 SD around the mean).
Figure 1.
Moderator models for self-reported vision. (a) Simple slopes predicting functional limitations using self-reported vision for high versus low scores on relationship satisfaction (1 SD ± mean). (b) Simple slopes predicting depressive symptomatology using self-reported vision for high versus low scores on relationship satisfaction (1 SD ± mean). (c) Simple slopes predicting functional limitations using self-reported vision for high versus low scores on supportive spouse behaviors (1 SD ± mean).
Step 3A in Table 2 also indicates that supportive spouse behaviors moderated the link between self-reported vision and functional limitations (B = 1.00, β = .11, t = 2.86, p < .01). Contrary to expectations, however, decomposition of the interaction term showed that poorer self-reported vision was unrelated to functional limitations at low levels of supportive spouse behaviors (B = 0.06, t = 0.31, p = n.s.), whereas at high levels, poorer self-reported vision was associated with more functional limitations (B = 0.83, t = 4.09, p < .001; see Figure 1c).
Moderation of the Effects of Visual Acuity (Objective Vision)
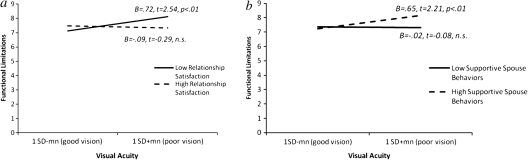
Compared with the results with self-reported vision, Step 3B in Table 2 shows weaker yet statistically significant support for marital quality as a moderator of the effects of visual acuity on functional limitations, ΔF(3,725) = 2.68, p < .05, ΔR2 = .009, and depressive symptomatology, ΔF(3,725) = 2.73, p < .05, ΔR2 = .009. As seen with self-reported vision, relationship satisfaction served to significantly buffer the impact of visual acuity on functional limitations (B = −0.49, β = −.09, t = −2.40, p < .05). The simple slopes analysis showed that, as expected, visual acuity was unrelated to functional limitations when relationship satisfaction was high (B = −0.09, t = −0.29, p = n.s.), but poor visual acuity significantly predicted more functional limitations when relationship satisfaction was low (B = 0.72, t = 2.54, p < .01). Figure 2a presents the simple slopes predicting functional limitations from visual acuity for high versus low relationship satisfaction (±1 SD around the mean) plotted at ±1 SD above and below the mean of visual acuity.
Figure 2.
Moderator models for visual acuity. (a) Simple slopes predicting functional limitations using visual acuity for high versus low scores on relationship satisfaction (1 SD ± mean). (b) Simple slopes predicting functional limitations using visual acuity for high versus low scores on supportive spouse behaviors (1 SD ± mean).
Supportive spouse behaviors also moderated the effects of visual acuity on functional limitations and depressive symptoms (B = 0.88, β = .07, t = 2.01, p < .05 and B = 1.79, β = .09, t = 2.57, p < .01, respectively, for the interaction terms). Again parallel to the findings seen with self-reported vision, poorer visual acuity was unrelated to functional limitations for low supportive spouse behaviors (1 SD below the mean) but was associated with more functional limitations for high levels (1 SD above the mean) of supportive spouse behaviors (B = −0.02, t = −0.08, p = n.s. and B = 0.65, t = 2.21, p < .01, respectively; see Figure 2b). The simple slopes analysis for depressive symptoms, however, yielded nonsignificant slopes predicting depressive symptoms from visual acuity for both high and low scores on supportive spouse behaviors (B = −0.66, t = −1.40, p = n.s. and B = 0.71, t = 1.52, p = n.s., respectively).
DISCUSSION
This study examined the moderating role played by marital quality in the path from visual function—assessed subjectively and objectively—to functional limitations, feelings of social isolation, and depressive symptomatology in a probability-based sample of older adults. Although previous studies have examined the buffering role of support from friends and family in the link between visual impairment and well-being in elders (e.g., McIlvane & Reinhardt, 2001), no study could be located that had examined the capacity of a good marriage to play such a mitigating role. And yet, the spousal relationship remains a key social tie in the late adulthood years (e.g., Antonucci et al., 2001), and one that is close or marked by better quality has been found to be an important resource for older adults (e.g., Bookwala, 2005; Bookwala & Franks, 2005; Bookwala & Jacobs, 2004; Umberson et al., 2006). The present study hypothesized that a marriage of good quality will buffer the negative impact of poor vision on older adults’ quality of life.
Preliminary analyses showed that poorer self-reported vision was consistently related to more functional limitations, feelings of social isolation, and depressive symptomatology and that poorer visual acuity was related to more functional limitations. This pattern of findings is consistent with other studies that have examined links to quality of life from self-rated visual function (e.g., Horowitz, 2004; Reinhardt, 1996) and objective assessments of distance vision (Jang et al., 2003). Consistent with study expectations and findings from other studies (e.g., Antonucci et al., 2001; Bookwala & Franks, 2005; Bookwala & Jacobs, 2004; Umberson et al., 2006), the present study also found that better marital quality, specifically a more satisfying marital relationship, predicted better quality of life in general.
Most central to the study’s goals was the test of the moderating role of a good marriage in the link from vision to quality of life. The results showed that higher marital satisfaction was a strong buffer of the effects of poorer self-reported vision on functional limitations and depressive symptomatology and a weaker but significant moderator of the effects of lower visual acuity on functional limitations. These findings show that a more satisfying marriage may be a key resource in older adults’ adaptation to poor visual function. We know from past research that a good spousal relationship can play a valuable role in enhancing quality of life (e.g., Antonucci et al., 2001; Bookwala & Jacobs, 2004; Walen & Lachman, 2000) and that a good quality marriage can mitigate the adverse effects of mid- and late life stressors such as physical disability or living with a cognitively impaired or depressed spouse (e.g., Bookwala & Franks, 2005; Mancini & Bonnano, 2006; Tower & Kasl, 1995; Tower et al., 1997). The present results extend these findings to the domain of visual function to show that a more satisfying marriage buffers the link between poor vision and quality of life in late adulthood.
Not all marital quality indicators, however, served to buffer the link from poor vision to better quality of life. Leisure or recreational time spent with one’s spouse did not moderate the negative role of poor vision in functional limitations, feelings of social isolation, and symptoms of depression. Moreover, supportive spouse behaviors moderated the link between poor vision and functional limitations in the direction opposite from that expected. More supportive spouse behaviors were associated with higher functional limitations in the case of poor vision (subjective and objective). A plausible explanation for this finding is that supportive behaviors from one’s spouse may be a response to functional limitations that accompany poorer visual ability such that older adults with poorer vision (and thus, more functional limitations) may be more likely to receive more supportive behaviors from their spouse. Some supporting evidence for this explanation is offered by Reinhardt and colleagues (2003) who found that more functional disability was linked to higher support from family members in a sample of visually impaired elders. They posit that interactions of family members are responsive in nature and are likely to be increased in times of need as in the case of a spouse who reports high visual impairment and functional limitations. It also is plausible, however, that heightened support from the spouse is overprotective or solicitous in nature. Studies of visually impaired elders have shown that overprotection or solicitous support can compromise adaptation and quality of life (e.g., Cimarolli & Boerner, 2005; Cimarolli, Reinhardt, & Horowitz, 2006). Thus, solicitous support may result in visually impaired elders being prevented by the spouse from carrying out day-to-day activities and thereby them experiencing more functional limitations. Finally, Antonucci and Akiyama (1995) pointed out that interpersonal relationships may exacerbate the effects of stressors when the support that is provided is ineffective. The supportive behaviors assessed in the NSHAP pertained to emotionally supportive behaviors rather than to material or instrumental support. It is plausible that emotionally supportive behaviors are ineffective in the context of poor vision and thus may not buffer or may even exacerbate difficulties in day-to-day function. It is essential that future studies undertake a more nuanced analysis of the role of different types of supportive behaviors (e.g., emotional support vs. instrumental support vs. solicitous support) in buffering versus exacerbating the effects of poor vision on functional limitations.
A noteworthy characteristic of the present findings is that the moderator models for self-reported vision controlled for the direct effects of visual acuity and vice versa. This approach is more rigorous because it examines the simultaneous effects of both perceived visual function and objective visual acuity. In addition, it takes into account discrepancies between individuals’ subjective assessments of their visual function and objective performance scores. An examination of the match between the two vision assessments (not reported earlier) showed that 91 respondents whose visual acuity fell in the moderately decreased to poor range rated their vision to be good to excellent and an additional 63 respondents whose visual acuity fell in the normal to good range rated their vision as fair or poor. Thus, approximately one fifth of the sample showed some discordance between their self-reported and objectively assessed vision. This is in keeping with the small (albeit significant) correlation (r = .24) obtained between self-reported vision and performance on the visual acuity test. Such discordance between subjective and objective assessments of vision has been reported in earlier research (Fors, Thorslund, & Parker, 2006) and thus including both assessments when available in statistical models is desirable. Including both subjective and objective measures of vision also can offer complementary information on visual ability and may serve to counter the limitations of the other (Horowitz, 2004). Horowitz (2004) pointed out that a performance-based measure of visual acuity does not assess impairments that may be experienced due to visual field, contrast sensitivity, and depth perception problems and thus can offer an objective but more exclusive estimate of visual problems. In contrast, she noted that a self-reported measure of visual ability is likely to capture more global evaluations of vision including trouble seeing, difficulty reading newspaper print, and difficulty seeing a familiar person across a room’s length that provide insight into the day-to-day implications of poor vision. As such, self-reported data on visual function are likely to offer a subjective but more inclusive estimate of visual problems. Future research may gain further by assessing even more specific aspects of visual function objectively (e.g., impairment in depth perception, extreme contrast or brightness sensitivity in addition to visual acuity as assessed in NSHAP) and subjectively (e.g., self-reported trouble with reading newspaper print and recognizing people at a small distance in addition to the global assessment of one’s vision as assessed in NSHAP) in the study of the effects of vision on quality of life and the capacity of a good marriage to alter these effects.
In sum, this study offers insight into the role of marital quality in protecting older adults from the negative role of vision impairment in functional limitations, feelings of social isolation, and depressive symptomatology. It does so via data from a representative sample of community-residing older adults in the United States with varying levels of visual function who participated in the NSHAP (S. Smith et al., 2009). The NSHAP is a rich source of data on the lives of older adults that includes assessments of several aspects of marital and similar partnered relationships, subjective and objective dimensions of visual function, and multiple dimensions of quality of life. These strengths notwithstanding, it is important to note the study’s limitations. First, the data are cross-sectional in nature and, as such, preclude unambiguous causal interpretations of relationships. For example, one could argue for the moderating role of quality of life in the link from vision impairment to marital quality. However, there is strong theoretical and empirical evidence for a reliable link from worse visual function to poorer quality of life (e.g., Bourque et al., 2007; Horowitz, 2004), and the stress-buffering hypothesis of social support (Cohen, 2004) provides a viable theoretical argument for treating the spousal relationship as the moderator in this link. Other studies demonstrating the moderating role of marital quality in the face of a diverse set of stressors experienced in late life (e.g., Bookwala, 2005; Bookwala & Franks, 2005; Tower & Kasl, 1995) further strengthen the rationale for treating the spousal relationship as a moderator in the well-established link between vision impairment and quality of life in late adulthood. Another limitation tied to the cross-sectional nature of the data is that the present study cannot speak to the issue of vision loss because prior levels of visual function in the sample are unknown. As such, it is not possible to assess the extent to which deterioration in vision plays a role in quality of life and the extent to which marital quality moderates the impact of such deterioration. As future waves of the NSHAP become available, however, it will be possible to assess these relationships longitudinally. Despite the cross-sectional nature of the data, the present study makes important and novel contributions to our understanding of the linkages among vision, marital quality, and quality of late life. It also adds to the growing literature on the protective role that a marriage of good quality can play in the overall promotion of health and well-being as individuals age.
FUNDING
The National Social Life, Health, and Aging Project is supported by the National Institutes of Health—the National Institute on Aging, Office of Women’s Health Research, Office of AIDS Research, and the Office of Behavioral and Social Science Research (5R01AG021487). Portions of this paper were presented at the 63rd annual meeting of the Gerontological Society of America, Atlanta, GA.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Allen KR, Blieszner R, Roberto KA. Families in the middle and later years: A review of research in the 1990s. Journal of Marriage and the Family. 2000;62:911–926. doi: 10.1111/j.1741-3737.2000.00911.x. [Google Scholar]
- Antonucci TC, Akiyama H. Convoys of social relations: Family and friendships within a life span context. In: Blieszner R, Bedford VH, editors. Handbook of aging and the family. Westport, CT: Greenwood Press; 1995. pp. 355–372. [Google Scholar]
- Antonucci TC, Lansford JE, Akiyama H. Impact of positive and negative aspects of marital relationships and friendships on well-being of older adults. Applied Developmental Science. 2001;5:68–75. doi:10.1207/S1532480XADS0502_2. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. doi:10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Birditt KS, Antonucci TC. Relationship quality profiles and well-being among married adults. Journal of Family Psychology. 2007;21:595–604. doi: 10.1037/0893-3200.21.4.595. doi:10.1037/0893-3200.21.4.595. [DOI] [PubMed] [Google Scholar]
- Bookwala J. The role of marital quality in physical health during the mature years. Journal of Aging and Health. 2005;17:85–104. doi: 10.1177/0898264304272794. doi:10.1177/0898264304272794. [DOI] [PubMed] [Google Scholar]
- Bookwala J. Blieszner, Bedford V. Handbook of Families and Aging. Marriage and like relationships in middle and late life. In R. Greenwood/ABC-CLIO: Westport, CT. [Google Scholar]
- Bookwala J, Fekete E. Psychological resources and affective well-being in never-married adults. Journal of Social and Personal Relationships. 2009;26:411–428. doi: 10.1177/0265407509339995. doi:10.1177/0265407509339995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookwala J, Franks MM. The moderating role of marital quality in older adults’ depressed affect: Beyond the `main effects’ model. Journal of Gerontology: Psychological Sciences. 2005;60:P338–P441. doi: 10.1093/geronb/60.6.p338. doi:10.1093/geronb/60.6.P338. [DOI] [PubMed] [Google Scholar]
- Bookwala J, Jacobs J. Age, marital processes, and depressed affect. The Gerontologist. 2004;44:328–338. doi: 10.1093/geront/44.3.328. doi:10.1093/geront/44.3.328. [DOI] [PubMed] [Google Scholar]
- Bookwala J, Lawson B. The Gerontologist. 2011. The effects of poor vision on depressive symptoms: A test of the activity restriction model. doi:10.1093/geront/gnr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque P, Leger C, Pushkar D, Beland F. Self-reported sensory impairment and life satisfaction in older French-speaking adults. Canadian Journal of Nursing Research. 2007;39:154–171. [PubMed] [Google Scholar]
- Bradbury TN. Assessing the four fundamental domains of marriage. Family Relations. 1995;44:459–468. [Google Scholar]
- Bradbury TN, Fincham FD, Beach SRH. Research on the nature and determinants of marital satisfaction: A decade in review. Journal of Marriage and the Family. 2000;62:964–980. doi:10.1111/j.1741-3737.2000.00964.x. [Google Scholar]
- Brody BL, Gamst AC, Williams RA, Smith AR, Lau PW, Dolnak D, Brown SI. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Opthalmology. 2001;108:1893–1900. doi: 10.1016/s0161-6420(01)00754-0. doi:10.1016/S0161-6420(01)00754-0. [DOI] [PubMed] [Google Scholar]
- Brown SL, Kawamura S. Relationship quality among cohabitors and marrieds in older adulthood. Social Science Research. 2011;39:777–786. doi: 10.1016/j.ssresearch.2010.04.010. doi:10.1016/j.ssresearch.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casten RJ, Rovner BW, Edmonds SE. The relationships among personality and vision- specific function among older people with impaired vision. Journal of Mental Health and Aging. 2001;7:325–334. [Google Scholar]
- Choi H, Marks NF. Transition to caregiving, marital disagreement, and psychological well-being. Journal of Family Issues. 2006;27:1701–1722. doi:10.1177/0192513X06291523. [Google Scholar]
- Chou K. Combined effect of vision and hearing impairment on depression in older adults: Evidence from the English Longitudinal Study of Ageing. Journal of Affective Disorders. 2008;106:191–196. doi: 10.1016/j.jad.2007.05.028. doi:10.1016/j.jad.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Cimarolli VR, Boerner K. Social support and well-being in adults who are visually impaired. Journal of Visual Impairment and Blindness. 2005;99:521–534. [Google Scholar]
- Cimarolli VR, Reinhardt JP, Horowitz A. Perceived overprotection: Support gone bad? The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2006;61:18–23. doi: 10.1093/geronb/61.1.s18. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. doi:10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Crews JE. The demographic, social, and conceptual contexts of aging and vision loss. Journal of the American Optometric Association. 1994;65:63–68. [PubMed] [Google Scholar]
- Crews JE, Jones GC, Kim JH. Double jeopardy: The effects of comorbid conditions among older people with vision loss. Journal of Visual Impairment and Blindness. 2006;100:824–848. (special supplement) [Google Scholar]
- Cutrona CE. Social support in couples: Marriage as a resource in times of stress. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- Federal Interagency Forum on Aging-Related Statistics. Older Americans 2008: Key indicators of well-being. Washington, DC: U.S. Government Printing Office; 2008. [Google Scholar]
- Femia EE, Zarit SH, Johansson B. The disablement process in very late life: A study of the oldest-old in Sweden. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2001;56:12–23. doi: 10.1093/geronb/56.1.p12. doi:10.1093/geronb/56.1.P12. [DOI] [PubMed] [Google Scholar]
- Fors S, Thorslund M, Parker MG. Do actions speak louder than words? Self-assessed and performance-based measures of physical and visual function among old people. European Journal of Ageing. 2006;3:15–21. doi: 10.1007/s10433-006-0021-5. doi:10.1007/s10433-006-0021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Iglesias H, Sellars B, Antonucci TC. Resilience in old age: Social relations as a protective factor. Research in Human Development. 2008;5:181–193. doi:10.1080/15427600802274043. [Google Scholar]
- Gunnel L, Nordholm L. Adaptation strategies, well-being, and activities of daily living among people with low vision. Journal of Visual Impairment and Blindness. 1999;93:434–446. [Google Scholar]
- Hatch SL. Conceptualizing and identifying cumulative adversity and protective resources: Implications for understanding health inequalities. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2005;60:130–134. doi: 10.1093/geronb/60.special_issue_2.s130. doi:10.1093/geronb/60.Special_Issue_2.S130. [DOI] [PubMed] [Google Scholar]
- Horowitz A. The prevalence and consequences of vision impairment in later life. Topics in Geriatric Rehabilitation. 2004;20:185–195. [Google Scholar]
- Jang Y, Mortimer JA, Haley WE, Small BJ, Chisolm TEH, Graves AB. The role of vision and hearing in physical, social, and emotional functioning among older adults. Research on Aging. 2003;25:172–191. doi:10.1177/0164027502250019. [Google Scholar]
- Judd CM, McClelland GH. Data analysis: A model comparison approach. New York, NY: Harcourt Brace Jovanovich; 1989. [Google Scholar]
- Kim HK, McKenry PC. The relationship between marriage and psychological well-being: A longitudinal analysis. Journal of Social Issues. 2002;23:885–911. doi:10.1177/019251302237296. [Google Scholar]
- LaForge RG, Spector WD, Sternberg J. The relationship of vision and hearing impairment to one-year mortality and functional decline. Journal of Aging and Health. 1992;4:126–148. doi:10.1177/089826439200400108. [Google Scholar]
- Liu H, Umberson D. The times they are a changin’: Marital status and health differentials from 1972 to 2003. Journal of Health and Social Behavior. 2008;49:239–253. doi: 10.1177/002214650804900301. doi:10.1177/002214650804900301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupsakko T, Mantyjarvi M, Kautiainen H, Sulkava R. Combined hearing and visual impairment and depression in a population aged 75 years and older. International Journal of Geriatric Psychiatry. 2002;17:808–813. doi: 10.1002/gps.689. doi:10.1002/gps.689. [DOI] [PubMed] [Google Scholar]
- Mancini AD, Bonnano GA. Marital closeness, functional disability, and adjustment in late life. Psychology and Aging. 2006;21:600–610. doi: 10.1037/0882-7974.21.3.600. doi:10.1037/0882-7974.21.3.600. [DOI] [PubMed] [Google Scholar]
- McIlvane JM, Reinhardt JP. Interactive effect of support from family and friends in visually impaired elders. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2001;56:374–382. doi: 10.1093/geronb/56.6.p374. doi:10.1093/geronb/56.6.P374. [DOI] [PubMed] [Google Scholar]
- O’Donnell C. The greatest generation meets its greatest challenge: Vision loss and depression in older adults. Journal of Visual Impairment and Blindness. 2005;99:197–208. [Google Scholar]
- O’Muircheartaigh C, Eckman S, Smith S. Statistical design estimation for the National Social Life, Health, and Aging Project (NSHAP) The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2009;64(S1):i12–i19. doi: 10.1093/geronb/gbp045. doi:10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Kempen GIJM, Penninx BWJH, Brilman EI, Beekman ATF, Van Sonderen E. Chronic medical conditions and mental health in older people: Disability and psychosocial resources mediate specific health effects. Psychological Medicine. 1997;27:1065–1077. doi: 10.1017/s0033291797005321. [DOI] [PubMed] [Google Scholar]
- Pienta AM, Hayward MD, Jenkins KR. Health consequences of marriage for the retirement years. Journal of Family Issues. 2000;21:559–586. doi:10.1177/019251300021005003. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Reinhardt J. The importance of friendship and family support in adaptation to chronic vision impairment. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1996;51:268–278. doi: 10.1093/geronb/51b.5.p268. doi:10.1093/geronb/51B.5.P268. [DOI] [PubMed] [Google Scholar]
- Reinhardt J, Boerner K, Benn D. Predicting individual change in support over time among chronically impaired older adults. Psychology and Aging. 2003;18:770–779. doi: 10.1037/0882-7974.18.4.770. doi:10.1037/0882-7974.18.4.770. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. doi:10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. doi:10.1037/0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Schumm LP, McClintock M, Williams S, Leitsch S, Lundstrom J, Hummel T. . ., Lindau ST. Assessment of sensory function in the National Social Life, Health, and Aging Project. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2009;64(S1):i76–i85. doi: 10.1093/geronb/gbp048. doi:10.1093/geronb/gbp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Fleeson W, Geiselmann B, Settersten RA, Kunzmann U. Sources of well-being in very old age. In: Baltes PB, Mayer KU, editors. The Berlin aging study: Aging from 70 to 100. Cambridge: Cambridge University Press; 1999. pp. 450–471. [Google Scholar]
- Smith S, Jaszczak A, Graber J, Lunden K, Leitsch S, Wargo E, O’Muircheartaigh C. Instrument development, study design implementation, and survey conduct for the National Social Life, Health, and Aging Project (NSHAP) The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2009;64(S1):i20–i29. doi: 10.1093/geronb/gbn013. doi:10.1093/geronb/gbn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Urbano J. A telephone group support program for the visually impaired elderly. Clinical Gerontologist: Journal of Aging and Mental Health. 1993;13:61–71. doi:10.1300/J018v13n02_05. [Google Scholar]
- Tower RB, Kasl SV. Depressive symptoms across older spouses and the moderating effect of marital closeness. Psychology and Aging. 1995;10:625–638. doi: 10.1037//0882-7974.10.4.625. doi:10.1037/0882-7974.10.4.625. [DOI] [PubMed] [Google Scholar]
- Tower RB, Kasl SV, Moritz DJ. The influence of spouse cognitive impairment on respondents’ depressive symptoms: The moderating role of marital closeness. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1997;52:270–278. doi: 10.1093/geronb/52b.5.s270. doi:10.1093/geronb/52B.5.S270. [DOI] [PubMed] [Google Scholar]
- Travis LA, Boerner K, Reinhardt JP, Horowitz A. Exploring functional disability in older adults with low vision. Journal of Visual Impairment and Blindness. 2004;98:534–545. [Google Scholar]
- Turner RJ, Noh S. Physical disability and depression: A longitudinal analysis. Journal of Health and Social Behavior. 1988;29:23–37. [PubMed] [Google Scholar]
- Umberson D, Liu H, Powers DA. Marital status, marital transitions, and body weight: Gender, race, and life course considerations. Journal of Health and Social Behavior. 2009;50:327–343. doi: 10.1177/002214650905000306. doi:10.1177/002214650905000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Williams K, Powers DA, Liu H, Needham B. You make me sick: Marital quality and health over the life course. Journal of Health and Social Behavior. 2006;47:1–16. doi: 10.1177/002214650604700101. doi:10.1177/002214650604700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science and Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. doi:10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Wahl H.-W., Schilling O, Oswald F, Heyl V. Psychosocial consequences of age-related visual impairment: Comparison with mobility-impaired older adults and long-term outcome. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 1999;54:304–316. doi: 10.1093/geronb/54b.5.p304. doi:10.1093/geronb/54B.5.P304. [DOI] [PubMed] [Google Scholar]
- Wahl H, Tesch-Romer C. Aging, sensory loss, and social functioning. In: Charness N, Parks D, Sabel B, editors. Communication, technology and aging: Opportunities and challenges for the future. New York, NY: Springer Publishing Co; 2001. pp. 108–126. [Google Scholar]
- Walen HR, Lachman ME. Social support and strain from partner, family, and friends: Costs and benefits for men and women in adulthood. Journal of Social and Personal Relationships. 2000;17:5–30. doi:10.1177/0265407500171001. [Google Scholar]
- Williamson GM, Christie J. Aging well in the 21st century: Challenges and opportunities. In: Lopez SJ, Snyder CR, editors. Oxford handbook of positive psychology. 2nd ed. New York: Oxford University Press; 2009. pp. 165–169. [Google Scholar]
- Williamson GM, Shaffer DR. The activity restriction model of depressed affect: Antecedents and consequences of restricted normal activities. In: Williamson GM, Shaffer DR, Parmelee PA, editors. Physical illness and depression in older adults: A handbook of theory, research, and practice. New York, NY: Plenum; 2000. pp. 173–200. [Google Scholar]
- World Health Organization. WHOQOL user manual. Geneva, Switzerland: WHO Programme on Mental Health; 1998. [Google Scholar]
- Xu Y, Burleson BR. The association of experienced spousal support with marital satisfaction: Evaluating the moderating effects of sex, ethnic culture, and type of support. Journal of Family Communication. 2004;4:123–145. [Google Scholar]