Abstract
Previous studies have shown that female preferences for male pheromones depend on the female’s reproductive condition and the dominance status of the male. However, it is unknown which olfactory system detects the odors that result in a preference for a dominant male. Therefore, in the present study, we asked whether dominant versus subordinate male urinary odors differentially activate the main and accessory olfactory systems in female (C57Bl/6j) mice by monitoring the induction of the immediate early gene, c-fos. A more robust induction of Fos was observed in female mice which had direct nasal contact with dominant male urinary odors in four specific segments of the accessory olfactory system, i.e., the posteroventral part of the medial amygdala, the bed nucleus of the stria terminalis, the medial part of the preoptic nucleus and the ventrolateral part of the ventromedial hypothalamus, compared to females that were exposed to subordinate male urine. This greater activation of the accessory olfactory pathway by dominant male urine suggests that there are differences in the nonvolatile components of dominant versus subordinate male urine that are detected by the vomeronasal organ. By contrast, subordinate male urinary odors induced a greater activation in the piriform cortex which is part of the main olfactory system, suggesting that female mice discriminate between dominant and subordinate male urine using their main olfactory system as well.
Keywords: olfaction, vomeronasal system, preferences, hormones
1. Introduction
In rodent species, body odors provide essential information about the sex, social and reproductive status of conspecifics (Brown, 1979). They induce hormonal changes and play a key role in mate recognition and partner preferences (reviewed in Bakker, 2003; Keller and Bakker, 2009; Keller et al., 2009). For instance, male urinary pheromones induce sexual maturation (Lombardi and Vandenbergh, 1977) and pregnancy block (Bruce, 1959; Lloyd-Thomas and Keverne, 1982) in female mice. These physiological effects of male pheromones are mediated through the vomeronasal organ (VNO) and subsequently the accessory olfactory system since lesions of the VNO prevented the occurrence of pregnancy block in female mice when exposed to an unfamiliar male (Lloyd-Thomas and Keverne, 1982). Furthermore, female mice must make direct nasal contact with non-volatile male body odors first before later showing any recognition/attraction to volatile components of male body odors. This suggests a role for the VNO and the accessory olfactory system in mate recognition (Hurst et al., 1998; Martinez-Garcia et al., 2009). However, it should be noted that odor-experienced female mice might use volatile odors alone to discriminate between different males, which would implicate the main olfactory system in odor preferences. Indeed, lesions of the main olfactory epithelium (MOE) by bilateral infusion of zinc sulfate into the nares disrupted male odor preferences and mate recognition in odor-experienced female mice whereas lesions of the VNO had no such effect (Keller et al., 2006a; Keller et al., 2006b). These results suggest that mate recognition likely depends on a combination of MOE and VNO input.
Studies of wild-caught house mice living in seminatural enclosures have shown that females’ male odor preferences are based on the females’ reproductive condition (estrous or nonestrous) and the dominance status of the male (Mossman and Drickamer, 1996). Thus in dominance odor tests, estrous females preferred odors from dominant males whereas nonestrous females exhibited no significant preferences for either subordinate or dominant male odors. We recently confirmed such a preference for dominant versus subordinate male odors in female laboratory (C57Bl/6j) mice (Veyrac and Bakker, 2008). We also found that this preference depended on the hormonal status and prior sexual experience of the female. Thus sexually naïve, female mice ovariectomized in adulthood needed to be treated with both estradiol and progesterone to show a significant preference for dominant over subordinate male odors, whereas sexually experienced females showed this preference when treated with estradiol alone (Veyrac and Bakker, 2008). This odor preference was based on volatile odors alone, since female subjects could not make any direct nasal contact with the odor sources. Thus, the dominance status of males is a criterion used by female mice for mate selection. However, at present it is still unknown which olfactory system detects the odors that result in a preference for a dominant male. Therefore, in the present study we asked whether dominant versus subordinate male urinary odors differentially activate the main and accessory olfactory systems in female mice, using the expression of the immediate early-gene, c-fos, as a marker of neuronal activation. Female subjects were first provided with mating experience with different males since more a pronounced preference for a dominant over a subordinate male was previously observed in females following sexual experience (Veyrac and Bakker, 2008).
2. Results
2.1.1 Accessory olfactory bulb
Exposure to either dominant or subordinate male urine induced Fos expression in both the granular (Figure 2A,C) and mitral cell layers of the AOB (Figure 2B,D). Kruskall-Wallis tests showed a significant effect of urine exposure on the induction of Fos in the anterior and posterior parts of the AOB (Granular anterior part p=0.0272; Mitral anterior part p=0.0294; Granular posterior part p=0.0164; Mitral posterior part p=0.0312). Subsequent Mann-Whitney comparisons showed no significant effects of type of urine on the number of Fos positive cells (p>0.05 dominant versus subordinate urine for all the AOB regions analyzed).
Figure 2.
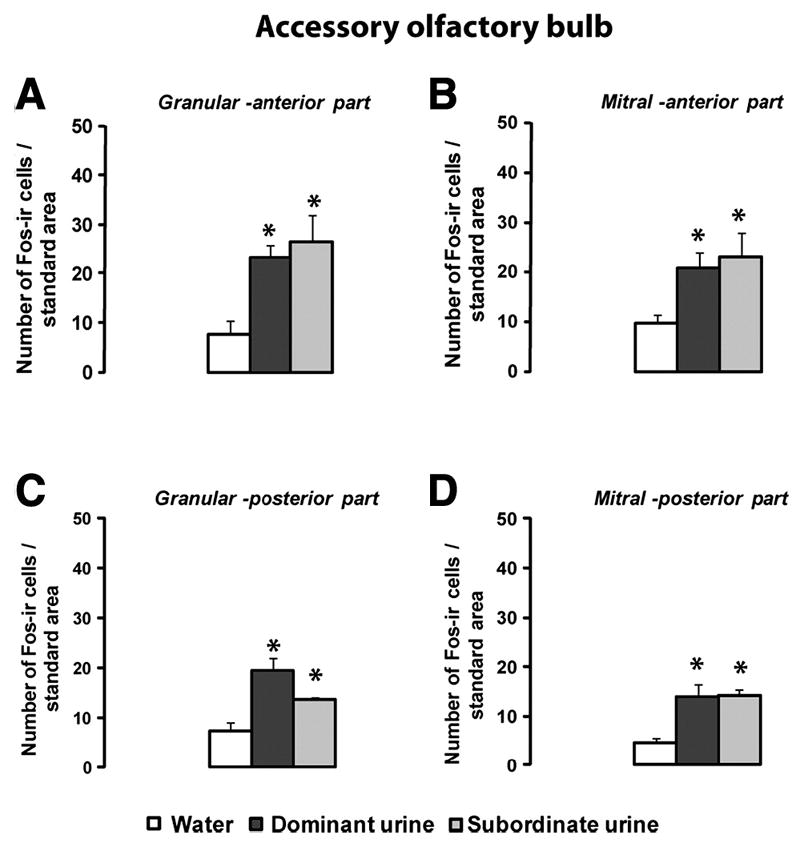
Fos expression in the accessory olfactory bulb. A: granular cell layer, anterior part of the AOB; B: mitral cell layer, anterior part of the AOB; C: granular cell layer, posterior part of the AOB, and D: mitral cell layer, posterior part of the AOB. Data are expressed as means ± SEM. *p<0.05 compared to water-exposed females; Water n=4; Dominant n=4-5 Subordinate n=3.
2.1.2 Accessory olfactory pathway
Exposure to dominant male urine induced a greater Fos expression in several brain regions of the accessory olfactory pathway, including the posteroventral part of the medial amygdala (MePV), the anterior medial part of the BNST, the medial part of the preoptic nucleus (MPN) and the ventrolateral part of the VMH, than exposure to subordinate male urine or water (Figure 3B,C,D and E; Kruskall-Wallis tests: MePV p=0.0064; BNST p= 0.049 ; MPN p=0.0273; VMH-VL p=0.0243). Thus, a greater Fos response was induced by dominant male urine compared to subordinate male urine in the MePV (Mann-Whitney test p= 0.0275), BNST (Mann-Whitney test p= 0.0339), MPN (Mann-Whitney test p=0.0273) and VMH-VL (Mann-Whitney test p=0.0493). By contrast, no significant differences in Fos expression was observed in the posterposterodorsal part of the medial amygdala of females exposed to either dominant or subordinate male urine (Kruskall-Wallis tests p=0.0185 and Mann-Whitney comparison Dominant versus subordinate urine p=0.4624). Likewise, no significant activation was observed after exposure to dominant male urine in the medial amygdala (MeA), the posterior part of the BNST or the dorsomedial part of the VMH (Table1).
Figure 3.
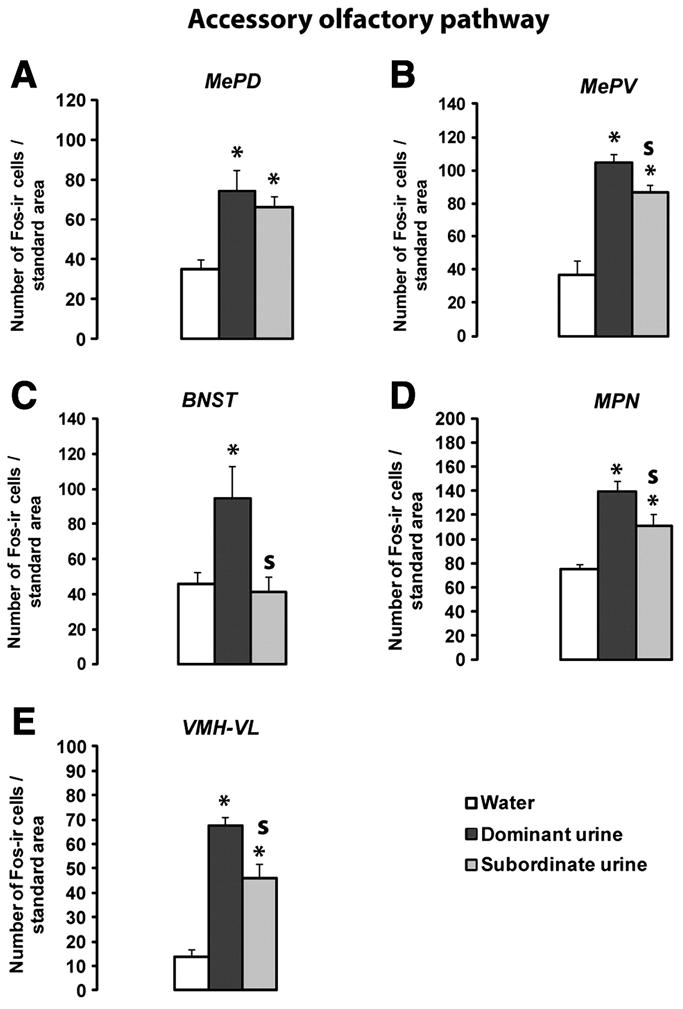
Fos expression in the accessory olfactory pathway. A: Posteroventral portion of the medial amygdala (MePV); B: posterodorsal portion of the medial amygdala (MePD); C: medial preoptic nucleus (MPN); D: ventrolateral portion of the ventromedial hypothalamus (VMH-VL); E: posteromedial portion of the bed nucleus of the stria terminalis (BNST-). Values are means ± SEM. *p<0.05 compared to water exposed females. S p<0.05: significantly different between dominant versus subordinate male urine exposed females; Water n=3-4; Dominant n=3-5; Subordinate n=3-4.
Table 1.
Fos expression in regions of the accessory and main olfactory pathways that did not show any significant activation after exposure to either dominant or subordinate male urine compared to water.
| Number of Fos-ir cells | MeA | BNST-Post | VMH-DM | ACo-Ant |
|---|---|---|---|---|
| Interaural coordinates | 2.74 mm | 3.58 mm | 2.46 mm | 2.74 mm |
| Water | 76.5 +/- 6.9 | 48.8 +/- 10.1 | 36.6 +/- 5.0 | 60.1 +/- 11.2 |
| Dominant urine | 80.6 +/- 9.4 | 64.3 +/- 6.2 | 37.2 +/- 5.3 | 72.8 +/- 4.4 |
| Subordinate urine | 82.2 +/- 8.1 | 57.3 +/- 6.0 | 31.3 +/- 5.3 | 59.1 +/- 13.1 |
| Kruskall-Wallis test | p=0.9175 | p=0.5836 | p=0.5568 | p=0.7901 |
Anterior part of the Medial amygdala (MeA); Posterior part of Bed Nucleus of the Stria Terminalis (BNST-Post), Dorsomedial part of the ventromedial hypothalamic nucleus (VMH-DM), anterior cortical amygdaloid nucleus, ACo-Ant. Values are means ± SEM. Non parametric Kruskall-Wallis tests were used to determine statistical differences between groups. Number of animals per group: Water n=4; Dominant n=5; Subordinate n=4. Interaural coordinates: distance (in mm) in front of the interaural line (Paxinos and Franklin, 2001).
2.2.1 Main olfactory bulb
Similar patterns of glomerular activation in the MOB were observed between females exposed to dominant male urine vs subordinate male urine (Figure 4A). As shown previously (Martel and Baum, 2007), the regions with the greatest number of urine odor-activated glomeruli (in red) included the rostral-lateral as well as the caudal-medial portions of the MOB. Point-by-point Mann Whitney U-tests (bottom panels Figure 4B) showed significant differences (red-yellow colors) between plots for clean vs. dominant male urine, and for water vs. subordinate male urine, but no significant differences between plots for dominant vs. subordinate male urine.
Figure 4.
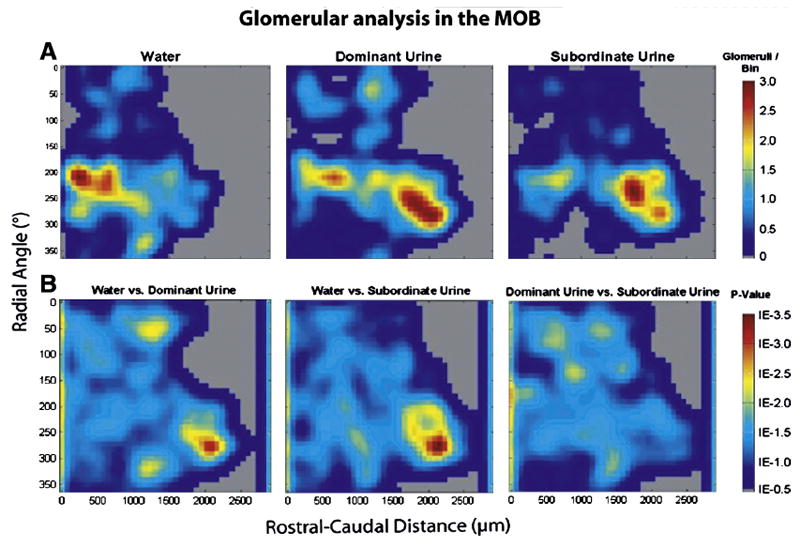
A: contour plots showing areas of main olfactory bulb (MOB) glomerular activation in females exposed to water (clean; n=4), dominant male urine (n=5), or subordinate male urine (n=4) The scale showing the number of activated glomeruli per bin, 10° radial angle is located to the right of each panel; B: Mann-Whitney U comparisons between clean vs dominant, clean vs subordinate, and dominant vs subordinate male urine. Significant p-values were determined using a false discovery rate (FDR) critical value of 0.025 (1E -1.6)
2.2.2 Main olfactory pathway
Exposure to either dominant or subordinate male urine induced Fos expression only in the posterior ACo which is part of the olfactory amygdala (Table1; Figure 5 A; Kruskall-Wallis test p=0.0375;) and the piriform cortex (Figure 5B Kruskall-Wallis test p=0.0064). However, Mann-Whitney tests showed that subordinate male urine induced a greater Fos expression in the piriform cortex than exposure to dominant urine (Figure 5B; subordinate versus dominant urine p=0.0143). Interestingly this effect was observed both in the anterior and posterior parts of the piriform cortex (Figure 5C anterior part: Kruskall-Wallis test p=0.0201, Mann-Whitney test subordinate versus dominant urine p=0.050; Figure 5D posterior part: Kruskall-Wallis test p=0.0048, Mann-Whitney test subordinate versus dominant urine p=0.0143)
Figure 5.
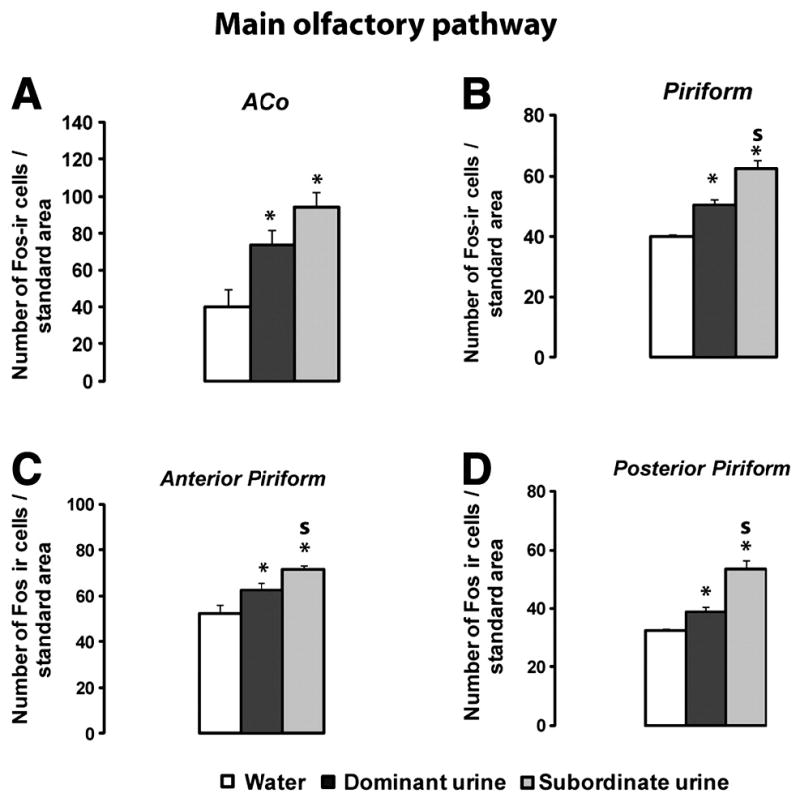
Fos expression in the main olfactory pathway. A: Anterior cortical medial amygdala (ACo); B: Piriform cortex; C: Anterior part of the piriform cortex; D: Posterior part part of the Piriform cortex. Values are means ± SEM. *p<0.05 compared to water (clean) exposed females. S p<0.05 compared to females exposed to dominant male urine; Water n=3-4; Dominant n=4-5; Subordinate n=3-4.
3. Discussion
Direct nasal exposure to either dominant or subordinate male urine differentially activated the main and accessory olfactory systems in female mice. A more robust stimulation of Fos expression was observed in female mice exposed to dominant male urinary odors in three specific segments of the accessory olfactory system, including the MePV, the BNST, and the MPN. In addition, a more robust induction of Fos was observed in the VMH-vl which receives olfactory inputs from the medial amygdala (Choi et al., 2005). By contrast, subordinate male urine induced a greater Fos response in one area of the main olfactory pathway, the piriform cortex.
3.1 Accessory olfactory system
Interestingly, urinary odors from dominant as opposed to subordinate males activated more robustly the MePV, the BNST, and the MPN in female mice. In many rodent species, the expression of sexual behaviors depends critically on the perception and identification of conspecific odors (Johnston and Mueller, 1990). Accumulating evidence shows that the behavioral response to these odors is mediated by a neural network of steroid hormone-sensitive forebrain nuclei, which includes the medial amygdala, BNST, and the MPN (Baum and Everitt, 1992; Pfaus and Heeb, 1997). The MePV is important in relaying olfactory information to the MPN. In female hamsters, lesions centered in the medial amygdala eliminated female preferences for male odors in a Y-maze (Petrulis and Johnston, 1999). The role of the MPN in the expression of female sexual behavior or male preferences is less clear. All available evidence suggests that the MPN plays an inhibitory role in female sexual behavior. Thus bilateral lesions of the MPN facilitate lordosis behavior (Powers and Valenstein, 1972).
The BNST may relay information from the olfactory bulbs to the neuroendocrine system, and in particular kisspeptin and GnRH neurons located in the hypothalamus. There are clear sex differences in projections from the BNST to the anteroventral preoptic region (AVPV), a region important for female reproductive functioning, with a stronger innervation of the AVPV in male compared to female rats (Gu et al., 2003). Treatment of newborn females with testosterone or neonatal castration of males reversed these sex differences, suggesting that these projections are organized perinatally by testosterone (Polston et al., 2004). These projections are thought to be inhibitory since they are GABA-ergic (they express the enzyme GAD). Thus exposure to dominant but not subordinate male urine may activate the reproductive neuroendocrine system in female mice. We recently observed that opposite-sex urinary odors induced Fos expression in kisspeptin neurons located in the rostral periventricular area of the third ventricle which includes the AVPV (RP3V; (Bakker et al., 2010). In that study, urine samples were pooled from different males of unknown dominance status. Future studies should determine whether dominance status influences the ability of male urinary odors to activate kisspeptin neurons in the RP3V.
The greater induction of Fos in the female VMHvl shown in response to dominant male urinary odors is also interesting since the VMH is important for the expression of female sexual behavior, including female social odor preferences. Thus it was shown (Robarts and Baum, 2007) that lesions of the VMH disrupted olfactory mate recognition and sexual receptivity in female ferrets. Furthermore, (Choi et al., 2005) showed that a neural circuit delineated by the transcription factor, Lhx6, conveys olfactory inputs of reproductive significance to the VMH in mice.
The greater activation of the accessory olfactory pathway by dominant male urine suggests that there may be differences in the nonvolatile constituents of dominant versus subordinate male urine that are detected by the VNO (Roberts et al., 2010). This could be due to differences in the profiles of major urinary proteins (MUP) since MUPs play an important role in conveying volatile molecules to the VNO as well as slowing down their release from male scent marks (Armstrong et al., 2005). However, the greater activation may also be due to a greater exposure to two terpenic constituents E,E,-alpha-farnesene and E-beta-farnasene that are produced in the preputial glands and are elevated in dominant male urine when compared to subordinate male urine or control males (Novotny et al., 1990).
3.2 Main olfactory system
No differences were observed in patterns of activation of MOB glomeruli by either dominant or subordinate male urine, suggesting that the same suite of MOE receptor neurons detects dominant and subordinate urinary odors and thus that any discrimination between these different categories of male odors is not made at the level of the MOE, but either at more proximal levels of the main olfactory system or using the VNO detection system. This result is surprising since previous studies showed statistically significant differences in the profiles of MOB glomerular activation following exposure to different urinary odor stimuli, such as urinary volatiles from ovariectomized females given estradiol alone versus those from ovariectomized females treated with estradiol plus progesterone (Martel et al., 2007), suggesting that urinary odors vary according to the hormonal status of the female. Likewise, Schaefer and co-workers (Schaefer et al., 2001; Schaefer et al., 2002) showed that distinct clusters of MOB glomeruli were activated in female mice exposed to urinary volatiles from males carrying different haplotypes of the major histocompatibility complex. These latter findings together with the present results suggest that the difference between the actions of dominant and subordinate male urine on fos expression in the forebrain olfactory pathway may reflect differences in the concentration rather than composition of pheromones contained in urine from the two types of males. It should be noted that the dominant and subordinate males used in the present study were derived from the same breeding colony (C57Bl/6j) and thus share the same MHC haplotype. By contrast, subordinate male urine induced a greater Fos response in the posterior piriform cortex, although it should be noted that dominant male urine also activated this brain area. This finding of a greater induction of Fos in the posterior piriform cortex by subordinate male urine does not necessarily mean that subordinate male odors activate the main olfactory system more than dominant male odors since one cannot distinguish between stimulatory and inhibitory neurons using the expression of Fos protein. It only shows that subordinate and dominant male urinary odors are differentially processed by the main olfactory system and thus suggests that female mice discriminate between these two urine types using their main olfactory system as well as the accessory olfactory system. However, this discrimination is probably not made at the level of the olfactory bulb since no differences were observed in MOB glomerular activation patterns. Thus, our finding (Veyrac and Bakker, 2008) of a preference for dominant over subordinate male volatile odors is probably the result of a complex interaction among the AOB, the MOB, and the hypothalamus, perhaps including the reproductive neuroendocrine system as well.
In summary, we observed a differential activation of the main and the accessory olfactory systems by dominant versus subordinate male urinary odors when female recipient mice were allowed direct nasal contact with the odors. This result is in line with our previous behavioral observation (Veyrac and Bakker, 2008) of a clear-cut preferences in female mice for urinary odors from dominant versus subordinate males.
4. Experimental Procedures
4.1 Animals
Male (n=9) and female (n=15) C57BL/6j mice aged 12 weeks at the beginning of the experiment were used. All mice were obtained from a local breeding colony at the University of Liège. Males and females were housed in separate climate-controlled units on a reversed 12/12 h light-dark cycle (lights off at 8am, lights on at 8pm). Food and water were available ad libitum.
All experiments were conducted in accordance with the guidelines set forth by the National Institutes of Health Guiding Principles for the Care and Use of Research Animals and were approved by the Ethical Committee for Animal Use of the University of Liège.
4.2 Establishing the dominance status of male mice
In order to determine males’ dominance status, paired encounters were conducted between 9 sexually naïve adult males using a protocol previously described (Mossman and Drickamer, 1996, Mak et al., 2007). Males were housed individually for 2 weeks before testing began and throughout the experiment. Encounters were conducted in opaque chambers with hardware plastic lids in which pairs of males were introduced and observed for 10 minutes. Behavioral patterns were observed and scores were given as follows: aggressive dominance (a score of 3) was defined as three consecutive attacks by one mouse (aggressive grooming, biting and chasing); passive dominance (a score of 2) was defined as consistent threatening displacement by one mouse including upright or sideways postures; a subordinate behavior (score of 0) was defined as retreat or fleeing by one mouse including “one back” position and crouching and a draw (a score of 1) was defined as no attacks or consistent displacement occurring on the part of either mouse. After completion of these tests, one dominant and one subordinate male were selected based on their average scores (which were calculated after nine encounters per subject). Thus the male with the highest score was considered to be dominant and the male with the lowest score was considered to be subordinate.
4.3 Urine collection
Urine from the dominant and the subordinate male was collected by holding the mouse by the scruff of the neck over a funnel. These urine samples were taken within one week after males’ dominance/subordinance status was established. It should be noted that urine was not collected on the day of testing itself to prevent putative pheromone content from being affected by the experience of victory for the dominant male versus defeat for the subordinate male. Furthermore, urine was always collected at the same time of the day (in the morning) to avoid any diurnal fluctuations in its composition, and care was taken that no fecal contamination occurred. Urine samples from the dominant and subordinate male were stored at -80 C, only defrosted once, pooled for each individual and then used in the experiment on the same day.
4.4 Surgery and hormonal treatment
Adult female mice were ovariectomized (OVX) under general anesthesia using a mixture of Ketamine (80mg/kg i.p. per mouse) and medetomidine (Domitor, Pfizer, 1mg/kg i.p. per mouse). Mice received atipamezole (Antisedan, Pfizer, 4mg/kg per mouse) at the end of surgery in order to accelerate their recovery. All females were implanted under general anesthesia with a 5-mm-long SILASTIC (inner diameter: 1.57mm; outer diameter: 2.41 mm; length: 5 mm) capsule containing 17-β-estradiol (OVX-E2) (diluted 1:1 with cholesterol) which produced circulating levels of estradiol similar to those observed during estrus in mice (Bakker et al., 2002). In order to provide sexual experience, sexually naïve OVX-E2 female mice received a s.c. injection of progesterone (500μg/mouse) 3 hours before they were paired with a sexually active male for 10 min in a Plexiglas chamber. All female mice were tested on separate occasions with at least five different males which had not been tested for their dominance status and which were not used later as urine donors. This ensured that females received different mating and social odor experiences with several as opposed to one particular male.
4.5.1 Neural Fos responses to urinary odors from a dominant versus a subordinate male
In order to determine whether urinary odors from a dominant male versus a subordinate male would differentially activate the main and accessory olfactory system in female recipient mice, sexually experienced, OVX, E2-treated female mice were exposed to either dominant or subordinate male urine or to water as a control.
Females were habituated daily for 1 week to the urine exposure procedure by applying deionized water directly onto subjects’ noses (following the protocol previously described in (Pierman et al., 2008). We chose to apply the urine directly onto the nose instead of giving subjects free access to the urine to avoid differences in central neural activation due to possible differences in time that subjects spent investigating the urinary odors. On the day of urine exposure, 3 groups of females (n=5 mice/group) were exposed to different stimuli: 1) 30μl of deionized water; 2) 30μl of dominant male urine or 3) 30μl of subordinate male urine. Ninety minutes later, female subjects were deeply anesthetized with an intraperitoneal injection of ketamine and medetomidine and perfused transcardially with 0.9% saline, followed by 4% cold paraformaldehyde in 0.1 M phosphate buffer. Brains were post-fixed overnight in the same solution, cryoprotected in a 30% sucrose solution in 0.1 M phosphate buffer, frozen in chilled isopentane (-55°C) and cryostat sectioned (Leica). For the olfactory bulb (OB), serial coronal sections (14μm) were mounted on superfrost Plus glass slides (Menzel-Glaser) and free-floating serial coronal sections of the forebrains (30μm) were saved in antifreeze solution at -20°C until processed for immunocytochemistry. Thus Fos immunocytochemistry for the OB was carried out on slide-mounted sections whereas Fos immunocytochemistry for the forebrain was carried out on free-floating sections prior to being mounted onto slides for later image analysis.
4.5.2 Immunocytochemistry
Every fourth section of the forebrain and sixth of the OB were processed for Fos immunoreactivity as previously described (Pierman et al., 2008). All OB and forebrain sections were processed for Fos-immunoreactivity in one run. Sections were pre-incubated for 3h at room temperature (RT) in 7.5% normal goat serum (NGS) in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 (Sigma). Sections were then incubated overnight with a rabbit polyclonal anti-c-Fos antibody (1/3000 in phosphate-buffered saline containing 0.1% Triton X-100 / 2% NGS; Santa Cruz SC-52) followed by an incubation for 1 h in a goat anti-rabbit biotinylated antibody (1/200 in phosphate-buffered saline containing 0.1% Triton X-100 / 2% NGS; Dako Cytomation). Endogenous peroxidases were blocked for 30 min in phosphate-buffered saline containing H2O2 at a final concentration of 3%. Sections were then processed with avidin–biotin-peroxydase complex (ABC Elite Kit; Vector Laboratory) for 45 min and reacted for 5 min with 3,3’diaminobenzidine tetrahydrochloride containing nickel chloride (Vector Laboratory). Sections were washed, mounted on gelatin-coated slices, dried, left in SafeSolv for 5 min (Labonord) and coverslipped using SafeMount (Labonord). For the OB, sections were treated with the same protocol except that they were incubated in Target Retrieval Solution (Dako) for 20 min at 95°C before the blocking solution.
4.5.3 Quantification of Fos-immunoreactive nuclei
Numbers of Fos-immunoreactive cells were counted in several brain areas included in the accessory and main olfactory projection pathway, as previously described (Pierman et al., 2008) by an experimenter who was blind to the experimental condition of the animals. Sections were digitized through a video camera (CCD camera, XC-77CE, Sony) attached to a microscope (Olympus MTV-3 - 20X and 40X objectives), and number of Fos immunoreactive cells was quantified with a PC-based image analysis system using the particle-counting protocol of the NIH Image program (Version 1.37; Wayne Rasband, NIH, Bethesda, MD, USA). Digital images were made binary and a manual threshold was used for discriminating the labeled cells from the background. With a 20x objective, exclusion thresholds were set at 10 to 100 pixels to remove from the counts dark objects that were not the same size as a cell nucleus (20 to 500 with a 40x objective). Brain structures were identified based on the mouse atlas (Paxinos and Franklin, 2001), and the computer field was placed in a standardized manner based on pre-defined anatomic landmarks in the sections (e.g., edge of the third ventricle or prominent fiber tracts) (Figure 1 and table 1). All Fos-immunoreactive cells were counted in the entire quantification field, and the numbers were averaged for both sides of the brain (one section for each brain hemisphere). We analyzed number of Fos-ir cells in both the accessory (accessory olfactory bulb, AOB; anterior, posteroventral and posterodorsal parts of the medial amygdala, MeA, MePV and MePD; anterior medial part and posterior part of the bed nucleus of the stria terminalis, BNST; medial part of the preoptic nucleus, MPN; dorsomedial and ventrolateral part of the ventromedial hypothalamic nucleus, VMH-DM and VMH-VL) and the main (piriform cortex, Pir; anterior and posterior cortical amygdaloid nucleus, ACo) olfactory system.
Figure 1.
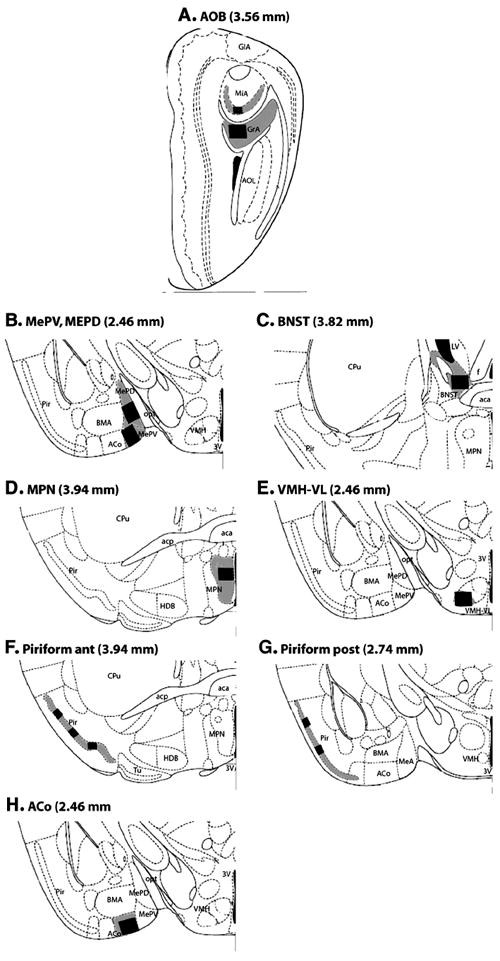
Drawings modified from the mouse brain atlas (Paxinos and Franklin, 2001) showing the location of forebrain regions in which Fos-immunoreactive cells were counted. The distance of each coronal brain slice rostral to the interaural line is given in parentheses for each panel. The counting areas are shown as a black rectangle. The two different sizes of rectangles correspond to the areas analyzed using either a 20x or 40x objective, respectively. A: Accessory olfactory bulb (AOB). B: Medial amygdaloid nucleus, postero-ventral (MePV) and postero-dorsal (MePD) parts. C: Medial part of the Anterior Bed Nucleus of the Stria Terminalis (BNST). D: Medial part of the Preoptic nucleus (MPN). E: Ventro lateral part of the Ventromedial hypothalamic nucleus (VMH-VL). F: Anterior part of the Piriform cortex. G: Posterior part of the Piriform cortex. H: Anterior cortical Amygdaloid nucleus (ACo). Additional abbreviations: MiA= accessory mitral cell layer; GlA= accessory glomerular layer; GrA= accessory granular layer; AOL= anteriolateral olfactory nucleus; 3V= third ventricle; BMA= basomedial amygdaloid nucleus; opt= optic tract; LV=lateral ventricle; f=fornix; aca= anterior commisure, anterior part ; CPu=caudate putamen; acp=anterior commisure, posterior part; HDB= horizontal limb diagonal band.
4.5.4 Mapping of Fos expression in the glomerural layer of the MOB
Patterns of glomerular activation in the MOB of females exposed to either water (control), dominant or subordinate male urine were analyzed. Slides with mounted sections of the olfactory bulb were sent to the laboratory of Dr. Michael Baum at Boston University where Fos expression was mapped in the glomerular layer of the MOB using the method previously described (Martel and Baum, 2007). Briefly, odor-induced MOB glomerular activation was mapped using Matlab software in conjunction with the GLOM-MAP program (Salcedo et al., 2005). The location of each activated glomerulus was mapped according to the radial angle from a central point of origin and rostral-caudal distance through the bulb. Activated glomeruli were defined as having 180° of continuous Fos activation or two 90° arcs of Fos activation in the periglomerular cells surrounding the glomerulus (Schaefer et al., 2001; Schaefer et al., 2002). Sections were analyzed in their precise rostral-caudal sequence (spaced by 84μm). The rostral and caudal limits for analysis were determined using anatomical landmarks, with the most rostral section being the first to contain clear mitral cell and external plexiform layers, and the most caudal section lying just posterior to the AOB. Using the thickness of each section (14μm) and its sequence within the sections to be analyzed, we were able to estimate the rostral-caudal location of each activated glomerulus. A central axis (0-180°) extending from the dorsal mitral cell layer to the ventral mitral cell layer was established for each section, and a central point of origin was then determined to be 1/3 the distance from the dorsal portion of the central axis in sections not containing the AOB, and 1/3 the distance from the granular cusp of the AOB in sections containing the AOB. This point of origin was used to determine the radial angle of an activated glomerulus relative to the central axis. The rostral-caudal distance and radial angle of each activated glomerulus provided coordinates with which the GLOM-MAP program allowed us to create 2-dimensional color contour plots that show the density of activated glomeruli throughout the MOB. Mapping was accomplished by capturing images of each section using a Nikon digital camera attached to an Olympus microscope. An investigator blind to the treatment of each subject identified and annotated each activated glomerulus using ACT1 imaging program. Annotated images were then imported into the GLOM-MAP OBS program, the central axis was established and the positions of the activated glomeruli were recorded. Using the statistical toolbox, GLOM-MAP GDB, these data were compiled and smoothed, generating individual contour plots, group averaged contour plots, and statistical comparisons between groups. Point by point Mann-Whitney U-tests were used to specify significantly different clusters of activated glomeruli. Significant p-values were determined using a false discovery rate (FDR) critical value of 0.025 (1E -1.6) that was adjusted for multiple Mann-Whitney tests comparisons. The use of a FDR reduced the occurrence of Type I statistical errors.
4.6 Statistics
All Fos data were expressed as mean number of Fos immunoreactive cells /standard area (+/-) SEM and were derived from 3-5 female mice for each experimental condition. Non parametric Kruskall-Wallis tests followed by Mann-Whitney two-tailed comparisons were used to determine whether exposure to either dominant or subordinate male urine or water differentially activated the accessory and main olfactory projection pathways. Only effects with a P-value lower than 0.05 are mentioned as significant in the results.
Acknowledgments
This research was supported by NIH grant (HD 044897) to JB and MJB, and by grants from the University of Liège C-06/89 to JB and AV. JB is a research associate at the Fonds National de la Recherche Scientifique (Belgium).
References
- Armstrong SD, Robertson DH, Cheetham SA, Hurst JL, Beynon RJ. Structural and functional differences in isoforms of mouse major urinary proteins: a male-specific protein that preferentially binds a male pheromone. Biochem J. 2005;391:343–350. doi: 10.1042/BJ20050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J. Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J Neuroendocrinol. 2003;15:615–621. doi: 10.1046/j.1365-2826.2003.01036.x. [DOI] [PubMed] [Google Scholar]
- Bakker J, Pierman S, Gonzalez-Martinez D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav. 2010;57:390–395. doi: 10.1016/j.yhbeh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Brown RE. Mammallian social odors: a critical review. Advances in the Study of Behavior. 1979;10:103–162. [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Gu G, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol. 2003;460:542–562. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Robertson DHL, Tolladay U, Beynon RJ. Proteins in urine scent marks of male house mice extend the longevity of olfactory signals. Anim Behav. 1998;55:1289–1297. doi: 10.1006/anbe.1997.0650. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Mueller UG. Olfactory but not vomeronasal mediation of scent marking by male golden hamsters. Physiol Behav. 1990;48:701–706. doi: 10.1016/0031-9384(90)90214-o. [DOI] [PubMed] [Google Scholar]
- Keller M, Bakker J. Pheromonal communication in higher vertebrates and its implication on reproductive function. Editorial Behav Brain Res. 2009;200:237–238. doi: 10.1016/j.bbr.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006a;31:315–323. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006b;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Lloyd-Thomas A, Keverne EB. Role of the brain and accessory olfactory system in the block to pregnancy in mice. Neuroscience. 1982;7:907–913. doi: 10.1016/0306-4522(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Lombardi JR, Vandenbergh JG. Pheromonally induced sexual maturation in females: regulation by the social environment of the male. Science. 1977;196:545–546. doi: 10.1126/science.557838. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26:463–475. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Keller M, Douhard Q, Bakker J, Baum MJ. Comparison of urinary odor-induced glomerular activation in the main olfactory bulb of aromatase knock-out and wild type female mice. Neurosci Lett. 2007;421:101–105. doi: 10.1016/j.neulet.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia F, Martinez-Ricos J, Agustin-Pavon C, Martinez-Hernandez J, Novejarque A, Lanuza E. Refining the dual olfactory hypothesis: pheromone reward and odour experience. Behav Brain Res. 2009;200:277–286. doi: 10.1016/j.bbr.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Mossman CA, Drickamer LC. Odor preferences of female house mice (Mus domesticus) in seminatural enclosures. J Comp Psychol. 1996;110:131–138. doi: 10.1037/0735-7036.110.2.131. [DOI] [PubMed] [Google Scholar]
- Novotny M, Harvey S, Jemiolo B. Chemistry of male dominance in the house mouse, Mus domesticus. Experientia. 1990;46:109–113. doi: 10.1007/BF01955433. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. Academic Press; SanDiego: 2001. [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44:397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Pierman S, Douhard Q, Bakker J. Evidence for a role of early oestrogens in the central processing of sexually relevant olfactory cues in female mice. Eur J Neurosci. 2008;27:423–431. doi: 10.1111/j.1460-9568.2007.06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Powers B, Valenstein ES. Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science. 1972;175:1003–1005. doi: 10.1126/science.175.4025.1003. [DOI] [PubMed] [Google Scholar]
- Robarts DW, Baum MJ. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm Behav. 2007;51:104–113. doi: 10.1016/j.yhbch.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chem Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22:9513–9521. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Bakker J. Society for Behavioral Neuroendocrinology. Gronigen: Pays Bas; 2008. Hormonal determinants of female mouse preferences for the dominant male. [Google Scholar]


