Abstract
Background/Aims
The role of Helicobacter pylori in gastroesophageal reflux disease remains still controversial and the effect of the organism on severity of reflux esophagitis have been rarely issued. The aim of this study was to investigate the relationship between H. pylori infection and reflux esophagitis, and especially the severity of reflux esophagitis.
Methods
We performed a cross-sectional case-control study of 5,616 subjects undergoing both upper endoscopy and H. pylori serology during health Check-up (2,808 cases vs age- and sex-matched controls). Smoking, alcohol, body mass index and waist circum - ference were added to a multiple regression model.
Results
Prevalence of H. pylori infection was lower in cases with reflux esophagitis than in controls (38.4% vs 58.2%, P < 0.001) and negative associations with H. pylori infection continued across the grade of esophagitis (46.7% in Los Angeles classification M [LA-M], 34.3% in LA-A or LA-B and 22.4% in LA-C or LA-D, P < 0.001). Positive serology for H. pylori independently reduced the risk of reflux esophagitis (adjusted OR, 0.44; 95% CI, 0.39-0.49). Notably, the negative associations continued across the grade of esophagitis with adjusted ORs of 0.63 in LA-M, 0.36 in LA-A or LA-B and 0.20 in LA-C or LA-D (P < 0.001).
Conclusions
In a age-sex matched Korean, H. pylori seropositivity was independently and inversely associated with the risk and severity of reflux esophagitis, suggesting the organism may have a protective role against gastroesophageal reflux disease.
Keywords: Esophagitis, Gastroesophageal reflux, Helicobacter pylori, Peptic, Risk factors
Introduction
The possible role of Helicobacter pylori in the etiology of gastroesophageal reflux disease (GERD) has been a topic of major interest and of great debate over the past years. The declining prevalence of H. pylori infection and related peptic ulcer disease has been paralleled by a rising trend of GERD incidence and its complications in Asia including Korea.1-4 Most epidemiologic data have shown lower H. pylori infection rates among patients suffering from GERD symptoms or esophagitis,5-9 but the sample size in these studies was small.
Recently a prospective cohort study with large scale in Korea also suggested H. pylori infection to have a strong negative association with reflux esophagitis,10 but the cases of reflux esophagitis in that study was 490 of not a large enough sample size and there was no mention about the relationship between the severity of reflux esophagitis and H. pylori. Two meta-analyses of observational studies established negative associations between the 2 conditions.11,12 All data cited above have prompted a speculation that the organism might exert a protective effect against the development of GERD. However, aforementioned meta-analyses revealed marked heterogeneity between studies; patients with GERD from Far East had a lower rate of H. pylori infection than those from Western Europe and North America, despite a higher prevalence of H. pylori infection in the general population.11
Other investigators failed to confirm this negative relation13,14 and a recently published population-based study in Norway did not find any association between H. pylori and reflux symptoms.15 GERD is regarded as a multifactorial disease. Hence, the reason for the controversy regarding a presumed relationship between H. pylori and GERD appears to be related to the lack of studies into the effects of the different variables in stratified populations.
To date, large-scale population-based studies on this topic adjusting for confounding factors are still needed. Given the high prevalence of both H. pylori infection and GERD in Korea4,16 with a multitude of patients suffering from both conditions simultaneously, the true relation between the 2 conditions and, by extension, the effect of H. pylori infection on severity of reflux esophagitis in general population seems to be of clinical relevance. Accordingly, the aim of this study was to determine the definite relationship between H. pylori infection and reflux esophagitis in a large Korean population.
Materials and Methods
Study Population
We conducted a cross-sectional case-control study. All subjects attended a routine medical Check-up including upper endoscopy and H. pylori serology at Seoul National University Hospital (SNUH) Healthcare System Gangnam Center between October 2004 and April 2007. Details have been previously explained.16 In brief, most of the study subjects resided in Seoul metropolitan area. Considering the unacceptably high prevalence of gastric cancer in Korea,17 majority of individuals older than 40 years received upper endoscopy as a part of a nationwide screening program in Korea. The sampling frame for cases consisted of all subjects with endoscopically identified esophagitis who have never seen the doctors with this matter. Exclusion criteria were as follows; incomplete responder to the questionnaires, prior histories of gastrointestinal surgery or gastric cancer, active or healing staged gastric or duodenal ulcer, past treatment of H. pylori and current use of anti-ulcer or anti-reflux medications. Controls were randomly selected from age- and sex-matched individuals with normal upper endoscopic findings without gastrointestinal symptoms. Study protocol was approved by Institutional Review Board of SNUH.
Definitions and Exposure Measurements
Reflux esophagitis was defined endoscopically if definite erosions (mucosal breaks) or minimal mucosal changes (erythema and/or whitish discoloration) were present. The severity of esophagitis was graded from M to D according to Los Angeles (LA) classification system with Japanese modifications.18 All subjects were requested to complete a comprehensive, structured questionnaires prior to endoscopic procedure. The questionnaires covered questions about prescripted medications taken at least 1 week during the past year and reflux symptoms (heartburn and/or acid regurgitation) including atypical symptoms (hoarseness, globus sensation, dyspepsia, epigastric soreness, chest pain and chronic cough). Several questions regarding lifestyle factors that can affect GERD were also included, such as current smoking (smoked regularly during the previous 12 months) and alcohol consumption (≥ 140 g/wk). All subjects underwent physical examinations on the day of endoscopy by trained personnel who used a written, systematic protocol with standardized instruments. Body mass index (BMI) was calculated from automatically measured weight and height (InBody 4.0, Biospace Co, Ltd, Seoul, Korea) and categorized as normal (< 23 kg/m2), overweight (23-24.9 kg/m2) or obese (≥ 25 kg/m2) according to the WHO Western Pacific Regional Office proposal.19 We examined the waist circumference as an alternative of abdominal obesity, which was measured at the WHO recommended site (middle level between the lowest rib margin and iliac crest).20
Helicobacter pylori Serology Examination
Diagnosis of H. pylori infection was based on the detection of serum H. pylori immunoglobulin G antibody (anti-H. pylori IgG) using a commercially available enzyme-linked immunosorbent assay (H. pylori-EIA-Well, Radim, Rome, Italy) which was previously validated in a nationwide Korean seroepidemiologic study.2 Anti-H. pylori IgG levels higher than 15 RU/mL were regarded as positive.
Statistical Methods
Continuous variables were expressed as mean ± SD. Nominal and ordinal variables were stated with percentages. Continuous data and categorical parameters were analyzed by Student's t test and χ2 test, respectively. The association between H. pylori serostatus and the presence of reflux esophagitis was investigated by multivariate analysis. We included additional variables with a known or probable association with GERD such as current smoking, alcohol consumption, BMI and waist circumference in the multiple logistic regression models. For each variable, relative risks were given in the form of odds ratio (OR) and 95% confidence interval (CI). All analyses were performed using the Statistical Package for the Social Sciences version 17.0 (SPSS INC, Chicago, IL, USA). A 2 tailed P-value of < 0.05 was considered statistically significant. Age- and sex-matching and statistical analysis was supported by SNUH Medical Research Collaborating Center.
Results
Characteristics of Study Population
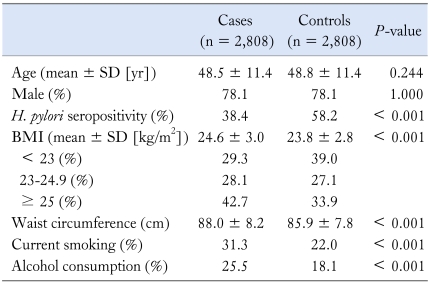
During the study period, 48,684 consecutive subjects visited our center. When the analysis was restricted to those undergoing both upper endoscopy and serologic test for H. pylori, 35,980 potential subjects were considered eligible. H. pylori infection was present in 57.9% and the overall prevalence of reflux esophagitis was 9.2%. Of them, 4,476 were excluded based on the responses to the questionnaires. We excluded additional 850 who were symptomatic or took medications for proton pump inhibitor and H. pylori eradication. Two hundred eighty-nine were also ruled out based on other positive endoscopic findings. A total of 5,616 subjects (2,808 cases with reflux esophagitis and 2808 age- and sex-matched asymptomatic controls) were eventually enrolled for analysis. Characteristics of the study population are presented in Table 1. Their mean age was 48.7 ± 11.4 years and 78.1% were men. Cases were more likely to have higher BMI and waist circumference (for each variable, P < 0.001). The proportions of current smokers and alcohol drinkers were significantly higher in cases than in controls (for each variable, P < 0.001).
Table 1.
Comparisons of Baseline Characteristics Between Cases and Age-, Sex-Matched Controls
H. pylori, Helicobacter pylori; BMI, body mass index.
Helicobacter pylori Infection and Risk for Reflux Esophagitis
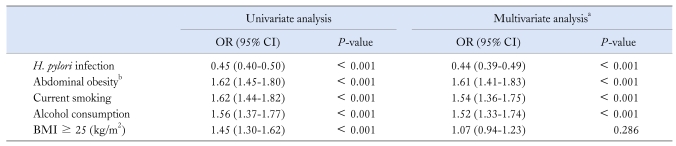
The prevalence of H. pylori infection was significantly lower in subjects with reflux esophagitis than in controls (38.4% vs 58.2%, P < 0.001). After adjusting for the effects of current smoking, alcohol consumption, BMI and waist circumference, positive serology of H. pylori negatively associated with the risk for reflux esophagitis (adjusted OR, 0.44; 95% CI, 0.39-0.49; P < 0.001). Multivariate analysis also revealed that current smoking, alcohol consumption and abdominal obesity independently increased the risk for reflux esophagitis. On the other hand, the association with BMI was no longer significant after multiple adjustments (Table 2).
Table 2.
Univariate and Multivariate Analyses on the Risk for Reflux Esophagitis
aAdjusted for all variables in the table, b> 90 cm in men and > 80 cm in women.
BMI, body mass index.
Helicobacter pylori Infection and Severity of Reflux Esophagitis
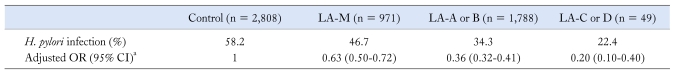
More severe esophagitis showed lower H. pylori infection rate: 46.7% in LA-M, 34.3% in LA-A or B and 22.4% in LA-C or D (P for trend < 0.001). It was noteworthy that the negative associations with H. pylori infection continued across the grades of esophagitis with adjusted ORs of 0.63 (95% CI, 0.50-0.72) in LA-M, 0.36 (95% CI, 0.32-0.41) in LA-A or B, and 0.20 (95% CI, 0.10-0.40) in LA-C or D (P < 0.001) (Table 3).
Table 3.
Risk Across the Severity Grades of Reflux Esophagitis in Relation to the Presence of Helicobacter pylori Infection
aAdjusted for smoking, alcohol, body mass index and waist circumference.
LA, Los Angeles classification; H. pylori, Helicobacter pylori.
Discussion
This is the study on the association between H. pylori infection and reflux esophagitis adjusting for many confounding variables for GERD in a large healthy population. A remarkably lower prevalence of H. pylori infection was noted in patients with reflux esophagitis (38.4% vs 58.2%). H. pylori seropositivity substantially (> 50%) reduced the risk of reflux esophagitis (adjusted OR, 0.44; 95% CI, 0.39-0.49) after adjusting for potential confounders. These results were similar to the results of the recent large scale study10; 34.9% (OR, 0.42; 95% CI, 0.34-0.51). Another interesting observation was that the negative associations with H. pylori infection continued across the grades of esophagitis (P < 0.001), which added weight to the hypothesis on the putative protective role of H. pylori in offering protection against the development and progression of GERD. There has been some evidence that H. pylori infected GERD patients have less severe reflux changes, though the number of cases available for analysis was small or only an intention-to-treat population with symptoms were enrolled.6,21,22
The proposed mechanism is gastric acid hyposecretion due to corpus predominant gastritis and subsequent atrophic changes followed by H. pylori infection.23-25 The more virulent type of H. pylori such as cytotoxin-associated gene A (cagA)-positive strain was postulated to promote the more intense gastric inflammation, exemplifying the risk of atrophy and thereby the further reduction in GERD risk.26,27 In addition, atrophic gastritis has been suggested to be a significant modifying factor for relationship between H. pylori and GERD; the regional distribution and severity of gastritis with corpus atrophy might be more important in a subset of patients with a predisposition to GERD, rather than just the presence or absence of H. pylori infection.5,27-30
Further elucidation of the complex relationships among H. pylori, gastric atrophy, esophageal acid exposure, esophagitis and the development of reflux symptoms is being held back by difficulties in clinical and technical methods used to identify virulence factors, histological gastritis patterns and the different host inflammatory conditions including cytokine kinetics at the same time.25 Apart from H. pylori infection, multivariate analysis reconfirmed that abdominal obesity as expressed by waist circumference independently increased the risk for reflux esophagitis as reported by our previous study.16 Although some researchers have proposed intriguing mechanism that H. pylori infection or eradication modify the natural history of GERD through weight changes,31,32 data on this theory are minimal. Our subanalysis found no significant association between H. pylori serology and obese indicators such as BMI and waist circumference (data not shown).
The negative associations in this study is consistent with some previous epidemiologic studies.5-10 Two recent systematic reviews have corroborated the association with pooled ORs of 0.60 (95% CI, 0.47-0.78)11 and 0.7 (95% CI, 0.63-0.78).12 Such negative association has not been consistently observed among different ethnic groups13,14 and more apparent among Eastern Asians than Western populations.11 A potential explanation for the contradictory results is that prevalence of H. pylori infection and distribution of virulent strains differ significantly among various geographic regions.11,25,33 In addition, ethnic or inter-individual variations in host response to H. pylori infection could be responsible for the conflicting results; some host genotypes, particularly those related to pro-inflammatory cytokines such as IL-1β and their receptor isotypes, may result in more severe gastric damage with consequent decrease in acid production.25,34,35 Also, variations in study design particularly regarding the selection of cases and controls make direct comparison of the preceding reports difficult. The negative correlation seems to be greater in studies assessing endoscopically proven esophagitis rather than symptomatic GERD.11 Furthermore, the control group in the published studies was heterogeneous and included symptomatic patients or the patients who received an endoscopy for another indication.6,11,26 Because patients with nonulcer dyspepsia or peptic ulcer disease are more likely to be colonized with H. pylori than the general population,36 such endoscopy controls would have a higher rate of H. pylori infection than average prevalence.
Our study has several advantages over other studies. First and foremost, the sample size is large, especially the size of cases is the largest to our knowledge (5,616 individuals, 2,808 cases of reflux esophagitis), even though there is one recent study in similar setting with larger scale (10,102 subjects, and 490 cases of reflux esophagitis),10 and controls were exactly matched for age and sex, which are risk factors of reflux esophagitis in other studies. This matching method allowed well-powered analysis of interactions. Second, according to what we searched the references, there was no study about the effect of H. pylori on severity of reflux esophagitis. Third, the cases and controls were chosen by stringent criteria with less risk of misclassification error. We enrolled the cases with esophagitis identified by endoscopy not based on reflux symptoms. Moreover, primary methodologic strength in the current study is a random sampling of asymptomatic healthy controls, which minimizes the selection bias and enables extrapolation to the prevalence of H. pylori infection in general population. Finally, Healthwatch version2.0,37 the large database in our center, fully computerizes a broad range of exposures including comprehensive drug history and makes it possible to control for potential confounders, thereby permitting a less biased estimate of the association.
Interpretation of our findings requires careful consideration from several aspects. First, cross-sectional design prevents any conclusion regarding causality between H. pylori and reflux esophagitis. Since H. pylori colonization most commonly begins in childhood, it is unlikely that the outcome (reflux esophagitis) preceded the exposure.38 Second, this study was conducted on the subjects at single healthcare center, so the pool of subjects might represent relatively high socioeconomic status and it's possible the enrolled person was more concerned about health. But this study design is more favorable to other study in tertiary center and our study population approached to the general population. Third, H. pylori infection status was evaluated by the serologic test alone. There was no clinical information available with regards to current or past H. pylori infection status in the serologic test. Nevertheless, serology is the most frequently used method for epidemiologic research and has been used to predict the prevalence of H. pylori infection in various populations with approved sensitivity and specificity. We also did not address cagA status nor did we evaluate the histologic gastritis pattern. Comparative studies examining the virulence factors in conjunction with systematic biopsies of gastric mucosa between GERD patients and non-reflux controls are therefore warranted.
In summary, we found that H. pylori seropositivity had strong inverse relationships with the risk and severity of reflux esophagitis in a large sample of Korean population after multiple confounding factor adjustments. These data support the hypothesis that H. pylori could be a protective factor against GERD. Our findings need to be confirmed by prospective clinical trials. As well, long-term interventional studies examining the effect of treating the infection on esophageal disease will provide clear information on which to base clinical decisions.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.El-Serag HB, Sonnenberg A. Opposing time trends of peptic ulcer and reflux disease. Gut. 1998;43:327–333. doi: 10.1136/gut.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan HJ, Goh KL. Changing epidemiology of Helicobacter pylori in Asia. J Dig Dis. 2008;9:186–189. doi: 10.1111/j.1751-2980.2008.00344.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim JI, Kim SG, Kim N, et al. Changing prevalence of upper gastrointestinal disease in 28893 Koreans from 1995 to 2005. Eur J Gastroenterol Hepatol. 2009;21:787–793. doi: 10.1097/MEG.0b013e32830e285a. [DOI] [PubMed] [Google Scholar]
- 5.Yamaji Y, Mitsushima T, Ikuma H, et al. Inverse background of Helicobacter pylori antibody and pepsinogen in reflux oesophagitis compared with gastric cancer: analysis of 5732 Japanese subjects. Gut. 2001;49:335–340. doi: 10.1136/gut.49.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendra S, Ackroyd R, Robertson IK, Ho JJ, Karim N, Kutty KM. Helicobacter pylori, ethnicity, and the gastroesophageal reflux disease spectrum: a study from the East. Helicobacter. 2007;12:177–183. doi: 10.1111/j.1523-5378.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen TS, Chang FY. The prevalence and risk factors of reflux esophagitis among adult Chinese population in Taiwan. J Clin Gastroenterol. 2007;41:819–822. doi: 10.1097/01.mcg.0000225658.30803.79. [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Kubo A, Levin TR, et al. Helicobacter pylori and gastroesophageal reflux disease: a case-control study. Helicobacter. 2008;13:352–360. doi: 10.1111/j.1523-5378.2008.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson LA, Murphy SJ, Johnston BT, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–739. doi: 10.1136/gut.2007.132662. [DOI] [PubMed] [Google Scholar]
- 10.Nam SY, Choi IJ, Ryu KH, Kim BC, Kim CG, Nam BH. Effect of Helicobacter pylori infection and its eradication on reflux esophagitis and reflux symptoms. Am J Gastroenterol. 2010;105:2153–2162. doi: 10.1038/ajg.2010.251. [DOI] [PubMed] [Google Scholar]
- 11.Raghunath A, Hungin AP, Wooff D, Childs S. Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ. 2003;326:737–743. doi: 10.1136/bmj.326.7392.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cremonini F, Di Caro S, Delgado-Aros S, et al. Meta-analysis: the relationship between Helicobacter pylori infection and gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;18:279–289. doi: 10.1046/j.1365-2036.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- 13.Wu JC, Sung JJ, Ng EK, et al. Prevalence and distribution of Helicobacter pylori in gastroesophageal reflux disease: a study from the East. Am J Gastroenterol. 1999;94:1790–1794. doi: 10.1111/j.1572-0241.1999.01207.x. [DOI] [PubMed] [Google Scholar]
- 14.Grande M, Cadeddu F, Villa M, et al. Helicobacter pylori and gastroesophageal reflux disease. World J Surg Oncol. 2008;6:74–80. doi: 10.1186/1477-7819-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordenstedt H, Nilsson M, Johnsen R, Lagergren J, Hveem K. Helicobacter pylori infection and gastroesophageal reflux in a population-based study (The HUNT Study) Helicobacter. 2007;12:16–22. doi: 10.1111/j.1523-5378.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 16.Chung SJ, Kim D, Park MJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–1365. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 17.Shin HR, Jung KW, Won YJ, Park JG 139 KCCR-affiliated Hospitals. 2002 Annual report of the Korea central cancer registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshihara Y. [Endoscopic findings of GERD] Nippon Rinsho. 2004;62:1459–1464. [Japanese] [PubMed] [Google Scholar]
- 19.WHO/IASO/IOTF. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia Pty Ltd; 2000. pp. 1–55. [Google Scholar]
- 20.World Health Organization. Obesity: prevention and managing, the global epidemic. Geneva: World Health Organization; 2000. Report of a WHO consultation on obesity; p. 9. [PubMed] [Google Scholar]
- 21.Wu JC, Sung JJ, Chan FK, et al. Helicobacter pylori infection is associated with milder gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14:427–432. doi: 10.1046/j.1365-2036.2000.00714.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu JC, Cheung CM, Wong VW, Sung JJ. Distinct clinical characteristics between patients with nonerosive reflux disease and those with reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5:690–695. doi: 10.1016/j.cgh.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 23.El-Omar EM, Oien K, El-Nujumi A, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997;113:15–24. doi: 10.1016/s0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- 24.Koike T, Ohara S, Sekine H, et al. Helicobacter pylori infection prevents erosive reflux oesophagitis by decreasing gastric acid secretion. Gut. 2001;49:330–334. doi: 10.1136/gut.49.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza RC, Lima JH. Helicobacter pylori and gastroesophageal reflux disease: a review of this intriguing relationship. Dis Esophagus. 2009;22:256–263. doi: 10.1111/j.1442-2050.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- 26.Vicari JJ, Peek RM, Falk GW, et al. The seroprevalence of cagA-positive Helicobacter pylori strains in the spectrum of gastroesophageal reflux disease. Gastroenterology. 1998;115:50–57. doi: 10.1016/s0016-5085(98)70364-6. [DOI] [PubMed] [Google Scholar]
- 27.Queiroz DM, Rocha GA, Oliveira CA, et al. Role of corpus gastritis and cagA-positive Helicobacter pylori infection in reflux esophagitis. J Clin Microbiol. 2002;40:2849–2853. doi: 10.1128/JCM.40.8.2849-2853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Serag HB, Sonnenberg A, Jamal MM, Inadomi JM, Crooks LA, Feddersen RM. Corpus gastritis is protective against reflux oesophagitis. Gut. 1999;45:181–185. doi: 10.1136/gut.45.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koike T, Ohara S, Sekine H, et al. Helicobacter pylori infection inhibits reflux esophagitis by inducing atrophic gastritis. Am J Gastroenterol. 1999;94:3468–3472. doi: 10.1111/j.1572-0241.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 30.McColl KE, Watabe H, Derakhshan MH. Role of gastric atrophy in mediating negative association between Helicobacter pylori infection and reflux oesophagitis, Barrett's oesophagus and oesophageal adenocarcinoma. Gut. 2008;57:721–723. doi: 10.1136/gut.2007.144774. [DOI] [PubMed] [Google Scholar]
- 31.Loffeld RJ. Helicobacter pylori, obesity and gastro-oesophageal reflux disease. Is there a relation? A personal view. Neth J Med. 2005;63:344–347. [PubMed] [Google Scholar]
- 32.Yang YJ, Sheu BS, Chang WL, Cheng HC, Yang HB. Increased body mass index after H. pylori eradication for duodenal ulcer predisposes to erosive reflux esophagitis. J Clin Gastroenterol. 2009;43:705–710. doi: 10.1097/MCG.0b013e3181948c45. [DOI] [PubMed] [Google Scholar]
- 33.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. H. pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 34.Queiroz DM, Guerra JB, Rocha GA, et al. IL1B and IL1RN polymorphic genes and Helicobacter pylori cagA strains decrease the risk of reflux esophagitis. Gastroenterology. 2004;127:73–79. doi: 10.1053/j.gastro.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 35.Ando T, El-Omar EM, Goto Y, et al. Interleukin 1B proinflammatory genotypes protect against gastro-oesophageal reflux disease through induction of corpus atrophy. Gut. 2006;55:158–164. doi: 10.1136/gut.2005.072942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danesh J, Lawrence M, Murphy M, Roberts S, Collins R. Systematic review of the epidemiological evidence on Helicobacter pylori infection and nonulcer or uninvestigated dyspepsia. Arch Intern Med. 2000;160:1192–1198. doi: 10.1001/archinte.160.8.1192. [DOI] [PubMed] [Google Scholar]
- 37.Chung SJ, Kim YS, Yang SY, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010;25:519–525. doi: 10.1111/j.1440-1746.2009.06147.x. [DOI] [PubMed] [Google Scholar]
- 38.Rowland M, Daly L, Vaughan M, Higgins A, Bourke B, Drumm B. Age-specific incidence of Helicobacter pylori. Gastroenterology. 2006;130:65–72. doi: 10.1053/j.gastro.2005.11.004. [DOI] [PubMed] [Google Scholar]