Abstract
Acetaminophen (APAP) overdose causes liver injury in humans and mice. DNA fragmentation is a hallmark of APAP-induced cell death, and nuclear translocation of apoptosis-inducing factor (AIF) correlates with DNA fragmentation after APAP overdose. To test the hypothesis that AIF may be a critical mediator of APAP-induced cell death, fasted male AIF-deficient Harlequin (Hq) mice and respective wild-type (WT) animals were treated with 200 mg/kg APAP. At 6 h after APAP, WT animals developed severe liver injury as indicated by the increase in plasma alanine aminotransferase (ALT) activities (8600 ± 1870 U/l) and 61 ± 8% necrosis. This injury was accompanied by massive DNA strand breaks in centrilobular hepatocytes (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling [TUNEL] assay) and release of DNA fragments into the cytosol (anti-histone ELISA). In addition, there was formation of reactive oxygen (increase in liver glutathione disulfide (GSSG) levels and mitochondrial protein carbonyls) and peroxynitrite (nitrotyrosine [NT] staining) together with mitochondrial translocation of activated c-jun-N-terminal kinase (P-JNK) and release of AIF from the mitochondria. In contrast, Hq mice had significantly less liver injury (ALT: 330 ± 130 U/l; necrosis: 4 ± 2%), minimal nuclear DNA damage, and drastically reduced oxidant stress (based on all parameters) at 6 h. WT and Hq mice had the same baseline levels of cyp2E1 and of glutathione. The initial depletion of glutathione (20 min after APAP) was the same in both groups suggesting that there was no relevant difference in metabolic activation of APAP. Thus, AIF has a critical function in APAP hepatotoxicity by facilitating generation of reactive oxygen in mitochondria and, after nuclear translocation, AIF can be involved in DNA fragmentation.
Keywords: acetaminophen, drug hepatotoxicity, cell death, DNA fragmentation, mitochondria, reactive oxygen species
Acetaminophen (APAP) overdose is currently the most frequent cause of acute liver failure in the United States (Larson et al., 2005). The mechanisms of toxicity include the formation of a reactive metabolite, depletion of cellular glutathione, and APAP -protein adduct formation (Cohen et al., 1997; Nelson, 1990). Although these mechanisms are critical for the toxicity, they are considered initiating events that require further amplification to cause necrotic cell death (Jaeschke and Bajt, 2006). Central to the process propagating to cell necrosis are mitochondria as a source of reactive oxygen species (ROS) and peroxynitrite formation (Cover et al., 2005; Jaeschke et al., 2003). Recent data provided evidence that this oxidant stress is responsible for activation of c-jun-N-terminal kinase (JNK) and translocation of its activated form, phosphorylated c-jun-N-terminal kinase (P-JNK) to mitochondria (Hanawa et al., 2008). Mitochondrial JNK, in turn, further promotes mitochondrial oxidant stress (Saito et al., 2010). The extensive formation of reactive oxygen and reactive nitrogen species inside the mitochondria is a critical factor in APAP-induced liver injury (Bajt et al., 2004; Knight et al., 2002; Ramachandran et al., 2011b) through triggering of the mitochondrial membrane permeability transition (MPT) and collapse of the mitochondrial membrane potential (Kon et al., 2004; Ramachandran et al., 2011a). Mitochondria are also involved in promoting nuclear DNA fragmentation. Mitochondrial intermembrane proteins including apoptosis-inducing factor (AIF) and endonuclease G can be released into the cytosol initially through a bax pore and later, after the MPT and matrix swelling, through rupture of the outer mitochondrial membrane (Bajt et al., 2008). After translocation to the nucleus, AIF and endonuclease G may cause DNA fragmentation (Bajt et al., 2006). Although nonspecific endonuclease inhibitors have been shown to reduce APAP-induced DNA damage and cell death (Shen et al., 1992), the functional significance of AIF or endonuclease G for APAP-induced liver injury remains unclear.
AIF is a mitochondrial protein that can be released during caspase-independent cell death (Susin et al., 1999). Because AIF is anchored through its N-terminus in the inner mitochondrial membrane, release of AIF requires proteolytic cleavage and permeabilization of the outer mitochondrial membrane (Joza et al., 2009; Norberg et al., 2010; Sevrioukova, 2011). The soluble form of AIF has nuclear localization sequences that enable its translocation to the nucleus (Joza et al., 2009; Norberg et al., 2010; Sevrioukova, 2011). AIF in the nucleus is involved in large-scale DNA fragmentation and chromatin condensation (Boujrad et al., 2007). However, the main physiological function of AIF appears to be in the mitochondria, where it acts as an NADH oxidase (Miramar et al., 2001). It was suggested that AIF is involved in oxidative phosphorylation through modulating the function of complex I (Vahsen et al., 2004). The importance of AIF is documented by the fact that AIF deficiency is embryonically lethal (Brown et al., 2006). However, the Harlequin (Hq) mouse, which has only about 10–20% of normal AIF levels, is viable and can function as a model of partial AIF deficiency (Klein et al., 2002). Although we have demonstrated that AIF is released together with endonuclease G from the mitochondria and both proteins translocate to the nucleus during APAP hepatotoxicity (Bajt et al., 2006), the exact function and pathophysiological relevance of AIF in APAP hepatotoxicity remains unclear. Therefore, we used the Hq mouse to investigate if AIF plays a relevant role in the pathophysiology of APAP-induced liver injury.
MATERIALS AND METHODS
Animals.
Male Hq mice (hemizygous for Aifm1Hq) and age-matched wild-type (WT) controls (CF-1 strain background) from the same colony with an average weight of 18–20 g were purchased from Jackson Laboratory (Bar Harbor, ME). The harlequin mutation has a retroviral insertion in the first intron of the AIF gene (Sevrioukova, 2011). This insertion leads to an 80% decrease in mRNA and protein levels of AIF relative to WT controls (Klein et al., 2002). All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food (certified rodent diet no. 8604, Harlan Teklad, Indianapolis, IN) and water. The experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research. All chemicals were purchased from Sigma Chemical Co. (St Louis, MO) unless stated otherwise.
Experimental design.
Overnight fasted mice were treated with 200 mg/kg APAP ip (dissolved in warm saline). Depending on the nature of the experiments, the animals were sacrificed between 20 min and 24 h after APAP treatment and blood was withdrawn from the vena cava into a heparinized syringe for the measurement of alanine aminotransferase (ALT) activities (Liquid ALT, Pointe Scientific, Inc., Canton, MI). The liver was removed and was rinsed in ice-cold saline; liver sections were fixed in 10% phosphate-buffered formalin for histological analyses. A portion of the liver was used for mitochondrial isolation and the remaining liver was snap-frozen in liquid nitrogen and stored at −80°C.
Biochemical assays.
Total soluble glutathione (GSH) and glutathione disulfide (GSSG) were measured in liver homogenate and mitochondria with a modified method of Tietze as described in detail (Jaeschke and Mitchell, 1990; Knight et al., 2001). Protein carbonyl content in the sample was measured using dinitrophenyl hydrazine (DNPH) as described (Sohal et al., 1993). Briefly, samples were treated with 10mM DNPH in 2 N HCl and incubated for 1 h at room temperature. After incubation, the proteins in the mixture were precipitated with 10% trichloro acetic acid and the precipitate was washed with a mixture of ethanol:ethylacetate (1:1). The precipitate was then dissolved in 6M guanidine HCl and the absorbance was read at 366 nm.
Histology and immunohistochemistry.
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut and stained with hematoxylin and eosin (H&E) for blinded evaluation of necrosis by the pathologist (Gujral et al., 2002). The percent of necrosis was estimated by evaluating the number of microscopic fields with necrosis compared with the cross-sectional area. Additional sections were also stained for nitrotyrosine (NT) -protein adducts with the DAKO LSAB peroxidase kit (K0679) (DAKO Corp., Carpinteria, CA) and an anti-NT antibody (Invitrogen, Carlsbad, CA) (Knight et al., 2002). For the terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) assay, sections of liver were stained with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN) as described in the manufacturer’s instructions (Gujral et al., 2002).
Isolation of subcellular fractions and Western blotting.
Mitochondria and cytosolic fractions were isolated by differential centrifugation as described (Cover et al., 2005). Western blotting was carried out as described in detail (Bajt et al., 2000) using the following antibodies: a rabbit anti-AIF antibody (Abcam, Cambridge, MA), a rabbit anti-bax antibody (Invitrogen), and rabbit anti-phospho-JNK and rabbit anti-endonuclease G polyclonal antibodies (Cell signaling Technology, Danvers, MA), a mouse antiproliferating cell nuclear antigen (anti-PCNA) monoclonal antibody (Santa Cruz) and rabbit anti-Smac and anti-cytochrome c polyclonal antibodies (Santa Cruz). A horseradish peroxidase–coupled antirabbit immunoglobulin G (IgG) (Santa Cruz) was used as secondary antibody. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech. Inc., Piscataway, NJ).
Statistics.
All results were expressed as mean ± SE. Comparisons between multiple groups were performed with one-way ANOVA or, where appropriate, by two-way ANOVA, followed by a post hoc Bonferroni test. When the data were not normally distributed, we used the Kruskal-Wallis Test (nonparametric ANOVA) followed by Dunn’s multiple comparisons test; p < 0.05 was considered significant.
RESULTS
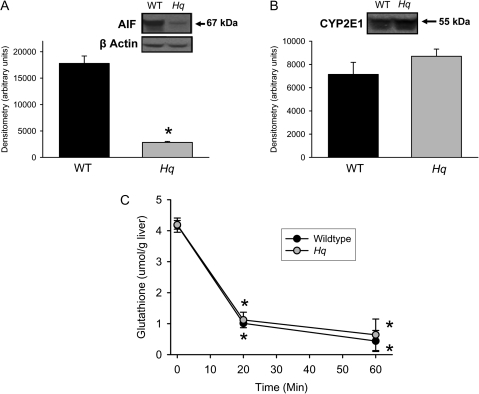
In order to evaluate the pathophysiological role of AIF in APAP hepatotoxicity, partial AIF-deficient Hq mice were used. Consistent with the original report (Klein et al., 2002), AIF protein levels in the liver were 84% lower in Hq mice compared with WT animals (Fig. 1A). In contrast, the hepatic protein expression levels of cytochrome P450 2E1 (cyp2E1), one of the key enzymes responsible for APAP activation (Gonzalez, 2007), were not significantly different between these groups of animals (Fig. 1B). Treatment of WT and Hq mice with a moderate overdose of APAP (200 mg/kg) resulted in a similar rapid decline of hepatic GSH levels within 20 min (WT: −76%; Hq: −73%) and a further reduction by 60 min (Fig. 1C). In the absence of cell injury, this initial GSH consumption reflects mainly N-acetyle-p-benzoquinone imine (NAPQI) formation (Jaeschke, 1990). Thus, the data indicate that the metabolic activation rate of APAP is similar in WT and in Hq mice.
FIG. 1.
Hepatic expression of AIF and cytochrome P450 2E1 in WT and AIF-deficient (Hq) mice. AIF (A) and cyp2e1 (B) levels were determined by western blot analysis. Densitometric analysis was performed in three animals per group. In addition, animals were either untreated or injected with 200 mg/kg APAP, liver samples were obtained between 0 and 60 min and the glutathione levels were analyzed (C). All data are expressed as mean ± SE of four animals per group or time point. *p < 0.05 (compared with WT or controls).
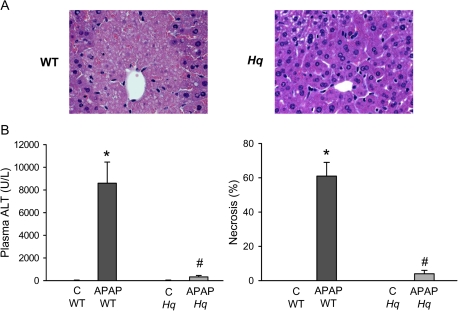
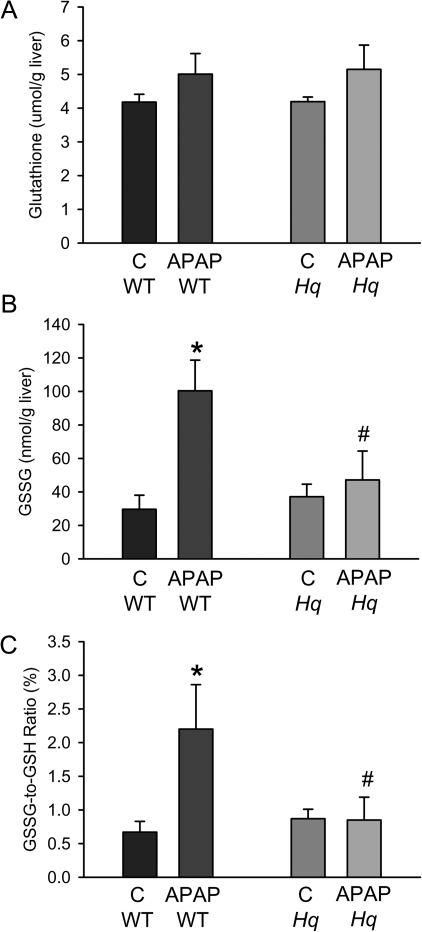
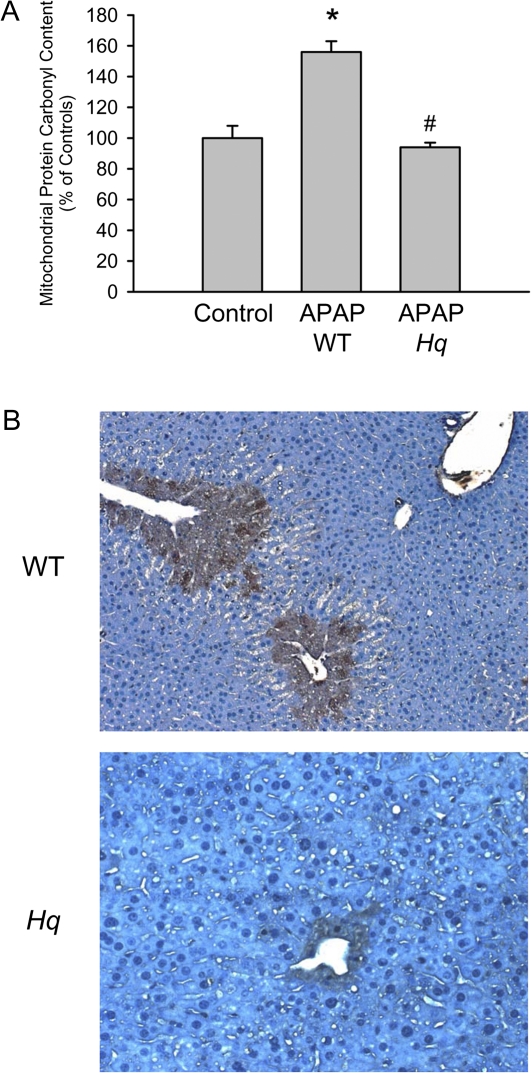
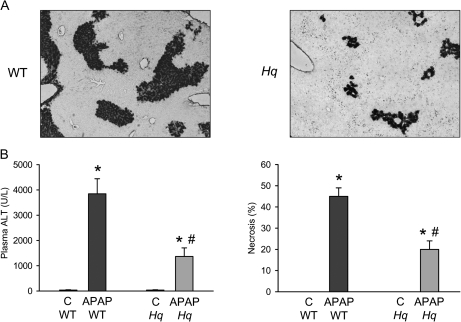
Treatment of WT mice with APAP (200 mg/kg) for 6 h induced substantial liver injury as indicated by the dramatic increase in plasma ALT activities and histological evidence of extensive centrilobular necrosis (Figs. 2A and 2B). In contrast, Hq mice were almost completely protected (Figs. 2A and 2B). To evaluate the oxidant stress in these animals, hepatic GSH and GSSG levels were measured. Despite the initial depletion (Fig. 1C), hepatic glutathione levels were completely recovered at 6 h after APAP treatment (Fig. 3A). However, the significant increase in GSSG levels and consequently the increase in the GSSG:GSH ratio reflects an oxidant stress in these animals (Figs. 3B and 3C). In Hq mice, the hepatic GSH content was similarly recovered as in WT animals (Fig. 3A). However, GSSG levels were not elevated and the GSSG:GSH ratio was similar to controls (Fig. 3B and 3C) indicating that APAP overdose did not induce a relevant oxidant stress in livers of Hq mice. This conclusion was confirmed by measurement of protein carbonyls in mitochondria as an indicator of mitochondrial oxidant stress (Ramachandran et al., 2011b) and with staining of liver sections for NT protein adducts as an indicator for peroxynitrite formation (Hinson et al., 1998). In WT mice, APAP treatment induced a significant increase in protein carbonyls, which was completely prevented in Hq mice (Fig. 4A). Similarly, the extensive centrilobular NT staining in APAP-treated WT animals was substantially reduced and limited to one- or two-cell layers around the central vein in Hq mice (Fig. 4B).
FIG. 2.
APAP-induced liver injury in WT and AIF-deficient (Hq) mice. (A) Representative H&E-stained sections are shown (×200). (B) Plasma ALT activities and the area of necrosis were measured as indicators for liver injury in untreated controls (C) or 6 h after administration of 200 mg/kg APAP. Data represent means ± SE of n = 6 animals per group. *p < 0.05 (compared with controls), **p < 0.05 (compared with WT/APAP).
FIG. 3.
Effect of APAP on hepatic glutathione content in WT and AIF-deficient (Hq) mice. Glutathione (GSH + GSSG) (Panel A) and GSSG (B) were measured in untreated controls (C) and 6 h after administration of 200 mg/kg APAP. The GSSG:GSH ratio was calculated for each sample (C). Data represent means ± SE of six animals per group. *p < 0.05 (compared with controls), **p < 0.05 (compared with WT/APAP).
FIG. 4.
APAP-induced oxidant and nitrosative stress in WT and AIF-deficient (Hq) mice. Protein carbonyls (A) were measured in hepatic mitochondria and liver sections were stained for NT protein adducts (B) at 6 h after 200 mg/kg APAP. For protein carbonyls data represent means ± SE of three animals per group. For NT staining, representative sections are shown (×100). *p < 0.05 (compared with controls), **p < 0.05 (compared with WT/APAP).
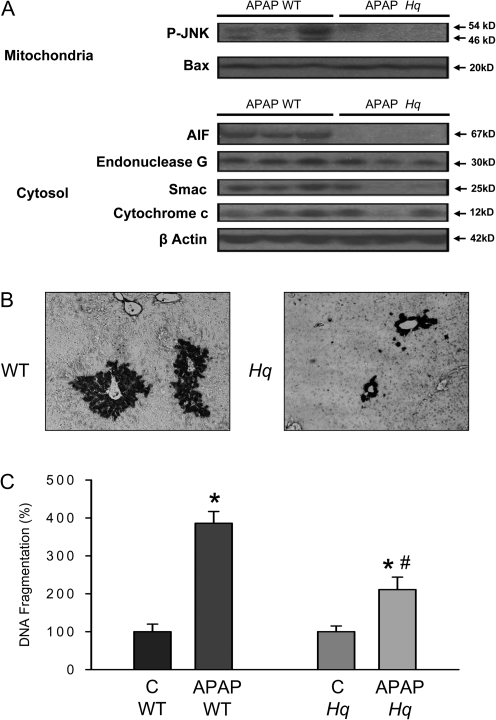
A consequence of the early mitochondrial oxidant stress is the activation of JNK and the translocation of P-JNK to mitochondria (Hanawa et al., 2008). Consistent with these findings, P-JNK was detected in mitochondria of WT animals 6 h after APAP (Fig. 5A); although quantitatively P-JNK translocation was less than previously reported (Hanawa et al., 2008), this may be due to the considerably lower dose of APAP used. However, no mitochondrial translocation of P-JNK was found in Hq mice (Fig. 5A). In addition, APAP-induced AIF, Smac, endonuclease G, and cytochrome c release into the cytosol in WT animals. Although the mitochondrial release of AIF and in part of Smac was eliminated, the release of endonuclease G was only moderately attenuated in Hq mice. The cytochrome c release was not significantly altered (Fig. 5A). In contrast to P-JNK, APAP-mediated mitochondrial bax translocation was similar in both WT and Hq mice (Fig. 5A). Nuclear DNA fragmentation after APAP overdose involves mitochondria-derived AIF and endonuclease G (Bajt et al., 2006). To investigate a potential impact of AIF-deficiency on nuclear DNA fragmentation, DNA strand breaks (TUNEL assay) and DNA fragments in the cytosol (antihistone ELISA) were analyzed. The TUNEL assay showed extensive DNA damage in cells around the central vein in WT animals (Fig. 5B). The TUNEL-positive cells correlated well with cells that stained positive for NT protein adducts (Fig. 4B) and were considered necrotic by H&E staining (Fig. 2A). Nuclear DNA fragmentation was also supported by the approximately fourfold increase in cytosolic DNA fragments (Fig. 5C). Hq mice had significantly less DNA damage as demonstrated by both TUNEL assay and the antihistone ELISA (Figs. 5B and 5C).
FIG. 5.
Translocation of cytosolic proteins to mitochondria, release of mitochondrial intermembrane proteins, and nuclear DNA fragmentation in WT and AIF-deficient (Hq) mice. (A) Western blot analysis of P-JNK and Bax in the mitochondria and AIF, endonuclease G, Smac, and cytochrome c in the cytosol. DNA strandbreaks (TUNEL Assay) (B), and DNA fragmentation (antihistone ELISA) (C) were determined. Samples were obtained from WT and AIF-deficient (Hq) mice either untreated (C) or 6 h after treatment with 200 mg/kg APAP. Representative animals (n = 3 per group) are shown for the western blots and representative tissue sections are shown for the TUNEL assay. DNA fragmentation data represent means ± SE of six animals per group. *p < 0.05 (compared with controls), **p < 0.05 (compared with WT/APAP).
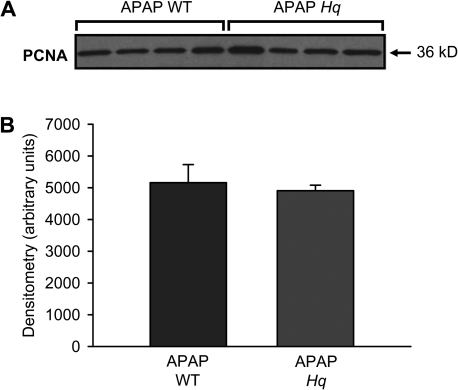
To rule out that tissue damage in Hq mice was not just delayed, liver injury and nuclear DNA fragmentation were evaluated in WT and Hq mice at 24 h after APAP (Fig. 6). The severe liver injury in WT animals was significantly reduced in Hq mice when judged by plasma ALT activities, area of necrosis, and by the TUNEL assay (Figs. 6A and 6B). However, the protection was not quite as effective as at 6 h suggesting that both a protection and a delay in injury were responsible for the eliminated early tissue damage. To determine if liver regeneration played a role in the protection seen in Hq mice, levels of the cell proliferation marker PCNA were evaluated in livers of untreated controls and at 24 h after APAP treatment. No PCNA was detectable in untreated animals (data not shown). In contrast, substantial PCNA expression was observed at 24 h after APAP treatment (Fig. 7). However, there was no significant difference between WT and Hq mice.
FIG. 6.
APAP-induced liver injury in WT and AIF-deficient (Hq) mice. DNA strand breaks (TUNEL assay) (A) and plasma ALT activities and the area of necrosis (B) were determined as indicators of APAP-induced liver injury in untreated controls (C) or 24 h after administration of 200 mg/kg AAP. Data represent means ± SE of five animals per group. *p < 0.05 (compared with controls), **p < 0.05 (compared with APAP/WT).
Fig. 7.
APAP-induced PCNA expression in WT and AIF-deficient (Hq) mice. (A) Western blot analysis of PCNA levels in liver homogenates from WT and Hq mice 24 h after treatment with 200 mg/kg APAP. (B) Densitometric analysis of the PCNA western blot. Data represents mean ± SE of four animals per group.
DISCUSSION
The objective of the current investigation was to assess the pathophysiological function of AIF in APAP hepatotoxicity. Our data clearly demonstrate that the lower AIF levels in Hq mice resulted in a dramatic reduction in liver injury at 6 and 24 h after APAP overdose in vivo. Thus, AIF plays a critical role in the mechanisms of APAP-induced liver injury.
Metabolic Activation of APAP in Hq Mice
Metabolic activation by the P450 system is an essential initiating event in the development of APAP hepatotoxicity (Nelson, 1990). The identical depletion kinetics of GSH, which is caused by NAPQI formation during the first 20 min after APAP administration (Jaeschke, 1990; Jaeschke et al., 2011), suggested that there was no difference in reactive metabolite formation between WT and Hq mice. This conclusion was further supported by the fact that both WT and Hq mice expressed similar levels of cyp2E1, which is the dominant cyp- activation APAP in mice and in humans (Gonzalez, 2007). Thus, the substantial protection against APAP hepatotoxicity observed in Hq mice was not caused by inhibition of NAPQI formation, the most upstream event in the pathophysiology.
AIF and Nuclear DNA Fragmentation
Our previous report demonstrated the release of mitochondrial intermembrane proteins including cytochrome c, second mitochondrial activator of caspases (Smac), endonuclease G, and AIF after APAP exposure (Bajt et al., 2006). However, only endonuclease G and AIF translocated to the nucleus. Furthermore, preventing mitochondrial damage by scavenging NAPQI or reactive oxygen and peroxynitrite protected the mitochondria and eliminated the release of intermembrane proteins (Bajt et al., 2006; Cover et al., 2005). Because it is well established that nuclear AIF is involved in large-scale DNA fragmentation and chromatin condensation (Boujrad et al., 2007), our first hypothesis focused on the endonuclease activity of AIF. The extensive reduction of nuclear DNA degradation as shown by the TUNEL assay (DNA strand breaks) and the antihistone ELISA (DNA fragments in the cytosol) supports the hypothesis that AIF is a critical mediator of nuclear DNA fragmentation during APAP hepatotoxicity. However, the observation that the APAP-induced mitochondrial oxidant stress was almost eliminated in Hq mice suggests that the protection might be upstream of the nuclear events.
An interesting observation of the study was that bax translocation to the mitochondria was not affected in the AIF-deficient mice. Bax can be found in mitochondria within 1 h after APAP treatment (Bajt et al., 2008). Although the initial bax activation may be in part caused by JNK activation, which is dependent on the early oxidant stress (Hanawa et al., 2008; Saito et al., 2010), the findings suggest that bax activation is independent of the AIF-controlled mitochondrial oxidant stress (discussed later). In addition, the mitochondrial bax pore can allow the release of intermembrane proteins such as AIF, Smac, endonuclease G, and cytochrome c (Bajt et al., 2008). However, AIF requires proteolytic cleavage before it can be released (Norberg et al., 2010). This requirement and the much lower levels of AIF in Hq mice may be the reason for the elimination of AIF release but only a moderate or no effect on endonuclease G and cytochrome c. This observation suggests that AIF has an impact on nuclear DNA damage either alone or in combination with endonuclease G.
AIF and Mitochondrial Oxidant Stress
A surprising finding of this investigation was the dramatic reduction of the mitochondrial oxidant stress in APAP-treated Hq mice. This conclusion is based on the measurement of three different parameters of oxidant stress and peroxynitrite formation. Elevated levels of GSSG, which is generated mainly inside the mitochondria after APAP (Jaeschke, 1990; Knight et al., 2001; Yan et al., 2010), mitochondrial protein carbonyls (Ramachandran et al., 2011b), and NT protein adducts (Hinson et al., 1998), which are also formed mainly in mitochondria (Cover et al., 2005), were observed after APAP in WT animals but not in Hq mice. These findings suggest that ROS formation is prevented in Hq mice rather than mitochondrial ROS being scavenged. This would indicate that AIF is involved in the formation of mitochondrial ROS after APAP overdose. The observation that the translocation of P-JNK to the mitochondria is prevented in Hq mice provides further support for this conclusion. APAP-induced JNK activation and translocation to the mitochondria is caused mainly by an early mitochondrial oxidant stress (Hanawa et al., 2008; Saito et al., 2010). In turn, the mitochondrial P-JNK translocation further promotes the mitochondrial oxidant stress (Saito et al., 2010). Based on these data, it can be concluded that partial AIF-deficiency in Hq mice clearly affects the APAP-induced generation of ROS in liver mitochondria.
It is now widely recognized that AIF has vital functions in regulating mitochondrial morphology and energy metabolism (Joza et al., 2009; Norberg et al., 2010; Sevrioukova, 2011). AIF is involved in the assembly of complex I of the respiratory chain and has NADH oxidase activity (Miramar et al., 2001; Vahsen et al., 2004). AIF deficiency in Hq mice results in reduced complex I protein expression and activity in mitochondria of the brain and retina but not in the liver or heart (Vahsen et al., 2004). The reduced oxidative phosphorylation capacity in brain and retina may contribute to neurodegeneration and blindness in older Hq animals (Klein et al., 2002). In terms of ROS formation, there are conflicting results. AIF deficiency has been shown to enhance mitochondrial ROS formation in Hep3B cells (Apostolova et al., 2006). However, liver and muscle mitochondria from mice with liver- or muscle-specific AIF deletion showed increased coupling of oxidative phosphorylation and no change in basal ROS formation (Pospisilik et al., 2007). Interestingly, brain mitochondria from Hq mice, which had a 50% reduction of complex I activity, did not show a difference in basal or complex I inhibitor–stimulated ROS production compared with mitochondria from WT animals (Chinta et al., 2009). However, ROS formation due to reversed electron transfer from complex II was inhibited by 50% in Hq mitochondria (Chinta et al., 2009). Although it remains to be investigated if the same mechanisms apply to liver mitochondria from Hq mice where complex I activity is not affected, these data suggest that AIF deficiency can attenuate ROS formation in mitochondria under certain stress conditions such as APAP-induced hepatotoxicity. It appears that AIF deficiency either impairs electron leakage at complex I, the most likely site of ROS formation in vivo (Murphy, 2009), or the electron input at this site is reduced. In support of the latter hypothesis, it was demonstrated that mitochondria from AIF-deleted muscle or liver prefer noncomplex I electron donors (Pospisilik et al., 2007).
Early promotion of regeneration after drug-induced liver injury can limit liver damage and accelerate recovery (Mehendale, 2005). However, based on the expression of PCNA, AIF-deficient Hq mice did not show evidence for increased regeneration compared with WT mice after APAP overdose. This suggests that the protection against APAP in Hq mice was unlikely caused by an effect on tissue repair processes.
In summary, our data demonstrated a profound protective effect of partial AIF deficiency against APAP hepatotoxicity. This protection correlated with a drastic reduction of mitochondrial ROS and peroxynitrite formation, suggesting that AIF is involved in ROS leakage of the electron transport chain in hepatocytes. In addition, AIF is involved in the nuclear DNA fragmentation. Given the critical role of mitochondrial oxidant stress and peroxynitrite in the formation of the MPT and cell necrosis, AIF could be a promising therapeutic target to limit APAP-induced liver injury.
FUNDING
National Institutes of Health (R01 DK070195, R01 AA12916 to H.J.); National Center for Research Resources (P20 RR016475, P20 RR021940), a component of the National Institutes of Health.
References
- Apostolova N, Cervera AM, Victor VM, Cadenas S, Sanjuan-Pla A, Alvarez-Barrientos A, Esplugues JV, McCreath KJ. Loss of apoptosis-inducing factor leads to an increase in reactive oxygen species, and an impairment of respiration that can be reversed by antioxidants. Cell Death Differ. 2006;13:354–357. doi: 10.1038/sj.cdd.4401776. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–2619. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- Brown D, Yu BD, Joza N, Bénit P, Meneses J, Firpo M, Rustin P, Penninger JM, Martin GR. Loss of Aif function causes cell death in the mouse embryo, but the temporal progression of patterning is normal. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9918–9923. doi: 10.1073/pnas.0603950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Rane A, Yadava N, Andersen JK, Nicholls DG, Polster BM. Reactive oxygen species regulation by AIF- and complex I-depleted brain mitochondria. Free Radic. Biol. Med. 2009;46:939–947. doi: 10.1016/j.freeradbiomed.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab. Dispos. 2007;35:1–8. doi: 10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem. Res. Toxicol. 1998;11:604–607. doi: 10.1021/tx9800349. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Knight T R, Bajt ML. The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicol. Lett. 2003;144:279–288. doi: 10.1016/s0378-4274(03)00239-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity—a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: not just an apoptosis-inducing factor. Ann. N.Y. Acad. Sci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Mehendale HM. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol. Pathol. 2005;33:41–51. doi: 10.1080/01926230590881808. [DOI] [PubMed] [Google Scholar]
- Miramar MD, Costantini P, Ravagnan L, Saraiva LM, Haouzi D, Brothers G, Penninger JM, Peleato ML, Kroemer G, Susin SA. NADH oxidase activity of mitochondrial apoptosis-inducing factor. J. Biol. Chem. 2001;276:16391–16398. doi: 10.1074/jbc.M010498200. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Norberg E, Orrenius S, Zhivotovsky B. Mitochondrial regulation of cell death: processing of apoptosis-inducing factor (AIF) Biochem. Biophys. Res. Commun. 2010;396:95–100. doi: 10.1016/j.bbrc.2010.02.163. [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic. Res. 2011a;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2011b;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova IF. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid. Redox Signal. 2011;14:2545–2579. doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca(2+)-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol. Appl. Pharmacol. 1992;112:32–40. doi: 10.1016/0041-008x(92)90276-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Vahsen N, Candé C, Brière JJ, Bénit P, Joza N, Larochette N, Mastroberardino PG, Pequignot MO, Casares N, Lazar V, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HM, Ramachandran A, Bajt ML, Lemasters JJ, Jaeschke H. The oxygen tension modulates acetaminophen-induced mitochondrial oxidant stress and cell injury in cultured hepatocytes. Toxicol. Sci. 2010;117:515–523. doi: 10.1093/toxsci/kfq208. [DOI] [PMC free article] [PubMed] [Google Scholar]