Abstract
Polybrominated diphenyl ether (PBDE) flame retardants are known to affect thyroid hormone (TH) regulation. The TH-regulating deiodinases have been implicated in these impacts; however, PBDE effects on the fish thyroid system are largely unknown. Moreover, the liver as a potential target of PBDE toxicity has not been explored in young fish. This study measured decabromodiphenyl ether (BDE-209) effects on TH regulation by measuring deiodinase activity in juvenile fathead minnows (Pimephales promelas). Dietary accumulations and debromination of BDE-209 were also measured, and the morphology of thyroid and liver tissues was examined. Juvenile fathead minnows (28 days old) received a 28-day dietary treatment of BDE-209 at 9.8 ± 0.16 μg/g of food at 5% of their body weight per day followed by a 14-day depuration period in which they were fed clean food. Chemical analysis revealed that BDE-209 accumulated in tissues and was metabolized to reductive products ranging from penta- to octaBDEs with 2,2′,4,4′,5,6′-hexabromodiphenyl ether (BDE-154) being the most accumulative metabolite. By day 28 of the exposure, rates of outer and inner ring deiodination (ORD and IRD, respectively) of thyroxine (T4) were each reduced by ∼74% among treatments. Effects on T4-ORD and T4-IRD remained significant even after the 14-day depuration period. Histological examination of treated fish showed significantly increased thyroid follicular epithelial cell heights and vacuolated hepatocyte nuclei. Enlarged biliary passageways may be the cause of the distinctive liver phenotype observed, although further testing is needed. Altogether, these results suggest that juvenile fish may be uniquely susceptible to thyroid disruptors like PBDEs.
Keywords: deiodinase, endocrine disruption, metabolism, polybrominated diphenyl ether, thyroid hormone
Decabromodiphenyl ether (BDE-209) is the fully brominated polybrominated diphenyl ether (PBDE) congener that constitutes > 97% of the PBDE mixture, decaBDE. It is the only PBDE commercial mixture still used today, primarily as an additive in high impact polystyrene in electronic casings and textile backcoatings. Increasing levels of BDE-209 continue to be measured in soils and sediments (de Wit et al., 2006; Hale et al., 2006), and studies indicate that BDE-209 is bioavailable to humans (Bi et al., 2007; Lunder et al., 2010) and wildlife (Gauthier et al., 2008; Law et al., 2008). Due to concerns about environmental persistence, bioaccumulation, and toxicity, decaBDE is now scheduled for phaseout in the United States by the end of 2013. However, environmental contamination is expected to continue as products containing decaBDE continue to be used, recycled, and discarded. In addition, decaBDE remains in use in other countries, presenting ongoing global contamination issues. BDE-209 may also break down photolytically (Stapleton and Dodder, 2008) and microbially (Gerecke et al., 2005) to more persistent lower–molecular weight PBDEs that have a greater potential for bioaccumulation and toxicity.
The liver is the major site of xenobiotic metabolism, and PBDEs have been shown to biotransform in animals to persistent and bioactive metabolites. Studies in fish have shown that metabolism occurs primarily via reductive debromination, with little to no formation of hydroxylated PBDEs (Kierkegaard et al., 1999; Roberts et al., 2011; Stapleton et al., 2004). This piscine metabolic pathway is distinguished from that of rodent and human metabolism whereby oxidative cytochrome P450–mediated pathways dominate to produce hydroxylated PBDEs (Germer et al., 2006; Richardson et al., 2008; Stapleton et al., 2009).
Although reductive debromination appears to be the major metabolic pathway in fish, there continues to be a poor understanding of the role of specific enzymes in PBDE metabolism. Studies conducted in our laboratory and by others suggest a possible role for iodothyronine deiodinase (DI) enzymes in catalyzing PBDE debromination in fish (Browne et al., 2009; Noyes et al., 2010; Stapleton et al., 2004). DI enzymes, of which three isoforms have been identified in fish (types 1, 2, and 3), are membrane-bound proteins that are associated with the endoplasmic reticulum and regulate intracellular thyroid hormone (TH) homeostasis (Eales et al., 1999). Transferase enzymes, such as glutathione S-transferases (GSTs), which frequently catalyze xenobiotic metabolism, have also been hypothesized to be involved in mediating PBDE and TH metabolism by conjugation processes. For example, American kestrels (Falco sparverius) exposed to PBDEs exhibited depressed circulating thyroxine (T4) and altered glutathione (GSH) homeostasis, suggesting a possible mediating role for GSTs (Fernie et al., 2005). However, in contrast with these findings, GSTs did not catalyze pentaBDE-99 debromination in cytosolic fractions from either Chinook salmon (Oncorhynchus tshawytscha) or common carp (Cyprinus carpio), implying that they may not be involved in PBDE metabolism in fishes (Browne et al., 2009).
PBDEs closely resemble the structure of THs, and increased scrutiny has focused on the potential for these contaminants to cause thyroid disruption. THs are essential for vertebrate growth, development, and reproduction. Early fish life stages may be uniquely susceptible to thyroid-perturbing xenobiotics because their thyroid systems are incompletely formed but are nonetheless crucial to development. Circulating levels of THs have been shown to decline in adult fathead minnows (Pimephales promelas) and lake trout (Salvelinus namaycush) exposed to tetraBDE-47 and a PBDE congener mix, respectively (Lema et al., 2008; Tomy et al., 2004). The PentaBDE commercial mixture and BDE-47 have also been shown to impair embryonic and larval fish development (Lema et al., 2007; Timme-Laragy et al., 2006).
Informative reviews by Browne and Eales, among others, propose that understanding xenobiotic effects on the fish thyroid system requires examination at the central hypothalamic-pituitary-thyroid (HPT) axis and in peripheral tissues (Brown et al., 2004; Eales and Brown, 1993). T4 is the prohormone of the more biologically active hormone 3,3′,5-triiodothyronine (T3), and its biosynthesis and regulation are under negative feedback control by the central HPT axis. In peripheral tissues, T4 is converted to T3 by outer ring deiodination (T4-ORD) mediated by DI enzymes. The fish thyroid, unlike the mammalian thyroid, is thought to be only a negligible source of circulating T3. Moreover, research has shown that plasma T3 levels in fish may not provide a meaningful index of thyroid status as some tissues (e.g., liver and gill) mostly use locally generated T3 for nuclear receptor–mediated activity (Maclatchy and Eales, 1992). These thyroidal aspects that are unique to fishes make it important to examine peripheral intracellular activity, in addition to changes at the central HPT axis, when evaluating xenobiotic effects on the fish thyroid system.
Given ongoing gaps in our understanding of PBDE metabolism and toxicity among fishes, objectives of this study were to measure PBDE accumulation and metabolism in juvenile fathead minnows (P. promelas) receiving dietary exposures of BDE-209 and to examine effects of these exposures on thyroid structure and function (as measured by DI activity) and liver morphology. BDE-209 effects on DI and GST activities were both evaluated in this study because results continue to be mixed as to their involvement in PBDE metabolism. Histological and morphometric evaluations of the thyroid and liver were performed to better understand the potential for PBDEs to impart structural changes that might be indicative of altered functioning of these key organ systems.
MATERIALS AND METHODS
Materials.
BDE-209 (98% pure) was purchased from Sigma-Aldrich (St Louis, MO). Unlabeled THs (T4, T3, rT3, 3,3′-T2, and 3,5-T2), reduced GSH, 1-chloro-2,4-dinitrobenzene (CDNB), and dithiothreitol (DTT) were also purchased from Sigma-Aldrich. Internal and surrogate standards included: 13C-2,2′,3,4,5,5′-hexachlorodiphenylether (CDE 141) and 13C-decabromodiphenyl ether (13C-BDE-209) (Wellington Labs, Guelph, Canada); 4′-fluoro-2,3′,4,6-tetrabromodiphenyl ether (F-BDE-69) (Chiron, Trondheim, Norway); and 13C-3,3′-diiodithyronine (13C-T4), 13C-T3, 13C-rT3, and 13C-T3 (Isotec, Miamisburg, OH). All solvents used were of high performance liquid chromatography grade.
Animals.
Approximately 750 seven-day-old fathead minnows (P. promelas) were purchased from Aquatox, Inc. (Hot Springs, AR) and randomly distributed into six 10-gallon glass tanks (approximately 125 fish per tank). Fish were 28 days old (DO) at the start of the study and were acclimated for 21 days prior to study commencement to a control diet of preserved brine shrimp (Artemia salina) nauplii (Drs. Foster and Smith, Rhinelander, WI). Further information concerning animal care can be found in the Supplementary data.
BDE-209 dietary exposure.
Fish in three tanks received a dietary exposure of 9.8 ± 0.16 μg of BDE-209/g wet weight (ww) of food at 5% of their body weight per day for 28 days. Untreated fish in the remaining three tanks received a control diet containing no BDE-209 at the 5% body weight per day feeding regimen. To monitor recovery, the 28-day exposure was followed by a 14-day depuration period in which all fish received control food containing no BDE-209. The BDE-209 amended food was prepared by dissolving BDE-209 in cod liver oil (TwinLab, UT) and spiking the cod oil solution onto preserved brine shrimp nauplii. To confirm dosages, BDE-209 amended and control diets were analyzed by gas chromatography mass spectrometry operated in electron capture negative ionization mode (GC/ECNI-MS). Ten fish were weighed from each tank at the start of the experiment and then weekly over the course of the study. The average mass per tank was used to calculate the feeding rate, and the feeding rate was adjusted to account for growth. Fish from treatment and control tanks were sampled on experimental days 0 (28-day-old fish), 14 (42-day-old fish), 28 (56-day-old fish), and 42 (70-day-old fish). Fish were euthanized using an overdose of MS-222, immediately frozen in liquid nitrogen, and stored at −80°C until further analysis.
PBDE extractions and analysis.
PBDEs were extracted from whole-body samples of fish collected from BDE-209-treated and control replicate tanks on days 0, 14, and 28 (Table 1). Procedures used to isolate PBDEs from fish tissues are described in the Supplementary data. All samples were analyzed using GC/ECNI-MS (Agilent models 6890N and 5975). Extracts were analyzed for a suite of 32 PBDE congeners ranging from tri- to decaBDE. The operating conditions for the GC/MS have been described previously (Stapleton et al., 2008). The homologue groups of PBDE metabolites were confirmed by analyzing some extracts by gas chromatography/electron impact-mass spectrometry (GC/EI-MS) and monitoring the molecular ion fragment M-2Br. Tri- through octaBDE congeners were quantified by monitoring bromide ions (m/z 79 and 81). The nonaBDEs and BDE-209 were quantified by monitoring m/z responses of 486.6 and 484.6, and the 13C-BDE-209 was quantified using m/z responses of 494.6 and 496.6.
TABLE 1.
Sampling Regimen for Study End Points Evaluated
| Study end point | Sample size | Sampling times |
| Measurements of PBDE bioaccumulation and metabolism | n = 3 tanks (12–15 fish pooled per tank) | Days 0, 14, and 28 |
| Preparation of microsomes and cytosol for enzymatic assays | n = 3 tanks (12–15 fish pooled per tank) | Days 0, 14, 28, and 42 |
| DI activity in microsomes | n = 3 fish pools | Days 0, 14, 28, and 42 |
| GST activity in microsomal and cytosolic fractions | n = 3 fish pools | Days 0, 14, 28, and 42 |
| Histological examinations | n = 3 (one individual per tank; three tanks) | Day 28 |
In vitro DI assay.
A series of in vitro assays were undertaken whereby exogenous T4 and 3,3′,5′-triiodothyronine (rT3) were incubated with whole-fish microsomes prepared from BDE-209-treated and control fathead minnows to examine changes in intracellular DI activity and TH metabolism. Whole-fish microsomal preparation procedures are described in the Supplementary Data. Fish were randomly pooled from each tank (within a treatment) across each sample time point to provide approximately 0.5 g of tissue per tank for microsome preparation (Table 1). Microsomes from BDE-209-treated and control groups (n = 3 fish pools; 12–15 fish per pool) across the four sampling time points (days 0, 14, 28, and 42) were incubated in glass test tubes with either 0.64μM of T4 or 0.77μM of rT3 to measure rates of ORD and IRD. All incubations contained 950 μl of incubation buffer and 50 μl of the appropriate microsomal fraction diluted to 10 mg/ml. The buffer used for all incubations consisted of 0.1 M potassium phosphate (K2HPO4) buffer with 10mM of DTT and 100μM of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) (pH 7.4). Incubations were conducted for 1.5 h in glass test tubes in a water bath at 25°C and 140 revolutions per minute oscillations. Negative controls (n = 3 pools) consisted of microsomes incubated with no exogenous TH. Buffer controls contained TH alone with no microsomal protein to correct for any substrate impurities and abiotic degradation. At the conclusion of the incubation period, 1 ml of ice-cold methanol was added to halt the reaction.
TH analysis.
TH formation rates mediated by endogenous DIs were determined using a liquid-liquid solid-phase extraction procedure and liquid chromatography tandem mass spectrometry (LC/MS/MS) analytical methodology recently developed in our laboratory (Wang and Stapleton, 2010). Rates of T3, rT3, 3,3′-T2, and 3,5-T2 production were measured using LC/MS/MS operated in positive electrospray ionization mode with recently published multiple reaction monitoring transitions and run parameters (Butt et al., 2011). Labeled internal standards, 13C-T4, 13C-rT3, 13C-T3, 13C-3,3′-T2 (Cambridge Isotope Laboratories, Andover, MA and Accustandard, New Haven, CT), were added to each standard and sample to quantify levels of T4, rT3, T3, and T2 hormones, respectively. Concentrations of THs were normalized to time and protein concentration to determine ORD and IRD rates.
Cytosolic and microsomal GST activity.
GST activity was determined in cytosolic and microsomal fractions of BDE-209-treated and control fish (Table 1) using previously published methods to measure the conjugation of GSH to CDNB to form glutathione-2,4-dinitrobenzene (Habig and Jakoby, 1981). Assay procedures and conditions are detailed in the Supplementary data.
Histological examination.
The thyroid follicles and livers of BDE-209-treated and control intact fish (n = 3; one individual per tank), euthanized on day 28, were examined histologically for morphological alterations. Methods used to prepare tissues for histological examination are outlined in the Supplementary Data. Frontal sections (2.5 μm thicknesses) of whole fish, selected at 50 μm intervals, were mounted on glass slides and stained with hematoxylin and eosin. Imaging and examinations were performed with a Nikon Eclipse E600 light microscope, Nikon DXM 1200 digital camera, and NIS-Elements 3.1 imaging software (Nikon, Melville, NY).
Morphometric analysis.
Ten unique follicles, randomly identified and selected in three treated and control fish, were measured. Specifically, epithelial cell heights (i.e., distance from basal to apical plasma membrane) were determined for four individual cells per fish at the widest diameter of each follicle. By design, the four cells were located approximately 90° from each other encompassing a total of 40 cells per fish. Mean cell heights were then calculated using the direct method established by Kalisnik et al. (1977). This method overcomes possible errors due to tangential planes through the epithelium. Morphometric analysis of livers from BDE-209-treated and control individuals (n = 3) involved capturing 20 nonoverlapping fields of 40 μm2 per field at ×400 magnification. The mean ratio (± SD) of vacuolated to total hepatocyte nuclei was established among these fields for each individual. After survey of hepatic structure, an array of specific alterations was selected for further analysis, including hepatocyte necrosis, glycogen/lipid inclusions, biliary passageway alterations, and inflammation sites (Hinton et al., 2008).
Quality assurance and data analysis.
Recoveries of F-BDE-69 and 13C-BDE-209 averaged 90 ± 1.4% and 71 ± 1.1%, respectively, during the GC/MS analysis. Small amounts of BDE-209 (3.9 ± 0.7 ng), BDE-207 (1.3 ± 0.3 ng), and BDE-47 (2.33 ± 0.1 ng) were detected in laboratory blanks (n = 3). Samples were blank corrected by subtracting mean blank values from fish sample results. Levels of the remaining tri- to nonaBDEs targeted under our method were below method detection limits (MDLs) in laboratory blanks and negative control samples. MDLs were defined as three times the SD of laboratory blanks (i.e., mean blank values + 3 × SD) and were typically measured at < 0.5 ng/g ww of tissue. For congeners not detected in blanks, the MDL was set at the laboratory instrumental detection limit (IDL). For the LC/MS/MS analysis, MDLs were calculated as three times the SD of T4 detected in negative controls and of T3 and rT3 in buffer controls. No 3,3′-T2 or 3,5-T2 hormones were detected in control samples, so the IDL was used as the MDL for these hormones. Trace quantities of T3 and rT3 were consistently detected in buffer control vials at levels of ∼0.5% and ∼0.1%, respectively, relative to T4 concentrations. These contaminants originated from T4 commercial material impurities; no abiotic transformation was observed during incubations. MDLs normalized to incubation conditions were as follows: T4 = 2.59 pmol/hr/mg protein; T3 and rT3 = 0.21 pmol/hr/mg protein; 3,3′-T2 and 3,5-T2 = 0.57 pmol/hr/mg protein. Differences in TH formation rates in T4- and rT3-incubated microsomes from BDE-209-treated versus control fish were analyzed for statistical significance using a Student’s t-test (Graphpad Prism 5.0, La Jolla, CA). Thyroid follicle measurements of treated and control fish were also evaluated using t-tests. Intergroup comparisons of changes in DI activity over fish age were determined using a one-way ANOVA analysis followed by a Bonferroni Multiple Comparison post hoc test (Graphpad Prism 5.0). Statistical significance was defined at the p < 0.05 level with high and very high statistical significance defined at p < 0.005 and p < 0.0005, respectively.
RESULTS
Growth
Fish growth was monitored throughout the experiment by taking weekly measurements of the mass of 10 fish randomly collected from each tank. Fish mass increased from ∼20 to ∼50 mg per fish over the course of the study. Fish growth rates for each tank were determined by fitting body weight measurements to the exponential model:
| (1) |
where b is the growth rate (slope; fish mass per time), t is the time in days, and a is a constant (Fisk et al., 1998). Concentrations of PBDEs in whole fish were corrected for growth dilution by multiplying fish concentrations by a factor of 1 + b · time. No significant differences in growth rates or lipid content were observed between BDE-209-treated and control groups. Additional results of growth and lipid measurements can be found in the Supplementary data.
BDE-209 Accumulation and Metabolism
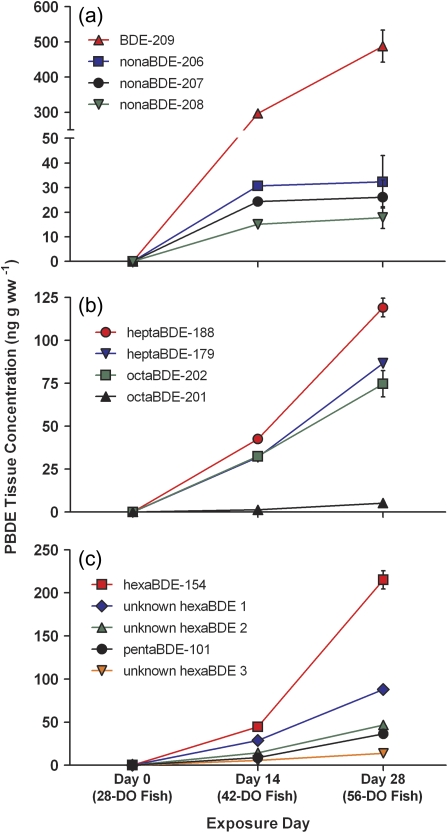
Figure 1 displays PBDE concentrations measured in fathead minnows exposed for 28 days to BDE-209-spiked Artemia sp. at a concentration of 9.8 ± 0.16 μg/g ww of food at 5% of their body weight per day. In addition to BDE-209 accumulation (488 ± 46 ng/g ww by day 28 of the exposure) (Fig. 1a), several lower PBDE congeners, ranging from pentaBDE-101 to octaBDEs, were formed via debromination of BDE-209 and increased in concentration over the exposure period (Figs. 1b and 1c). PentaBDE-101 was the lowest molecular weight congener detected, and hexaBDE-154 was the metabolite measured at the highest concentration at approximately 215 ± 11 ng/g ww of tissue by the end of the 28-day exposure. Three additional hexaBDE congeners were also detected but could not be identified with available PBDE standards. Two hepta- and octaBDE congeners (BDE-179, BDE-188, BDE-201, and BDE-202) along with the three nonaBDE congeners (BDE-206, BDE-207, and BDE-208) were also detected. DecaBDE contains small amounts of the nonaBDEs as impurities, and GC/ECNI-MS analysis of the BDE-209-treated food showed levels of the nonaBDEs at approximately 1.6% of the BDE-209-spiked food. No other congeners were detected in the BDE-209-treated food; MDLs for the penta- to octaBDEs were < 0.2 ng/g ww of food.
FIG. 1.
Concentrations (nanograms per gram wet weight) of BDE-209 and major metabolites measured in whole carcasses of fathead minnows receiving a 28-day dietary exposure of BDE-209 at 9.8 ± 0.16 μg/g ww of Artemia sp. at 5% of fish body weight per day (n = 3; mean ± SEM). Approximately 12–15 fish were pooled from three tanks (n = 3; mean ± SEM) across the exposure period (days 0, 14, and 28).
To ensure that metabolites detected were not due to low-level accumulations of minor impurities in the BDE-209-treated food, the maximum mass that could be accumulated in fish was calculated assuming they were present in food at concentrations equal to detection limits. For example, the MDL for BDE-154 in the BDE-209-treated food was 0.15 ng/g ww of food, so if BDE-154 was in the treated food at levels just below detection limits, the maximum mass that would be predicted to be present in pooled fathead minnows at day 28 of the exposure would not exceed 0.32 ng (i.e., 0.15 ng/g ww · 0.075 g of food per fish pool per day · 28 day exposure). The total mass of BDE-154 detected in BDE-209-treated fish (n = 3 pools) exceeded 60 ng after the 28-day exposure, making it infeasible for these accumulations to be attributable to low-level food contaminations.
Based on the suite of metabolites identified, approximately 5.8% of the BDE-209 exposure was estimated to be bioavailable to juvenile fathead minnows in this study. This percentage was calculated by estimating the average body burden of metabolites in fathead minnows at the end of the 28-day exposure as follows:
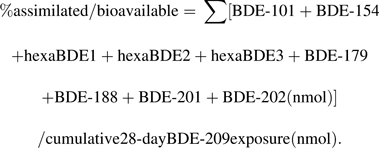 |
(2) |
The cumulative BDE-209 exposure over the 28-day exposure was estimated to be ∼0.45 nmol per fish (or ∼429 ng of BDE-209 per fish), and the summed metabolites detected at day 28 were ∼0.026 nmol per fish.
Intracellular DI activity
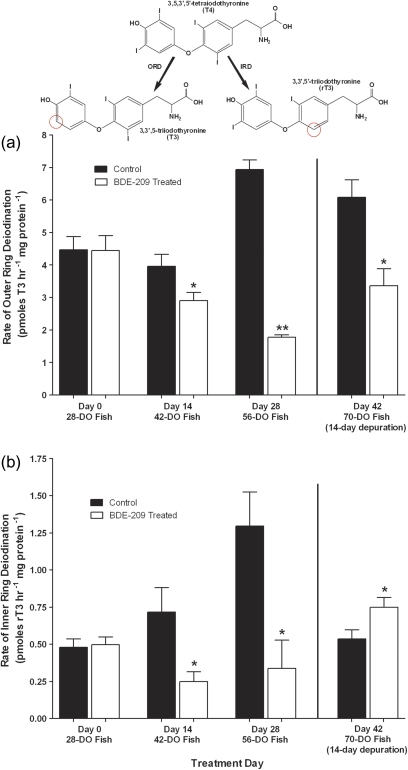
Figure 2 displays rates of T4-ORD and T4-IRD measured in microsomes prepared from BDE-209-treated and control individuals. At day 14 of the BDE-209 exposure, T4-ORD and T4-IRD activities were significantly inhibited among treated individuals by ∼27% and ∼66%, respectively. By day 28 of the exposure, deiodination rates were substantially inhibited among BDE-209-treated individuals with highly significant (p < 0.005) and significant (p < 0.05) reductions of ∼74% in both T4-ORD and T4-IRD, respectively. Some apparent recovery of T4-ORD was observed over the 14-day depuration period, although activity was still significantly inhibited by ∼45% compared with controls. Significant increases in T4-IRD activity were also detected in treatment groups over the 14-day depuration period. Formation rates of 3,3′-T2 (from the sequential loss of two iodine atoms from T4) were measured with no statistically significant differences observed between treatments and controls (data not shown).
FIG. 2.
ORD (a) and IRD (b) measured in microsomes from BDE-209-treated and control fish incubated with 0.64μM of thyroxine (T4) for 90 min at 25°C (n = 3; mean ± SEM). Fish that were 28 DO were exposed to 9.8 ± 0.16 μg/g of BDE-209 in their food at 5% of their body weight per day for 28 days followed by a 14-day depuration period to monitor recovery. Approximately 12–15 fish were pooled from three tanks (n = 3; mean ± SEM) across each sampling time point (days 0, 14, 28, and 42). One asterisk denotes deiodination activity that was significantly different from controls (p < 0.05), and two asterisks indicate high statistical significance (p < 0.005).
Microsomes from BDE-209-treated and control groups were also incubated with 0.77μM of rT3 as fathead minnows have three different DI isoforms that have different affinities for different TH substrates. Microsomes from treated individuals showed statistically significant increases (∼41%) in rT3-ORD activity at day 28 only. This significant increase in rT3-ORD at day 28 of the exposure was followed by a ∼56% reduction in rT3-ORD at the conclusion of the 14-day depuration period. Age-specific differences in T4-ORD and T4-IRD among controls were observed. A one-way ANOVA followed by a Bonferroni Multiple Comparison post hoc test revealed significant increases (p < 0.05) in T4-ORD activity among control individuals starting at day 28 of the exposure that continued over the 14-day depuration period. T4-IRD was also significantly (p < 0.05) increased among controls at day 28 relative to the other sampling time points.
GST Activity
No significant (p > 0.05) differences in GST activity were measured in either cytosolic or microsomal fractions prepared from BDE-209 treatment groups relative to controls over the exposure period. Further results of the GST analysis can be found in the Supplementary data.
Morphological Alterations
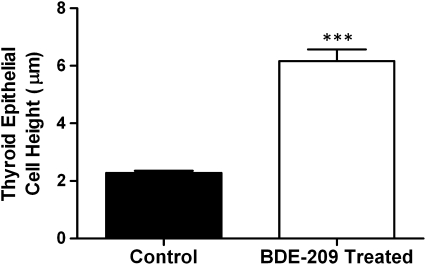
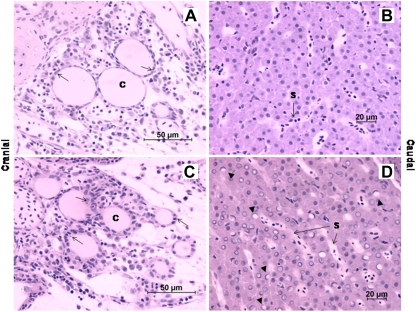
Results of high-resolution microscopy (Figs. 3 and 4) show that juvenile fathead minnows exposed to BDE-209 for 28 days exhibited increased thyroid follicular epithelial cell height (p < 0.0005) and colloid depletions relative to controls. Most teleosts, including fathead minnows, do not have individual thyroid glands but rather have several nonencapsulated follicles dispersed mainly at the base of the branchial arches near the ventral aorta. Whereas follicles from control individuals had predominantly squamous epithelium with well-formed colloids (Fig. 4a), follicles from BDE-209-treated individuals presented a cuboidal to low columnar epithelium (Fig. 4c). In addition, qualitative examination of thyroid sections indicated that increases in epithelial cell height in BDE-209-treated individuals were accompanied by varying degrees of irregularity in follicle outlines and decreasing colloid. No changes in tissue vascularity were apparent, and colloid vacuoles were not detected among treated individuals. However, substantial increases in inflammatory cells were observed in tissue surrounding follicles of treated fish.
FIG. 3.
Epithelial cell height of thyroid follicles from juvenile fathead minnows exposed to BDE-209 for 28 days and in control individuals receiving clean food (n = 3; one individual/tank; mean ± SEM). Measurements were made by identifying 10 unique follicles in each of the three treated and control fish. Cell height determinations were made at the widest follicle diameter by measuring the height of four cells located approximately 90° from each other for a total of 40 cells per fish. Three asterisks indicate very high statistical significance (p < 0.0005).
FIG. 4.
Light micrographs (oil immersion, ×600, hematoxylin and eosin staining) of juvenile fathead minnow thyroid follicles and liver tissue from control and BDE-209-treated individuals at exposure day 28 (n = 3; one individual per tank). (A) Thyroid follicles from a control fish; (B) liver tissue from a control fish; (C) thyroid follicles from a fish exposed to BDE-209 via the diet for 28 days; (D) liver tissue from a fish exposed to BDE-209 via the diet for 28 days. C = follicular colloid; arrows = follicular epithelium; S = sinusoid containing nucleated red blood cells; black triangles = ringed or crescent-shaped nuclei with apparent continuity to interhepatic biliary passages.
Hepatocytes of control individuals (Fig. 4b) presented a normal phenotype in which cytoplasm contained large areas of eosinophilic staining that contrasted with smaller perinuclear cuffs of basophilia overlying rough endoplasmic reticulum. Nuclei of hepatocytes in controls stained with uniform basophilia (i.e., were dark purple). In contrast, a readily apparent altered phenotype was observed in livers of juvenile fish exposed to BDE-209. Hepatocyte nuclei from treated individuals contained white vacuolated regions and peripheral basophilic areas as rings or crescents (Fig. 4d). A quantitative analysis of 20 nonoverlapping fields among BDE-209-treated individuals revealed that vacuolated nuclei constituted 48 ± 12% (mean ± SD) of total hepatocyte nuclei counted. No vacuolated nuclei were observed in control individuals. Hepatocytes of treated individuals appeared structurally intact and well defined with no indication of apoptosis or nuclear lysis. Moreover, despite the chronic duration of the study, no foci of inflammation were observed.
DISCUSSION
BDE-209 Accumulation and Metabolism
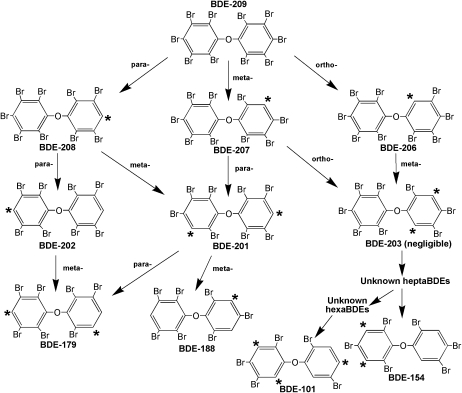
BDE-209 accumulated in juvenile fathead minnows and was readily debrominated to PBDE congeners with fewer bromine atoms (Fig. 1). Based on the major metabolites detected, Figure 5 presents a predicted pathway of reductive debromination. An analysis of this pathway suggests that reductive debromination was dominated by cleavage of bromine atoms from meta- and para-substituted positions, which is consistent with debromination patterns observed in incubations of carp liver microsomes with PBDEs (Roberts et al., 2011). Debromination patterns in treated individuals were also consistent with results observed in common carp (C. carpio) exposed to BDE-209 via the diet, whereby the dominant metabolites detected were also penta- to octaBDEs (Stapleton et al., 2004). This suggests possible family-specific commonality in PBDE metabolism as both carp and fathead minnows are members of the family Cyprinidae. However, we detected BDE-209 accumulation in fathead minnows, whereas no BDE-209 bioaccumulation was observed in juvenile carp.
FIG. 5.
Predicted debromination pathway of BDE-209 in 28-day-old fathead minnows (Pimephales promelas) exposed to 9.8 ± 0.16 μg/g ww of Artemia sp. at 5% of their body weight per day for 28 days. Asterisks indicate the predicted site of debromination.
Formation of hexaBDEs and pentaBDE-101 appeared to occur rapidly, given the negligible levels of predicted intermediate metabolites measured. These data suggest that BDE-209 metabolism in cyprinids is relatively rapid but may stop at the pentaBDEs with no appreciable metabolism to lower–molecular weight congeners. Longer exposure periods would be needed to fully evaluate this hypothesis as it does not appear that steady-state conditions were reached in this study. With regard to BDE-209 bioavailability, whereas the percent bioavailable in fathead minnows was limited to 5.8%, it was substantially higher than BDE-209 bioavailability measurements in common carp (Stapleton et al., 2004) and rainbow trout (Kierkegaard et al., 1999), which were less than 0.5%. In addition, the BDE-209 cumulative exposure over the 28-day treatment (∼429 ng per fish) and the percent bioavailability measured here are environmentally relevant. For example, BDE-209 levels in river, estuarine, and marine sediments have been measured at thousands of ng/g dry weight (Mai et al., 2005; Sellstrom et al., 1998; Vane et al., 2010).
Alterations in Deiodination Activity
It is possible that some or all three DI isoforms were inhibited in treated fathead minnows as both T4-ORD and T4-IRD declined, especially by day 28, where severely depressed T4-ORD and T4-IRD were measured. Type 1 and 2 isoforms of DI (D1 and D2) catalyze T4-ORD to produce the active T3 hormone, whereas D1 and type 3 isoforms of DI (D3) catalyze T4-IRD to inactive rT3. Thus, D1 can be involved in both ORD and IRD of T4. A 14-day recovery period in which fish received control food containing no BDE-209 resulted in some possible recovery of T4-ORD activity, although it was still significantly less than activity in controls. The decreased T4-ORD and T4-IRD observed in treated fish may be attributable to PBDEs mimicking THs, triggering direct downregulations of messenger RNA (mRNA) expression of genes encoding DIs. Recent in vitro testing in our laboratory using microsomal fractions prepared from carp liver tissue provides evidence of a possible competitive binding interaction between THs and PBDEs for DIs (Noyes et al., 2010).
If PBDEs are acting as TH mimics, the depressed DI activity measured in this study could also be linked to increased activity of TH-metabolizing enzymes, such as uridine diphosphate glucuronosyl transferases and sulfotransferases. TH-conjugating enzymes, and other classes of enzymes involved in TH metabolism, have not been examined in detail in fish. However, there is evidence of alterations of conjugating enzymes in mammals exposed to PBDEs (Richardson et al., 2008; Szabo et al., 2009; Zhou et al., 2002), and in one study, these alterations were concomitant with decreased expression and activity of DIs (Szabo et al., 2009). Further testing is needed to examine BDE-209 effects on transferase enzymes in fish.
In addition to BDE-209 potentially acting as a competitive substrate, DIs could have distinctive substrate specificities for the various reductive metabolites measured in this study, thereby differentially competing with THs to affect deiodination rates. Very little is known about the deiodination kinetics of THs in fishes in the presence of PBDEs. In addition, widespread differences in Michaelis-Menton kinetics (Km and Vmax values) have been shown across teleosteans further complicating interpretations (Leatherland et al., 1990). More work is needed to better understand the potential for differential competitive interactions of PBDE congeners on TH deiodination.
An analysis of DI activity among control individuals sampled over the course of the study revealed significant (p < 0.05) increases in both T4-ORD and T4-IRD at day 28 of the study when fish were 56 DO and that the elevated T4-ORD continued over the 14-day depuration period. The increase in T4-ORD activity measured at this age coincided with substantial inhibitions of T4-ORD and altered T4-IRD among BDE-209-exposed fish. The juvenile fish phase is conventionally considered a period of fish growth and gonadal maturation, and TH disturbances have been shown to impair reproductive functioning (Cyr and Eales, 1996; Lanno and Dixon, 1994; Timmermans et al., 1997). Previous results have shown that tetraBDE-47 caused reductions in mature spermatozoa and spawning among adult fathead minnows (Lema et al., 2008; Muirhead et al., 2006), and these impairments were concomitant with depressed plasma T4 and elevated thyroid-stimulating hormone (TSH)β mRNA expression (Lema et al., 2008). In contrast to these findings, no changes in gonadal development were indicated in juvenile zebrafish exposed to tetraBDE-47, although hypoactivity was measured that could be related to neurodevelopmental impairments (Chou et al., 2010). Altogether, marked perturbations in DI activity observed among 56- and 70-day-old fathead minnows exposed to BDE-209, in relation to increases in DI activity across control groups of the same age, suggest that BDE-209 and/or its metabolites could alter important developmental pathways in juvenile fish, particularly those linked to gonadal maturation and reproduction.
The statistically significant increases in rT3-ORD, resulting in elevated formation rates of 3,3′-T2, stands in some contrast to effects in T4-incubated microsomes where declines in ORD were observed. The purpose for choosing T4 and rT3 as incubation substrates was to help delineate DI isoforms in fathead minnows potentially altered by exposure to BDE-209. D1 has been shown to have a substrate preference for rT3, whereas D2 has been shown to have a substrate preference for T4 (Mol et al., 1998). It is possible that D1 was upregulated and D2 was downregulated in BDE-209-exposed fish. The upregulation in D1 could be a compensatory response to marked inhibition of D2 but is insufficient to fully compensate for the D2 inhibition in treated individuals. Additional work will be needed to better understand relative responses of DI isoforms in fish exposed to PBDEs.
Morphological Alterations
The thickening of the follicular epithelium and qualitative changes detected in thyroid tissues (i.e., irregular follicle outlines, colloid depletions, and increase in inflammatory cells) of BDE-209-treated fish are hallmarks of thyroid overstimulation and injury (Eales and Brown, 1993). The follicular epithelium hypertrophy observed may have resulted from reduced levels of circulating T4 over the exposure period. This hypothesis of depressed plasma TH will require further verification by measuring circulating T4 levels, which was not possible during this study. However, previous studies have shown that dietary exposures to PBDEs depress circulating T4 in fish (Lema et al., 2008; Tomy et al., 2004), rodents (Kuriyama et al., 2007; Rice et al., 2007; Richardson et al., 2008), and birds (Fernie et al., 2005).
Unlike the mammalian thyroid system, the fish thyroid system may not be centrally driven through the HPT axis. Rather, the HPT of fishes may govern predominantly circulating T4 homeostasis through TSH stimulation from the pituitary (Eales and Brown, 1993; Maclatchy and Eales, 1992). Depressions in plasma T4 will stimulate the pituitary to release TSH that then activates the thyroid to compensate for depressed TH levels. This feedback condition can lead to thickening of the follicular epithelium with extended stimulation. Depressed circulating T4 among BDE-209-treated individuals could have contributed to decreased levels of T4 in peripheral tissues that subsequently produced declines in intracellular T4-ORD and T4-IRD observed in our DI assay through the 28-day exposure.
In addition to notable changes in the thyroid, a profoundly altered liver phenotype characterized by a large number of vacuolated hepatocellular nuclei was measured among fish exposed to BDE-209. We hypothesize that these vacuolated nuclei, which were not observed in controls, may be attributable to impingements caused by enlarged “intrahepatic” biliary passageways. In fish, hepatocytes are arranged as tubules clustered around an interhepatic biliary passageway with their apices directed toward the central biliary canaliculus and/or bile preductules (Hinton et al., 2008). Cyprinids, like fathead minnows, also have finger-like indentations, conventionally termed intrahepatic canaliculi that extend into hepatocytes, acting as continuous extensions of the interhepatic canaliculi (Vogt and Segner, 1997; Yamamoto, 1965). A careful review of these high-resolution images showed continuity between the vacuole detected in the nuclei of BDE-209-treated fish and interhepatic biliary passageways, suggesting that these areas are connected. These types of biliary dilations and enlargements have also been observed in Japanese medaka (Oryzias latipes) exposed to aqueous α-naphthylisothiocyanate, a well-described hepatotoxicant (Hardman et al., 2008). The possible relationship of vacuoles observed in the present study to canaliculi, both intercellular and so-called intracellular, requires higher-resolution examination with transmission electron microscopy.
Further examination of hepatotoxicity among fishes exposed to PBDEs is merited, given the profound structural abnormalities observed here. For example, enlarged biliary passageways, which may be a BDE-209 clearance mechanism, could be accompanied by increased expression or activity of TH efflux transporters, leading to increased hormone excretions. Previous studies have shown that mRNA expression of multidrug resistance–associated proteins 2 and 3 (Mrp2 and Mrp3), which are major efflux transporters of glucuronides out of cells, was increased in rodents exposed to tetraBDE-47 and the pentaBDE commercial mixture DE-71 (Richardson et al., 2008; Szabo et al., 2009). In addition, multidrug resistance protein (Mdr1a), which encodes P-glycoproteins (P-gps) that are efflux transporters of glucuronides and THs into the bile, was also increased in rodents exposed to DE-71 (Szabo et al., 2009). Little is known about the activity of these membrane-bound transporters in PBDE-exposed fish and further investigation is warranted.
CONCLUSIONS
Results of this study provide a more integrated understanding of the potential for BDE-209 to impair thyroid systems of juvenile fathead minnows and other fish species. BDE-209 was readily metabolized by juvenile fish to lower PBDE congeners dominated by penta- to octaBDEs. Marked perturbations of intracellular TH formation rates among treated fish suggest impaired deiodination activity with only limited recovery after a 14-day depuration period. These perturbations in deiodination were accompanied by significant thyroid follicle damage and liver alterations. However, although the morphological findings observed in this study were pervasive, they will require further examination with increased resolution, across multiple doses of BDE-209, and with greater sample sizes to verify and elucidate effects observed here. Further study is also needed to describe toxicological mechanisms that underly PBDE-related thyroid disruptions. Altogether, these results suggest that BDE-209 may be affecting the fish thyroid system at multiple levels, including in peripheral tissues and central HPT axis, and that juvenile fish may be uniquely susceptible to developmental and reproductive abnormalities when exposed to thyroid disruptors like PBDEs.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Environmental Health Sciences (R01ES016099; U.S. Environmental Protection Agency STAR Fellowship (FP917145).
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health, or the U.S. Environmental Protection Agency.
References
- Bi XH, Thomas GO, Jones KC, Qu WY, Sheng GY, Martin FL, Fu JM. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ. Sci. Technol. 2007;41:5647–5653. doi: 10.1021/es070346a. [DOI] [PubMed] [Google Scholar]
- Brown SB, Adams BA, Cyr DG, Eales JG. Contaminant effects on the teleost fish thyroid. Environ. Toxicol. Chem. 2004;23:1680–1701. doi: 10.1897/03-242. [DOI] [PubMed] [Google Scholar]
- Browne EP, Stapleton HM, Kelly SM, Tilton SC, Gallagher EP. In vitro hepatic metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE 99) in Chinook Salmon (Oncorhynchus tshawytscha) Aquat. Toxicol. 2009;92:281–287. doi: 10.1016/j.aquatox.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Wang DL, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid regulating deiodinases in human liver. Toxicol. Sci. 2011 doi: 10.1093/toxsci/kfr117. Advance Access published on May 11, 2011; doi:10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CT, Hsiao YC, Ko FC, Cheng JO, Cheng YM, Chen TH. Chronic exposure of 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) alters locomotion behavior in juvenile zebrafish (Danio rerio) Aquat. Toxicol. 2010;98:388–395. doi: 10.1016/j.aquatox.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev. Fish Biol. Fisher. 1996;6:165–200. [Google Scholar]
- de Wit CA, Alaee M, Muir DCG. Levels and trends of brominated flame retardants in the Arctic. Chemosphere. 2006;64:209–233. doi: 10.1016/j.chemosphere.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Eales JG, Brown SB. Measurement and regulation of thyroidal status in teleost fish. Rev. Fish Biol. Fisher. 1993;3:299–347. [Google Scholar]
- Eales JG, Brown SB, Cyr DG, Adams BA, Finnson KR. Deiodination as an index of chemical disruption of thyroid hormone homeostasis and thyroidal status in fish. In: Henshel DS, Black MC, Harrass MC, editors. Environmental Toxicology and Risk Assessment: Standardization of Biomarkers for Endocrine Disruption and Environmental Assessment: Eighth Volume, ASTM STP 1364. West Conshohocken, PA: American Society for Testing and Materials; 1999. pp. 136–164. [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouillard KG, Ritchie IJ. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicol. Sci. 2005;88:375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Fisk AT, Norstrom RJ, Cymbalisty CD, Muir DCG. Dietary accumulation and depuration of hydrophobic organochlorines: bioaccumulation parameters and their relationship with the octanol/water partition coefficient. Environ. Toxicol. Chem. 1998;17:951–961. [Google Scholar]
- Gauthier LT, Hebert CE, Weseloh DVC, Letcher RJ. Dramatic changes in the temporal trends of polybrominated diphenyl ethers (PBDEs) in herring gull eggs from the Laurentian Great Lakes: 1982-2006. Environ. Sci. Technol. 2008;42:1524–1530. doi: 10.1021/es702382k. [DOI] [PubMed] [Google Scholar]
- Gerecke AC, Hartmann PC, Heeb NV, Kohler HPE, Giger W, Schmid P, Zennegg M, Kohler M. Anaerobic degradation of decabromodiphenyl ether. Environ. Sci. Technol. 2005;39:1078–1083. doi: 10.1021/es048634j. [DOI] [PubMed] [Google Scholar]
- Germer S, Fery Y, van der Ven L, Piersma AH, Kamyschnikow A, Schrenk D. Induction of cytochrome P450 enzymes in rat liver and rat primary hepatocytes by polybrominated diphenyl ethers. Toxicol. Lett. 2006;164:S159–S160. [Google Scholar]
- Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferase. In: Jacoby WB, editor. Detoxification and Drug Metabolism: Conjugation and Related Systems. New York, NY: Academic Press; 1981. pp. 398–405. [DOI] [PubMed] [Google Scholar]
- Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 2006;64:181–186. doi: 10.1016/j.chemosphere.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Hardman R, Kullman S, Yuen B, Hinton DE. Non invasive high resolution in vivo imaging of alpha-naphthylisothiocyanate (ANIT) induced hepatobiliary toxicity in STII medaka. Aquat. Toxicol. 2008;86:20–37. doi: 10.1016/j.aquatox.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DE, Segner H, Au DWT, Kullman SW, Hardman RC. Liver toxicity. In: Di Giulio RT, Hinton DE, editors. The Toxicology of Fishes. Boca Raton, FL: CRC Press; 2008. pp. 327–400. [Google Scholar]
- Kalisnik M, Jakopin P, Sustarsic J. On the methodology of thyroid epithelial cell thickness determination. J. Microsc. 1977;110:157–162. doi: 10.1111/j.1365-2818.1977.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Kierkegaard A, Balk L, Tjarnlund U, De Wit CA, Jansson B. Dietary uptake and biological effects of decabromodiphenyl ether in rainbow trout (Oncorhynchus mykiss) Environ. Sci. Technol. 1999;33:1612–1617. [Google Scholar]
- Kuriyama SN, Wanner A, Fidalgo-Neto AA, Talsness CE, Koerner W, Chahoud I. Developmental exposure to low-dose PBDE-99: tissue distribution and thyroid hormone levels. Toxicology. 2007;242:80–90. doi: 10.1016/j.tox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Lanno RP, Dixon DG. Chronic toxicity of waterborne thiocyanate to the fathead minnow (Pimephales promelas)—a partial life-cycle study. Environ. Toxicol. Chem. 1994;13:1423–1432. [Google Scholar]
- Law RJ, Herzke D, Harrad S, Morris S, Bersuder P, Allchin CR. Levels and trends of HBCD and BDEs in the European and Asian environments, with some information for other BFRs. Chemosphere. 2008;73:223–241. doi: 10.1016/j.chemosphere.2008.02.066. [DOI] [PubMed] [Google Scholar]
- Leatherland JF, Reddy PK, Yong AN, Leatherland A, Lam TJ. Hepatic 5′-monodeiodinase activity in teleosts in vitro—a survey of 33 species. Fish Physiol. Biochem. 1990;8:1–10. doi: 10.1007/BF00004426. [DOI] [PubMed] [Google Scholar]
- Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environ. Health Perspect. 2008;116:1694–1699. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema SC, Schultz IR, Scholz NL, Incardona JP, Swanson P. Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE 47) Aquat. Toxicol. 2007;82:296–307. doi: 10.1016/j.aquatox.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young US children than in their mothers. Environ. Sci. Technol. 2010;44:5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- Maclatchy DL, Eales JG. Intracellular and extracellular sources of T3 binding to putative thyroid-hormone receptors in liver, kidney, and gill nuclei of immature rainbow trout, Oncorhynchus mykiss. J. Exp. Zool. 1992;262:22–29. doi: 10.1002/jez.1402620105. [DOI] [PubMed] [Google Scholar]
- Mai BX, Chen SJ, Luo XJ, Chen LG, Yang QS, Sheng GY, Peng PG, Fu JM, Zeng EY. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ. Sci. Technol. 2005;39:3521–3527. doi: 10.1021/es048083x. [DOI] [PubMed] [Google Scholar]
- Mol KA, Van der Geyten S, Burel C, Kuhn ER, Boujard T, Darras VM. Comparative study of iodothyronine outer ring and inner ring deiodinase activities in five teleostean fishes. Fish Physiol. Biochem. 1998;18:253–266. [Google Scholar]
- Muirhead EK, Skillman D, Hook SE, Schultz IR. Oral exposure of PBDE-47 in fish: toxicokinetics and reproductive effects in Japanese medaka (Oryzias latipes) and fathead minnows (Pimephales promelas) Environ. Sci. Technol. 2006;40:523–528. doi: 10.1021/es0513178. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Kelly SM, Mitchelmore CL, Stapleton HM. Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquat. Toxicol. 2010;97:142–150. doi: 10.1016/j.aquatox.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol. Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol. Appl. Pharm. 2008;226:244–250. doi: 10.1016/j.taap.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Roberts S, Noyes PD, Gallagher EP, Stapleton HM. Species-specific differences and structure-activity relationships in the debromination of PBDE congeners in three fish species. Environ. Sci. Technol. 2011;45:1999–2005. doi: 10.1021/es103934x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellstrom U, Kierkegaard A, de Wit C, Jansson B. Polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from a Swedish river. Environ. Toxicol. Chem. 1998;17:1065–1072. [Google Scholar]
- Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ. Sci. Technol. 2004;38:112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Dodder NG. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ. Toxicol. and Chem. 2008;27:306–312. doi: 10.1897/07-301R.1. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Allen JG, McClean MD, Webster TF. Measurement of polybrominated diphenyl ethers on hand wipes: estimating exposure from hand-to-mouth contact. Environ. Sci. Technol. 2008;42:3329–3334. doi: 10.1021/es7029625. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ. Health. Perspect. 2009;117:197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PRS, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol. Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Levin ED, Di Giulio RT. Developmental and behavioral effects of embryonic exposure to the polybrominated diphenylether mixture DE-71 in the killifish (Fundulus heteroclitus) Chemosphere. 2006;62:1097–1104. doi: 10.1016/j.chemosphere.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Timmermans LPM, Chmilevsky DA, Komen H, Schipper H. Precocious onset of spermatogenesis in juvenile carp (Cyprinus carpio L., teleostei) following treatment with low doses of L-thyroxine. Eur. J. Morphol. 1997;35:344–353. doi: 10.1076/ejom.35.5.344.13085. [DOI] [PubMed] [Google Scholar]
- Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environ. Sci. Technol. 2004;38:1496–1504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- Vane CH, Ma YJ, Chen SJ, Mai BX. Increasing polybrominated diphenyl ether (PBDE) contamination in sediment cores from the inner Clyde Estuary, UK. Environ. Geochem. Health. 2010;32:13–21. doi: 10.1007/s10653-009-9261-6. [DOI] [PubMed] [Google Scholar]
- Vogt G, Segner H. Spontaneous formation of intercellular bile canaliculi and hybrid biliary-pancreatic canaliculi in co-culture of hepatocytes and exocrine pancreas cells from carp. Cell Tissue Res. 1997;289:191–194. doi: 10.1007/s004410050865. [DOI] [PubMed] [Google Scholar]
- Wang DL, Stapleton HM. Analysis of thyroid hormones in serum by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010;397:1831–1839. doi: 10.1007/s00216-010-3705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Some observations on fine structure of intrahepatic biliary passages in goldfish (Carassius auratus) Z. Zellforsch. Mik. Ana. 1965;65:319–329. doi: 10.1007/BF00345633. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KA. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.