Abstract
The loss of tuberin, the tuberous sclerosis-2 (Tsc-2) gene product, is associated with cytoplasmic mislocalization of p27 in uterine leiomyomas derived from Eker rats (Tsc-2EK/+) and in human metastatic renal cell carcinoma tissue. Signaling associated with cytoplasmic mislocalization of p27 in renal cancer is relatively unknown. Renal tumors derived from 2,3,5-tris-(glutathion-S-yl)hydroquinone (TGHQ)-treated Tsc-2EK/+ rats, and null for tuberin, display elevated nuclear and cytosolic p27, with parallel increases in cytosolic cyclin D1 levels. Similar changes are observed in TGHQ-transformed renal epithelial cells derived from Tsc-2EK/+ rats (QTRRE cells), which, in addition to the cytoplasmic mislocalization of p27 and cyclin D1, exhibit high ERK, B-Raf, and Raf-1 kinase activity. Renal tumor xenografts, derived from subcutaneous injection of QTRRE cells into nude mice, also display increases in cytosolic mislocalization of p27 and cyclin D1. Dibutyryl cAMP and/or phosphodiesterase inhibitors (PIs; pentoxifylline or theophylline) increase Rap1B activation, B-Raf kinase activity, and cytosolic p27/cyclin D1 protein levels in QTRRE cells. Inhibition of Raf kinases with either sorafenib or B-Raf small interfering RNA (siRNA) caused a mitogen-activated protein kinase–mediated downregulation of p27. Moreover, decreases in cyclin D1 were also associated with p27 siRNA knockdown in QTRRE cells. Finally, theophylline-mediated increases in p27 and cyclin D1 were attenuated by sorafenib, which modulated Raf/MEK/ERK signaling. Collectively, these data suggest that the cAMP/Rap1B/B-Raf pathway modulates the expression of p27 and the cytoplasmic mislocalization of p27-cyclin D1 in tuberous sclerosis gene–regulated-renal cancer. Therefore, the loss of tuberin and engagement of the cAMP pathway may independently direct p27-cyclin D1 cytosolic stabilization during renal tumor formation.
Keywords: B-raf, p27, cAMP, cyclin D1, renal cell carcinoma, quinol-thioether
Tuberous sclerosis complex (TSC) is an autosomal dominant hereditary disease, with a high rate of spontaneous mutations in Tsc-1 and Tsc-2 genes (Kida et al., 2005). Mutations in TSC genes occur during human renal tumor formation (Bernstein and Robbins, 1991; Bjornsson et al., 1996), and loss of heterozygosity (LOH) at the Tsc-2 locus occurs in renal tumors of TSC and sporadic renal cell carcinoma (RCC) patients (Carbonara et al., 1996; Henske et al., 1995). The tumor suppressor tuberin, the protein product of Tsc-2, can affect subcellular localization and protein accumulation of the cyclin-dependent kinase inhibitor p27 (kip) (Rosner and Hengstschlager, 2004; Short et al., 2008). Tuberin also complexes with p27 to promote its nuclear localization (Rosner et al., 2007) and inhibit its ubiquitin-mediated degradation by Skp1/cullin/F-box (SCF) proteosome (Rosner et al., 2008; Rosner and Hengstschlager, 2004).
Nuclear p27 typically associates with distinct cyclin-dependent kinase (CDK)-cyclin complexes to initiate cell cycle arrest. Complex interactions between CDKs, cyclins, and p27 govern the G1-S phase transition of the cell cycle. During the G1 checkpoint, D-type cyclins (D1, D2, and D3) bind and activate CDK4/6 (Malumbres and Barbacid, 2009). Due to the instability of the cyclin D1-CDK4 complex, p27 interaction is necessary for complex assembly and stabilization (Cheng et al., 1999; Sherr and Roberts, 1999). Thus, p27 is an extremely versatile CDK inhibitor, in that it can regulate nuclear cell cycle exit and proliferation (Blain, 2008; Vervoorts and Luscher, 2008). Nuclear p27 is typically characterized as a tumor suppressor, but recent developments have revealed the duality of p27 as an oncogene in certain cancers, subsequent to its cytoplasmic mislocalization.
In human kidney, breast, colon, ovarian, thyroid, and esophageal cancers, cytoplasmic mislocalization of p27 correlates with an aggressive tumor type and poor prognosis (Alkarain et al., 2004; Ciaparrone et al., 1998; Hennenlotter et al., 2008; Masciullo et al., 2000; Motti et al., 2005; Pantuck et al., 2007; Singh et al., 1998; Viglietto et al., 2002). Analysis of Tsc2 null uterine leiomyomas from the Eker rat (Tsc-2EK/+), Tsc2−/− mouse embryonic fibroblasts (MEFs), and microscopic kidney lesions in Tsc2+/−/p27+/+ mice and Tsc2+/−/p27+/− mice, all revealed cytoplasmic mislocalization of p27 (Short et al., 2008). The molecular mechanisms facilitating the cytoplasmic retention of p27, and its ability to evade targeted degradation, remain complex. Degradation of p27 is necessary for the G1 to S phase transition to occur and is initiated by phosphorylation of p27 by cyclin E- and A-CDK2, translocating p27 to the cytoplasm for SCF E3 ubiquitin ligation (Carrano et al., 1999; Sheaff et al., 1997; Sutterluty et al., 1999; Vlach et al., 1997). Binding of p27 to cyclin D-CDK4 complexes protects p27 from degradation by the SCFSKP E3-ubiquitin-ligase complex (Kamura et al., 2004). As p27Kip1 has never been found unbound in vivo (Vervoorts and Luscher, 2008) and cytoplasmic p27-cyclin D-CDK4 complexes have intact kinase activity (Blain et al., 1997; James et al., 2008), an oncogenic function of p27 may reside in its maintenance of constitutive p27-cyclin D-CDK4 activity. Cyclin D1 is overexpressed in a variety of cancers (Kim and Diehl, 2009). Moreover, in tuberin null renal tumors and renal cells derived from Tsc-2EK/+ rats, the loss of tuberin expression is associated with an increase in cyclin D1 protein levels (Soucek et al., 1997; Yoon et al., 2002) and with an increase in cyclin D1 gene expression (Patel et al., 2003).
The signaling driving p27 expression, localization, and stabilization in RCC is relatively unknown. In a variety of cell types, the cyclic adenosine monophosphate (cAMP) pathway is associated with increased levels of p27 (Alderson and Hama, 2009; Bond et al., 2008; da Silva et al., 2008; Paris et al., 2006). In retinal, schwannoma, and neuronal cells, the cAMP mediated increase in p27 is also linked to cell cycle exit and differentiation (Alderson and Hama, 2009; da Silva et al., 2008; Paris et al., 2006). Increases in cAMP also mediate activation of Rap1 GTPase-activating protein 1 (Rap1) and subsequent downstream activation of B-Raf (Garcia et al., 2001; Grewal et al., 2000; Vossler et al., 1997). Rap activation of ERK is dependent on the expression levels of B-Raf. In cell lines lacking B-Raf expression, Rap1 inhibits Raf-1/ERK activation (Dugan et al., 1999; Okada et al., 1998; Schmitt and Stork, 2001). These data suggest that cAMP signaling can regulate p27 expression and Rap-GTP/B-Raf activation of the mitogen-activated protein kinase (MAPK) cascade.
In the present study, we examined the relationship between cAMP, the B-Raf/ERK MAPK cascade, and p27 in tuberin-deficient renal tumor models: 2,3,5-tris-(glutathion-S-yl)hydroquinone (TGHQ)-induced renal tumors in Tsc-2EK/+rats, TGHQ-transformed Tsc-2EK/+ cells (QTRRE), and QTRRE renal tumor xenografts in nude mice. Our data identify cAMP as a modulator of Rap/B-Raf activity, B-Raf MAPK expression of p27, and p27-cyclin D1 cytosolic mislocalization in tuberous sclerosis renal carcinogenesis.
MATERIALS AND METHODS
Cell cultures
Tuberin-negative quinol-thioether-transformed rat renal epithelial (QTRRE-2, QTRRE-3) cells were established from primary renal epithelial cells (Yoon et al., 2001). Human kidney cells (HK2) and LLC-PK1 (porcine proximal tubule epithelial cells) were obtained from the American Type Culture Collection (Manassas, VA). HK2 and QTRRE cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (1:1) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS). LLC-PK1 cells were maintained in DMEM supplemented with 10% FBS. Cells were grown at 37°C in a humidified atmosphere of 5% CO2.
Renal tumor xenograft nude mice
QTRRE-2 and QTRRE-3 cell tumorigenicity in athymic National Cancer Institute female nude mice was performed as described previously (Patel et al., 2003). Briefly, 5- to 6-week-old mice were purchased from Taconic (Hudson, NY) and were maintained on a 12-h light/dark cycle and allowed food and water ad libitum. The animals were divided into three groups (n = 2 per group). Once QTRRE-2 or -3 cells reached log phase growth stage in culture, they were harvested and resuspended in matrigel (BD Biosciences, San Jose, CA). The animals were injected subcutaneously at one site/animal with 5 × 106 cells/0.2 ml matrigel per site. When the size of a tumor reached 10–15 mm in diameter, the mice were euthanized, and sections of tumor were fixed in 10% phosphate-buffered formalin and embedded in paraffin or snap frozen in liquid nitrogen and stored at −80°C.
Animal dosing and tissue preparation
Male Eker rats (wild-type, Tsc-2+/+, and mutant, Tsc-2EK/+), 8 weeks old, were obtained from the University of Texas MD Anderson Cancer Center, Smithville, TX. The animals were housed according to a 12-h light/dark cycle and allowed food and water ad libitum. TGHQ was synthesized as previously described and used at > 98% purity, as determined by high performance liquid chromatography (Lau et al., 1988). The rats were divided into four subgroups: (1) Tsc-2EK/+ control, (2) Tsc-2EK/+ TGHQ-treated, (3) Tsc-2+/+ control, and (4) Tsc-2+/+ TGHQ treated. The rats were administered TGHQ (2.5 μmol/kg in 0.5 ml of 1× PBS, ip) 5 days a week for 4 months, the dose increasing to 3.5 μmol/kg at 4 months for an additional 4 months, according to previously established protocol (Lau et al., 2001). Control rats were administered PBS only. The TGHQ dosing solution was prepared fresh in 1× PBS daily. The animals were euthanized by CO2 asphyxiation. For histological studies, a mid-sagital longitudinal section of the left kidneys was fixed in 10% phosphate-buffered formalin and paraffin embedded. For biochemical assays, the outer stripe of the outer medulla (OSOM, regions from which tumors were derived), cortex, and renal tumors were excised, frozen immediately in liquid nitrogen, and stored at −80°C.
Nuclear and cytoplasmic extraction
Fractionation of (1) renal tumor xenografts derived from subcutaneous injection of QTRRE-2 or -3 cells in nude mice, (2) kidney sections from vehicle-treated nude mice, (3) renal tumors from TGHQ-treated Tsc-2EK/+ rats, (4) OSOM dissected from vehicle-treated Tsc-2EK/+ rats, or (5) HK2 cells was performed using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL) according to the manufacturer's protocol. For the fractionation of QTRRE-3 or HK2 cells, cells were seeded at a density of 3 × 105 cells per well in six-well plates. At 80–90% confluency, cells were treated with 3.6mM pentoxifylline or 3.3mM theophylline for 24 h in DMEM/F12 with 10% FBS or 50μM sorafenib for 1.5 h in DMEM/F12 with 2% FBS. Following drug treatments, cells were harvested with trypsin and washed with PBS. Cell fractionation was performed according to the manufacturer's protocol.
Western blot analysis
The OSOM of TGHQ or vehicle-treated Tsc-2EK/+ and Tsc-2+/+ rats, TGHQ-Tsc-2EK/+ rat renal tumors or QTRRE mouse xenograft tumors, and QTRRE-3 or HK2 cells were homogenized with 100 μl ice-cold lysis buffer (Cell Signaling Technology, Inc., Beverly, MA) containing 1mM Pefabloc SC (Roche) and Complete Protease Inhibitor Cocktail tablets (Roche). Protein lysates were then subjected to heat in a boiling water bath for 5 min prior to loading into 10 or 12% gels and were then separated via SDS-polyacrylamide gel electrophoresis (PAGE) with 10% resolving gels and 4% acrylamide stacking gels. Proteins were then transferred to polyvinylidene difluoride (PVDF; Bio-Rad Laboratories, Hercules, CA) membranes. Membranes were blocked with 5% milk. Primary antibodies used were cyclin D1 (A-12), B-Raf (H-145), Raf-1 (C-20), SP1 (E3), p27 (F-8), Rap1B (Santa Cruz Biotechnologies); p42/44, phospho-p42/44 (T202/Y204) (20G11) (Cell Signaling Technologies); and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Ambion, Austin, TX). The secondary immunoglobulin conjugated with horseradish peroxidase (Santa Cruz Biotechnology, CA) was used at a 1:3000 dilution. The blots were visualized with Amersham ECLTM Western Blotting Detection Reagents (GE Healthcare, UK) and protein expression was determined by densitometric analysis with protein expression normalized to GADPH.
Rap1 activation assay
Rap1 activation was determined as described (van Triest and Bos, 2004). The GST-Ral GDS-RBD fusion protein was isolated from Escherichia coli strain BL21. A 10% suspension of glutathione-agarose beads was pre-coupled to 100 μl of cleared GST-Ral GDS-RBD lysate for 1 h on a tumbler at 4°C. HK2 cells and QTRRE-3 cells were treated with 3.6mM pentoxifylline (Sigma) for 24 h. Total cell lysates were isolated using Cell Lysis Buffer (Cell Signaling Technology, Inc., Beverly, MA). For each sample, equal quantities of total cell lysate were incubated with the GST-Ral GDS-RBD protein and glutathione-agarose beads slurry for 1.5 h on a tumbler at 4°C. After coupling, beads were washed 4 times with Cell Lysis Buffer and bound proteins were eluted in 15 μl of XT Sample Buffer (Bio-Rad). Precipitates were subjected to 12% SDS-PAGE followed by transfer onto PVDF membranes, which were subsequently incubated overnight with a 1:1000 dilution of Rap1B (Santa Cruz Biotechnology), then washed and incubated with 1:3000 dilution of goat immunoglobulin conjugated with horseradish peroxidase (Santa Cruz Biotechnology, CA). The blots were visualized with Amersham ECLTM Western Blotting Detection Reagents (GE Healthcare, UK).
B-Raf and Raf-1 kinase activity assay
At 80–90% confluency, QTRRE cells were treated with 3.6mM pentoxifylline or 3.3mM theophylline for 24 h in DMEM/F12 with 10% FBS. Cells were lysed with Cell Lysis Buffer, as described above, and 500 μg of total cell lysate was immunoprecipitated using B-Raf and Raf-1 polyclonal antibodies (Santa Cruz Biotechnology, CA) bound to protein A/G-agarose beads (Pierce Biotechnology Inc., IL). Kinase activity of the immunoprecipitates was determined using B-Raf or Raf-1 Kinase Cascade Assay Kits (Upstate Biotechnology) as previously reported (Yoon et al., 2004) and according to the manufacturer’s protocol. The incorporated [γ-33P]ATP was quantified by liquid scintillation spectroscopy.
Immunostaining
QTRRE cells grown on glass coverslips were treated with 3.6mM pentoxifylline or 3.3mM theophylline for 24 h in DMEM/F12 with 10% FBS. Cells were fixed with 4% paraformaldehyde, permeabilized with acetone, and blocked with 10% goat serum in 1% BSA/PBS. Cells were incubated with anti-p27 (Santa Cruz Biotechnology, CA) overnight at 4°C, followed by incubation with secondary Alexa 546 goat anti-mouse IgG (Molecular Probes, Eugene, OR) and TO-PRO-3 (Invitrogen) for 1 h at room temperature (RT). Cells were mounted with vectashield (Vector Laboratories, Burlingame, CA). The slides were imaged using a 60× water immersion plan-apochromat objective on an LSM 510 multiphoton/confocal laser-scanning microscope (Carl Zeiss, Inc., Thornwood, NY).
siRNA transfection
QTRRE cells were transfected at 50–60% confluence. For QTRRE transfection, 100nM B-Raf, Raf-1, p27, or cyclin D1 ON_TARGETplus SMARTpool siRNA, or siCONTROL Non-Targeting siRNA #5 (Dharmacon RNA Technologies, NY) was combined with serum-free DMEM/F12 media (100 μl) and incubated for 5 min at RT. In parallel, DharmaFECT #2 (5 μl) was incubated in serum-free DMEM/F12 (200 μl) for 5 min at RT. Small interfering RNA (siRNA) solution (100 μl) was then combined with the DharmaFECT #2 solution (200 μl) and incubated for 20 min at RT. The siRNA-DharmaFECT complex solution was added directly to each well, mixed gently, and incubated for 24, 48, 72, or 96 h at 37°C in a CO2 incubator.
Statistics
Data are expressed as means ± SD. Mean values were compared using a Student’s t-test, two tails, with unequal variances.
RESULTS
TGHQ Increases p27 Levels in Tsc-2+/+ and Tsc-2EK/+ Rats and in QTRRE Tumor Xenografts from Nude Mice
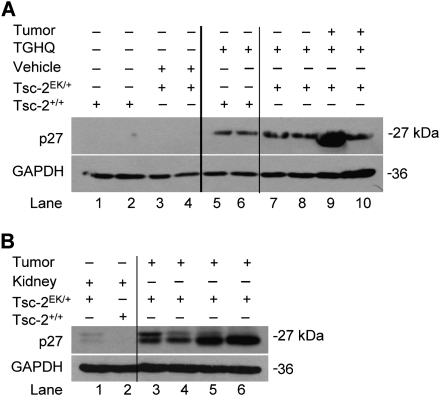
Tsc-2EK/+ rats carry a single autosomal mutation on one allele of the tuberous sclerosis tumor suppressor gene that predisposes these rats to the development of spontaneous renal cell tumors, and at a very high incidence (Eker et al., 1981; Kobayashi et al., 1995; Yeung et al., 1994). The majority of renal cell tumors in Tsc-2EK/+ rats originate from within renal proximal tubules, and these tumors are histologically similar to renal tumors in humans (Everitt et al., 1992; Walker, 1998). Western blot analysis was performed on the kidney lysates obtained from the OSOM region of the Tsc-2EK/+ and Tsc-2+/+ rats treated for 8 months with TGHQ or vehicle. Densitometric analysis of p27 expression, normalized to GADPH, revealed that neither group exhibit detectable levels of p27 (Fig. 1A lanes 1–4). Interestingly, TGHQ treatment alone was sufficient to induce a substantial increase in p27 in the OSOM of 8-month Tsc-2+/+ rats (Fig. 1A lanes 5–6). An increase in p27 protein levels was observed within the OSOM of TGHQ-Tsc-2EK/+ rats treated with TGHQ for 8 months (Fig. 1A lane 7–8), and a substantial increase was seen in one of the renal tumors (Fig. 1A lane 9). Similarly, kidneys of vehicle-treated nude mice did not display detectable levels of p27 (Fig. 1B lane 2), but kidneys of nude mice with subcutaneous renal tumor xenografts showed a slight increase in p27 (Fig. 1B lane 1). Renal tumor xenografts derived from QTRRE cell lines express elevated levels of p27 (Fig. 1B lanes 3–6).
FIG. 1.
Increased p27 levels in TGHQ-treated Tsc-2+/+ and Tsc-2EK/+ rats and QTRRE tumor xenografts in nude mice. (A) Whole tissue lysates from the OSOM or renal tumors of vehicle- or TGHQ-treated Tsc-2+/+ and Tsc-2EK/+ rats were immunoblotted for p27 and GAPDH. (B) Whole tissue lysates from the kidneys of nude mice treated with vehicle (lane 2) or with subcutaneous renal tumor xenografts (lane 1) and whole tissue lysates generated from renal tumor xenografts derived by subcutaneous injection of QTRRE cells in nude mice (lanes 3–6) were immunoblotted for p27 and GAPDH. Every other lane represents a duplicate animal from the same treatment.
Loss of Tsc2 Correlates with Cytoplasmic Localization of p27 and Cyclin D1 in Tsc2 Null Cells and Renal Tumors
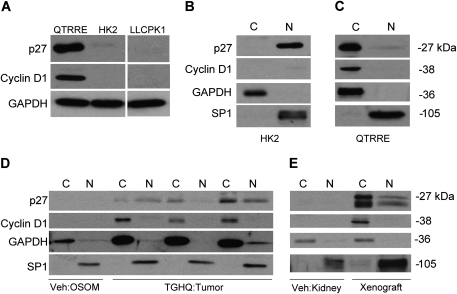
QTRRE cells, which are null for tuberin, express high levels of pERK1/2 (Yoon et al., 2004) and high levels of cyclin D1 and p27 relative to Tsc-2+/+ normal human kidney cells (HK2) or Tsc-2+/+ porcine LLC-PK1 proximal tubule cells (Fig. 2A). We have previously shown that LLC-PK1 cells are an appropriate control model when compared with the QTRRE cells, with respect to the relevant signaling pathways following Tsc-2 mutation (Yoon et al., 2004). We analyzed the localization pattern of p27 in HK2 and QTRRE cells. Nuclear-cytoplasmic extraction of HK2 cells revealed nuclear localization of p27 and nondetectable levels of cyclin D1 (Fig. 2B), whereas in tumorigenic QTRRE cells, p27 and cyclin D1 were primarily localized to the cytosolic fraction (Fig. 2C). We next examined the cytosolic localization of p27 and cyclin D1 in renal tumors from TGHQ-treated Tsc-2EK/+ rats and in renal tumor xenografts formed following subcutaneous injection of QTRRE cells in nude mice. Nuclear-cytoplasmic extraction of the OSOM from vehicle-treated Tsc-2EK/+ rats did not display detectable levels of p27 or cyclin D1 (Fig. 2D) in either fraction. In contrast, renal tumors derived from Tsc-2EK/+ rats treated with TGHQ for 8 months revealed an increase in nuclear and cytoplasmic p27 (Fig. 2D). The cytoplasmic localization of p27 correlated with increased levels of cytosolic cyclin D1 (Fig. 2D). Similarly, renal tumor xenografts derived from QTRRE cells in nude mice expressed elevated levels of cytosolic and nuclear p27 (Fig. 2E) and an increase in cytoplasmic cyclin D1 (Fig. 2E). Kidneys from vehicle-treated nude mice did not display detectable levels of p27 or cyclin D1 (Fig. 2E lane 2) in either fraction.
FIG. 2.
Cytoplasmic localization of p27 in tuberin-null renal tumors. (A) Whole cell lysates from QTRRE, HK2, and LLC-PK1 cells were immunoblotted for p27, cyclin D1, and GAPDH. Cytosolic [C] and nuclear [N] lysates were generated from (B) HK2 cells, (C) QTRRE cells, (D) kidney OSOM of 8-month vehicle-treated Tsc-2EK/+ rats or tumors from 8-month TGHQ-treated Tsc-2EK/+ rats, and (E) vehicle-treated kidneys of nude mice or renal tumor xenografts derived by subcutaneous injection of QTRRE cells in nude mice. Samples were immunoblotted for p27, cyclin D1, GAPDH (cytosolic fractionation control), and SP1 (nuclear fractionation control).
cAMP-Mediated Rap1 and B-Raf Activation
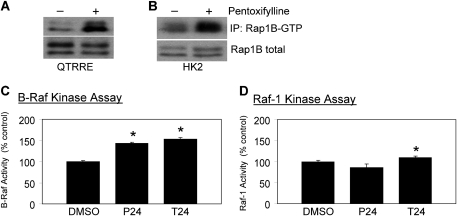
Increases in cAMP have been implicated in the activation of Rap1 and in the subsequent downstream activation of B-Raf in rat neuronal cells and in human acute megakaryoblastic leukemia cells (Garcia et al., 2001; Grewal et al., 2000; Vossler et al., 1997). The influence of cAMP on Rap1 activation in QTRRE and HK2 cells was therefore examined, and consistent with other cell models, Rap1B-GTP was elevated in both QTRRE and HK2 cells following treatments that increase cAMP concentrations (Figs. 3A and 3B).
FIG. 3.
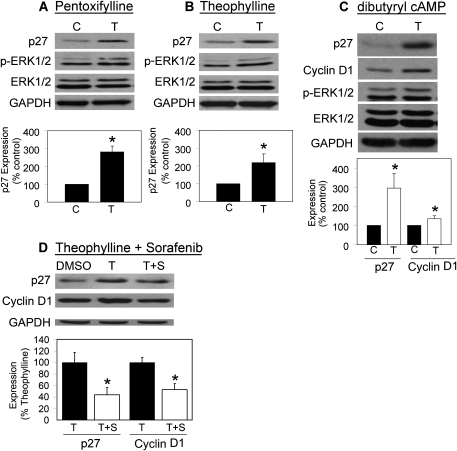
Phosphodiesterase inhibition activates Rap1 and B-Raf in QTRRE cells. (A) QTRRE or (B) HK2 cells were treated with pentoxifylline (3.6mM) for 24 h. Rap1B-GTP was precipitated with GST-Ral GDS-RBD bound to glutathione-sepharose beads from 500 μg of QTRRE or HK2 total cell lysate, and after removal of the beads, identified with a Rap1B antibody following SDS-PAGE and Western blotting. Rap1B immunoblots of the same total cell lysates were performed to confirm equal expression of total Rap1B between DMSO- and pentoxifylline-treated lysates. (C) and (D), QTRRE cells were treated with pentoxifylline (P, 3.6mM), theophylline (T, 3.3mM), or DMSO for 24 h. Equal amounts of lysates were subjected to (C) B-Raf kinase activity assay or (D) Raf-1 kinase activity assay. B-Raf and Raf-1 activity were determined as described in “Materials and Methods”. Values represent the mean ± SD (n = 3). A significant difference was seen between DMSO and pentoxifylline or theophylline treatments in QTRRE cells at *p < 0.05.
Rap1 can bind both B-Raf and Raf-1, but Rap1 binding results in activation of B-Raf and inhibition of Raf-1 (Dugan et al., 1999; Garcia et al., 2001; Okada et al., 1998; Peyssonnaux and Eychene, 2001; Vossler et al., 1997). B-Raf (Fig. 3C) and Raf-1 (Fig. 3D) activation by cAMP in QTRRE cells was determined by kinase-specific activity assays following phosphodiesterase inhibition with pentoxifylline or theophylline. Pentoxifylline increased B-Raf kinase activity by approximately 40% (Fig. 3C) but did not result in a statistically significant change in Raf-1 kinase activity (Fig. 3D). Similarly, cAMP preservation with theophylline resulted in a 50% increase in B-Raf kinase activity (Fig. 3C), and a nominal 9% increase in Raf-1 kinase activity (Fig. 3D).
B-Raf Regulates p27 Protein Levels
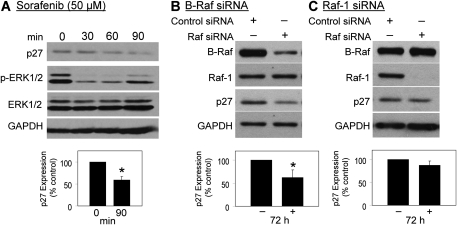
To investigate whether Raf kinases contribute to high p27 protein levels, p27 was determined by Western blotting following treatment of QTRRE cells with the Raf kinase inhibitor sorafenib (50μM). Sorafenib caused a time-dependent decrease in p27, with a 40% reduction by 90 min. There is a sustained decrease in pERK1/2 between 30 and 60 min with recovery beginning to occur around 90 min (Fig. 4A). The recovery of ERK phosphorylation is likely due to several factors, including changes in the protein stability via binding of p27 to cyclin D1 influencing the kinetics of ERK phosphorylation. Thus, although ERK phosphorylation appears to be recovering, the propagation of this signaling event may still be manifest.
FIG. 4.
Effect of sorafenib and Raf siRNA on p27 expression in QTRRE cells. (A) QTRRE cells were treated with sorafenib (50μM) for 30, 60, and 90 min. QTRRE cells were transfected with (B) 100nM B-Raf or (C) Raf-1 ON_TARGETplus SMARTpool siRNA or siCONTROL Non-Targeting siRNA #5 (Dharmacon RNA Technologies, NY) for 72 h. Whole cell lysates were subjected to Western blotting with antibodies specific to p27, pERK1/2, ERK1/2, and GAPDH. Values represent the mean ± SD (n = 3). A significant difference in p27 protein levels was seen between control (DMSO) and sorafenib or B-Raf siRNA–treated cells at *p < 0.01.
Similar to most FDA approved small molecule inhibitors, sorafenib is not selective for its primary target, Raf kinases; in a kinase-binding assay, sorafenib bound 10% of 384 kinases tested, with affinities within tenfold of that for Raf kinases (Karaman et al., 2008). Therefore, to confirm, as well as identify which Raf isoform is responsible for MAPK regulation of p27 protein levels, QTRRE cells were transfected with B-Raf and Raf-1 siRNA. Real-time PCR analysis of both Raf isoforms, following 48 h siRNA treatment, revealed an approximate 95% decrease in Raf messenger RNA (mRNA) levels (data not shown). Western blot analysis revealed a 75% knockdown B-Raf concomitant with a 40% decrease in p27 protein levels (Fig. 4B). In contrast, almost complete knockdown of Raf-1 had no effect on p27 levels (Fig. 4C). Both Raf siRNA’s were target specific, and neither Raf siRNA produced a significant change in GAPDH protein expression. These data, combined with the increase in Rap/B-Raf activation following cAMP stimulation in QTRRE cells (Fig. 3), prompted us to subsequently determine the connection between cAMP signaling and p27 expression.
cAMP Signaling Regulates Expression and Cytoplasmic Localization of p27
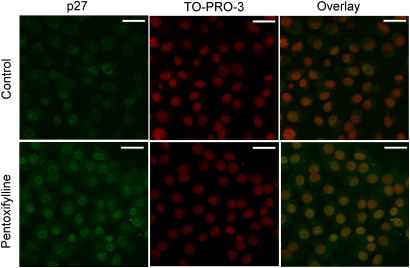
cAMP regulates p27 protein levels in a variety of cell types (Alderson and Hama, 2009; Bond et al., 2008; da Silva et al., 2008; Kowalczyk et al., 2009; Paris et al., 2006). To examine whether cAMP modulation influences p27 protein levels in QTRRE cells, we probed for p27 and p-ERK1/2 following treatment with phosphodiesterase inhibitors. Protein levels of p27 increased 2.8- and 2.2-fold following incubation with pentoxifylline (Fig. 5A) or theophylline (Fig. 5B), respectively, with an accompanying increase in the higher molecular weight band of p-ERK1/2. Pentoxifylline mediated increases in p27 were confirmed by confocal microscopy (Fig. 6). To investigate whether cAMP treatment can directly increase p27 protein levels, QTRRE cells were treated with dibutyryl cAMP for 19 h, which produced a 2.9-fold increase in p27 protein levels and a slight increase in the upper molecular weight band of p-ERK (Fig. 5C). Incubation with dibutyryl cAMP also caused a 1.4-fold increase in cyclin D1 (Fig. 5C). Finally, sorafenib attenuated increased p27 and cyclin D1 levels after theophylline pretreatment by 57 and 48%, respectively (Fig. 5D).
FIG. 5.
Phosphodiesterase inhibition and dibutyryl cAMP regulates p27 expression and protein stability in QTRRE cells. QTRRE cells were treated with (A) pentoxifylline (3.6mM), (B) theophylline (3.3mM), (C) dibutyryl cAMP (1 mM), or (D) theophylline (3.3mM) for 24 h followed by sorafenib (50μM) for 90 mins. Cells treated with vehicle (DMSO or medium) served as controls. Equivalent whole cell lysates were subjected to Western blotting with antibodies specific to p27, cyclin D1, pERK1/2, ERK1/2, or GAPDH. Values represent the mean ± SD (n = 3) for pentoxifylline and theophylline treatments and the mean ± SD (n = 4) for dibutyryl cAMP. A significant difference in p27 and/or cyclin D1 protein levels was seen between DMSO (C) and pentoxifylline, theophylline, or dibutyryl cAMP treatments (T) in QTRRE cells at *p < 0.05.
FIG. 6.
Effect of pentoxifylline on p27 protein levels in QTRRE cells. QTRRE cells treated with pentoxifylline for 24 h were examined by immunocytochemistry with an anti-p27 antibody, TO-PRO-3 to detect nuclei, and an overlay of the two stains. Immunofluorescence was detected by confocal microscopy with a 60× water immersion plan-apochromat objective; white scale bars are 20 microns.
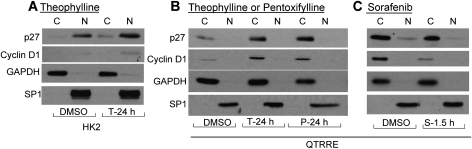
To evaluate the effect of cAMP on the subcellular compartmental localization of p27, nuclear-cytoplasmic extraction of wild-type HK2 cells and tumorigenic QTRRE cells was performed. Treatment of HK2 cells with theophylline resulted in an increase in nuclear and cytosolic p27 and a modest increase in nuclear cyclin D1 (Fig. 7A). In contrast to HK2 cells, in QTRRE cells, pentoxifylline or theophylline caused an increase in cytosolic p27 (Fig. 7B). Furthermore, phosphodiesterase inhibition produced a corresponding increase in cytosolic cyclin D1 (Fig. 7B). Sorafenib treatment resulted in undetectable levels of nuclear p27 and a concomitant 25% decrease in cytosolic p27 (Fig. 7C) that corresponded with a 50% decreases in cytosolic cyclin D1 (Fig. 7C) as determined by densitometric analysis with protein expression normalized to GADPH.
FIG. 7.
Cytoplasmic mislocalization of p27 and cyclin D1 modulated by cAMP-MAPK signaling. (A) cytosolic [C] and nuclear [N] lysates were generated from HK2 cells treated with theophylline (3.3mM) or DMSO for 24 h. (B) and (C) cytosolic [C] and nuclear [N] lysates were generated from QTRRE cells treated with (B) theophylline (3.3mM), pentoxifylline (3.6mM), or DMSO for 24 h or (C) sorafenib (50μM) or DMSO for 1.5 h. Equal amounts of extracted lysates were subjected to SDS-PAGE and immunoblotting with antibodies specific to p27, cyclin D1, GAPDH (cytosolic fractionation control), or SP1 (nuclear fractionation control).
Cytoplasmic Stabilization of Cylcin D1 Is Codependent on p27 Cytosolic Relocalization
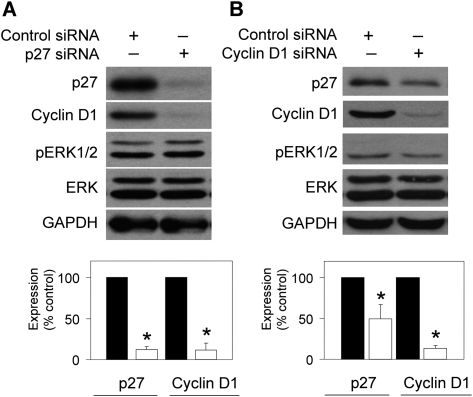
In MEFs, lack of p27 expression is associated with decreased cyclin D1 protein levels (Cheng et al., 1999). Furthermore, p27 deficiency results in cyclin D1-CDK4 complex instability (Cheng et al., 1999; Sherr and Roberts, 1999). To investigate which protein is a necessary component of p27-cyclin D1 cytosolic relocalization and stabilization, we knocked down p27 or cyclin D1 with siRNA. Following a 48-h treatment with p27 siRNA in QTRRE cells, there was an equivalent 88% decrease in both p27 and cyclin D1 protein levels (Fig. 8A); similarly, a 48-h treatment of cyclin D1 siRNA resulted in a 50% decrease in p27 protein levels (Fig. 8B).
FIG. 8.
p27 stabilizes cyclin D1 in the cytoplasm of QTRRE cells. QTRRE cells were transfected with (A) 100nM p27 ON_TARGETplus SMARTpool siRNA, (B) 100nM cyclin D1 ON_TARGETplus SMARTpool siRNA or siCONTROL Non-Targeting siRNA #5 (Dharmacon RNA Technologies, NY) for 48 h. Whole cell lysates were subjected to Western blotting with antibodies specific to p27, cyclin D1, pERK1/2, ERK1/2, and GAPDH. Values are means ± SD (n = 3). A significant difference was seen between control siRNA and p27 siRNA–treated cells at *p < 0.01.
DISCUSSION
We have established that modulation of B-Raf kinase activity directly alters p27 protein levels and promotes the cytoplasmic relocalization of p27 in TGHQ-mediated tuberous sclerosis RCC. The cytoplasmic mislocalization and increased expression of both p27 and cyclin D1 were documented in (1) tuberin null renal tumors from TGHQ-treated Tsc-2EK/+ rats, (2) renal tumor xenografts derived by the subcutaneous injection of QTRRE (null of tuberin) cells in nude mice (Figs. 1 and 2), and (3) in tumorigenic QTRRE cells (compared to HK2 and LLC-PK1 cells) (Fig. 2). Moreover, treatment of Tsc-2+/+ rats for 8 months with TGHQ was sufficient to induce increased p27 expression within the OSOM (Fig. 1). Modulation of cAMP and MAPK cascades revealed that cAMP activation of Rap-GTP/B-Raf (Fig. 3) mediates increased p27 protein expression (Figs. 4–6) and its mislocalization to the cytoplasm (Fig. 7) in tuberin null QTRRE and human HK2 cells (Fig. 7). Finally, we established that p27-cyclin D1 complex formation stabilizes the proteins within the cytosol of QTRRE cells; siRNA targeting either p27 or cyclin D1 in QTRRE cells resulted in a substantial decrease in both proteins (Fig. 8). Similarly, manipulation of p27 protein levels in QTRRE cells by phosphodiesterase inhibitors (Figs. 5A and 5B and 7B) and dibutyryl cAMP (Fig. 5C) caused parallel increases in p27 and cyclin D1. Sorafenib caused parallel decreases in p27 and cyclin D1 in the absence (Figs. 4A and 7C) or presence of theophylline-enhanced cAMP effects (Fig. 5D).
Tuberin null renal tumors derived from Eker rats (Tsc-2EK/+) (Yoon et al., 2002), tuberin null QTRRE cells [44] and renal tumor xenografts derived from tuberin null QTRRE cells, all display cytoplasmic mislocalization of p27, consistent with reports that Tsc2 null uterine leiomyomas and Tsc2−/− MEFs display cytoplasmic localization of p27 (Short et al., 2008). Tuberin has a dual function in the regulation of p27, regulating both its nuclear localization and stability. Tuberin complex formation with p27 inhibits 14-3-3-mediated cytoplasmic retention of p27 and promotes nuclear localization of p27 (Rosner et al., 2007). Complex formation also stabilizes p27 protein levels by inhibiting degradation of p27 by the SCF-type E3 ubiquitin proteosome (Rosner and Hengstschlager, 2004). Consequently, loss of tuberin expression should increase degradation of p27. However, in contrast, the loss of tuberin in QTRRE cells and in renal tumors derived from TGHQ-treated Tsc-2EK/+ rats produced an unanticipated increase in total p27 protein levels. We therefore suggest that p27-cyclin D1 complex formation in the cytoplasm protects p27 from targeted degradation by the SCF proteosome. The localization and degradation of p27 is complex and highly dependent on its phosphorylation status at a number of key residues (Vervoorts and Luscher, 2008). For example, in Go, phosphorylation on either S10 by dual specificity tyrosine phosphorylation–regulated kinase 1B (DYRK1B/MIRK) or T198 by AKT or ribosomal protein S6 kinase alpha-1/2 (RSK1/2) stabilizes p27 in the nucleus (Besson et al., 2006; Deng et al., 2004; Kossatz et al., 2006; Kotake et al., 2005). In G1, modification of p27 by AKT or RSK1/2 on T198 also allows for p27-cyclin D-CDK complex formation in the cytosol (Fujita et al., 2002, 2003; Kossatz et al., 2006). The modulation of these phosphorylation sites may be critical in cytoplasmic p27-cyclin D1 complex formation in RCC and must be further explored.
Treatment of tuberin null QTRRE cells with phosphodiesterase inhibitors or dibutyryl cAMP resulted in a parallel increase in p27 and cyclin D1 protein levels, suggesting that cAMP signaling is a key regulator of p27 expression and stabilization (Fig. 5). cAMP is an effective inducer of p27 expression in a variety of cell types, including schwannoma, vascular smooth muscle, neural, and skin cells (Alderson and Hama, 2009; Bond et al., 2008; da Silva et al., 2008; Kowalczyk et al., 2009; Paris et al., 2006). cAMP also regulates p27 transcription in neuronal cells (Shin et al., 2009). Since phosphodiesterase inhibitors increased p27 levels in both tuberin negative and positive cells, tuberin status may be an independent factor in cAMP regulation of p27 expression. Furthermore, in neuronal progenitor, retinal, schwannoma, and skin cells, a cAMP mediated increase in p27 promoted differentiation and/or cell cycle exit (Alderson and Hama, 2009; da Silva et al., 2008; Kowalczyk et al., 2009; Paris et al., 2006). In contrast, increases in p27 in tuberin-deficient QTRRE cells did not result in cell cycle exit but rather an increase in the cytoplasmic p27/cyclin D1 complex. Therefore, concomitant increases in p27 and cyclin D1 suggest reciprocal protein stabilization. Moreover, p27 or cyclin D1 siRNA knockdown in QTRRE cells resulted in concomitant decreases in cyclin D1 or p27, respectively; which suggests increased protein stability via complex formation. When p27 is bound to cyclin D-CDK4 complexes, it is protected from degradation by the SCFSKP2 E3-ubiquitin-ligase complex (Kamura et al., 2004). Similarly, due to the instability of the cyclin D1-CDK4 complex, p27 interaction is necessary for complex assembly and stabilization (Cheng et al., 1999; Sherr and Roberts, 1999). Complexes of p27-cyclin D-CDK4 exhibit intact kinase activity (Blain et al., 1997; James et al., 2008) and may therefore play a crucial role in tumor formation.
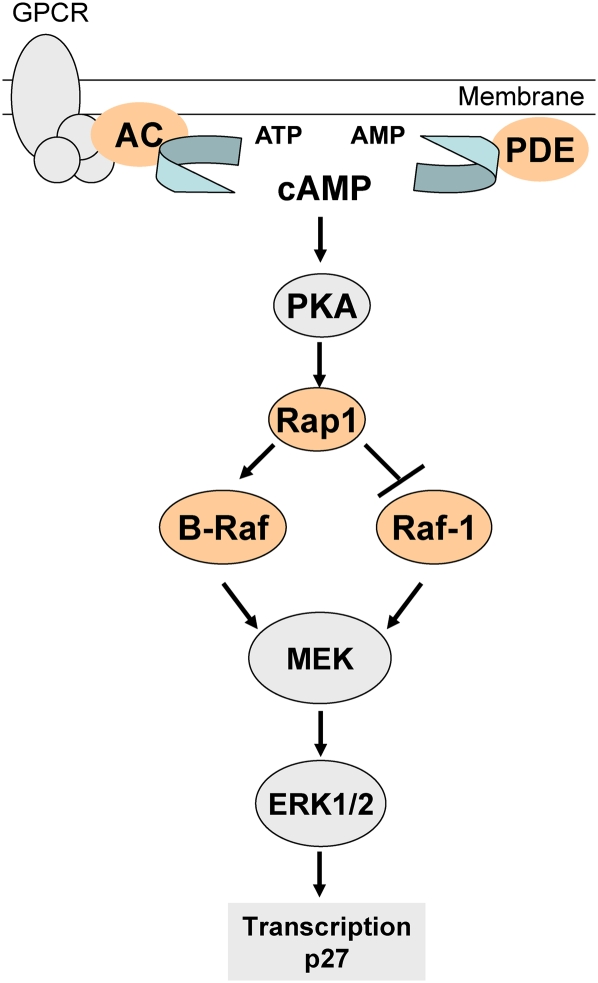
We also show that phosphodiesterase inhibitors activate Rap-GTP and B-Raf kinase activity but have negligible effects on Raf-1 kinase activity (Fig. 3). These data are consistent with findings that Rap activation of ERK is dependent on B-Raf expression (Dugan et al., 1999; Okada et al., 1998; Schmitt and Stork, 2001). In B-Raf-negative NIH 3T3 cells, cAMP inhibition of the Raf-1/ERK MAPK cascade is mediated through Rap1 (Dugan et al., 1999; Okada et al., 1998; Schmitt and Stork, 2001). Therefore, we propose that cAMP is modulating the transcriptional activation of p27 through the Rap/B-Raf MAPK cascade (Fig. 9). Furthermore, an almost complete knockdown of Raf-1 with siRNA did not result in a significant change in p27 protein levels (Fig. 4C); these data, in combination with the Rap-GTP/B-Raf kinase activity assay (Fig. 3), strongly suggest that cAMP is only activating the B-Raf MAPK cascade and not Raf-1. In tumorigenic QTRRE cells, Raf-1 regulates cyclin D1 protein translation through ERK cross talk with eukaryotic translation initiation factor 4E-binding protein-1 (4EBP1) (Cohen, Gard, Nagle, Dietrich, Monks, and Lau, unpublished data). In contrast, inhibition of B-Raf has far less of an effect on cyclin D1 as Raf-1 and has no effect on 4EBP1 phosphorylation. Moreover, Raf inhibition with sorafenib did not cause a decrease in cyclin D1 mRNA levels, suggesting that protein translation and/or stability may be responsible for the observed decreases in cyclin D1 protein levels (Cohen, Gard, Nagle, Dietrich, Monks, and Lau, unpublished data). Consequently, we suspect that B-Raf regulates p27 transcription, and the increase in p27 protein also involves complex formation with cyclin D1 thereby inhibiting p27 degradation. These data highlight cAMP as an effective regulator of B-Raf MAPK–mediated induction of p27 protein levels in a tuberous sclerosis renal tumor model (Fig. 9).
FIG. 9.
Rap1-GTP/B-Raf MAPK regulation of p27 in QTRRE cells. Adenylate cyclase (AC) converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP), and phosphodiesterases (PDE) revert cAMP to AMP. cAMP mediates protein kinase A (PKA) activation of Rap1. Rap1-GTP activates B-Raf kinase activity and inhibits Raf-1. B-Raf phosphorylates and activates the MEK/ERK MAPK cascade. ERK1/2 regulates transcription of p27.
The von Hoppel-Lindau (VHL) tumor suppressor gene regulates cell cycle arrest in a manner similar to Tsc-2, via stabilization of p27Kip1 (Pause et al., 1998). The VHL tumor suppressor gene is a primary target for mutations in human RCC (Gnarra et al., 1994; Shuin et al., 1994) but does not contribute to rodent renal tumorigenesis (Kikuchi et al., 1995; Walker et al., 1996). Tsc-2 is mutated in human (Wagner and Linehan, 1996; Yu et al., 1986) and rodent (Kobayashi et al., 1995, 1999; Onda et al., 1999; Yeung et al., 1994) RCC. Although Tsc-2 is the primary target for RCC in our Eker rat model, both Tsc-2 and VHL result in predominately clear cell RCC pathology and share converging signaling proteins, including p27. Recent developments have revealed the duality of p27 as both a tumor suppressor and as an oncogene in certain cancers (Sicinski et al., 2007). As a tumor suppressor, reduced p27Kip1 levels in primary cancers are highly correlated with decreased patient survival (Short et al., 2008; Sicinski et al., 2007). This duality of p27 is also displayed in RCC. Over 370 human RCC tissues were immunostained with p27 to determine its subcellular localization; staining identified nuclear p27 in 78% of the tumors and cytoplasmic localization in 46% of the tumors (Pantuck et al., 2007). Furthermore, cytoplasmic p27 staining was higher in metastatic RCC and was identified as a predictor of disease-specific survival time, with cytoplasmic mislocalization predicting a poor prognosis (Pantuck et al., 2007). Therefore, the induction of p27 expression may have a variable outcome depending on the tumor grade.
Cytoplasmic mislocalization of p27 occurs in a variety of human cancers that correlate with a high tumor grade and poor survival rate (Alkarain et al., 2004; Ciaparrone et al., 1998; Hennenlotter et al., 2008; Masciullo et al., 2000; Motti et al., 2005; Pantuck et al., 2007; Singh et al., 1998; Viglietto et al., 2002). Depending on the tissue specificity and pathology of the tumor, p27 expression may be either low in the total cell lysate or elevated in the cytosol due to cytoplasmic localization (Short et al., 2008; Sicinski et al., 2007). Recently, transformed keratinocytes treated with phosphodiesterase inhibitors increased their sensitivity to growth inhibition by glucocorticoids (Kowalczyk et al., 2009). The use of phosphodiesterase inhibitors as a method to induce p27 expression, and ultimately cell cycle exit, may be a useful tool to treat low-grade tumors but may exacerbate high-grade tumor formation. Therefore, further characterization of the relationship between cAMP pathway activation and p27 cytoplasmic localization will shed light on the role of p27 in renal tumor formation.
Our data identified B-Raf MAPK as an effective modulator of p27 expression, which furthers our understanding of the mechanisms of action of sorafenib in treating human RCC. Studies performed in human RCC 786-0 and Caki-1 cells suggest that neither VHL status nor the mammalian target of rapamycin (mTOR) cascade play a direct role in the cytoplasmic relocalization p27 (Kim et al., 2009). Therefore, we suspect that the B-Raf/MAPK cascade is the primary pathway regulating p27 protein expression and cytoplasmic stabilization. Finally, the correlation between the cytoplasmic localization of p27 and an aggressive tumor type during human RCC highlights the necessity for an appropriate rodent model mirroring these characteristics. Our tuberin-deficient renal tumor models not only have similar clear cell pathology but also display cytoplasmic p27-cyclin D1, which makes it an ideal model to further characterize p27 as a predictive biomarker in RCC.
FUNDING
National Institutes of Health (GM039338 to S.S.L.); National Institutes of Environmental Health Sciences, Southwest Environmental Health Sciences Center (P30ES006694 to S.S.L.); National Institute of Environmental Health Sciences Training Grants (T32ES007091 to J.D.C., and T32ES016652 to N.J.M.).
Acknowledgments
We are grateful to J.L. Bos (University of Utrecht, the Netherlands for his generous gift of the GST-RalGDS construct, and Drs Laurence Hurley and Justin Dietrich, Division of Medicinal Chemistry, for providing us with sorafenib to use in our experiments. We thank Mr Christopher Kuhlman for his assistance with preparation of figures. The Zeiss LSM 510 Meta confocal microscope was provided by the Arizona Research Laboratories, Division of Biotechnology, Imaging Facility. We acknowledge the assistance with the image capture provided by Mr Douglas Cromey, of the Southwest Environmental Health Sciences Center Cellular Imaging Facility Core.
References
- Alderson NL, Hama H. Fatty acid 2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T schwannoma cells. J. Lipid Res. 2009;50:1203–1208. doi: 10.1194/jlr.M800666-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkarain A, Jordan R, Slingerland J. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J. Mammary Gland Biol. Neoplasia. 2004;9:67–80. doi: 10.1023/B:JOMG.0000023589.00994.5e. [DOI] [PubMed] [Google Scholar]
- Bernstein J, Robbins TO. Renal involvement in tuberous sclerosis. Ann. N. Y. Acad. Sci. 1991;615:36–49. doi: 10.1111/j.1749-6632.1991.tb37746.x. [DOI] [PubMed] [Google Scholar]
- Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson J, Short MP, Kwiatkowski DJ, Henske EP. Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features. Am. J. Pathol. 1996;149:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- Blain SW. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle. 2008;7:892–898. doi: 10.4161/cc.7.7.5637. [DOI] [PubMed] [Google Scholar]
- Blain SW, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin A-Cdk2 and cyclin D2-Cdk4. J. Biol. Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- Bond M, Wu YJ, Sala-Newby GB, Newby AC. Rho GTPase, Rac1, regulates Skp2 levels, vascular smooth muscle cell proliferation, and intima formation in vitro and in vivo. Cardiovasc. Res. 2008;80:290–298. doi: 10.1093/cvr/cvn188. [DOI] [PubMed] [Google Scholar]
- Carbonara C, Longa L, Grosso E, Mazzucco G, Borrone C, Garre ML, Brisigotti M, Filippi G, Scabar A, Giannotti A, et al. Apparent preferential loss of heterozygosity at TSC2 over TSC1 chromosomal region in tuberous sclerosis hamartomas. Genes Chromosomes Cancer. 1996;15:18–25. doi: 10.1002/(SICI)1098-2264(199601)15:1<18::AID-GCC3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB. Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res. 1998;58:114–122. [PubMed] [Google Scholar]
- da Silva AG, Campello-Costa P, Linden R, Sholl-Franco A. Interleukin-4 blocks proliferation of retinal progenitor cells and increases rod photoreceptor differentiation through distinct signaling pathways. J. Neuroimmunol. 2008;196:82–93. doi: 10.1016/j.jneuroim.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Deng X, Mercer SE, Shah S, Ewton DZ, Friedman E. The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G(0) by Mirk/dyrk1B kinase. J. Biol. Chem. 2004;279:22498–22504. doi: 10.1074/jbc.M400479200. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Kim JS, Zhang Y, Bart RD, Sun Y, Holtzman DM, Gutmann DH. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J. Biol. Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- Eker R, Mossige J, Johannessen JV, Aars H. Hereditary renal adenomas and adenocarcinomas in rats. Diagn. Histopathol. 1981;4:99–110. [PubMed] [Google Scholar]
- Everitt JI, Goldsworthy TL, Wolf DC, Walker CL. Hereditary renal cell carcinoma in the Eker rat: a rodent familial cancer syndrome. J. Urol. 1992;148:1932–1936. doi: 10.1016/s0022-5347(17)37087-8. [DOI] [PubMed] [Google Scholar]
- Fujita N, Sato S, Katayama K, Tsuruo T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 2002;277:28706–28713. doi: 10.1074/jbc.M203668200. [DOI] [PubMed] [Google Scholar]
- Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 2003;278:49254–49260. doi: 10.1074/jbc.M306614200. [DOI] [PubMed] [Google Scholar]
- Garcia J, de Gunzburg J, Eychene A, Gisselbrecht S, Porteu F. Thrombopoietin-mediated sustained activation of extracellular signal-regulated kinase in UT7-Mpl cells requires both Ras-Raf-1- and Rap1-B-Raf-dependent pathways. Mol. Cell Biol. 2001;21:2659–2670. doi: 10.1128/MCB.21.8.2659-2670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat. Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Horgan AM, York RD, Withers GS, Banker GA, Stork PJ. Neuronal calcium activates a Rap1 and B-Raf signaling pathway via the cyclic adenosine monophosphate-dependent protein kinase. J. Biol. Chem. 2000;275:3722–3728. doi: 10.1074/jbc.275.5.3722. [DOI] [PubMed] [Google Scholar]
- Hennenlotter J, Ohneseit PA, Simon P, Merseburger AS, Serth J, Kuehs U, Kramer M, Hartmann JT, Stenzl A, Kuczyk MA. PTEN and p27Kip1 are not downregulated in the majority of renal cell carcinomas–implications for Akt activation. Oncol. Rep. 2008;19:1141–1147. [PubMed] [Google Scholar]
- Henske EP, Neumann HP, Scheithauer BW, Herbst EW, Short MP, Kwiatkowski DJ. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995;13:295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- James MK, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol. Cell Biol. 2008;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, Yoshida M, Nakayama K, Nakayama KI. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat. Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Kida Y, Yamaguchi K, Suzuki H, Kanda E, Ando M, Ohashi K, Funata N, Saito H. Tuberous sclerosis, associated with renal cell carcinoma and angiomyolipoma, in a patient who developed endstage renal failure after nephrectomy. Clin. Exp. Nephrol. 2005;9:179–182. doi: 10.1007/s10157-005-0356-9. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Kobayashi E, Nishizawa M, Hamazaki S, Okada S, Hino O. Cloning of the rat homologue of the von Hippel-Lindau tumor suppressor gene and its non-somatic mutation in rat renal cell carcinomas. Jpn. J. Cancer Res. 1995;86:905–909. doi: 10.1111/j.1349-7006.1995.tb02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jonasch E, Alexander A, Short JD, Cai S, Wen S, Tsavachidou D, Tamboli P, Czerniak BA, Do KA, et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin. Cancer Res. 2009;15:81–90. doi: 10.1158/1078-0432.CCR-08-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J. Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat. Genet. 1995;9:70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- Kossatz U, Vervoorts J, Nickeleit I, Sundberg HA, Arthur JS, Manns MP, Malek NP. C-terminal phosphorylation controls the stability and function of p27kip1. EMBO J. 2006;25:5159–5170. doi: 10.1038/sj.emboj.7601388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y, Nakayama K, Ishida N, Nakayama KI. Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J. Biol. Chem. 2005;280:1095–1102. doi: 10.1074/jbc.M406117200. [DOI] [PubMed] [Google Scholar]
- Kowalczyk P, Kinjo T, Kowalczyk M, Walaszek Z, Hanausek M, Slaga TJ. Effect of phosphodiesterase antagonists on glucocorticoid mediated growth inhibition in murine skin cell lines. Eur. J. Pharmacol. 2009;610:29–36. doi: 10.1016/j.ejphar.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Lau SS, Fang M, Wong JW. Effects of composting process and fly ash amendment on phytotoxicity of sewage sludge. Arch. Environ. Contam. Toxicol. 2001;40:184–191. doi: 10.1007/s002440010162. [DOI] [PubMed] [Google Scholar]
- Lau SS, Hill BA, Highet RJ, Monks TJ. Sequential oxidation and glutathione addition to 1,4-benzoquinone: correlation of toxicity with increased glutathione substitution. Mol. Pharmacol. 1988;34:829–836. [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Masciullo V, Ferrandina G, Pucci B, Fanfani F, Lovergine S, Palazzo J, Zannoni G, Mancuso S, Scambia G, Giordano A. p27Kip1 expression is associated with clinical outcome in advanced epithelial ovarian cancer: multivariate analysis. Clin. Cancer Res. 2000;6:4816–4822. [PubMed] [Google Scholar]
- Motti ML, Califano D, Troncone G, De Marco C, Migliaccio I, Palmieri E, Pezzullo L, Palombini L, Fusco A, Viglietto G. Complex regulation of the cyclin-dependent kinase inhibitor p27kip1 in thyroid cancer cells by the PI3K/AKT pathway: regulation of p27kip1 expression and localization. Am. J. Pathol. 2005;166:737–749. doi: 10.1016/S0002-9440(10)62295-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Matsuda M, Anafi M, Pawson T, Pessin JE. Insulin regulates the dynamic balance between Ras and Rap1 signaling by coordinating the assembly states of the Grb2-SOS and CrkII-C3G complexes. EMBO J. 1998;17:2554–2565. doi: 10.1093/emboj/17.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/-) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantuck AJ, Seligson DB, Klatte T, Yu H, Leppert JT, Moore L, O'Toole T, Gibbons J, Belldegrun AS, Figlin RA. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- Paris M, Wang WH, Shin MH, Franklin DS, Andrisani OM. Homeodomain transcription factor Phox2a, via cyclic AMP-mediated activation, induces p27Kip1 transcription, coordinating neural progenitor cell cycle exit and differentiation. Mol. Cell Biol. 2006;26:8826–8839. doi: 10.1128/MCB.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Ma N, Monks TJ, Lau SS. Changes in gene expression during chemical-induced nephrocarcinogenicity in the Eker rat. Mol. Carcinog. 2003;38:141–154. doi: 10.1002/mc.10153. [DOI] [PubMed] [Google Scholar]
- Pause A, Lee S, Lonergan KM, Klausner RD. The von Hippel-Lindau tumor suppressor gene is required for cell cycle exit upon serum withdrawal. Proc. Natl. Acad. Sci. U S A. 1998;95:993–998. doi: 10.1073/pnas.95.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol. Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- Rosner M, Freilinger A, Hanneder M, Fujita N, Lubec G, Tsuruo T, Hengstschlager M. p27Kip1 localization depends on the tumor suppressor protein tuberin. Hum. Mol. Genet. 2007;16:1541–1556. doi: 10.1093/hmg/ddm103. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschlager M. Skp2 inversely correlates with p27 and tuberin in transformed cells. Amino Acids. 2009;37:257–262. doi: 10.1007/s00726-008-0141-7. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hengstschlager M. Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2. J. Biol. Chem. 2004;279:48707–48715. doi: 10.1074/jbc.M405528200. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJ. Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1. Mol. Cell Biol. 2001;21:3671–3683. doi: 10.1128/MCB.21.11.3671-3683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shin MH, Mavila N, Wang WH, Vega Alvarez S, Hall MC, Andrisani OM. Time-dependent activation of Phox2a by the cyclic AMP pathway modulates onset and duration of p27Kip1 transcription. Mol. Cell Biol. 2009;29:4878–4890. doi: 10.1128/MCB.01928-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short JD, Houston KD, Dere R, Cai SL, Kim J, Johnson CL, Broaddus RR, Shen J, Miyamoto S, Tamanoi F, et al. AMP-activated protein kinase signaling results in cytoplasmic sequestration of p27. Cancer Res. 2008;68:6496–6506. doi: 10.1158/0008-5472.CAN-07-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuin T, Kondo K, Torigoe S, Kishida T, Kubota Y, Hosaka M, Nagashima Y, Kitamura H, Latif F, Zbar B, et al. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54:2852–2855. [PubMed] [Google Scholar]
- Sicinski P, Zacharek S, Kim C. Duality of p27Kip1 function in tumorigenesis. Genes Dev. 2007;21:1703–1706. doi: 10.1101/gad.1583207. [DOI] [PubMed] [Google Scholar]
- Singh SP, Lipman J, Goldman H, Ellis FH, Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–1735. [PubMed] [Google Scholar]
- Soucek T, Pusch O, Wienecke R, DeClue JE, Hengstschlager M. Role of the tuberous sclerosis gene-2 product in cell cycle control. Loss of the tuberous sclerosis gene-2 induces quiescent cells to enter S phase. J. Biol. Chem. 1997;272:29301–29308. doi: 10.1074/jbc.272.46.29301. [DOI] [PubMed] [Google Scholar]
- Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- van Triest M, Bos JL. Pull-down assays for guanoside 5′-triphosphate-bound Ras-like guanosine 5′-triphosphatases. Methods Mol. Biol. 2004;250:97–102. doi: 10.1385/1-59259-671-1:97. [DOI] [PubMed] [Google Scholar]
- Vervoorts J, Luscher B. Post-translational regulation of the tumor suppressor p27(KIP1) Cell Mol. Life Sci. 2008;65:3255–3264. doi: 10.1007/s00018-008-8296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–5344. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Wagner JR, Linehan WM. Molecular genetics of renal cell carcinoma. Semin. Urol. Oncol. 1996;14:244–249. [PubMed] [Google Scholar]
- Walker C. Molecular genetics of renal carcinogenesis. Toxicol. Pathol. 1998;26:113–120. doi: 10.1177/019262339802600113. [DOI] [PubMed] [Google Scholar]
- Walker C, Ahn YT, Everitt J, Yuan X. Renal cell carcinoma development in the rat independent of alterations at the VHL gene locus. Mol. Carcinog. 1996;15:154–161. doi: 10.1002/(SICI)1098-2744(199602)15:2<154::AID-MC8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc. Natl. Acad. Sci. U S A. 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HS, Monks TJ, Everitt JI, Walker CL, Lau SS. Cell proliferation is insufficient, but loss of tuberin is necessary, for chemically induced nephrocarcinogenicity. Am. J. Physiol. Renal Physiol. 2002;283:F262–F270. doi: 10.1152/ajprenal.00261.2001. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Monks TJ, Walker CL, Lau SS. Transformation of kidney epithelial cells by a quinol thioether via inactivation of the tuberous sclerosis-2 tumor suppressor gene. Mol. Carcinog. 2001;31:37–45. doi: 10.1002/mc.1037. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Ramachandiran S, Chacko MA, Monks TJ, Lau SS. Tuberous sclerosis-2 tumor suppressor modulates ERK and B-Raf activity in transformed renal epithelial cells. Am. J. Physiol. Renal Physiol. 2004;286:F417–F424. doi: 10.1152/ajprenal.00234.2003. [DOI] [PubMed] [Google Scholar]
- Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J. Natl. Cancer Inst. 1986;77:351–356. [PubMed] [Google Scholar]