Abstract
Exposure to the pyrethroid pesticide deltamethrin has been demonstrated to cause apoptosis both in vitro and in vivo. However, the molecular pathways leading to deltamethrin-induced apoptosis have not been established. To identify these pathways, SK-N-AS neuroblastoma cells were exposed to deltamethrin (100nM–5μM) for 24–48 h. Deltamethrin produced a time- and dose-dependent increase (21–300%) in DNA fragmentation, an indicator of apoptosis. Data demonstrate that the initiation of DNA fragmentation resulted from interaction of deltamethrin with Na+ channels and consequent calcium influx, as tetrodotoxin and the intracellular Ca2+ chelator BAPTA-AM completely prevented apoptosis. DNA fragmentation was accompanied by increased caspase-9 and -3 activities and was abolished by specific caspase-9 and -3 inhibitors. However, deltamethrin did not increase cytosolic cytochrome c levels, indicating that the mitochondrial pathway was likely not involved. Additional studies demonstrated that deltamethrin exposure activated caspase-12 activity and that pharmacological inhibition and siRNA knockdown of calpain prevented deltamethrin-induced DNA fragmentation, thus indicating a role for the endoplasmic reticulum (ER) stress pathway. This was confirmed by the observation that inhibition of eIF2α abolished deltamethrin-induced DNA fragmentation. Together, these data demonstrate that deltamethrin causes apoptosis through its interaction with Na+ channels, leading to calcium overload and activation of the ER stress pathway. Because ER stress and the subsequent unfolded protein response have been observed in a number of neurodegenerative diseases, these data provide mechanistic information by which high-level exposure to pyrethroids may contribute to neurodegeneration.
Keywords: pyrethroid, pesticide, neurodegeneration, ER stress, siRNA, calpain, caspases, apoptosis, eIF2α, cytosolic calcium, sodium influx, sodium channel
Pyrethroid insecticides are widely used to control a broad range of insect-pests in agriculture, public health, veterinary medicine, and residential settings, accounting for about 25% of the worldwide insecticide market (Casida and Quistad, 1998). In recent years, the use of pyrethroids has been increasing because restrictions have been placed on many of the organophosphorus insecticides. Although exposure of the general population is thought to be generally low, high levels of exposure have been observed in pesticide applicators (Calvert et al., 2003; Kimata et al., 2009), and pyrethroid-related poisoning reports to poison control centers have increased over recent years (Power and Sudakin, 2007). Furthermore, residual neurological symptoms in individuals highly exposed to pyrethroids have been reported (Muller-Mohnssen, 1999). These findings raise concern over the potential long-term neurotoxic effects of high-level exposures.
Pyrethroids have been generally classified into two subclasses, type I and type II, based on high dose exposure. Type I pyrethroids produce the T-syndrome, which is characterized by whole body tremor and type II pyrethroids produce the CS-syndrome, which is characterized by choreoathetosis and salivation (Breckenridge et al., 2009; Soderlund et al., 2002). The mechanism by which pyrethroids are thought to exert neurotoxicity is by prolonging the opening of Na+ channels (Narahashi, 1996). This prolonged opening of Na+ channels results in persistent depolarization, leading to repetitive firing, and if the exposure is high enough, seizures, paralysis, and death (Bradberry et al., 2005). However, recent attention has focused on potential alternative targets that may be involved in pyrethroid toxicity (Breckenridge et al., 2009). Specifically, various lines of evidence have suggested possible roles for voltage-gated calcium channels, chloride channels, GABAA receptors, and the mitochondrial electron transport chain in the acute manifestation of neurotoxicity elicited by pyrethroids (Breckenridge et al., 2009; Soderlund et al., 2002). Although these studies have provided important insight into potential mechanisms by which pyrethroids produce their characteristic syndromes following high-level exposure, little attention has focused on the long-term neurological and pathological effects of such exposures.
Recently, attention has focused on the potential relationship between pyrethroid exposure and neurodegeneration (Abdel-Rahman et al., 2004; Bloomquist et al., 2002; Elwan et al., 2006; Tayebati et al., 2009). As with the classic pyrethroid poisoning–induced syndromes, the mechanism(s) by which pyrethroids result in neurodegeneration is not clear. However, higher level, but nonlethal, exposure to the type II pyrethroid insecticide deltamethrin has been shown to cause apoptosis both in vitro (Elwan et al., 2006; Wu et al., 2003) and in vivo (Wu and Liu, 2000a,b). Although apoptosis is important for the regulation of normal physiological functionthroughout life, excessive apoptosis contributes to pathological cell death observed in several neurodegenerative diseases including Alzheimer's disease (AD) and Parkinson's disease (PD) (Arduino et al., 2009). Thus, the ability of pyrethroid insecticides to cause apoptosis may contribute to the potential for high-level exposures to contribute to neurodegeneration. However, the mechanism by which pyrethroids, and in particular deltamethrin, induces apoptosis has not been established.
Here, we sought to identify the molecular mechanism and pathway by which deltamethrin causes apoptosis. The data reveal that deltamethrin initiates the apoptotic cascade through interaction with Na+ channels, leading to calcium overload and initiation of the apoptotic cascade. However, the mitochondrial-mediated pathway did not appear to be involved as there was no release of cytochrome c release from the mitochondria or alteration of mitochondrial membrane potential. Further studies identified the role of calpain and the endoplasmic reticulum (ER) stress pathway as mediators of deltamethrin-induced apoptosis. Given the prominent role of apoptosis, calpain, and the ER stress pathway in neurodegeneration (Culmsee and Landshamer, 2006; Vosler et al., 2008), these data provide mechanistic information as to how high-level exposure to pyrethroids could result in neurodegeneration.
MATERIALS AND METHODS
Materials.
Deltamethrin (lot # 392-7B, purity: 99%) was purchased from Chem Service Inc. (West Chester, PA). Cell culture supplies, including minimum essential medium (MEM) with Earl’s salts and L-glutamine, phosphate-buffered saline (PBS) 1× without calcium and magnesium, MEM nonessential amino acids 100×, sodium pyruvate (100mM), trypsin EDTA (0.05% trypsin, 0.53mM EDTA in Hank's Balanced Salt Solution (HBSS), without sodium bicarbonate, calcium, and magnesium) 1×, and fetal bovine serum (FBS) were purchased from Mediatech, Inc. (Herndon, VA). Cytotoxicity Detection Kit (lactate dehydrogenase, LDH) and the Cell Death Detection ELISAPLUS assay kit were obtained from Roche Applied Science (Indianapolis, IN). Caspase-9 substrate (Ac-LEHD-7-amino-4-methylcoumarin [AMC]), specific caspase-3 inhibitor (Z-DEVD-FMK), caspase-9 inhibitor (Z-LEHD-FMK), and calpain inhibitor (PD150606) were obtained from Alexis Biochemicals (San Diego, CA). Caspase-3 substrate (Ac-DEVD-AMC) was purchased from Bachem Americas, Inc. (Torrance, CA). Digitonin, Ethylene glycol-bis (2-aminoethylether)-N, N, N′, N′-tetraacetic acid (EGTA), Tris-HCl, selective intracellular Ca2+ chelator (BAPTA-AM), and EDTA 2Na were obtained from Sigma (St. Louis, MO). The ER stress inhibitor salubrinal (eIF2α inhibitor) was obtained from EMD chemicals (Gibbstown, NJ). The caspase-12 activity assay kit was purchased from BioVision, Inc. (Mountain View, CA) and mito chondrial isolation kit was purchased from Pierce (Rockford, IL). Materials for Western blot were purchased from Invitrogen (Carlsbad, CA). Purified mouse anti-cytochrome c was purchased from BD Biosciences-Pharmingen (San Diego, CA). All other chemicals and reagents were obtained from Fisher Scientific (Pittsburgh, PA).
Cell culture.
SK-N-AS human neuroblastoma cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in MEM containing 10% fetal bovine serum, 2mM L-glutamine, 5mM sodium pyruvate, 5mM nonessential amino acids, 50 IU/ml penicillin, and 50 μg/ml streptomycin. SK-N-AS cells were chosen as a model based on previous observations that deltamethrin targets the dopamine system (Bloomquist et al., 2002; Elwan et al., 2006), and these cells have dopaminergic characteristics, including expression of tyrosine hydroxylase and the dopamine transporter (Wang and Bannon, 2005). SK-N-AS cells also express several Na+ channel isoforms that are sensitive to deltamethrin (data not shown) and have been used to investigate mechanisms of apoptosis (Day et al., 2009).
Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. Three days after plating in a flask, cells were resuspended in MEM medium and then plated at 2 × 104 cells per well into 96-well plate. A stock solution of 10mM deltamethrin was prepared in absolute ethanol (EtOH), and dilutions of deltamethrin were made in MEM medium and added to the cells. The final concentration of EtOH was less then 0.5% for all experiments. Cells were exposed to EtOH in concentration equivalent to that used in highest concentration of deltamethrin as a vehicle control. This concentration had no effect on any of the parameters studied.
LDH assay.
The cytotoxic effect of deltamethrin on SK-N-AS cells was monitored as previously described (Elwan et al., 2006) by measuring LDH leakage into the culture media with a cytotoxicity detection kit (Roche Applied Scientific). Cells were incubated with different concentrations of deltamethrin (0–50μM) in MEM medium for 24 and 48 h at 37°C. After the treatment, 100 μl of cell-free media was added to 100 μl of the catalyst-dye mixture (supplied with kit) in a 96-well plate and then incubated at room temperature for 30 min. The LDH activity was measured at 490 nm with Spectramax microplate reader (Molecular Devices).
Determination of DNA fragmentation.
DNA fragmentation was determined with the Cell Death Detection ELISAPLUS assay kit (Roche Applied Science) according to Elwan et al. (2006). Briefly, SK-N-AS cells (2 × 104 cells/well) were exposed to 0–5μM deltamethrin for 24 and 48 h in the presence or absence of Z-DEVD-FMK (50μM), Z-LEHD-FMK (50μM), tetrodotoxin (TTX) (1μM), BAPTA-AM (5μM), PD150606 (10μM), or salubrinal (50μM). At the end of treatment, media was removed, cells were washed once with PBS and lysed. The supernatants were applied to a streptavidin-coated 96-well microtiter plates and incubated with 80 μl of immunoreagent (mixture of anti-histone-biotin and anti-DNA-POD). Following incubation, the amount of nucleosomes retained by anti-DNA-POD in the immunocomplex was spectrophotometrically determined with 2,2′-azino-di(3-ethoxybenzyl thiazoline sulfonate) at 405nm using Spectramax microplate reader (Molecular Devices).
Caspase activity assay.
Caspase-3 and caspase-9 activities were determined according to Kaul et al. (2003) with some modifications. Briefly, SK-N-AS cells (1 × 106 cells/ml) were grown in 6-well plate and treated with 0–5μM deltamethrin for 24–48 h. After treatment, cells were lysed with 0.8 ml lysis buffer (50mM Tris-HCl, 1mM EDTA, 10mM EGTA, 10μM digitonin, pH 7.4) for 20 min at 37°C. Lysates were then centrifuged at 10,000 rpm for 15 min and supernatants were incubated with 50μM Ac-DEVD-AMC or Ac-LEHD-AMC at 37°C for 60 min. Caspase activity was then determined by measuring the levels of released AMC using a Spectramax spectrofluorometer with excitation at 380 nm and emission at 460 nm. The activity was calculated as fluorescence units/milligram protein/hour and expressed as percentage of control. Activity of caspase-12 was assayed with kit and protocol supplied by BioVision, Inc.
Measurement of intracellular Na+ ([Na+]i).
Cells were rinsed once with PBS and then incubated with 10μM CoroNa™ Green-AM (catalog # C36676, Invitrogen) in oxygenated Krebs-Ringer-HEPES buffer (125 mM NaCl, 4.8 mM KCl, 2.6 mM CaCl2, 1.2 mM Mg SO4, 25 mM HEPES, 0.1% sucrose and 0.1% BSA, pH 7.4) for 1 h at 37°C. Dye loaded cells were rinsed twice with sucrose and bovine serum albumin (BSA)-free KRH buffer and then Na+ influx was determined after adding deltamethrin by measuring fluorescence intensity every 30 s over 60 min with excitation 485 nm and emission 515 nm.
Measurement of intracellular calcium ([Ca2+]i).
SK-N-AS cells were seeded on a 96-well plate at a density of 2 × 104 cells per well. After 12 h, cells were rinsed once with PBS and then incubated with 5μM Calcium Green-1-AM ester (catalog # C3012, Invitrogen) in oxygenated KRH buffer for 1 h at 37°C. After loading the calcium dye, cells were rinsed twice with sucrose and BSA-free KRH buffer then warmed up in a Spectramax spectrofluorometer for 10 min at 37°C. The test compounds were added to the cells after obtaining a stable baseline. Intracellular Ca2+ was determined by measuring fluorescence intensity in every 5 s over 60 min at excitation 506 nm and emission 531 nm.
Measurement of mitochondrial membrane potential (ΔΨm).
Rhodamine 123 (Rh123) (catalog # R302, Invitrogen), a cell-permeant cationic green-fluorescent dye was use to determine mitochondrial ΔΨm. Briefly, cells were plated at 2.5 × 104 cells per well in a black clear bottom 96-well plates and treated with 0, 1, 5, 10, and 25μM deltamethrin for 1, 12, 24, and 48 h. At the end of treatment, cells were incubated with 10μM Rh123 in phenol red free MEM medium for 30 min at 37°C and then washed once with PBS. The fluorescence intensity was measured with excitation at 485 nm and emission at 530 nm using a Spectramax microplate reader (Molecular Devices).
Western immunoblotting.
SK-N-AS cells (2 × 107) were exposed to deltamethrin (0–5μM) for 24 and 48 h at 37°C. Cells were washed once with PBS and then cytosolic fractions were extracted using mitochondria isolation kit (Pierce) for cytochrome c expression. For CHOP/GADD153 expression, cells were harvested in lysis buffer (catalog # 1067-100, BioVision, Inc.) with 0.1% protease inhibitor and kept on ice for 20 min. Samples were centrifuged at 14,000 × g for 10 min and supernatants were collected. Protein concentrations were quantified using the bicinchoninic acid assay (Smith et al., 1985), and 20 μg of protein sample was loaded per lane on 4–12% NuPAGE Novex Bis-Tris Mini Gels (Invitrogen). After electrophoresis, proteins from the gels were blotted to polyvinylidene difluoride membranes (Invitrogen). The membranes were incubated in 7.5% nonfat milk in 0.1% Tween 20 containing Tris buffered saline (TTBS) for 1 h at room temperature to block nonspecific protein binding sites. The membranes were then incubated overnight at 4°C with 1:2000 diluted anti-cytochrome c monoclonal primary antibody (catalog # 556433, BD Pharmigen) or a polyclonal antibody to CHOP/GADD153 (catalog # sc-575, Santa Cruz, CA). After being washed three times with TTBS, the membranes were incubated with horseradish peroxidase–conjugated secondary antibodies for 1 h at room temperature. The specific antibody bound protein was detected by SuperSignal® West Dura Extended Duration Substrate (Thermo Scientific Pierce) using Alpha Innotech Fluorochem (San Leandro, CA) imaging system and stored as a digital image. Membranes were then stripped for 15 min at room temperature with Pierce Stripping Buffer and re-probed with a monoclonal α-tubulin antibody to confirm equal protein loading in each lane.
siRNA transfection.
Transfection was performed according to the manufacturer's (Invitrogen) protocol. Twenty-four hours after culturing in antibiotic free MEM media, cells (50–60% confluence) were transfected with 15nM siRNA (Santa Cruz, CA) using a lipid-based transfection reagent (Lipofectamine RNAiMAX, Invitrogen) in order to knockdown calpain-1. A scrambled siRNA sequence was employed in parallel wells to control for nonspecific effects of transfection. At 24 h posttransfection, cells were exposed to 5μM deltamethrin for an additional 24 h and used for Western blot and DNA fragmentation assays.
Statistical analysis.
Statistical analysis was performed using Prism 5.01 software (Graphpad software, San Diego, CA). All experiments were performed at least 3 times on separate day using different vials of frozen cell stocks. On individual days, experiments were performed in duplicate or triplicate and averaged to form a single experimental unit.
Data are presented as mean ± SEM. All analyses were performed on raw data using analysis of variance. Bonferroni's or Tukey's post hoc multiple comparison tests were performed where appropriate. Statistical significances were determined at level of p < 0.05.
RESULTS
Deltamethrin Induces Dose-Dependent Cell Death and Apoptosis in SK-N-AS Neuroblastoma Cells
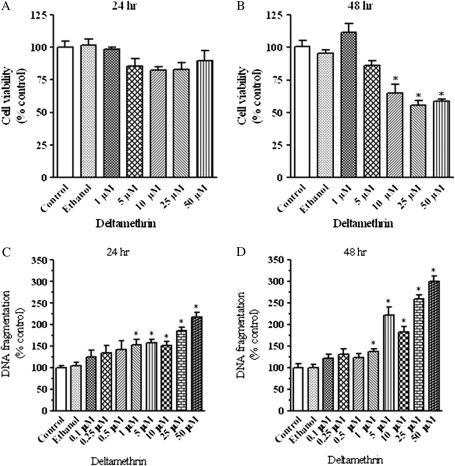
To determine the time-course and dose-response of deltamethrin-induced cell death, SK-N-AS neuroblastoma cells were exposed to various concentrations (0–50μM) of deltamethrin for 24 or 48 h. Overt cell death was determined by measuring LDH release from cells into incubation medium. Exposure to deltamethrin resulted in time- and dose-dependent cell death at concentrations of 10μM and above (Figs. 1A and 1B). Similar results were observed using alamar blue as an indicator of cell death (data not shown). However, DNA fragmentation, an indicator of apoptosis, increased in a dose- and time-related manner following exposure to deltamethrin (100nM–50μM), ranging from 24 to 218% at 24 h after exposure (Fig. 1C) and from 21 to 300% after 48 h of exposure (Fig. 1D).
FIG. 1.
Deltamethrin produces a dose- and time-related increase in cell death and apoptosis Deltamethrin-induced cell death at 24 (A) and 48 h (B), as determined by LDH release and apoptosis at 24 (C) and 48 h (D), as determined by DNA fragmentation ELISA in SK-N-AS neuroblastoma cells. Data represent mean ± SEM from three to six separate experiments, each performed in triplicate and presented as percentage of control. * indicates significantly different from control (p < 0.05).
Role of Sodium Channels and Calcium Homeostasis in Deltamethrin-Induced Apoptosis
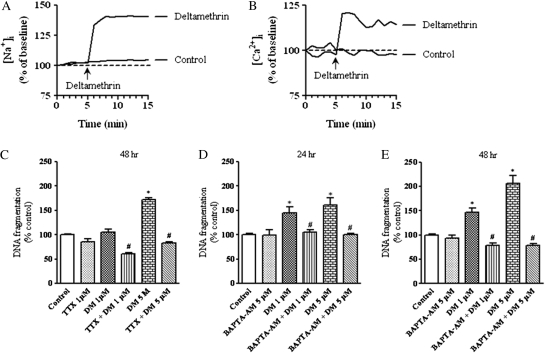
Because prolonged opening of Na+ channels by pyrethroids increases cellular Ca2+ influx and subsequent cell depolarization (Narahashi, 1996), and elevation of intracellular Ca2+ concentration has been reported to cause neuronal cell death (Arduino et al., 2009), we sought to assess the relative contributions of sodium channel activation and calcium influx in deltamethrin-induced apoptosis. To determine whether deltamethrin increased cytosolic Na+ and Ca2+, cells were treated with 5μM deltamethrin, and Na+ and Ca2+ influx was measured every 5 s with CoroNa Green-AM and Calcium Green-1-AM, respectively. Deltamethrin (5μM) caused a rapid increase in Na+ (40 ± 8%, p < 0.05) and Ca2+ influx (21 ± 0.3%, p < 0.05), which remained elevated over the course of monitoring (Figs. 2A and 2B).
FIG. 2.
Requirement of sodium channel activation and intracellular calcium for deltamethrin-induced apoptosis. Representative traces of ([Na+]i) (A) and Ca2+ (B) influx in SK-N-AS cells. Pretreatment with the Na+ channel antagonist TTX (1μM) prevented deltamethrin-induced DNA fragmentation (C). Chelation of intracellular calcium by BAPTA-AM prevented deltamethrin-induced DNA fragmentation (D and E). Data represent mean ± SEM of three to five individual experiments, each performed in triplicate and expressed as percentage of control. * indicates significantly different from control and # indicate significant differences between deltamethrin-treated cells and deltamethrin plus TTX or BAPTA-AM-treated cells (p < 0.05).
To determine the role of Na+ flux in deltamethrin-induced apoptosis, cells were cotreated with TTX, a selective Na+ channel blocker, and DNA fragmentation was determined following deltamethrin exposure for 24 and 48 h. Cotreatment with 1μM TTX completely blocked the deltamethrin-induced DNA fragmentation (Fig. 2C), demonstrating the requirement of deltamethrin interaction with Na+ channels for the initiation of apoptosis. To determine the role of intracellular Ca2+ in deltamethrin-induced apoptosis, cells were pretreated with 5μM of BAPTA-AM, a chelator of intracellular Ca2+ ([Ca2+]i) for 30 min before adding the deltamethrin. Pretreatment with BAPTA-AM completely abolished DNA fragmentation induced by deltamethrin (Figs. 2D and 2E), demonstrating the role of elevated [Ca2+]i in deltamethrin-induced apoptosis.
Activation of Caspase-3 and -9 by Deltamethrin in SK-N-AS Neuroblastoma Cells
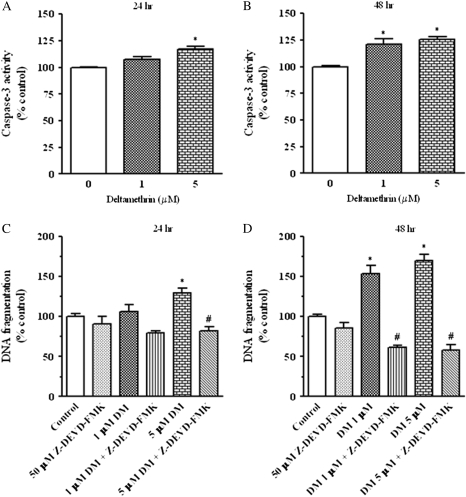
To assess the downstream mediators of deltamethrin-induced DNA fragmentation, we first examined the involvement of caspase-3, which has been recognized to play an important role in the execution phase of neuronal and nonneuronal cell apoptosis (Cohen, 1997). Deltamethrin exposure caused a modest dose- and time-dependent increase in caspase-3 activity (Figs. 3A and 3B). At 24 h postexposure to 5μM deltamethrin, there was a 17% increase in caspase-3 activity, whereas 21 and 26% increases were observed with 1 and 5μM deltamethrin at 48 h following exposure (Fig. 3B). To directly assess the role caspase-3 in deltamethrin-induced apoptosis, cells were cotreated with 50μM Z-DEVD-FMK, a caspase-3 inhibitor, which completely abolished deltamethrin-induced DNA apoptosis (Figs. 3C and 3D).
FIG. 3.
Role of caspase-3 in deltamethrin-induced apoptosis. Exposure to deltamethrin increased the activation of caspase-3 in a dose- and time-dependent manner (A and B). Deltamethrin-induced DNA fragmentation was completely prevented by the caspase-3 inhibitor Z-DEVD-FMK (C and D). Data represent mean ± SEM of four individual experiments, each performed in triplicate and expressed as percentage of control. * indicates significantly different from control and # indicate significant differences between deltamethrin-treated cells and deltamethrin plus Z-DEVD-FMK-treated cells (p < 0.05).
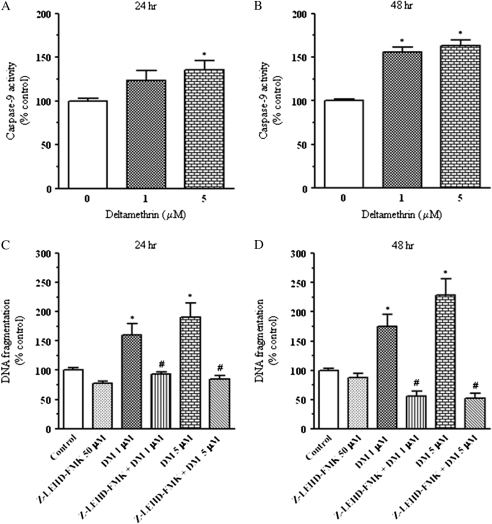
Caspase-9 is an intracellular cysteine proteases known to cleave and activate caspase-3, resulting in DNA fragmentation and eventual apoptosis (Cohen, 1997; Kitazawa et al., 2003). At 24 and 48 h after treatment, SK-N-AS cells exposed to deltamethrin showed a time- and concentration-dependent activation of caspase-9 activity (Figs. 4A and 4B). Treatment with 1 or 5μM deltamethrin for 24 h increased caspase-9 activity by 24 and 36%, respectively (Fig. 4A). In contrast, exposure to 1 and 5μM deltamethrin for 48 h resulted in 56 and 63% increase in caspase-9 activation, respectively (Fig. 4B). When cells were cotreated with 50μM Z-LEHD-FMK, a caspase-9 inhibitor, deltamethrin-induced apoptosis was prevented (Figs. 4C and 4D).
FIG. 4.
Role of caspase-9 in deltamethrin-induced apoptosis. Exposure to deltamethrin increased the activation of caspase-9 in a dose- and time-dependent manner (A and B). Inclusion of the caspase-9 inhibitor, Z-LEHD-FMK (50μM), prevented deltamethrin-induced DNA fragmentation (C and D). Data represent mean ± SEM of four individual experiments, each performed in triplicate and expressed as percentage of control. * indicates significantly different from control and # indicate significant differences between deltamethrin-treated cells and deltamethrin plus Z-LEHD-FMK-treated cells (p < 0.05).
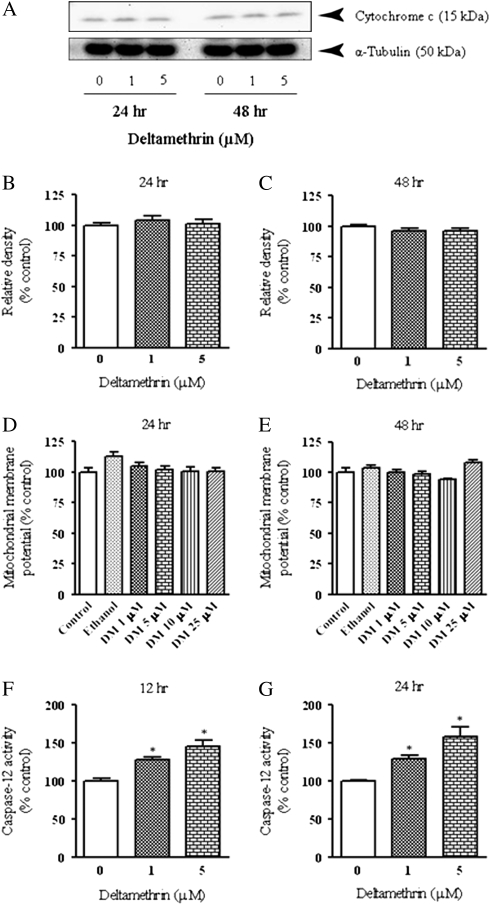
Deltamethrin Does Not Cause Mitochondrial Cytochrome c Release
Based on the ability of deltamethrin to induce caspase-9 and -3 activity, we measured the release of cytochrome c from mitochondria into cytosol, which is an important signal for apoptosome formation and caspase-9 activation (Gorman et al., 2000). No changes in the levels of cytosolic cytochrome c were observed at 24 or 48 h after deltamethrin exposure (Figs. 5A–C). Furthermore, we observed no significant alteration in mitochondrial membrane potential (Figs. 5D and 5E), suggesting an alternate pathway not initiated by mitochondrial cytochrome c release was likely responsible for deltamethrin-induced apoptosis.
FIG. 5.
Deltamethrin activates caspase-12 without altering the function of mitochondria. Western blot analysis of cytochrome c with α-tubulin immunoblot as loading control (A). Representative blots are presented and data are expressed as relative pixel density in bar graphs (B and C). Effects of deltamethrin on mitochondrial membrane potential at 24 (D) and 48 h (E). Activation of caspase-12 at 12 (F) and 24 h (G) after deltamethrin exposure. The values represent mean ± SEM from at least three individual experiments, each performed in triplicate. Data are expressed as percentage of vehicle control. * indicates significantly different from control (p < 0.05).
Activation of Caspase-12 and Calpain by Deltamethrin in SK-N-AS Cells
As an alternate mechanism, calcium-induced caspase-12 can directly activate caspase-9, without release of cytoslic cytochrome c from mitochondria, as part of the ER stress pathway leading to apoptosis (Morishima et al., 2002; Nakagawa and Yuan, 2000; Rao et al., 2001). Therefore, we examined whether caspase-12 was activated during deltamethrin exposure to SK-N-AS cells. As shown in Figures 5F and 5G, deltamethrin significantly increased the activation of caspase-12 in a dose- and time-dependent manner. Caspase-12 was activated (28–45%) as early as 12 h after deltamethrin exposure (Fig. 5F) and was increased to 30–58% following 24 h of exposure (Fig. 5G).
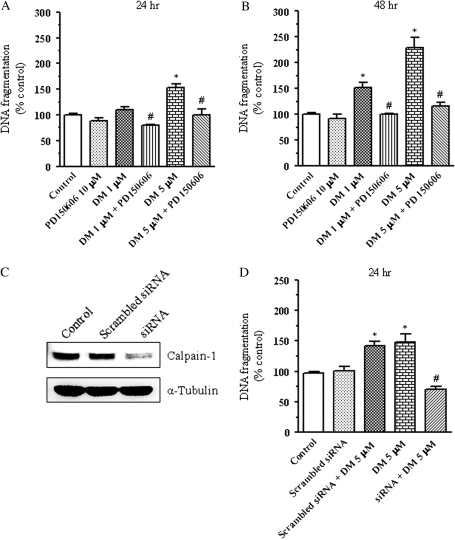
Activation of calpain, a calcium-dependent cysteine protease, is known to activate caspase-12 when stimulated by release of intracellular calcium from the ER (Nakagawa and Yuan, 2000). To determine whether calpain was activated during deltamethrin-induced apoptosis, DNA fragmentation was measured following cotreatment with PD150606, a specific calpain inhibitor. PD150606 completely prevented deltamethrin-induced DNA fragmentation (Figs. 6A and 6B). To further substantiate the functional role of calpain in deltamethrin-induced apoptotic cell death, we examined the effects of siRNA knockdown of calpain-1 on deltamethrin-induced DNA fragmentation. Treatment of SK-N-AS cells for 24 h with calpain-1 siRNA reduced calpain-1 protein expression by 71% (Fig. 6C). Knockdown of calpain-1 by siRNA prior to treatment with deltamethrin abolished deltamethrin-induced DNA fragmentation (Fig. 6D), confirming an integral role for calpain in deltamethrin-induced apoptosis and providing additional evidence for a role of the ER stress pathway.
FIG. 6.
Role of calpain in deltamethrin-induced apoptosis. Pretreatment with the calpain inhibitor PD150606 (10μM) prevented deltamethrin-induced DNA fragmentation (A and B). Treatment of SK-N-AS cells for 24 h with calpain-1 siRNA reduced calpain-1 protein expression (C). Knockdown of calpain-1 by siRNA prior to treatment with deltamethrin abolished deltamethrin-induced DNA fragmentation (D). Data present mean ± SEM of four individual experiments, each performed in triplicate and expressed as percentage of control. * indicates significantly different from control and # indicate significant differences between deltamethrin-treated cells and deltamethrin plus PD150606-treated cells or deltamethrin-treated siRNA transfected cells (p < 0.05).
Activation of the ER Stress Pathway by Deltamethrin Leads to Apoptosis
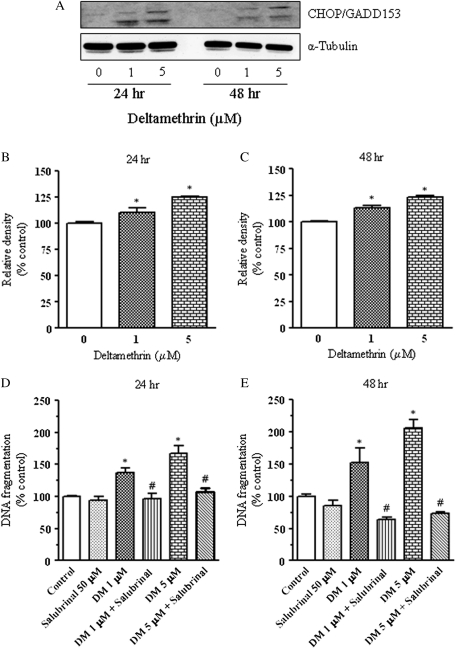
To confirm that deltamethrin-induced apoptosis via the ER stress pathway, we determined the effect of deltamethrin on the induction of CHOP/GADD153, an indicator of ER stress (Xu et al., 2005). Western blot analysis revealed the significant induction of CHOP/GADD153 protein at both 24 and 48 h after deltamethrin exposure (Figs. 7A–C). Cotreatment of SK-N-AS cells with salubrinal, an inhibitor of eIF2α, which is an integral component of the ER stress pathway (Boyce et al., 2005), prevented DNA fragmentation, confirming that deltamethrin causes apoptosis through activation of the ER stress pathway (Figs. 7D and 7E).
FIG. 7.
Role of the ER stress pathway in deltamethrin-induced apoptosis. CHOP/GADD153 expression following deltamethrin exposure was determined by Western blot analysis (A). Relative pixel densities from representative blots are presented in bar graphs (B and C). Deltamethrin-induced DNA fragmentation was completely prevented by salubrinal (eIF2α inhibitor) (D and E). Data represent mean ± SEM of four individual experiments, each performed in triplicate and expressed as percentage of control. * indicates significantly different from control, and # indicates significant differences between deltamethrin-treated cells and deltamethrin plus salubrinal-treated cells (p < 0.05).
DISCUSSION
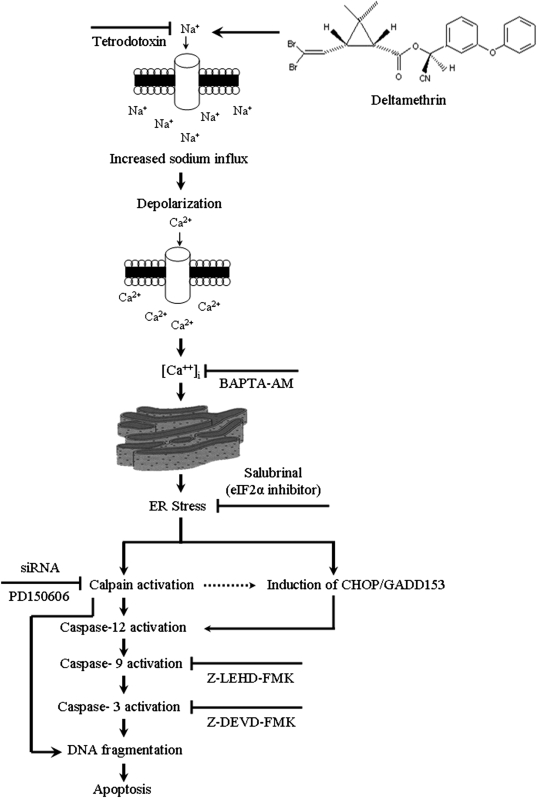
In this study, we investigated the molecular mechanisms by which exposure to the pyrethroid insecticide deltamethrin induces apoptosis. The results demonstrate that deltamethrin-induced apoptosis in SK-N-AS cells through the ER stress pathway. Specifically, deltamethrin exposure initiated a series of cell death signaling events that are dependent on interaction with voltage-gated Na+ channels, resulting in elevation of intracellular Ca2+ and activation of calpain and caspase-12 leading to DNA fragmentation (Fig. 8).
FIG. 8.
Schematic model describing the mechanism of deltamethrin-induced apoptosis. Deltamethrin increases Na+ influx by delaying the closing of Na+ channels, leading to depolarization and increased Ca2+ influx. This initiates the ER stress pathway as evidenced by induction of CHOP/GADD153 and activation of calpain. Calpain may translocate to the nucleus and initiate DNA fragmentation and also activate caspase-12, which in turn activates caspase-9 and caspase-3, leading to DNA fragmentation and apoptosis.
Previously, it has been reported that deltamethrin causes apoptosis in primary cortical neurons and SK-N-MC neuroblastoma cells (Wu and Liu, 2000a,b; Wu et al., 2000; Elwan et al., 2006). However, the mechanism(s) and apoptotic pathways by which deltamethrin causes apoptosis has not been established. Here, we demonstrate that exposure of SK-N-AS dopaminergic cells to higher concentrations (10μM and above) for 48 h led to overt cell death. However, lower concentrations (100nM–5μM) of deltamethrin caused DNA fragmentation, a hallmark of apoptosis, at 24 h of exposure, similar to that observed in the aforementioned studies. Although there are few studies that report brain levels of deltamethrin following in vivo exposures, two recent papers describing pharmacokinetic modeling of deltamethrin in rats found that brain levels of deltamethrin following oral gavage of 2–10 mg/kg deltamethrin ranged from 0.1 to 0.4 μg/g (Godin et al., 2010; Kim et al., 2010). These doses are below those which cause overt symptoms of pyrethroid intoxication and the highest brain concentrations produced are roughly in the low nanomolar range. However, higher doses would be expected to produce levels closer to the range employed in this study. Furthermore, the presence of serum in our media, which may reduce the amount of deltamethrin available to interact with the cell, makes it likely that the dose of deltamethrin that reaches the cell is lower than what we applied. It should be noted that the pharmacokinetic studies found that the models predicted twofold greater peak deltamethrin concentration in humans compared to rats (Godin et al., 2010) and that developing rats accumulated twice as much deltamethrin in the brain compared to adults (Kim et al., 2010). This increased accumulation in the developing animal became even more evident as the dose increased. These findings suggest that in high-level exposure situations, developing animals and humans may be more at risk than is predicted based on rodent studies.
As a first step in identifying the mechanism and pathways by which deltamethrin causes apoptosis, we focused on the role of Ca2+. In neuronal cells, Ca2+ plays an integral role in activation and/or enhancement of cell death pathways, including those involved in apoptosis (Berliocchi et al., 2005). Pyrethroids, including deltamethrin, have been demonstrated to depolarize cells by prolonging the opening of voltage-gated Na+ channels (Narahashi, 1996), subsequently increasing [Ca2+]i. Similarly, recent data in primary cortical neurons using sodium-binding benzofuran isophthalate as the Na+ indicator and fluo-3 as the [Ca2+]i indicator reported that pyrethroids, including deltamethrin, significantly increased [Na+]i and [Ca2+]i, which could be blocked with the sodium channel blocker TTX (Cao et al., 2008, 2011). Here, acute application of deltamethrin (5μM) rapidly caused increased Na+ and Ca2+ influx into the SK-N-AS cells and cotreatment with TTX completely prevented DNA fragmentation. This finding is in agreement with studies demonstrating that hypoxia and veratridine application activate Na+ channels, leading to activation of caspase-3 and apoptosis that can be blocked by TTX (Banasiak et al., 2004). We also found that the intracellular Ca2+ chelator BAPTA-AM completely prevented the deltamethrin-induced DNA fragmentation, similar to that observed in veratridine-induced apoptotic cell death in bovine chromaffin cells (Jordan et al., 2000). Although there is some evidence that deltamethrin can directly interact with Ca2+ channels and increase their function (Breckenridge et al., 2009; Clark and Symington, 2008), our data are in agreement with those of Cao et al. (2011) that the rise in Ca2+ is secondary to the activation of Na+ channels because TTX blocks deltamethrin-induced apoptosis. Taken together, these data suggest that application of Na+ channel agonists, such as deltamethrin and veratridine, causes apoptosis through activation of Na+ channels and subsequent elevation of [Ca2+]i.
Activation of caspase-3 is a critical event in the execution phase of apoptosis involving pesticide exposure (Choi et al., 2010; Kitazawa et al., 2003; Ramachandiran et al., 2007; Sherer et al., 2003). Although the activation of caspase-3 by pyrethroids has not been studied in detail, one study found that very high doses (100–200μM) of deltamethrin increased caspase-3 activity in HepG2 cells (Das et al., 2008), suggesting that caspase-3 may be the final effector caspase in deltamethrin-induced apoptosis. Here, we found that deltamethrin only modestly increased caspase-3 activity. However, pharmacological inhibition of this activation by z-DEVD-fmk prevented apoptosis. This discrepancy may be explained by the fact that z-DEVD-fmk can inhibit additional proteases, such as caspase-9 and calpain (Berger et al., 2006; Knoblach et al., 2004). Thus, the protective effect of z-DEVD-fmk against deltamethrin-induced apoptosis may be the result of its inhibitory effects on upstream proteases.
In the classic intrinsic pathway, caspase-9 is activated by release of cytochrome c from the mitochondria into the cytosol, and caspase-9 can then activate caspase-3 as the final effector of apoptosis (Gorman et al., 2000; Lee and Wei, 2000). Here, we observed increased caspase-9 activity following deltamethrin exposure and found that pharmacological inhibition of caspase-9 prevented DNA fragmentation. However, we also found that caspase-9 activation occurred in the absence of elevated levels of cytochrome c in the cytosol, indicating that deltamethrin-induced apoptosis is likely initiated through a mitochondrial-independent pathway. Previously, one study found that in vivo deltamethrin exposure reduced mitochondrial cytochrome c levels (Chen et al., 2007). However, levels in the cytosol were not determined and there were no loading control included in the Western blots. Thus, the potential role of cytochrome c release in deltamethrin-induced apoptosis requires additional study.
In addition to the classic intrinsic and extrinsic pathways of apoptosis, it has become recognized that activation of the ER stress pathway can contribute to initiation of apoptosis (Verkhratsky, 2005). The ER plays an integral role in the storage of Ca2+ to regulate intracellular Ca2+ homeostasis, and release of calcium stores from the ER can lead to activation of caspase-12, which can then activate caspase-9 and -3, leading to apoptosis (Verkhratsky, 2005). Here, we found that deltamethrin exposure increased casapse-12 activity in a dose- and time-related manner, suggesting that the ER stress pathway may play a role in deltamethrin-induced apoptosis. Additionally, the increased caspase-12 activity occurred earlier and at a greater magnitude than caspase-9, suggesting that activation of the ER stress pathway. Although there are currently no data in the literature pertaining to Na+ channel agonists and activation of caspase-12, data have demonstrated that conditions such as in vitro neuronal ischemia results in a parallel rise in cytosolic Na+ and Ca2+ leading to activation of caspase-12 (Chen et al., 2008). Additionally, the Na+ channel agonist veratridine has been found to cause release of ER Ca2+, suggesting that Na+ influx may play an important role in regulating intracellular calcium stores (Nikolaeva et al., 2005). Our data support these observations and suggest that activation of Na+ influx and the subsequent rise in cytosolic Ca2+ following deltamethrin activates caspase-12 through liberation of ER Ca2+stores.
Release of Ca2+ from the ER can result in activation of caspase-12 following cleavage by calpain initiating apoptosis in the ER stress pathway (Morishima et al., 2002; Nakagawa and Yuan, 2000). Furthermore, a study in Caenorhabditis. elegans, demonstrated that overexpression of the C. elegans Na+ channel induced Ca2+ release from the ER, calpain activation, and cell death (Bianchi et al., 2004). In this study, we found that the calpain inhibitor PD150606 and the knockdown of calpain-1 by siRNA completely prevented deltamethrin-induced DNA fragmentation, indicating calpain-dependent apoptotic cascade in deltamethrin-induced apoptosis. Calpain inhibition has also been demonstrated to protect against veratridine-induced apoptosis in bovine chromaffin cells (Jordan et al., 2000). Over activation of calpain due to ER stress has been reported to cause neuronal cell death in ischemic brain injury, neuromuscular degeneration, and AD (Saido et al., 1994; Saito et al., 1993; Tsuji et al., 1998; Van den Bosch et al., 2002). Thus, high-level exposure to deltamethrin may have the potential to cause neurodegeneration through activation of calpain.
The data generated thus far in this study have indicated that deltamethrin induces apoptosis through the ER stress pathway. Disruption of the delicate Ca2+ balance in the ER can cause transcription of genes to activate the ER stress-mediated apoptotic pathway (Xu et al., 2005). The induction of the transcription factor CHOP/GADD153 has been observed during unfolded protein response and is associated with induction of ER stress-mediated apoptosis (Oyadomari and Mori, 2004). CHOP, a member of the C/EBP family of bZIP transcription factors, is typically expressed at very low basal levels but increased under conditions of ER stress, where it can decrease expression of the anti-apoptotic molecule Bcl-2 (Xu et al., 2005). We found that deltamethrin treatment caused an early significant induction of CHOP that paralleled DNA fragmentation, providing further evidence that deltamethrin causes apoptosis through the ER stress pathway. During initiation of the ER stress pathway, CHOP can be induced by eIF2α, is phosphorylated by PKR-like ER kinase, and leads to inactivation of mRNA translation in an attempt to reduce ER stress (Xu et al., 2005). Here, we found that sulubrinal, an inhibitor of eIF2α, abolished deltamethrin-induced DNA fragmentation, providing further evidence to support the role of the ER stress pathway in deltamethrin-induced apoptosis.
Taken together, the data presented in this study establish a pathway of apoptosis initiated by deltamethrin exposure (Fig.8). Specifically, deltamethrin activates the ER stress pathway through its interaction with Na+ channels and subsequent calcium overload, leading to initiation of the apoptotic cascade through calpain-1 activation of caspase-12. We also observe a later increase in caspase-9 and a modest increase in caspase-3. These data suggest that deltamethrin-induced DNA fragmentation and apoptosis may occur in two stages, the first of which involves ER stress and calpain activation and the second involving activation of the caspase-cascade. Indeed, calpain has been found to translocate to the nucleus and initiate DNA fragmentation, possible by activating a DNAse (Rami et al., 2000). This may also explain why complete protection from deltamethrin-induced apoptosis was observed with the peptide-based inhibitors employed here because these compounds can also inhibit calpain activity (Knoblach et al., 2004).
Because the ER stress pathway and calpain activation has been implicated a number of neurodegenerative diseases including prion disease, AD, amyotrophic lateral sclerosis (ALS), and PD, these data provide insight into the mechanism by which high-level exposure to pyrethroid pesticides may cause long-term neurological effects and may have the potential to cause neurodegeneration. Although there are few data available on the association between high-level pyrethroid exposure and neurodegeneration in the human population, there is a case report indicating that chronic high-level inhalation pyrethroids produced a motor neuron disorder simulating ALS (Doi et al., 2006). Thus, additional epidemiological and clinical data are needed to determine what association, if any, such exposures have with the development of neurodegenerative diseases.
FUNDING
National Institutes of Health (R01ES015991, P30ES005022). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Health.
References
- Abdel-Rahman A, Abou-Donia S, El-Masry E, Shetty A, Abou-Donia M. Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. J. Toxicol. Environ. Health A. 2004;67:163–192. doi: 10.1080/15287390490264802. [DOI] [PubMed] [Google Scholar]
- Arduino DM, Esteves AR, Cardoso SM, Oliveira CR. Endoplasmic reticulum and mitochondria interplay mediates apoptotic cell death: relevance to Parkinson's disease. Neurochem. Int. 2009;55:341–348. doi: 10.1016/j.neuint.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- Berger AB, Sexton KB, Bogyo M. Commonly used caspase inhibitors designed based on substrate specificity profiles lack selectivity. Cell Res. 2006;16:961–963. doi: 10.1038/sj.cr.7310112. [DOI] [PubMed] [Google Scholar]
- Berliocchi L, Bano D, Nicotera P. Ca2+ signals and death programmes in neurons. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:2255–2258. doi: 10.1098/rstb.2005.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat. Neurosci. 2004;7:1337–1344. doi: 10.1038/nn1347. [DOI] [PubMed] [Google Scholar]
- Bloomquist JR, Barlow RL, Gillette JS, Li W, Kirby ML. Selective effects of insecticides on nigrostriatal dopaminergic nerve pathways. Neurotoxicology. 2002;23:537–544. doi: 10.1016/s0161-813x(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Bradberry SM, Thanacoody HK, Watt BE, Thomas SH, Vale JA. Management of the cardiovascular complications of tricyclic antidepressant poisoning: role of sodium bicarbonate. Toxicol. Rev. 2005;24:195–204. doi: 10.2165/00139709-200524030-00012. [DOI] [PubMed] [Google Scholar]
- Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS, Symington S, Clark JM, et al. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology. 2009;30(Suppl. 1):S17–S31. doi: 10.1016/j.neuro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Calvert GM, Mehler LN, Rosales R, Baum L, Thomsen C, Male D, Shafey O, Das R, Lackovic M, Arvizu E. Acute pesticide-related illnesses among working youths, 1988-1999. Am. J. Public Health. 2003;93:605–610. doi: 10.2105/ajph.93.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, George J, Gerwick WH, Baden DG, Rainier JD, Murray TF. Influence of lipid-soluble gating modifier toxins on sodium influx in neocortical neurons. J. Pharmacol. Exp. Ther. 2008;326:604–613. doi: 10.1124/jpet.108.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Shafer TJ, Murray TF. Mechanisms of pyrethroid insecticide-induced stimulation of calcium influx in neocortical neurons. J. Pharmacol. Exp. Ther. 2011;336:197–205. doi: 10.1124/jpet.110.171850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol. 1998;43:1–16. doi: 10.1146/annurev.ento.43.1.1. [DOI] [PubMed] [Google Scholar]
- Chen D, Huang X, Liu L, Shi N. Deltamethrin induces mitochondrial membrane permeability and altered expression of cytochrome C in rat brain. J. Appl. Toxicol. 2007;27:368–372. doi: 10.1002/jat.1215. [DOI] [PubMed] [Google Scholar]
- Chen X, Kintner DB, Luo J, Baba A, Matsuda T, Sun D. Endoplasmic reticulum Ca2+ dysregulation and endoplasmic reticulum stress following in vitro neuronal ischemia: role of Na+-K+-Cl− cotransporter. J. Neurochem. 2008;106:1563–1576. doi: 10.1111/j.1471-4159.2008.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Abel G, Klintworth H, Flavell RA, Xia Z. JNK3 mediates paraquat- and rotenone-induced dopaminergic neuron death. J. Neuropathol. Exp. Neurol. 2010;69:511–520. doi: 10.1097/NEN.0b013e3181db8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Symington SB. Neurotoxic implications of the agonistic action of CS-syndrome pyrethroids on the N-type Ca(v) 2.2 calcium channel. Pest. Manag. Sci. 2008;64:628–638. doi: 10.1002/ps.1573. [DOI] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem. J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Landshamer S. Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr. Alzheimer Res. 2006;3:269–283. doi: 10.2174/156720506778249461. [DOI] [PubMed] [Google Scholar]
- Das PC, Streit TM, Cao Y, Rose RL, Cherrington N, Ross MK, Wallace AD, Hodgson E. Pyrethroids: cytotoxicity and induction of CYP isoforms in human hepatocytes. Drug Metabol. Drug Interact. 2008;23:211–236. doi: 10.1515/dmdi.2008.23.3-4.211. [DOI] [PubMed] [Google Scholar]
- Day WT, Wu CH, Safa AR. Etoposide induces protein kinase Cδ- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol. Pharmacol. 2009;76:632–640. doi: 10.1124/mol.109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi H, Kikuchi H, Murai H, Kawano Y, Shigeto H, Ohyagi Y, Kira J. Motor neuron disorder simulating ALS induced by chornic inhalation of pyrethroid insecticides. Neurology. 2006;67:1894–1895. doi: 10.1212/01.wnl.0000244489.65670.9f. [DOI] [PubMed] [Google Scholar]
- Elwan MA, Richardson JR, Guillot TS, Caudle WM, Miller GW. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol. Appl. Pharmacol. 2006;211:188–197. doi: 10.1016/j.taap.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin SJ, DeVito MJ, Hughes MF, Ross DG, Scollon EJ, Starr JM, Setzer RW, Conolly RB, Tornero-Velez R. Physiologically based pharmacokinetic modeling of deltamethrin: development of a rat and human diffusion-limited model. Toxicol. Sci. 2010;115:330–343. doi: 10.1093/toxsci/kfq051. [DOI] [PubMed] [Google Scholar]
- Gorman AM, Ceccatelli S, Orrenius S. Role of mitochondria in neuronal apoptosis. Dev. Neurosci. 2000;22:348–358. doi: 10.1159/000017460. [DOI] [PubMed] [Google Scholar]
- Jordan J, Galindo MF, Calvo S, Gonzalez-Garcia C, Cena V. Veratridine induces apoptotic death in bovine chromaffin cells through superoxide production. Br. J. Pharmacol. 2000;130:1496–1504. doi: 10.1038/sj.bjp.0703451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur. J. Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kim KB, Anand SS, Kim HJ, White CA, Fisher JW, Tornero-Velez R, Bruckner JV. Age, dose, and time-dependency of plasma and tissue distribution of deltamethrin in immature rats. Toxicol. Sci. 2010;115:354–368. doi: 10.1093/toxsci/kfq074. [DOI] [PubMed] [Google Scholar]
- Kimata A, Kondo T, Ueyama J, Yamamoto K, Yoshitake J, Takagi K, Suzuki K, Inoue T, Ito Y, Hamajima N, et al. Comparison of urinary concentrations of 3-phenoxybenzoic acid among general residents in rural and suburban areas and employees of pest control firms. Int. Arch. Occup. Environ. Health. 2009;82:1173–1178. doi: 10.1007/s00420-009-0424-7. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Alroy DA, Nikolaeva M, Cernak I, Stoica BA, Faden AI. Caspase inhibitor z-DEVD-fmk attenuates calpain and necrotic cell death in vitro and after traumatic brain injury. J. Cereb. Blood Flow Metab. 2004;24:1119–1132. doi: 10.1097/01.WCB.0000138664.17682.32. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J. Biomed. Sci. 2000;7:2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Muller-Mohnssen H. Chronic sequelae and irreversible injuries following acute pyrethroid intoxication. Toxicol. Lett. 1999;107:161–176. doi: 10.1016/s0378-4274(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell. Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996;79:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Nikolaeva MA, Mukherjee B, Stys PK. Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J. Neurosci. 2005;25:9960–9967. doi: 10.1523/JNEUROSCI.2003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Power LE, Sudakin DL. Pyrethrin and pyrethroid exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J. Med. Toxicol. 2007;3:94–99. doi: 10.1007/BF03160917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandiran S, Hansen JM, Jones DP, Richardson JR, Miller GW. Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: oxidation of thioredoxin and caspase-3 activation. Toxicol. Sci. 2007;95:163–171. doi: 10.1093/toxsci/kfl125. [DOI] [PubMed] [Google Scholar]
- Rami A, Agarwal R, Botez G, Winckler J. μ-Calpain activation, DNA fragmentation, and synergistic effects of caspase and calpain inhibitors in protecting hippocampal neurons from ischemic damage. Brain Res. 2000;866:299–312. doi: 10.1016/s0006-8993(00)02301-5. [DOI] [PubMed] [Google Scholar]
- Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol. Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814–822. [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson's disease. J. Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Di Tullio MA, Ricci A, Amenta F. Influence of dermal exposure to the pyrethroid insecticide deltamethrin on rat brain microanatomy and cholinergic/dopaminergic neurochemistry. Brain Res. 2009;1301:180–188. doi: 10.1016/j.brainres.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Shimohama S, Kimura J, Shimizu K. m-Calpain (calcium-activated neutral proteinase) in Alzheimer's disease brains. Neurosci. Lett. 1998;248:109–112. doi: 10.1016/s0304-3940(98)00348-6. [DOI] [PubMed] [Google Scholar]
- Van den Bosch L, Van Damme P, Vleminckx V, Van Houtte E, Lemmens G, Missiaen L, Callewaert G, Robberecht W. An alpha-mercaptoacrylic acid derivative (PD150606) inhibits selective motor neuron death via inhibition of kainate-induced Ca2+ influx and not via calpain inhibition. Neuropharmacology. 2002;42:706–713. doi: 10.1016/s0028-3908(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bannon MJ. Sp1 and Sp3 activate transcription of the human dopamine transporter gene. J. Neurochem. 2005;93:474–482. doi: 10.1111/j.1471-4159.2005.03051.x. [DOI] [PubMed] [Google Scholar]
- Wu A, Li L, Liu Y. Deltamethrin induces apoptotic cell death in cultured cerebral cortical neurons. Toxicol. Appl. Pharmacol. 2003;187:50–57. doi: 10.1016/s0041-008x(02)00032-7. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y. Apoptotic cell death in rat brain following deltamethrin treatment. Neurosci. Lett. 2000a;279:85–88. doi: 10.1016/s0304-3940(99)00973-8. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y. Deltamethrin induces delayed apoptosis and altered expression of p53 and bax in rat brain. Environ. Toxicol. Pharmacol. 2000b;8:183–189. doi: 10.1016/s1382-6689(00)00039-9. [DOI] [PubMed] [Google Scholar]
- Wu A, Ren T, Hu Q, Liu Y. Deltamethrin induces altered expression of P53, Bax and Bcl-2 in rat brain. Neurosci. Lett. 2000;284:29–32. doi: 10.1016/s0304-3940(00)00952-6. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]