Abstract
Background/Aims
Patients with type II diabetes mellitus (DM) were known to have higher prevalence of gastroesophageal reflux disease (GERD). Recent studies have shown that neuropathy has positive role on the development of GERD in type II DM, although its pathogenesis has not been fully understood yet. The aim of this study was to investigate whether neuropathy really contribute to the development of GERD and typical GERD symptoms in patients with type II DM in Korea.
Methods
One hundred and nineteen patients with type II DM who had given informed consents were enrolled. All patients underwent electromyography to check the presence of peripheral neuropathy, face-to-face interview to evaluate their typical GERD symptoms and esophagogastroduodenoscopy to look for the presence of erosive esophagitis. Ninety-five patients were finally included for this study and they were divided according to the presence or absence of the peripheral neuropathy.
Results
The mean age of 95 patients was 59.3 ± 9.1 years and the mean disease duration of DM was 9.3 ± 5.9 years. Typical GERD symptoms were similarly found in both groups with and without peripheral neuropathy (23.6% vs 22.8%, P = 0.921). Erosive esophagitis was more prevalent in patients with neuropathy than in those without neuropathy (31.5% vs 10.5%, P = 0.022).
Conclusions
In patients with type II DM, peripheral neuropathy is an independent risk factor for the erosive esophagitis. However, peripheral neuropathy did not contribute to the presence of the typical GERD symptoms.
Keywords: Diabetes mellitus, Type 2; Erosive esophagitis; Gastroesophageal reflux disease; Neuropathy
Introduction
Up to 75% of patients with diabetes mellitus (DM) are known to experience gastrointestinal (GI) symptoms in clinical practice.1 However, the exact prevalence of gastroesophageal reflux disease (GERD) in patients with DM has been on debate. A recent study reported that about 25% of the patients with type II DM had heartburn, acid regurgitation or other symptoms associated with GERD.2 Another study showed that the prevalence of GERD symptoms in DM patients was 41%,3 which was greater than the reported prevalence in general population according to the literature.4 A Korean study reported that the prevalence of erosive esophagitis among patients with DM undergone esophagogastroduodenoscopy (EGD) due to various GI symptoms was 18%.5
It was known that GI symptoms in patients with DM might be associated with its complications, particularly neuropathy, and with poor glycemic control.6 Some studies have shown that many of the GI symptoms in DM patients suggest motor dysfunction and that the neuropathy has positive role on the development of GERD symptoms in type II DM.7-11 However, this concept is still on debate and the pathogenesis of GERD in type II DM has not been fully understood yet.
This study was done to investigate whether neuropathy contributes to the development of erosive esophagitis and typical GERD symptoms in Korean patients with type II DM.
Materials and Methods
Study Population
Patients who had given informed consent with a history of at least 5 years of type II DM and age between 30 and 80 years were prospectively enrolled at Endocrinology Center of Korea University Hospital from August to December of 2008.
Patients were excluded if they had history of angina pectoris, arrhythmia, chronic obstructive pulmonary disease, asthma, sinusitis or previous upper GI surgery. Patients with recent or ongoing medication history of angiotensin-converting enzyme inhibitors, nitrites, β-blockers, calcium channel antagonists, anticholinergics or non-steroidal anti-inflammatory drugs were also excluded.
Study Protocol
The study protocol was approved by Institutional Review Board of Korea University Anam Hospital in August of 2008 (AN08089-001).
Height, weight, waist circumference, duration of DM and hemoglobin A1C (HbA1C) level were evaluated. All patients underwent 3 steps of investigations: electromyography (EMG), interview to evaluate typical symptoms of GERD and EGD.
Diagnosis of diabetes mellitus neuropathy
Patients underwent nerve conduction study and needle EMG for the evaluation of neuropathy. The latency, amplitude and nerve conduction velocity of motor and sensory nerves were recorded on the median and ulnar nerves of upper extremities and peroneal, posterior tibial and sural nerves of lower extremities. Peripheral neuropathy was defined as at least 2 different nerves were found to be abnormal in decreased amplitude or delayed nerve conduction velocity. Patients were divided into 2 groups based on the presence or absence of peripheral neuropathy.
Diagnosis of gastroesophageal reflux disease
During face-to-face interviews, patients were asked whether they had typical GERD symptoms of at least 1 episode a week using a reflux symptom checklist based on a previously validated GERD questionnaire.12,13 Typical GERD symptoms were heartburn and regurgitation. Heartburn was defined as pain or burning sense behind the sternum and regurgitation as acidic taste in the mouth or throat with upward movement of materials from the stomach. Symptom-based diagnosis of GERD was made when a patient had a history of at least weekly typical GERD symptoms that appeared 3 months or earlier before the enrollment.
Diagnosis of erosive esophagitis
Without knowing the result from EMG, expert endoscopists performing more than 1,000 endoscopies a year looked for the esophageal mucosal breaks during EGD. Esophagitis was graded using the Los Angeles classification.14 Cases with hiatal hernia or cases with esophageal candidiasis were excluded. Hiatal hernia was diagnosed when the location of gastroesophageal junction was noted to be at least 2 cm proximal to the impression of the diaphragm at EGD.15 Esophageal candidiasis was diagnosed when characteristic white pseudomembranous or plaque-like adherent to esophageal mucosa was observed by EGD without biopsy confirmation.
Statistical Methods
Statistical analysis was performed using SPSS software (SPSS 12.0 for Windows, SPSS Inc, Chicago, IL, USA). Data were expressed as mean ± SD, number of patients in each category and percentage of total patients in that group. Continuous variables were tested with Student t test and difference in the prevalence between the 2 groups with and without neuropathy were tested with Chi-square test. To evaluate the ability of the variables of interest to predict risk factors for erosive esophagitis in type II DM, a logistic regression analysis model with multivariate adjusted analysis was used. Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). P < 0.05 was selected as a significant level.
Results
From 119 initially recruited patients, 19 patients were excluded after history taking due to their past medical histories and 5 patients were excluded after endoscopy due to the presence of esophageal candidiasis (3 cases) and hiatal hernia (2 cases) and 95 patients were finally included for this study.
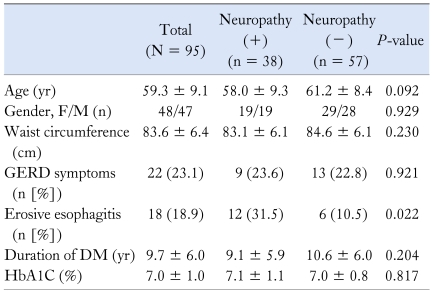
Mean age of the study subjects (N = 95) was 59.3 ± 9.1 years, female/male ratio was 48/47 and the mean disease duration of DM was 9.3 ± 5.9 years. Mean HbA1C level was 7.0% ± 1.0% and the mean waist circumference was 83.6 ± 6.4 cm. Typical GERD symptoms were noted in 23.1% and erosive esophagitis, all in Los Angeles A or B grade, was found in 18.9% (Table 1).
Table 1.
Characteristics of the Patients With Type II Diabetes Mellitus With/Without Neuropathy
GERD, gastroesophageal reflux disease; DM, diabetes mellitus; HbA1C, hemoglobin A1C.
Data were expressed as mean ± SD, number of patients in each category and percentage of total patients in that group.
Thirty-eight cases (40%) had peripheral neuropathy. There was no significant difference in characteristics such as age, gender, waist circumference, disease duration of DM and HbA1C level between the 2 groups with and without neuropathy (Table 1). The prevalence of typical GERD symptoms in patients with neuropathy was similar to that in those without neuropathy (23.6% vs 22.8%, P = 0.921; Table 1). Erosive esophagitis in patients with neuropathy was 3 times more frequent than in patients without neuropathy (31.5% vs 10.5%, P = 0.022; Table 1).
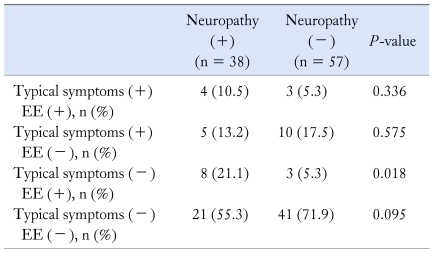
Proportions of patients having erosive esophagitis with typical GERD symptoms were not significantly different between the 2 groups with and without peripheral neuropathy (10.5% vs 5.3%, P = 0.336; Table 2). Numbers of cases with typical GERD symptoms without erosive esophagitis, ie, non-erosive reflux disease (NERD), were also not significantly different (13.2% vs 17.5%, P = 0.575; Table 2). However, patients with erosive esophagitis without typical GERD symptoms, ie, asymptomatic erosive esophagitis, were more commonly found in the group with neuropathy than in those without neuropathy (21.1% vs 5.3%, P = 0.018; Table 2). Among GERD patients who had typical GERD symptoms and/or erosive esophagitis, asymptomatic erosive esophagitis (21.1%) was the most commonly found entity in the neuropathy group, whereas NERD (17.5%) was the most commonly found entity in the non-neuropathy group (Table 2).
Table 2.
Status of Typical Reflux Symptoms and Erosive Esophagitis in Patients With Type II Diabetes Mellitus With/Without Neuropathy (N = 95)
EE, erosive esophagitis.
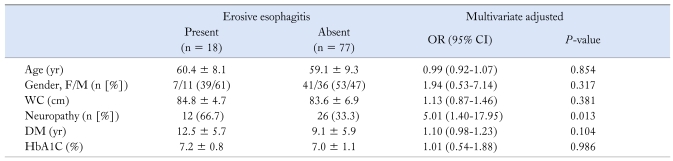
According to the multivariate analysis, age, gender, waist circumference, disease duration of DM and HbA1C level were not associated with the presence of erosive esophagitis. Neuropathy was the only significantly associated factor with an adjusted OR (95% CI) of 5.01 (1.40-17.95) (P = 0.013, Table 3).
Table 3.
Multivariate Analysis of Characteristics of the Patients With Type II Diabetes Mellitus With/Without Erosive Esophagitis (N = 95)
WC, waist circumference; DM, diabetes mellitus; HbA1C, hemoglobin A1C.
Data were expressed as mean ± SD, number of patients in each category and percentage of total patients in that group.
Discussion
GI symptoms are relatively common in clinical practice in patients with type II DM.1 In the present study, the proportion of patients experiencing at least weekly typical GERD symptoms in type II DM was 23.1%, which seems to be far higher than the reported prevalence of GERD from the general Korean population.16,17
It was reported that DM patients with neuropathy had significantly more GI symptoms than those without neuropathy.2,18 A recent study showed that the prevalence of heartburn was significantly higher in type II DM with neuropathy than in without neuropathy.3 In contrast to these reports, the present study showed that there was no significant difference in the proportions of patients experiencing typical GERD symptoms between the 2 groups of type II DM with and without neuropathy.
We could find only one Korean study published on the prevalence of erosive esophagitis among patients with DM who had undergone EGD due to various GI symptoms.5 However, the study did not mention the types of DM. And the study investigated the prevalence of erosive esophagitis irrespective of the GERD symptoms and therefore, NERD cases were not includeed.
Endoscopic examination has high specificity, but low sensitivity, for a diagnosis of GERD.19 It is clear that endoscopy is the only method of investigation to make a diagnosis of erosive esophagitis and grading its severity. According to a recent report, prevalence of erosive esophagitis in secondary and tertiary hospitals in Korea was 11.8%.20 In the present study, prevalence of erosive esophagitis by EGD in type II DM patients was 18.9%. This result is similar to a previous observation that 20% of the patients had erosive esophagitis in patients with refractory diabetic gastroparesis.21 In patients with neuropathy, the prevalence of erosive esophagitis was three times higher than those without neuropathy (31.5% vs 10.5%, P = 0.022). These findings were consistent with a previous study,11 which demonstrated an abnormal gastroesophageal reflux in diabetic patients with cardiovascular autonomic neuropathy. It has been hypothesized that the greater incidence of transient lower esophageal sphincter relaxation that is responsible for the gastroesophageal reflux is related to diabetic autonomic neuropathy.11 Autonomic nervous disorder in DM might cause a certain degree of gastric dysmotility that may increase intragastric distension and lead to a higher incidence of transient lower esophageal sphincter relaxation.
This study evaluated peripheral neuropathy instead of cardiovascular autonomic neuropathy in diabetic patients by needle EMG. It has been known that peripheral nerves consist of larger motor nerve fibers than the autonomic nerve fibers.22 Therefore, patients with peripheral neuropathy might mean that they had already damaged autonomic nerve fibers. Some observations have shown that autonomic neuropathy, as assessed by cardiovascular autonomic function, is closely associated with symptoms and signs of peripheral neuropathy in diabetes.9,10 Patients with peripheral neuropathy, reflected by reduced nerve conduction velocity, have high possibility of cardiovascular autonomic dysfunction in diabetics.23
In this study, asymptomatic erosive esophagitis was more frequently found in those with neuropathy (21.1%) than in those without neuropathy (5.3%), while symptomatic erosive esophagitis was not different in frequency between the 2 groups. This observation could be partly explained with an assumption that perception of the symptom of heartburn or regurgitation might be blunted in type II DM patients with neuropathy in addition to a probable contribution of neuropathy to the development of GERD. Some studies supported this assumption that acute changes in the blood glucose concentration have been shown to impair autonomic nerve function24 and lower significantly in pain threshold in diabetic patients.25 However, this assumption leaves a room to more intensive experimental study.
Major roles of EGD in the management of GERD patients are to diagnose and treat complications of GERD, especially peptic strictures, and to prove the presence of Barrett's esophagus.26 Considering our result that as much as one fifth of the diabetic patients with peripheral neuropathy had asymptomatic reflux esophagitis, EGD might be included as one of the initial evaluations in this subset of patients.
We have not included the drug history in this study and medications such as oral hypoglycemic agents, including metformin and/or insulin, which might have influenced symptoms of GERD. A large-scale population-based prospective study including more variables such as drug history, psychotic factors, etc. is needed.
In summary, the prevalence of erosive esophagitis in type II DM patients with neuropathy was higher than in those without neuropathy, while the proportions of patients experiencing typical GERD symptoms were not different between the 2 groups. Prevalence of asymptomatic erosive esophagitis was higher in the group with neuropathy than in those without neuropathy. Therefore, asking a DM patient whether he or she has typical GERD symptoms may not be sufficient for a diagnosis of GERD, especially in those with neuropathy, and EGD may be recommended to prove the presence of erosive reflux esophagitis in those patients.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Chandran M, Chu NV, Edelman SV. Gastrointestinal disturbances in diabetes. Curr Diab Rep. 2003;3:43–48. doi: 10.1007/s11892-003-0052-7. [DOI] [PubMed] [Google Scholar]
- 2.Nishida T, Tsuji S, Tsujii M, et al. Gastroesophageal reflux disease related to diabetes: analysis of 241 cases with type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19:258–265. doi: 10.1111/j.1440-1746.2003.03288.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Pitchumoni CS, Chandrarana K, Shah N. Increased prevalence of symptoms of gastroesophageal reflux diseases in type 2 diabetics with neuropathy. World J Gastroenterol. 2008;14:709–712. doi: 10.3748/wjg.14.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis. 1976;21:953–956. doi: 10.1007/BF01071906. [DOI] [PubMed] [Google Scholar]
- 5.Park KH, Yoon SB, Jo MH, et al. [Clinical characteristics and analysis of risk factor for gastroesophageal reflux disease in diabetic patient] J Korean Diabetes Assoc. 2005;29:358–366. [Korean] [Google Scholar]
- 6.Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–611. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 7.Locke GR., 3rd Epidemiology of gastrointestinal complications of diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:711–716. [PubMed] [Google Scholar]
- 8.Murray FE, Lombard MG, Ashe J, et al. Esophageal function in diabetes mellitus with special reference to acid studies and relationship to peripheral neuropathy. Am J Gastroenterol. 1987;82:840–843. [PubMed] [Google Scholar]
- 9.Lluch I, Hernández A, Real JT, et al. Cardiovascular autonomic neuropathy in type 1 diabetic patients with and without peripheral neuropathy. Diabetes Res Clin Pract. 1998;42:35–40. doi: 10.1016/s0168-8227(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 10.Young RJ, Zhou YQ, Rodriguez E, Prescott RJ, Ewing DJ, Clarke BF. Variable relationship between peripheral somatic and autonomic neuropathy in patients with different syndromes of diabetic polyneuropathy. Diabetes. 1986;35:192–197. doi: 10.2337/diab.35.2.192. [DOI] [PubMed] [Google Scholar]
- 11.Lluch I, Ascaso JF, Mora F, et al. Gastroesophageal reflux in diabetes mellitus. Am J Gastroenterol. 1999;94:919–924. doi: 10.1111/j.1572-0241.1999.987_j.x. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Chun HJ, Keum B, et al. An electron microscopic study - correlation of gastroesophageal reflux disease and laryngopharyngeal reflux. Laryngoscope. 2010;120:1303–1308. doi: 10.1002/lary.20918. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Lee EJ, Chun HJ, et al. Electron microscopic study of intercellular space: correlation analysis of bronchial asthma and gastroesophageal reflux disease. J Gastroenterol Hepatol. 2011;26:104–107. doi: 10.1111/j.1440-1746.2010.06410.x. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 15.Boyce HW. Endoscopic definitions of esophagogastric junction regional anatomy. Gastrointest Endosc. 2000;51:586–592. doi: 10.1016/s0016-5107(00)70295-1. [DOI] [PubMed] [Google Scholar]
- 16.Cho YS, Choi MG, Jeong JJ, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Asan-si, Korea. Am J Gastroenterol. 2005;100:747–753. doi: 10.1111/j.1572-0241.2005.41245.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeong JJ, Choi MG, Cho YS, et al. Chronic gastrointestinal symptoms and quality of life in the Korean population. World J Gastroenterol. 2008;14:6388–6394. doi: 10.3748/wjg.14.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spångéus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol. 1999;34:1196–1202. doi: 10.1080/003655299750024706. [DOI] [PubMed] [Google Scholar]
- 19.Richter JE. Severe reflux esophagitis. Gastrointest Endosc Clin N Am. 1994;4:677–698. [PubMed] [Google Scholar]
- 20.Hwang JK, Kim J, Hong SG, et al. [A prospective multicenter study on the prevalence and symptoms of erosive reflux esophagitis in secondary and tertiary hospitals in Korea] Korean J Gastroenterol. 2009;53:283–291. doi: 10.4166/kjg.2009.53.5.283. [Korean] [DOI] [PubMed] [Google Scholar]
- 21.Parkman HP, Schwartz SS. Esophagitis and gastroduodenal disorders associated with diabetic gastroparesis. Arch Intern Med. 1987;147:1477–1480. [PubMed] [Google Scholar]
- 22.Heimans JJ, Bertelsmann FW, Van Rooy JC. Large and small nerve fiber function in painful diabetic neuropathy. J Neurol Sci. 1986;74:1–9. doi: 10.1016/0022-510x(86)90186-3. [DOI] [PubMed] [Google Scholar]
- 23.Lanting P, Faes TJ, Ijff GA, Bertelsmann FW, Heimans JJ, van der Veen EA. Autonomic and somatic peripheral nerve function and the correlation with neuropathic pain in diabetic patients. J Neurol Sci. 1989;94:307–317. doi: 10.1016/0022-510x(89)90239-6. [DOI] [PubMed] [Google Scholar]
- 24.Yeap BB, Russo A, Fraser RJ, Wittert GA, Horowitz M. Hyperglycemia affects cardiovascular autonomic nerve function in normal subjects. Diabetes Care. 1996;19:880–882. doi: 10.2337/diacare.19.8.880. [DOI] [PubMed] [Google Scholar]
- 25.Thye-Rønn P, Sindrup SH, Arendt-Nielsen L, Brennum J, Hother-Nielsen O, Beck-Nielsen H. Effect of short-term hyperglycemia per se on nociceptive and nonnociceptive thresholds. Pain. 1994;56:43–49. doi: 10.1016/0304-3959(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DA, Fennerty MB. Heartburn severity underestimates erosive esophagitis severity in elderly patients with gastroesophageal reflux disease. Gastroenterology. 2004;126:660–664. doi: 10.1053/j.gastro.2003.12.001. [DOI] [PubMed] [Google Scholar]